Highlights
-
•
Monosodium glutamate induced testicular damage due to oxidative stress.
-
•
Using of selenium and/or vitamin E to alleviate the toxicity of monosodium glutamate especially on testis.
-
•
Increasing of MDA levels in MSG treated group while reduction in SOD, CAT and GPx activities.
Abbreviations: MSG, monosodium glutamate; vit E, vitamin E; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdhyde; GPx, glutathione peroxidase
Keywords: Monosodium glutamate, Vitamin E, Selenium, Oxidative stress, Testis
Abstract
Monosodium glutamate (MSG) has been recognized as flavor enhancer that adversely affects male reproductive systems. The present study was carried out to evaluate the potential protective role of vitamin E (vit E) or selenium against MSG induced oxidative stress and histopathological changes in testis tissues of rats. Mature male Wistar rats weighing 150–200 g BW were allocated to evenly twelve groups each group of ten animals, the first group was maintained as control group, the 2nd, 3rd and 4th groups were administered MSG in three different dose levels (low, medium and high) (6, 17.5 and 60 mg/kg BW), the 5th and 6th groups were given vit E in two doses (low and high) (150 and 200 mg/kg), the 7th and 8th groups were administered selenium in two doses (low and high) (0.25 and 1 mg/kg) daily via gavage for a period of 30 days. Meanwhile the 9th and 10th groups were given combinations of MSG (high dose) and vit E while, the 11th and 12th groups were given MSG (high dose) plus selenium in two recommended doses for each one. Monosodium glutamate caused an elevation in lipid peroxidation level parallel with significant decline in SOD, CAT as well as GPx activities in testis tissues. Administration of vit E or selenium to MSG-treated groups declined lipid peroxidation, increased SOD, CAT, GPx activities. Selenium or vit E significantly reduced MSG induced histopathological changes by the entire restoration of the histological structures and the testicular antioxidant status to great extent in treated rats. In conclusion, supplementation of selenium or vit E could ameliorate the MSG induced testicular toxicity to great extent and reduce the oxidative stress on testis tissues.
1. Introduction
Monosodium glutamate (MSG), a white crystalline powder, is the sodium salt of a naturally occurring non-essential amino acid, glutamic acid [1]. MSG is commonly marketed as a flavor enhancer and is used as a food additive particularly in West African and Asian dishes [2]. Generally, MSG is accepted as a safe food additive that needs no specified average daily intake or an upper limit intake [3]. However, inadvertent abuse of this food additive may occur because of its abundance, mostly without labeling, in many food ingredients [4].
MSG – is the sodium salt of glutamic acid [5]. MSG contains 78% of glutamic acid, 22% of sodium and water [3]. Glutamate is one of the most common amino acids found in nature and is the main component of many proteins and peptides of most tissues. Glutamate is also produced in the body and plays an essential role in human metabolism. MSG is a widely used flavor enhancing food additive that may be present in packaged foods without appearing on the label. This flavor enhancer, not very long ago, was isolated in the laboratory, and identified as MSG. Modern commercial MSG is produced by fermentation of starch, sugar, beet sugarcane or molasses [6].
Some reports indicated that MSG was toxic to human and experimental animals [7], [8]. MSG could produce symptoms such as numbness, weakness, flushing, sweating, dizziness and headaches. In addition, ingestion of MSG has been alleged to cause or exacerbate numerous conditions, including asthma, urticaria, atopic dermatitis, ventricular arrhythmia, neuropathy and abdominal discomfort [9]. MSG has a toxic effect on the testis by causing a significant oligozoospermia and increases abnormal sperm morphology in a dose-dependent fashion in male Wistar rats [10]. It has been implicated in male infertility by causing testicular hemorrhage, degeneration and alteration of sperm cell population and morphology [11], [12], [13].
Antioxidants have been reported to play a significant role in the protection against lipid peroxidation. Vitamin E is antioxidants that are thought to have a protective effect by either reducing or preventing oxidative damage. Lipid soluble vit E prevents lipid peroxidation chain reactions in cellular membranes by interfering with the propagation of lipid radicals [14]. It is well known as non-enzymatic antioxidant [15], [16]. Vitamin E inhibits peroxidation of membrane lipids by scavenging lipid peroxyl radicals [17], [18], and it also inhibits oxidative damage in several tissues by heavy metals and pesticides in experimental animals [17], [19].
Selenium plays an important role in many biological processes and it can protect against a number of diseases. It reduced sperm abnormalities by chemicals [20]. Selenium (Se), is an important antioxidant nutrient, is essential for normal testicular development, spermatogenesis and spermatozoa motility and functions [21]. Ursini et al. [22] reported that Se supplementation in sub fertile men with low Se status could improve sperm motility and increase the chance of successful conception. Selenium has also been demonstrated to have the protective effects against the toxicity of metals in the male reproductive system of experimental animals [23]. In spite of some studies on the effects of Se on spermatogenesis on rodent testis, little is known about the effects of Se on testicular testosterone synthesis.
It has been suggested that both Se and vit E have a protective role in peroxide damage to the sperm cell [24].
In the last few years, fear had increased due to the adverse reactions and toxicity of MSG, with few and limited literature regarding the biochemical and histological studies of the damage in testis treatment of animals with MSG. So, the present study was designed to investigate the effects of MSG on the testicular tissue of mature male rats. In addition, to the best of our knowledge, no comprehensive study concerning the protective effect of vit E and Se on MSG-induced inhibition of spermatogenesis and exhibited spermatogenic arrest which may be one of the main reasons affecting on reproductive performance. The main objective of the present work is to study the effect of Se or vit E and their role in amelioration of the testicular toxicity induced by MSG and reduction of the oxidative stress on testis tissues which may improve the reproductive performance.
2. Materials and methods
2.1. Animals
This study was performed on 120 mature male Wistar rats, weighing about 150–200 g BW. Animals were obtained from the animal house of Faculty of Veterinary Medicine, Zagazig University in Egypt. They were breeding in a well-ventilated room with the temperature ranging between 22 and 25 °C and maintained under standardized conditions away from any stressful conditions with 12/12 light and dark cycle with free access to humidity and were fed dry balanced meal for experimental animals provided by the General Organization for Grain Silos and Flour Mills in Jeddah, with a constant source of water. All experimental procedures and animal maintenance were conducted in accordance with the accepted standards of animal care per cage (Council of Europe, European convention for the protection of vertebrate animals [25]). We have followed the European community Directive (86/609/EEC) and national rules on animal care. One group served as control. Animals were weighed and randomly allocated into 12 groups (10 rats each) as following.
2.2. Chemicals
Monosodium glutamate (C5H9NO4·Na) with purity 99% NT was sold in most open markets under the license of Ajinomoto Co. Inc., Tokyo, Japan. A stock solution was prepared by dissolving of 60 g of MSG crystals in 1000 ml of distilled water. The dose schedule was so adjusted that the amount of MSG administration per animal was as per their respective weight. Vitamin E was supplied by Merck (Germany) and selenium tablets were supplied by Wassen Company.
2.3. Experimental protocols
Rats were divided into twelve groups, each consisting of ten rats. Group 1 – control rats treated with 1 mg/kg BW corn oil per day; Group 2 – MSG – low dose treated rats (6 mg/g BW per day in distilled water) [26]; Group 3 – MSG – medium dose treated rats (17.5 mg/g BW per day in distilled water); Group 4 – MSG – high dose treated rats (60 mg/g BW per day in distilled water); Group 5 – vit E treated rats (low dose; 150 mg/kg BW per day in corn oil) [27]; Group 6 – vit E-treated rats (high dose; 200 mg/kg BW per day in corn oil) [28]; Group 7 – Se-treated rats (low dose; 0.25 mg/kg BW per day in distilled water) [29]; Group 8 – Se-treated rats (high dose; 1.0 mg/kg BW per day in distilled water). Group 9 – MSG (high dose; 60 mg/kg BW) plus vit E (low dose; 150 mg/kg BW per day, respectively); Group 10 – MSG was treated with high dose of MSG and vit E (high dose; 200 mg/kg BW per day, respectively); Group 11 – MSG (high dose of MSG with Se at low dose; 0.25 mg/kg BW per day); Group 12 – MSG; the animals in this group was treated with high dose of MSG and high dose of Se (1.0 mg/kg BW per day). The doses were administered in the morning (between 9.30 and 10.30 h) to non-fasted rats.
The first day, when the animals were treated was considered experimental day 0. At the end of the 30 days of treatment, all animals were scarified and dissected. The testis tissues were quickly processed for light microscope investigations and biochemical examinations.
2.4. Tissues homogenates preparation and estimation of antioxidant capacities parameters;
The excised testicular tissue was washed with distilled water for the removal of blood, and later the fatty parts were removed. Tissues were homogenized in ice-cold 50 mM sodium phosphate buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA). The supernatant was separated by means of centrifugation at 5000 rpm for 20 min at 4 °C. The supernatant was used for the analyses of all biochemical parameters.
2.4.1. Lipid peroxidation assay
TBARS content was evaluated by using the thiobarbituric acid (TBA) test as described by Ohkawa et al. [30]. After incubation of testis homogenates with TBA at 95 °C, TBARS reacts to form a colored complex. Absorbance was measured spectrophotometrically at 532 nm to determine the TBARS content. The level is expressed as nmol/mg protein.
2.4.2. Measurement of superoxide dismutase (SOD)
SOD activity was measured according to the method described according to Marklund and Marklund [31] by assaying the auto oxidation of pyrogallol at 440 nm for 3 min. One unit of SOD activity was calculated as the amount of protein that caused 50% pyrogallol autooxidation inhibition. A blank without homogenate was used as a control for non-enzymatic oxidation of pyrogallol in Tris–EDTA buffer (50 mM Tris, 10 mM EDTA, pH 8.2). The SOD activity is expressed as U/mg protein.
2.4.3. Measurement of catalase (CAT)
CAT activity was measured according to the method described by Aebi [32] by assaying the hydrolysis of H2O2 and the resulting decrease in absorbance at 240 nm over a 3 min period at 25 °C. Before determination of the CAT activity, samples were diluted 1:9 with 1% (v/v) Triton X-100. CAT activity is expressed as mmol/mg protein.
2.4.4. Measurement of glutathione peroxidase (GPx)
GPx activity was measured using H2O2 as substrate according to the method described by Paglia and Valentine [33]. The reaction was monitored indirectly as the oxidation rate of NADPH at 240 nm for 3 min. A blank without homogenate was used as a control for non-enzymatic oxidation of NADPH upon addition of hydrogen peroxide in 0.1 M Tris buffer, pH 8.0. Enzyme activity was expressed as nmol/mg protein.
2.5. Histopathology
For histopathological examination, testis tissues were dissected and fixed in neutral buffered formalin solution. Then samples were processed by using a graded ethanol series, and embedded in paraffin. The paraffin sections were cut into 5 μ-thick slices and stained with hematoxylin and eosin for histological examination [34].
2.6. Statistical analysis
Data were collected, arranged and reported as mean ± standard error of mean (SEM) of twelve groups, and then analyzed using the computer program (SPSS/version 15.0). The statistical method was one way analyzes of variance ANOVA test, and if significant differences between means were found, Duncan's multiple range test (Whose significant level was defined as P < 0.05) according to [35] to estimate the effect of different treated groups.
3. Results
3.1. Lipid peroxidation (MDA) levels
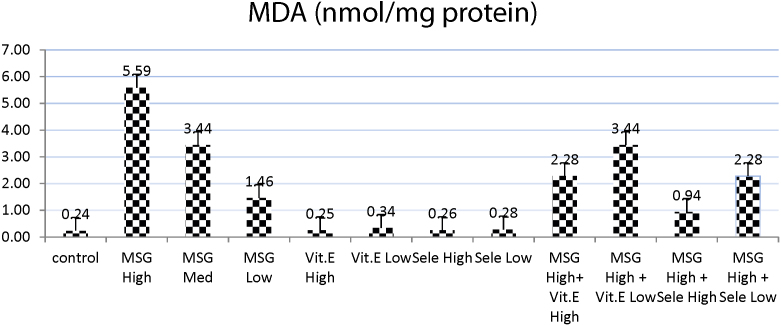
MDA level was significantly elevated in MSG (high dose) treated group as compared with normal control group, followed by significant increase in both MSG (medium and low dose treated groups) with respect to normal control group. Meanwhile, groups treated with MSG (high dose) co-administered with either vit E (High or low dose) afforded significant increase in MDA level when compared with normal control group, while elicited significant decrease when compared with MSG treated groups either (high, medium or low doses). The MSG (high dose) plus Se either at high or low dose afforded significant decrease in MDA level as compared with MSG-treated groups in three doses and these were the best ameliorative results that succeeded in decreasing MDA levels after treatment of rats with MSG co-administered with Se.
On the other hand, the Se-treated groups either in high or low dose elicited non-significant increase in MDA levels as compared to control group. Meanwhile, vit E (high dose) elicited a significant decrease in MDA level as compared to normal control group, while vit E (high dose) elicited non-significant changes in MDA level as compared to control group (Table 1 and Fig. 1).
Table 1.
Effects of monosodium glutamate (MSG), vitamin E, selenium and their combinations with MSG on antioxidant parameters capacities.
| Groups | MDA (nmol/mg protein) | CAT (mmol/mg protein) | SOD (U/mg protein) | GPx (nmol/mg protein) |
|---|---|---|---|---|
| (1) Control group | 0.24 ± 0.11f | 0.86 ± 0.12a | 9.35 ± 0.36ab | 5.67 ± 0.63ab |
| (2) MSG high dose | 5.42 ± 0.13a | 0.35 ± 0.10d | 2.63 ± 0.45h | 2.10 ± 0.85g |
| (3) MSG medium dose | 3.54 ± 0.14b | 0.24 ± 0.07f | 3.25 ± 0.22g | 3.25 ± 0.24f |
| (4) MSG low dose | 1.52 ± 0.07d | 0.45 ± 0.02e | 4.21 ± 0.11f | 3.75 ± 0.41f |
| (5) Vitamin E high dose | 0.25 ± 0.02f | 0.85 ± 0.23a | 9.20 ± 0.96b | 5.55 ± 0.74b |
| (6) Vitamin E low dose | 0.30 ± 0.05e | 0.81 ± 0.04ab | 9.02 ± 0.35bc | 5.24 ± 0.36c |
| (7) Selenium high dose | 0.26 ± 0.02f | 0.87 ± 0.15a | 9.28 ± 0.42b | 5.50 ± 0.36b |
| (8) Selenium low dose | 0.29 ± 0.07f | 0.80 ± 0.15ab | 8.89 ± 0.42c | 5.21 ± 0.65c |
| (9) MSG high dose + vit E high group | 2.33 ± 0.01c | 0.63 ± 0.18c | 6.75 ± 0.36d | 3.99 ± 0.10e |
| (10) MSG high dose + vit E low group | 3.47 ± 0.03b | 0.52 ± 0.14d | 6.47 ± 0.36e | 3.47 ± 0.66ef |
| (11) MSG high dose + selenium high dose | 0.92 ± 0.12e | 0.45 ± 0.32e | 7.25 ± 0.21d | 4.21 ± 0.84d |
| (12) MSG high dose + selenium low dose | 1.54 ± 0.19cd | 0.53 ± 0.19d | 7.10 ± 0.58d | 4.17 ± 0.34d |
Means within the same column in each category carrying different litters are significant at (P ≤ 0.05) using Duncan's multiple range tests, where the highest mean value has symbol (a) and decreasing in value were assigned alphabetically.
Fig. 1.
Effects of treatment of monosodium glutamate (high, med, low doses), vit. E (high, low doses), Se (high, low doses) and their combinations with MSG on MDA contents in the testis tissues of rats. Data represents the means ± SD of seven samples. (MSG, monosodium glutamate, vit E, vitamin E; H dose, high dose; M dose, medium dose; L dose, low dose; Se, selenium) Values are mean ± SD of seven rats in each group.
3.2. Catalase (CAT) activity
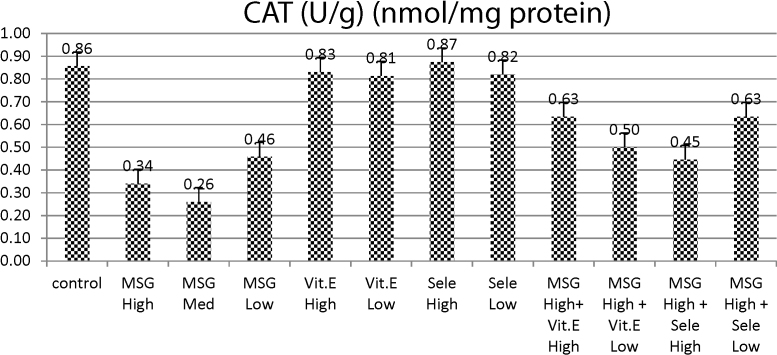
Table 1 and Fig. 2 revealed that the administration of MSG in three doses (high, medium and low dose) to rats induced highly significant decrease in CAT activities as compared to control group. Meanwhile rats treated with either vit E (in low or high dose) and/or Se (in either low or high dose) exhibited non-significant changes in CAT activity when compared with control group. On the other hand, MSG (high dose) treated group co-administered with vit E (high or low dose) and MSG (high dose) followed by administration of Se either (high or low dose) elicited slight decrease in CAT activity as compared with normal control group, but afforded significant increase in CAT activities as compared with MSG-treated groups either in (high, medium or low doses) as this effect was much less intense in groups treated with either MSG with vit E or Se.
Fig. 2.
Effects of treatment of monosodium glutamate (high, med, low doses), vit. E (high, low doses), Se (high, low doses) and their combinations with MSG on CAT contents in the testis tissues of rats. Data represents the means ± SD of seven samples. (MSG, monosodium glutamate; vit E, vitamin E; H dose, high dose; M dose, medium dose; L dose, low dose; Se, selenium). Values are mean ± SD of seven rats in each group.
3.3. Superoxide dismutase (SOD) activity
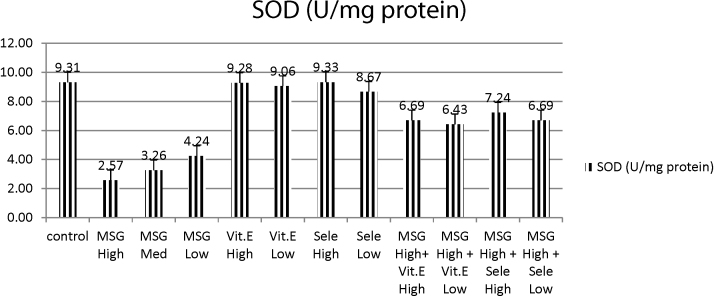
It was obvious from table that treatment of normal rats with MSG in three doses (high, medium or low) elicited significant decrease in SOD activity as compared with control group, at the same time, the administration of vit E in either high or low doses afforded non-significant decrease in SOD activity as compared to normal control group. However, Se-treated group at low dose afforded significant decrease in SOD activity as compared to control group. Meanwhile, Se-treated animals at high dose exhibited non-significant decrease in SOD activity when compared with control group (Table 1 and Fig. 3).
Fig. 3.
Effects of treatment of monosodium glutamate (high, med, low doses), vit. E (high, low doses), Se (high, low doses) and their combinations with MSG on SOD content in the testis tissues of rats. Data represents the means ± SD of seven samples. (MSG, monosodium glutamate; vit E, vitamin E; H dose, high dose; M dose, medium dose; L dose, low dose; Se, selenium) Values are mean ± SD of seven rats in each group.
At the meantime, MSG treated groups in (high, medium and low doses) afforded significant increase in SOD as compared to normal control group; meanwhile they elicited significant decrease with respect to normal control group.
3.4. Glutathione peroxidase (GPx)
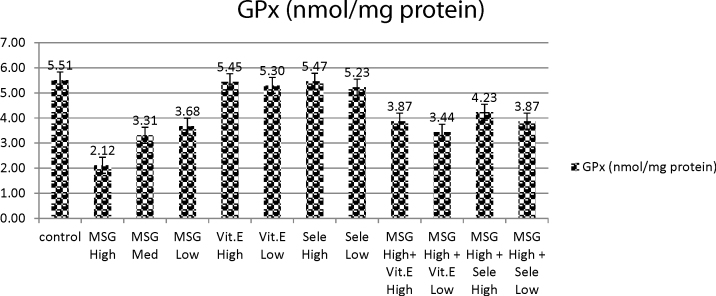
Administration of MSG in three doses (high, medium or low doses) induced significant decrease in GPx activity as compared to control group. On the same time, vit E (high dose) treated group and Se (high dose) treated group elicited non-significant changes in GPx activity as compared to control group. On the other hand, vit E (low dose) and Se (low dose) elicited slight significant decrease in GPx activity as compared to normal control group. Meanwhile, MSG (high dose) followed by administration of either vit E (high or low dose) or followed by Se at high or low dose elicited slight decrease in GPx activity with respect to normal control group (Table 1 and Fig. 4).
Fig. 4.
Effects of treatment of monosodium glutamate, vit. E, Se and their combinations with MSG on GPX content in the testis tissues of rats. Data represents the means ± SD of seven samples. (MSG, monosodium glutamate; vit E, vitamin E; H dose, high dose; M dose, medium dose; L dose, low dose; Se, selenium). Values are mean ± SD of seven rats in each group.
3.5. Histopathological investigation
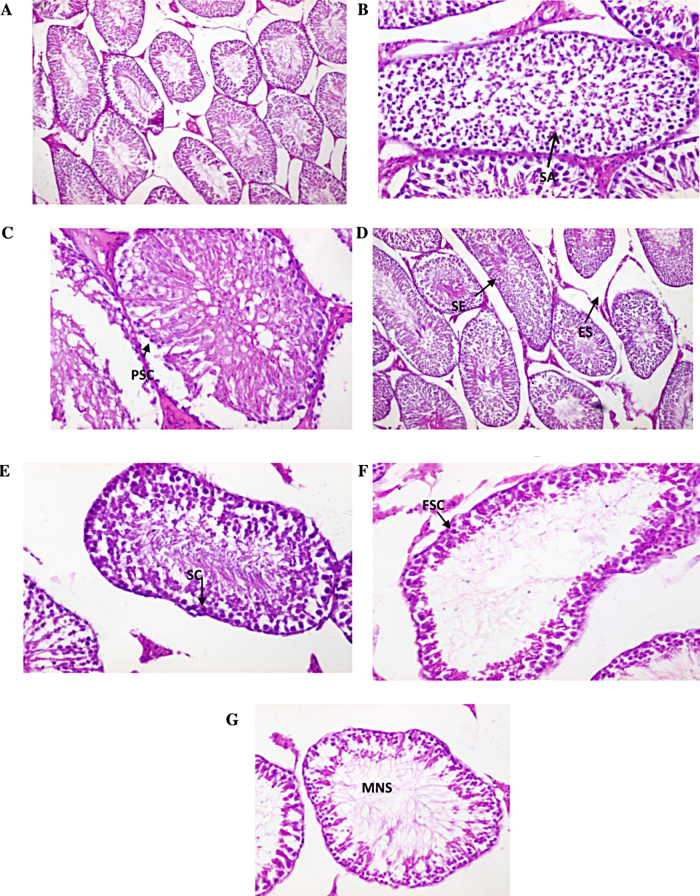
At the end of the 30 days of treatment, the spermatogenic cells in the seminiferous tubules appeared to have normal histological structure in control group. Testis of the male control treated rats appears to be oval in shape and showed normal seminiferous tubules – surrounded by few edematous stroma containing small groups of leydig cells (Fig. 5A). In the MSG-treated group with high dose, seminiferous tubules were observed filled by spermatogenic cells with few sperm formation and showing seminiferous tubules filled by spermatogonia only with few sperm formation (Fig. 5B and C). Meanwhile, in group 3 (vit E treated group high dose) there were testicular tissues showed normal seminiferous tubules surrounded by small groups of leydig cells (Fig. 5D). On the other hand, in group 4 (Se-treated group high dose), testicular tissues showed seminiferous tubules lined by layers of spermatogenic cells up to sperm formation (Fig. 5E). While, group 5 treated with (MSG + vit E at high dose) showed seminiferous tubules lined by few layers of spermetrogenic cells and few sperms (Fig. 5F). While group 6 treated with (MSG + Se at high dose) showed seminiferous tubules lined by few layers of spermetrogenic cells and moderate number sperms (Fig. 5G).
Fig. 5.
(A) Testicular section of control rats 100×. (B and C) Testicular sections of MSG (high dose) treated rats showing (B) seminiferous tubules filled by spermatogenic cells up to spermatid only with no sperm formation (SA, spermatogenic arrest). (C) Showing seminiferous tubules filled by primary spermatogonia only with no sperm formation (spermatogenic arrest) (PSC, primary spermatogenic cells). (D) Vit. E treated rats (high dose) testicular tissues showing normal seminiferous tubules surrounded by edematous stroma containing small groups of leydig cells (SE, seminiferous tubules; ES, edematous stroma). (E) Selenium treated rats (high dose) Showing seminiferous tubules lined by layers of spermatogenic cells up to sperm formation and surrounded by thin basement membrane (SC, spermatogenic cells). (F) MSG (high dose) + vit. E (high dose) showing seminiferous tubules lined by few layers of spermetrogenic cells and few sperms (FSC, few spermatogenic cells). (G) MSG (high dose) + selenium (high dose) Showing seminiferous tubules lined by few layers of spermetrogenic cells and moderate number sperms (MNS, moderate number of sperm).
4. Discussion
Lipid peroxidation is one of the main processes of oxidative damage, which plays a critical role in the toxicity of many xenobiotic (Ongjanovic et al. [75]). It was evaluated by assessment of TBARS [36]. In the present study, TBARS levels also increased in the MSG treated rats. It is known that MSG produces reactive oxygen species. Therefore, antioxidant enzymes could play a crucial role on MSG toxicity [37]. Our results was in harmony with Tezcan et al. [38] who declared that MDA is one of the final decomposition of lipid peroxidation and it is also formed as a product of the cyclooxygenase reaction in prostaglandin metabolism and this assure our finding who conclude the presence of oxidative stress in rats treated with MSG in which there was high level of MDA.
In agreement with previous study, the susceptibility of spermatozoa to oxidative stress as a consequence of the abundance of unsaturated fatty acids in the sperm plasma membrane and a very low concentration of cytoplasmic antioxidants is well known [39].
We demonstrated that the major reason for damage of testicular tissues is the increasing level of lipid peroxidation and these findings was in parallel with Aitken et al. [40] who reported that the increased lipid peroxidation led to oxidative damage to sperms DNA, alter membrane functions, impair motility and possibly have a significant effect on the development of spermatozoa. Possibly, the toxic effects of MSG on the spermatozoa physiological and biochemical parameters might be related to the increased production of free radicals in the rat reproductive organs.
There is a defense system which consists of antioxidant enzymes such as GPx, SOD and CAT [41], [42], [43]. The present investigation revealed that MSG caused significant decrease in SOD, CAT and GPx activities and these findings are greatly in accordance with Fábio et al. [37] who reported reduction in both SOD and GPx after administration of MSG and significant amelioration in these parameters after combination with Quercetin. These enzymes are also considered as an important indicator of the balance status between the first and second step of the enzymatic antioxidant pathway [44]. The testis, epididymis, sperm and seminal plasma contain high activities of antioxidant enzymes [45]. Whereas SOD catalyzes the conversion of superoxide radicals to hydrogen peroxide, CAT converts hydrogen peroxide into water [46]. Therefore, SOD–CAT system provides the first defense system against oxidative stress and these enzymes work together to eliminate active oxygen species [47], [48].
Glutathione peroxidases are antioxidant selenoenzymes that are present in the cytosol of cells. The major function of these enzymes, which use glutathione (GSH) as a substrate, is to reduce soluble hydrogen peroxide and alkyl peroxidases [43]. GPx converts hydrogen peroxide into water in the presence of oxidated glutathione [49].
In this study, the cleared decrease of SOD, CAT and GPx enzymes in MSG treated group may be due to the consumption during the breakdown of free radicals and high level of H2O2 or the inhibition of these enzymes by these radicals. Thus, the changes in oxidative defense systems and increase the level of oxidants in the testis tissues associated with MSG exposure leading to increased lipid peroxidation.
MSG may also affect male reproductive function (Alalwani et al. [77]). In this study MSG caused several histopathological changes like spermatogenic arrest, edema, and hypospermia. It may be related to oxidative effects of MSG on testis cell membrane and also testis tissues.
Oxidative damage primarily occurs via production of reactive oxygen species such as superoxide anion, peroxides, and it can damage to lipids, proteins and DNA. Therefore, it may cause to loss of enzymatic activity and structural integrity of enzymes and activate inflammatory processes [50]. It is suggested that toxic effects of MSG lead to alterations in the structural integrity of mitochondrial inner membrane, resulting in the depletion of mitochondrial GSH levels and increased formation of hydrogen peroxide by the mitochondrial electron transport chain (Sener et al. [74]). Oxidative stress plays an important role in the etiology of defective sperm formation, function, sperm count profile and male infertility [19], [51].
The maturation arrest observed in the present study which is represented by few numbers of spermatogenic layers and few sperms in the group treated with MSG was reinforced by El-Wessemy [52] who correlated this arrest to the testosterone inhibition which caused stopping of spermatogenesis. Previous researches have explained the mechanisms by which MSG inhibited the spermatogenesis in the current experiment. Glutamate receptors are present in different tissues: the hypothalamus, spleen, thymus, liver, kidneys, endocrine system, ovaries, etc. [53].
Our results came in harmony with other studies that proved the presence of functional glutamate transporters and receptors in testes of rats [54], [55] in mice. One of the mechanisms may be a direct effect of MSG via glutamate receptors and transporters on the epithelial cells of the seminiferous tubules.
Selenium can strengthen antioxidant ability by enhancing activities of antioxidant enzymes and by increasing contents of the antioxidants [56]. Inorganic Se such as sodium selenite is commonly used with vit. E for supplementation in animals diagnosed with Se deficiency or in animals residing in Se deficient areas [57]. In this study, the protective efficacy of selenium on MSG toxicity may be due to its antioxidant effects.
Selenium is present in biological systems as selenoproteins, which characteristically are oxidoreductases. These selenoenzymes have a variety of activities [58] and many of them, including the GPx and the thioredoxin reductases, have oxidant defense functions. Under conditions of selenium deficiency, tissue levels of these enzymes fall and oxidative stress conditions develop [59]. This increases the susceptibility of cells to certain types of oxidative and this is greatly was in harmony with our results as the oxidative stress level was low in Se-treated group while it was higher in group treated with MSG.
Our results are in agreement with Rao and Sharma [60] who had reported that co-administration of mercuric chloride and vit E was protective effect in their study. Because the major criterion of irreversibility of cell injury is damage to the plasma membrane, vit E becomes essential in the protection against chemical insult [61].
In the present study, vit E showed protective effect against MSG. This effect may be due to impaired absorption of MSG in the gastrointestinal tract and/or its antioxidant effect [62]. Vitamin E prevents oxidative damage to sensitive membrane lipids by destroying hydroperoxide formation, acting in conjunction with Se, and protects cellular membranes and lipid containing organelles from peroxidative damage by oxidative stress [63].
5. Conclusion
In this work, biochemical and histopathological alterations observed in testis tissues of rats exposed to MSG. In addition to this, Se or vit E ameliorated the MSG induced testicular toxicity to great extent and reduce the oxidative stress on testis tissues and thus instead of damaging testicular tissues, it enhanced male reproductive performance.
Conflict of interest
None declared.
Transparency document
Footnotes
Available online 22 October 2014
References
- 1.Furst P., Stehle P. What are the essential elements needed for the determination of amino acid requirements in humans? J. Nutr. 2004;34:1558–1565. doi: 10.1093/jn/134.6.1558S. [DOI] [PubMed] [Google Scholar]
- 2.Farombi E.O., Onyema O.O. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and guercetin. Hum. Exp. Toxicol. 2006;125:251–259. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 3.Samuels A. The toxicity/safety of MSG: a study in suppression of information. Account. Res. 1999;6:259–310. doi: 10.1080/08989629908573933. [DOI] [PubMed] [Google Scholar]
- 4.Egbuonu A.C., Obidoa O., Ezeokonkwo C.A., Ezeanyika L.U., Ejikeme P.M. Hepatotoxic effects of low dose oral administration of monosodium glutamate in male albino rats. Afr. J. Biotechnol. 2009;8:3031–3035. [Google Scholar]
- 5.Eweka O.E. Histological studies of the effects of monosodium glutamate on the kidney of adult Wistar rats. Internet J Health. 2007;6(2):2. [Google Scholar]
- 6.Walker R., Lupien J. The safety evaluation of monosodium glutamate. J. Nutr. 2000;130:1049S–1052S. doi: 10.1093/jn/130.4.1049S. [DOI] [PubMed] [Google Scholar]
- 7.Biodun D., Biodun A. Spice or poison? Is monosodium glutamate safe for human consumption. Natl. Concord. 1993:4–5. [Google Scholar]
- 8.AL-Harbi M.S., El-Shenawy N.S., Al-Weail N.O.S. Effect of monosodium glutamate on oxidative damage in the male mice: modulatory role of vitamin C. Adv. Food Sci. 2014 (in press) [Google Scholar]
- 9.Geha R., Beiser A., Ren C., Patterson R., Grammer L., Ditto A. Review of allergic reaction to monosodium glutamate and outcome of a multicenter double blind placebo-controlled study. J. Nutr. 2001;130:1032S–1038S. doi: 10.1093/jn/130.4.1058S. [DOI] [PubMed] [Google Scholar]
- 10.Onakewhor J., Oforofuo I., Singh S. Chronic administration of monosodium glutamate induces oligozoospermia and glycogen accumulation in Wistar rat testes. Afr. J. Reprod. Health. 1998;2(2):190–197. [Google Scholar]
- 11.Nayanatara A., Vinodini N., Damadar G., Ahemed B., Ramaswamy C., Shabarinath M., Bhat M. Role of ascorbic acid in monosodium glutamate mediated effect on testicular weight sperm morphology and sperm count in rat testis. J. Chin. Clin. Med. 2008;3:1–5. [Google Scholar]
- 12.Das R., Ghosh S. Long-term effects of monosodium glutamate on spermatogenesis following neonatal exposure in albino mice – a histological study. Nepal Med. Coll. J. 2010;12:149–153. [PubMed] [Google Scholar]
- 13.Igwebuike U., Ochiogu I., Ihedinihu B., Ikokide J., Idika I. The effects of oral administration of monosodium glutamate (MSG) on the testicular morphology and cauda eipididymal sperm reserves of young and adult male rats. Vet. Arch. 2011;81:525–534. [Google Scholar]
- 14.Rinne T., Mutschler E., Wimmer-Greinecker G., Moritz A., Olbrich H.G. Vitamins C and E protect isolated cardiomyocytes against oxidative damage. Int. J. Cardiol. 2000;75:275–281. doi: 10.1016/s0167-5273(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 15.Al-Attar A.M. Antioxidant effect of vitamin E treatment on some heavy metals-induced renal and testicular injuries in male mice. Saudi J. Biol. Sci. 2011;18:63–72. doi: 10.1016/j.sjbs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzun F.G., Kalender S., Durak D., Demir F., Kalender Y. Malathion-induced testicular toxicity in male rats and the protective effect of vitamins C and E. Food Chem. Toxicol. 2009;47:1903–1908. doi: 10.1016/j.fct.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.El-Demerdash F.M., Yousef M.I., Kedwany F.S., Baghdadi H.H. Cadmium induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem. Toxicol. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Uzunhisarcikli M., Kalender Y. Protective effects of vitamins C and E against hepatotoxicity induced by methyl parathion in rats. Ecotoxicol. Environ. Saf. 2011;74:2112–2118. doi: 10.1016/j.ecoenv.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Acharya U.R., Mishra M., Patro J., Panda M.K. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod. Toxicol. 2008;25:84–88. doi: 10.1016/j.reprotox.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Fahmy M.A., Hassan N.H.A., Farghaly A.A., Hassan E.E.S. Studies on the genotoxic effect of beryllium chloride and the possible protective role of selenium/vitamins A, C and E. Mutat. Res. 2008;652:103–111. doi: 10.1016/j.mrgentox.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Messaoudi I., Banni M., Said L., Said K., Kerkeni A. Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem. Biol. Interact. 2010;188:94–101. doi: 10.1016/j.cbi.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Ursini F., Heim S., Kiess M., Maiorino M., Roveri A., Wissing J., Flohe L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 23.Said L., Banni M., Kerkeni A., Said K., Messaoudi I. Influence of combined treatment with zinc and selenium on cadmium induced testicular pathophysiology in rat. Food Chem. Toxicol. 2010;48:2759–2765. doi: 10.1016/j.fct.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Marin-Guzman J., Mahan D.C., Whitmoyer R. Effect of dietary selenium and vitamin E on the ultrastructure and ATP concentration of boar spermatozoa, and the efficacy of added sodium selenite in extended semen on sperm motility. J. Anim. Sci. 2000;78:1544–1550. doi: 10.2527/2000.7861544x. [DOI] [PubMed] [Google Scholar]
- 25.Council of Europe . 2006. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. CETS No. 123. Appendix A of the Convention: (1986, Adopted 2005). Guidelines for Accommodation and Care of Animals (Article 5 of the Convention) Approved by the Multilateral Consultation. Available at: http://conventions.coe.int/Treaty/EN/Treaties/PDF/123-Arev.pdf. [Google Scholar]
- 26.El-Meghawry E.A., Osman H.E., Daghestani M.H. The effect of vitamin C administration on monosodium glutamate induced liver injury: an experimental study. Exp. Toxicol. Pathol. 2013;65(5):513–521. doi: 10.1016/j.etp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Seren G., Gürgen E., Erdogan D., Elmas G., Kaplanoglu G.T., Öze G. Chemoprotective effect of ascorbic acid, α-tocopherol, and selenium on cyclophosphamide-induced toxicity in the rat ovarium. Nutrition. 2013;29:777–784. doi: 10.1016/j.nut.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Paul M.V., Abhilash M., Varghese M.V., Alex M., Nair R.H. Protective effects of α-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicol. Mech. Methods. 2012;22(8):625–630. doi: 10.3109/15376516.2012.714008. [DOI] [PubMed] [Google Scholar]
- 29.Koyuturk M., Yanardag R., Bolkent S., Tunali S. The potential role of combined anti-oxidants against cadmium toxicity on liver of rats. Toxicol. Ind. Health. 2007;23:393–401. doi: 10.1177/0748233707081907. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 32.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 33.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–165. [PubMed] [Google Scholar]
- 34.Carleton H.M. 4th ed. Oxford University Press; London, New York, Toronto: 1967. Carleton's Histological Technique. [Google Scholar]
- 35.Snedecor G.W., Cochran W.G. 8th ed. Iowa State University; Ames: 1982. Statistical Methods. [Google Scholar]
- 36.Qiao D., Seidler F.J., Slotkin T.A. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Fábio R., Seiva F., Gustavo L., Chuffa A., Braga C.P., Paulo J., Amorim A., Fernandes A.A.H. Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem. Toxicol. 2012;50:3556–3561. doi: 10.1016/j.fct.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Tezcan E., Atmaca M., Kuloglu M., Ustundag B. Free radicals in patients with posttraumatic stress disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2003;253:86–91. doi: 10.1007/s00406-003-0413-x. [DOI] [PubMed] [Google Scholar]
- 39.Jones R., Manna T., Sherina R.J. Peroxidative breakdown of phospholipids in human spermatozoa: spermicidal effects of fatty acids, oxidized peroxides and protective reaction of seminal plasma. Fertil. Steril. 1979;32:257–265. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 40.Aitken R.J., Clarkson J., Fisher S. Generation of reactive oxygen species, lipid peroxidation and human sperm function. Biol. Reprod. 1989;40:183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 41.Celik I., Suzek H. Effects of subacute exposure of dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicol. Environ. Saf. 2009;72:905–908. doi: 10.1016/j.ecoenv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Uzun F.G., Demir F., Kalender S., Bas H., Kalender Y. Protective effect of catechin and quercetin on chlorpyrifos-induced lung toxicity in male rats. Food Chem. Toxicol. 2010;48:1714–1720. doi: 10.1016/j.fct.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 43.Demir F., Uzun F.G., Durak D., Kalender Y. Subacute chlorpyrifos-induced oxidative stress in rat erythrocytes and the protective effects of catechin and quercetin. Pestic. Biochem. Phys. 2011;99:77–81. [Google Scholar]
- 44.Jihen E.H., Imed M., Fatima H., Abdelhamid K. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: effects on the oxidative stress. Ecotoxicol. Environ. Saf. 2009;72:1559–1564. doi: 10.1016/j.ecoenv.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Aruldhas M.M., Subramanian S., Sekar P., Vengatesh G., Chandrahasan G., Govindarajulu P., Akbarsha M.A. Chronic chromium exposure-induced changes in testicular histoarchitecture are associated with oxidative stress: study in a non-human primate (Macaca radiata Geoffroy) Hum. Reprod. 2005;20:2801–2813. doi: 10.1093/humrep/dei148. [DOI] [PubMed] [Google Scholar]
- 46.Mansour S.A., Mossa A.T.H. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and protective effect of zinc. Pestic. Biochem. Phys. 2009;93:34–39. [Google Scholar]
- 47.El-Demerdash F.M. Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem. Toxicol. 2011;49:1346–1352. doi: 10.1016/j.fct.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Wafa T., Amel N., Issam C., Imed C., Abdelhedi M., Mohamed H. Subacute effects of 2,4-dichlorophenoxyacetic herbicide on antioxidant defense system and lipid peroxidation in rat erythrocytes. Pestic. Biochem. Phys. 2011;99:256–264. [Google Scholar]
- 49.Kanbur M., Eraslan G., Silici S. Antioxidant effect of propolis against exposure to propetamphos in rats. Ecotoxicol. Environ. Saf. 2009;72:909–915. doi: 10.1016/j.ecoenv.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Özyurt H., Söğüt S., Yıldırım Z., Kart L., Iraz M., Armutçu F., Ismail T., Özen S., Uzun A., Akyol Ö. Inhibitory effect of caffeic acid phenethyl ester on bleomycine-induced lung fibrosis in rats. Clin. Chim. Acta. 2004;339:65–75. doi: 10.1016/j.cccn.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Shen H., Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic. Biol. Med. 2000;28:529–536. doi: 10.1016/s0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 52.El-Wessemy A.M. The role of vitamin C in alleviating tamoxifen induced histological and ultrastructural changes in the testes of albino mice. Egypt J. Zool. 2007;48:303–326. [Google Scholar]
- 53.Gill S., Pulido O. Kulwer Academic/Plenum Publisher; New York: 2005. Glutamate Receptors in Peripheral Tissue Excitatory Transmission Outside the CNS. [Google Scholar]
- 54.Takarada T., Hinoi E., Balcar V., Taniura H., Yoneda Y. Possible expression of functional glutamate transporters in the rat testis. J. Endocrinol. 2004;181:233–244. doi: 10.1677/joe.0.1810233. [DOI] [PubMed] [Google Scholar]
- 55.Hu J., Yang N., Ma Y., Jiang J., Zhang J., Fei J., Guo L. Identification of glutamate transporters and receptors in mouse testis. Acta Pharmacol. Sin. 2004;25:366–371. [PubMed] [Google Scholar]
- 56.Su L., Wabg M., Yin S.T., Wang H.L., Chen L., Sun L.G., Ruan D.Y. The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicol. Environ. Saf. 2008;70:483–489. doi: 10.1016/j.ecoenv.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 57.Tiwary A.K., Stegelmeier B.L., Panter K.E., James L.F., Hall J.O. Comparative toxicosis of sodium selenite and selenomethionine in lambs. J. Vet. Diagn. Invest. 2006;18:61–70. doi: 10.1177/104063870601800108. [DOI] [PubMed] [Google Scholar]
- 58.Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigo R. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 59.Burk R.F. Selenium (Se) and antioxidant mechanisms. Free Radic. Res. 2006;40:S50. [Google Scholar]
- 60.Rao M.V., Sharma P.S.N. Protective effect of vitamin E against mercuric chloride reproductive toxicity in male mice. Reprod. Toxicol. 2001;15:705–712. doi: 10.1016/s0890-6238(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 61.Bansal A.K., Bansal M., Soni G., Bhatnagar D. Protective role of vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005;156:101–111. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Giadinis N., Koptopoulus G., Roubies N., Siarkou V., Papasteriades A. Selenium and vitamin E effect on antibody production of sheep vaccinated against enzootic abortion (Chlamydia psittaci) Comp. Immunol. Microbiol. Infect. Dis. 2000;23:129–137. doi: 10.1016/s0147-9571(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 63.Gupta S., Gupta H.K., Soni J. Effect of vitamin E and selenium supplementation on concentrations of plasma cortisol and erythrocyte lipid peroxides and the incidence of retinal fetal membranes in crossbred dairy cattle. Theriogenology. 2005;64:1273–1286. doi: 10.1016/j.theriogenology.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Sener G., Sehirli A.O., Ayanoglu-Dulger G. Melatonin protects against mercury (II)-induced oxidative tissue damage in rats. Pharmacol. Toxicol. 2003;93:290–296. doi: 10.1111/j.1600-0773.2003.pto930607.x. [DOI] [PubMed] [Google Scholar]
- 75.Ongjanovic L.B.I., Markovic L., Dor.d-evic S.D., Trbojevic N.Z., tajn I.S.A., Saicic L.Z.S. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q10 and Vitamin E. Reprod. Toxicol. 2010;29:191–197. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Alalwani D.A. Monosodium glutamate induced testicular lesions in rats (histological study) Middle East Fertil. Soc. J. 2013 (in press) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.