Abstract
Zearalenone (ZEA) is a secondary fungal metabolite produced mainly by a Fusarium graminearum. To clarify the toxicokinetics, and residues of ZEA and its major metabolites α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL) in chickens, ZEA was then administered intravenously (iv) or orally (po) to broiler chickens at a dosage of 1.2 mg/kg body weight. The concentrations of ZEA, α-ZOL and β-ZOL in the plasma and various tissues were quantified using LC–MS/MS. The plasma concentrations of ZEA were measurable up to 2 h after iv and po administration, and the concentrations of α-ZOL and β-ZOL were detected up to 4 h after both types of administration. A two-compartment model was developed to describe the toxicokinetic of ZEA in broilers. The values of t1/2β and Vd were 1.36 ± 0.29 h and 6.40 ± 0.89 l/kg, respectively. The absolute oral bioavailability was 29.66 ± 5.6%. ZEA, α-ZOL and β-ZOL were measurable in the vital organs after po administration. These results suggest that ZEA is absorbed from the gastrointestinal tract and it has ability to penetrate into the various tissues of broiler chickens.
Keywords: Zearalenone, α-Zearalenol, β-Zearalenol, Toxicokinetics, Residues, Broilers
1. Introduction
Zearalenone (ZEA), 6-(10-hydroxy-6-oxo-trans-1-undecenyl)-β-resorcyclic acid lactone, is a myco-estrogen with non-steroidal chemical structure produced by a variety of Fusarium fungi mainly by Fusarium graminearum. Contamination of ZEA in various agricultural crops has been observed worldwide especially in maize, wheat, oat and barley [13], [21], [27]. The most important toxic effect of ZEA is its estrogenic effect, which induces impaired fertility and abnormal fetal development in farm animals [4]. Several publications reported that ZEA induced hepatocarcinogenesis, nephropathy and hematotoxicity in rodents and milk reduction in cows [1], [14], [18], [26]. Regarding the animal susceptibility to the toxic effects of ZEA, pigs are the most susceptible to the estrogenic effects of ZEA [2], [6]. Thereafter, the contribution of the fate of ZEA to the susceptibility difference is unknown, because it has extensively been examined in pigs but not in other animals [2], [29].
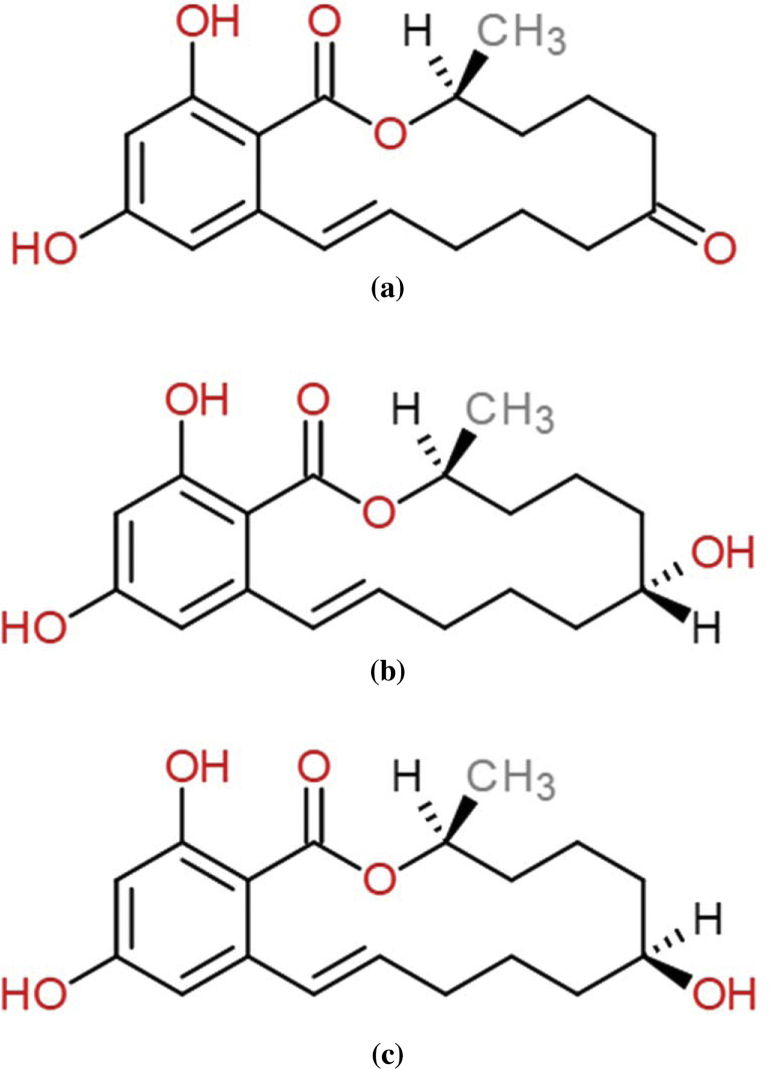
The liver is the major organ responsible for metabolism of ZEA to at least five stereoisomeric metabolites including α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), α-zearalanol (α-ZAL), β-zearalanol (β-ZAL) and zearalanone (ZAN) [17]. These metabolites are also produced by the fungi and contaminate crops such as corn stems [3] and rice [22]. In addition, recent report has shown the occurrence of α- and β-zearalenol in corn by-product, corn silage and soy meal [23]. Among these metabolites, α-zearalenol is a major hepatic metabolite of ZEA in various species especially rats and ruminants [10], [17]. The estrogenic activity of α-ZOL is 3–100 times higher than that of ZEA [3], [9], [11], [16], [28]. The results of three different bioassays using estrogen receptor gene activation revealed that α-zearalenol has 17 times as strong as α-ethinyl oestradiol [12]. Consequently, α-ZOL was found to cause reproductive dysfunctions in domestic livestock [6] (Fig. 1).
Fig. 1.
Chemical structures of zearalenone (a), α-zearalenol (b) and β-zearalenol (c).
The toxicokinetics and the corresponding tissue residues of ZEA have been studied in livestock, but limited information is available for broilers particularly, tissue residues in broiler chickens. Recently, Osselaere et al. [20] have reported the toxicokinetic characteristics of ZEA after intravenous administration however, the information on oral bioavailability, tissue residues and metabolism of ZEA are not available in broiler chickens. Adequate information on the disposition and the residue depletion of ZEA is also needed to evaluate possible differences in toxicity among species. Thus, we studied the fate, the residues and the metabolites of ZEA in broilers, based on the pharmacokinetic parameters and toxin depletion observed in various tissues.
2. Materials and methods
2.1. Toxins and chemicals
ZEA, α-ZOL and β-ZOL were purchased from Wako Chemical Co. (Tokyo, Japan). Other reagents and chemicals of an analytical grade were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Purified water was produced using the Milli-Q water purification system from Millipore, Inc. (Bedford, MA, USA). ZEA was dissolved with 10% dimethyl sulfoxide (DMSO, Wako Pure Chemical Industry Ltd., Tokyo, Japan) in physiologic saline to the final concentration of 4 mg/ml for administration.
2.2. Animals
Thirty-five 3-week-old female broilers (average weight 1.56 ± 2.32 kg) were obtained from Commercial chicken farm (CP Group of companies, Saraburi Province, Thailand). The experimental animals were housed in individual stainless-steel cages at the Laboratory Animal Facility, Faculty of Veterinary Medicine, Kasetsart University and they were acclimatized to the environment for 1 wk. The animals were fed with a commercial diet and drinking water ad libitum. All experimental procedures carried out on the animals in this study were ethically approved by the Animal Ethics Research Committee of the Faculty of Veterinary Medicine, Kasetsart University.
2.3. Experimental design for toxicokinetic study
Ten broiler chickens were weighed and then divided into two groups (n = 5). After overnight fasting, each group was administered ZEA intravenously (iv) or orally (po) at a dosage of 1.2 mg/kg bw. In this study, the dosage of ZEA was determined based on the results of preliminary study information. From our preliminary study, two dosages of ZEA at 0.6 and 1.2 mg/kg bw were examined in broiler chickens, we found that the dosage of 1.2 mg/kg bw of ZEA was no adverse effects observed. The level of ZEA and its metabolites was detected more clearly than the lower dosage. Taken together, this dosage was similar to the studies in pigs and goats [8], [15]. Blood samples were collected from the wing veins of each animal with heparinized syringes at 0, 5, 15 and 30 min and at 1, 2, 4, 8 and 12 h after ZEA administration. The plasma was separated by centrifugation (1968 × g) for 15 min. All of the plasma was frozen at −20 °C until analysis.
2.4. Experimental design for tissue residue study
Twenty-five broilers were administered ZEA orally at a dosage of 1.2 mg/kg of bw. The remaining five broilers served as controls and were orally administered DMSO in 0.9% physiologic saline. Animals were sacrificed with thiopentone sodium at a dosage of 20 mg/kg bw by iv administration. Tissue samples, including liver, kidney, muscle, intestine and excreta, were collected at 1, 3, 9 and 12 h after po administration (n = 5), respectively. All samples were frozen at −20 °C until analysis.
2.5. Extraction and clean-up procedure
The extraction method of ZEA and its metabolites in plasma, tissues and excreta was performed as described previously [25]. Briefly, 1 ml of broiler plasma was mixed with 6 ml of ammonium acetate buffer (pH 4.8); it was then incubated for 15 h at 37 °C with 25 μl of glucuronidase/arylsulfatase solution before adding 6 ml of phosphate buffer (PB, pH 7.4). The sample was then centrifuged at 1926 × g for 15 min. The supernatant was applied to immune-affinity column (IAC, Easi-Extract® Zearalenone, R-Biopharm Rhone Ltd., Dmstadt, Germany) after the IAC was preconditioned with 10 ml of PB. The IAC was rinsed with 15 ml of purified water. The analytes were eluted with 1.5 ml of acetonitrile and evaporated to dryness under a nitrogen stream at 40 °C on a heating block. The residue was redissolved with 150 μl of water–methanol mixtures (50:50, v/v) and then analyzed by liquid chromatography tandem mass spectrometry (LC–MS/MS).
A quantity of 1 g of tissue sample, ground and dried at 65 °C was extracted in 50 ml of water-methanol (50/50, v/v) for 60 min. The sample was then centrifuged at 1926 × g for 10 min. A 20 ml of supernatant was collected and mixed with 40 ml of buffer solution acetic acid-ammonium acetate, pH 4.8, it was then incubated for 15 h at 37 °C with 80 μl of a solution of glucuronidase/arylsulfatase and adjusted to pH 4.0 with glacial acetic acid. The solution was loaded into a Chromabond C18 column (Fisher Scientific Ltd., UK) after it was preconditioned with 10 ml of methanol and 10 ml of Milli-Q water. Thereafter, the columns loaded with the sample were rinsed with 5 ml of Milli-Q water and 5 ml of a methanol–water (30:70, v/v) mixture. The analytes were eluted with 1.25 ml of methanol. The eluate was mixed with 15 ml of PB and applied to immune-affinity column (IAC, Easi-Extract® Zearalenone, R-Biopharm Rhone Ltd., Dmstadt, Germany) after the IAC was preconditioned with 10 ml of PB. The IAC was rinsed with 15 ml of purified water. The analytes were eluted with 1.5 ml of acetonitrile and evaporated to dryness under a nitrogen stream at 40 °C on a heating block. The residue was redissolved with 150 μl of water–methanol mixtures (50:50, v/v) and then analyzed by LC–MS/MS.
Two grams of excreta were extracted with 40 ml of methanol–water (50:50, v/v). After centrifugation, 20 ml of supernatant was mixed with 40 ml of 0.05 M ammonium acetate buffer pH 4.8. This solution was incubated for 15 h at 37 °C with 80 μl of glucuronidase/arylsulfatase solution. All the following steps were performed as described above for clean-up of tissue samples. The eluate was evaporated to dryness under a nitrogen stream. The residue was redissolved in 250 μl of water–methanol mixtures (50:50, v/v) and then analyzed by LC–MS/MS.
2.6. LC parameters
The LC analysis was performed using an Agilent 1200 series system consisting of a binary high-pressure gradient pump, a vacuum solvent degassing unit, an automatic sample injector and a column thermostat (Agilent Technologies, Waldbronn, Germany). Separation was achieved by a Poroshell 120 EC-C18 column (2.7 μm, 3.0 mm × 50 mm) (Agilent Technologies, Palo, Alto, CA, USA) The column was maintained at a temperature of 45 °C. The LC mobile phase program consisted of a binary gradient of the 0.1% acetic acid in water (mobile phase A) and acetonitrile (mobile phase B). The composition started out at 30% mobile phase B and increased linearly to 70% mobile phase B by 6.4 min. The mobile phase then returned to 30% acetonitrile by 7 min, and the column was equilibrated for 3 min. The flow rate was 200 μl/min; the injection volume was 10 μl.
2.7. MS parameters
Mass spectrometry was performed using an Agilent Technologies 6460 triple quard mass spectrometer equipped with an electrospray ionization (ESI) source and Agilent MassHunter Workstation Software version 1.2. ESI-MS/MS was operated at unit mass resolution in multiple reaction monitoring (MRM) negative ion mode with the following settings: nebulizer gas pressure (NEB): 45 psi, gas flow 5.0 l/ml, gas temperature 300 °C, and Capillary voltage: −3500 V. The following transitions were used: ZEA: m/z 317.1 > 175 and 317.1 > 131, α-ZOL: 319.15 > 275.1, and for β-ZOL: 319.15 > 275.1.
2.8. Fortification procedure
To evaluate recovery, ZEA and its metabolites α-ZOL and β-ZOL were added to samples of blank plasma and tissues to yield final ZEA, α-ZOL and β-ZOL concentrations of 1, 5, 10, 50, 100, and 200 ng/ml (ng/g), respectively. The spiked samples were then analyzed in duplicate as described in the extraction/cleanup procedures. The mean (±SD) recoveries were follows; ZEA: 95.16 ± 4.61%, 84.56 ± 3.21%, 86.27 ± 5.18%, 83.63 ± 2.85%, 92.12 ± 3.52 and 101.63 ± 3.36%, α-ZOL: 90.22 ± 5.11%, 82.05 ± 4.15%, 88.13 ± 4.68%, 85.19 ± 4.06%, 93.15 ± 4.13 and 102.18 ± 4.24%, β-ZOL: 91.21 ± 4.11%, 85 ± 4.13%, 87.35 ± 4.26%, 84.24 ± 3.16%, 94.42 ± 4.12 and 103.61 ± 3.12% in the plasma, liver, kidney, muscle, small intestine and excreta, respectively. The limit of detection (LOD) of ZEA, α-ZOL and β-ZOL was 1 ng/ml (ng/g) in piglet plasma, excreta and various tissues. The limit of quantification of ZEA, α-ZOL and β-ZOL was 2–2.5 ng/ml (ng/g). The r2 value of the ZEA, α-ZOL and β-ZOL calibration curves was 0.992–0.996. The precision and accuracy indicated the method was repeatable. The intra- and inter-day precisions were <12%.
2.9. Toxicokinetic parameter calculations
The concentration of ZEA in experimental broiler chickens with respect to time was pharmacokinetically analyzed using a two-compartment model with the PK Solutions 2.0™ Program (Summit Research Services, Montrose, CO, USA), where was the peak concentration at initial time, AUC0–∞ was the area under the curve, t1/2β was the elimination half-life, t1/2α was the distribution half-life, Vd(area) was the volume of distribution, Cl was the plasma clearance, MRT was the mean residence time, Kel was the elimination rate constant, and K12 and K21 were the micro-rate constants. The absolute oral bioavailability (F) was calculated using the following equation:
2.10. Statistical analysis
The plasma concentration curves and tissue residues of ZEA, α-ZOL and β-ZOL are shown as the mean (±SD, n = 5) of the values for the broiler chickens sampled. Toxicokinetic parameters are also shown as the mean (±SD, n = 5).
3. Results
3.1. Plasma concentration and toxicokinetic parameters
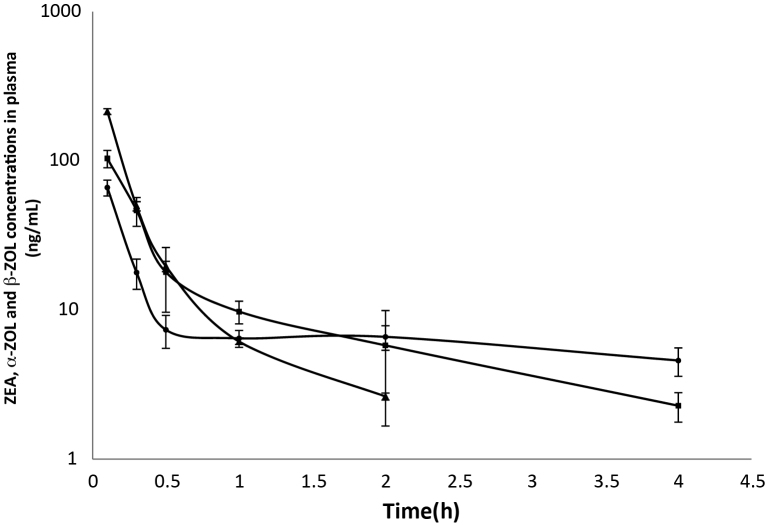
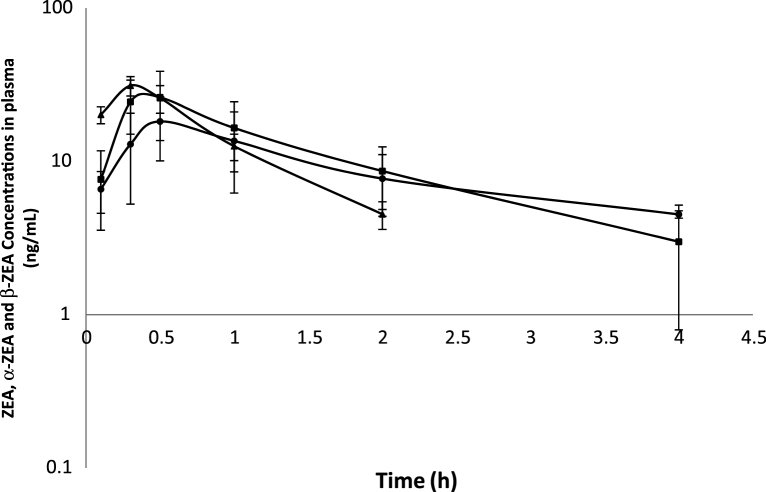
The determination of ZEA, α-ZOL and β-ZOL concentrations showed that they were detectable in the plasma of broiler chickens following a single iv or po administration of ZEA. The semi-logarithmic plots of the mean (±SD) plasma concentration–time curves of ZEA, α- ZOL and β-ZOL at a dosage of 1.2 mg/kg bw in broiler chickens following iv and po administrations are shown in Fig. 2, Fig. 3, respectively. ZEA was measurable from 5 min to 2 h, whereas α-ZOL and β-ZOL were detectable from 5 min to 4 h after iv and po administration of ZEA, respectively. The plasma profile displayed a rapid decrease in the ZEA concentration with time in both groups, as was to be expected following iv administration. The ZEA disposition fit an open 2-compartment pharmacokinetic model because ZEA concentration declined rapidly in a biphasic pattern, indicating fast distribution and elimination (Fig. 2) Following iv administration, the values for the t1/2β, Vd, Cl, and MRT were 1.36 ± 0.29 h, 6.40 ± 0.89 l/kg, 0.34 ± 0.03 l/h/kg and 2.10 ± 0.41 h, respectively. The mean (±SD) toxicokinetic parameters of ZEA in plasma after a single iv administration are summarized in Table 1. The maximum plasma concentration (Cmax) of ZEA was 15.90 ± 4.50 ng/ml at 15 min after po administration. The absolute oral bioavailability was 29.66%. ZEA, α-ZOL and β-ZOL were also detected in excreta up to 12 h following po administration (Table 1).
Fig. 2.
Mean values (±SD) of zearalenone (ZEA), α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL) concentrations in plasma broiler chickens after intravenous injection of ZEA at a dosage of 1.2 mg/kg BW; (■) α-ZOL, (●) β-ZOL, and (▴) ZEA (n = 5).
Fig. 3.
Mean values (±SD) of zearalenone (ZEA), α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL) concentrations in plasma broiler chickens after oral administration of ZEA at a dosage of 1.2 mg/kg BW; (■) α-ZOL, (●) β-ZOL, and (▴) ZEA (n = 5).
Table 1.
Mean ± SD value of the toxicokinetic parameters of zearalenone (ZEA) following a single intravenous or oral administration at a dosage of 1.2 mg/kg bw in broiler chickens (n = 5).
| Toxicokinetic parameters (unit) | Value |
|---|---|
| Intravenous administration | |
| Kel (h) | 0.53 ± 0.11 |
| K12 (h−1) | 1.33 ± 0.10 |
| K21 (h−1) | 0.68 ± 0.17 |
| t1/2α (h) | 0.10 ± 0.01 |
| t1/2β (h) | 1.36 ± 0.29 |
| CL (L/h/kg) | 0.34 ± 0.03 |
| Vd (L/kg) | 6.40 ± 0.89 |
| MRT (h) | 2.1 ± 0.41 |
| Oral administration | |
| Cmax (ng/ml) | 15.9 ± 4.5 |
| Tmax (min) | 15.0 ± 0.0 |
| Fpo (%) | 29.66 ± 5.6 |
Kel, elimination rate constant; K12, K21, micro-rate constants; t1/2α, distribution half-life; t1/2β, elimination half-life; CL, clearance; Vd, volume of distribution; MRT, mean residence time; Cmax, maximum concentration; Tmax, time at maximum concentration; Fpo, oral bioavailability.
The LC–MS/MS profile for various tissues, including the liver, kidney, muscle and small intestine, showed that ZEA was measurable up to 1 h in liver, kidney and small intestine, whereas it was not detectable in muscle of broiler chickens after po administration (Table 2). The maximum level of ZEA was 114.7 ± 13.1 ng/g at 1 h in small intestine after po administration. α-ZOL and β-ZOL were detectable up to 12 h in liver, kidney and small intestine, whereas they were detectable up to 1 h in muscle after po administration. All of ZEA, α-ZOL and β-ZOL were measurable up to 12 h in excreta of broiler chickens (Table 2).
Table 2.
Mean ± SD residue concentrations of zearalenone (ZEA), a-zearalenone (α-ZOL) and b-zearalenone (β-ZOL) in various tissues following a single oral administration at 1.2 mg/kg bw of ZEA in broiler chickens (n = 5).
| Time (h) | ZEA and it major metabolites concentration (ng/g) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver |
Kidney |
Small intestine |
Muscle |
Excreta |
|||||||||||
| ZEA | α-ZOL | β-ZOL | ZEA | α-ZOL | β-ZOL | ZEA | α-ZOL | β-ZOL | ZEA | α-ZOL | β-ZOL | ZEA | α-ZOL | β-ZOL | |
| 1 | 3.52 ± 1.9 | 105.2 ± 43.8 | 30.9 ± 13.7 | 3.55 ± 1.5 | 77.99 ± 23.1 | 36.6 ± 9.1 | 114.7 ± 13.1 | 626.1 ± 135.6 | 198.3 ± 60.2 | ND | 2.55 ± 0.2 | 2.40 ± 0.7 | 218.6 ± 20.1 | 399.7 ± 45.1 | 385.6 ± 25.2 |
| 3 | ND | 47.54 ± 24.1 | 37.3 ± 6.9 | ND | 11.28 ± 1.3 | 26.7 ± 6.8 | ND | 200.8 ± 48.5 | 103.9 ± 12.2 | ND | ND | ND | 199.8 ± 20.8 | 468.3 ± 67.8 | 338.1 ± 28.4 |
| 9 | ND | 31.09 ± 12.3 | 25.0 ± 6.6 | ND | 6.76 ± 3.0 | 16.6 ± 4.5 | ND | 91.6 ± 15.1 | 65.0 ± 11.6 | ND | ND | ND | 264.8 ± 27.8 | 1164.9 ± 78.1 | 209.7 ± 17.1 |
| 12 | ND | 7.84 ± 3.3 | 24.4 ± 2.1 | ND | 1.63 ± 0.3 | 4.8 ± 1.8 | ND | 24.6 ± 11.1 | 26.2 ± 6.1 | ND | ND | ND | 114.3 ± 58.2 | 1286.5 ± 144 | 119.4 ± 17.2 |
ND, not detected.
4. Discussion
To date, there are several publications for toxicokinetics and metabolism of ZEA in pigs and goats by single bolus of ZEA at 1 and 1.2 mg/kg bw, respectively. In this study, an intravenous or oral administration of ZEA at 1.2 mg/kg bw, the dose similar to their studies, we used to compare the fate of ZEA metabolism among broilers, goats and pigs [8], [15]. The present study used LC–MS/MS with electrospray ionization to determine the concentrations of ZEA and its active metabolites α-ZOL and β-ZOL in the plasma, excreta and various tissues following iv or po administration in broiler chickens. In the present study, no adverse effects were observed following the administration of ZEA in broiler chickens. Following iv administration, the t1/2β indicates the overall rate of elimination and allows the prediction of ZEA accumulation with a value for ZEA of 1.36 h in broiler chickens. The mean residence time was 2.10 h after iv administration. The ZEA appears to have been excreted rapidly in broiler chickens. The t1/2β of ZEA obtained in broilers of the present study was shorter than in pigs (2.63 h) [5] and goats (28.58 h) [8]. The Vd of ZEA in broiler chickens was 6.40 l/kg after iv administration. These results suggested that ZEA has penetrated to various tissues, although it appears to have been excreted rapidly after iv administration in broiler chickens. Regarding the values of Vd and Cl of ZEA in other animal species, they were 10.48 l/kg and 0.048 l/min/kg in pigs [5], whereas they were 7.32 l/kg and 0.003 l/min/kg in goats [8], respectively. Thus, the difference in susceptibility to the estrogenic effects of ZEA between ruminant and non-ruminant species cannot be explained by circulating ZEA and its metabolites [2], [6]. Furthermore, a large proportion of α-ZOL and β-ZOL was found in the plasma of broiler chickens. α-ZOL and β-ZOL were detectable in the plasma from 5 min to 4 h after iv administration of ZEA. The results suggested that ZEA was transformed rapidly into α-ZOL and β-ZOL in plasma of broiler chickens.
Following oral administration, a large proportion of ZEA was changed into α-ZOL and β-ZOL in the plasma and various tissues of broiler chickens following ZEA administration, indicating that ZEA was absorbed and metabolized rapidly. In addition, α-ZOL and β-ZOL were detectable up to 12 h in liver, kidney and small intestine, whereas they were detectable up to 1 h in muscle after po administration. The absolute oral bioavailability of ZEA was 29.66 ± 5.6%; it was higher in broilers than in rats (2.7%) [24]. The level of α-ZOL and β-ZOL in each tissue followed as small intestine > liver > kidney and muscle. The proportion of α-ZOL was higher than β-ZOL in broiler chickens. Accordingly, these findings are in general agreement with other studies where α-ZOL was reported to be the major metabolite of ZEA [2], [5], [7], [19], [29]. This also indicates that ZEA has the ability to penetrate into the various tissues of broilers as well. The LC–MS/MS profile of excreta showed a large proportion of α-ZOL and β-ZOL after the administration of ZEA in broiler chickens, however the proportion of α-ZOL was higher than β-ZOL. These findings clearly show that ZEA is excreted largely in the form of α-ZOL in the excreta of broiler chickens.
5. Conclusion
The study demonstrated here is the toxicokinetic profile of ZEA. ZEA and its active metabolites α-ZOL and β-ZOL were measurable in plasma, excreta and various tissues of broiler chickens after a single iv or po administration of ZEA. α-ZOL appears to be the major metabolite of ZEA in broiler chickens. Based on the toxicokinetic information, ZEA is absorbed rapidly from the gastrointestinal tract and extensively penetrates into various tissues. ZEA can be excreted largely in the form of α-ZOL in the excreta of broiler chickens.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgement
This study was supported by the Kasetsart University Research and Development Institute, Bangkok, Thailand.
References
- 1.Abbes S., Ouanes Z., ben Salah-Abbes J., Houas Z., Oueslati R., Bacha H., Othman O. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by zearalenone in mice. Toxicon. 2006;47:567–574. doi: 10.1016/j.toxicon.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Biehl B.M., Prelusky D.B., Koritz G.D., Hartin K.E., Buck W.B., Trenholm H.L. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol. Appl. Pharmacol. 1993;121:152–159. doi: 10.1006/taap.1993.1140. [DOI] [PubMed] [Google Scholar]
- 3.Bottalico A., Visconti A., Logrieco A., Solfrizzo M., Mirocha C.J. Occurrence of zearalenols (Diastereomeric Mixture) in corn stalk rot and their production by associated Fusarium species. Appl. Environ. Microbiol. 1985;49:547–551. doi: 10.1128/aem.49.3.547-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd P.A., Wittliff J.L. Mechanism of Fusarium mycotoxin action in mammary gland. J. Toxicol. Environ. Health. 1978;4:1–8. doi: 10.1080/15287397809529638. [DOI] [PubMed] [Google Scholar]
- 5.Dänicke S., Swiech E., Buraczewska L., Ueberschar K.H. Kinetics and metabolism of zearalenone in young female pigs. J. Anim. Physiol. Anim. Nutr. 2005;89:268–276. doi: 10.1111/j.1439-0396.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Diekman M.A., Green M.L. Mycotoxins and reproduction in domestic livestock. J. Anim. Sci. 1992;70:1615–1627. doi: 10.2527/1992.7051615x. [DOI] [PubMed] [Google Scholar]
- 7.Döll S., Dänicke S., Valenta H., Flachowsky G. In vitro studies on the evaluation of mycotoxin detoxifying agents for their efficacy on deoxynivalenol and zearalenone. Arch. Anim. Nutr. 2004;58:311–324. doi: 10.1080/00039420412331273268. [DOI] [PubMed] [Google Scholar]
- 8.Dong M., He X.J., Tulayakul P., Li J.Y., Dong K.S., Manabe N., Nakayama H., Kumagai S. The toxic effects and fate of intravenously administered zearalenone in goats. Toxicon. 2010;55:523–530. doi: 10.1016/j.toxicon.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Everette D.J., Perry C.J., Scott K.A., Martin B.W., Terry M.K. Estrogenic potencies of resorcyclic acid lactone and 17β-estradiol in female rats. J. Toxicol. Environ. Health. 1987;20:435–443. doi: 10.1080/15287398709530995. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick D.W., Hassen A.M., Arbuckle L.D. Effect of dietary protein on zearalenone metabolism and toxicity in the rat. Nutr. Res. 1988;8:663–667. [Google Scholar]
- 11.Hagler W.M., Mirocha C.J., Pathre S.V., Behrens J.C. Identification of naturally occurring isomer of zearalenol produced by Fusarium roseum “Gibbosum” in rice culture. Appl. Environ. Microbiol. 1979;37:849–853. doi: 10.1128/aem.37.5.849-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Guevel R., Pakdel F. Assessment of oestrogenic potency of chemicals used as growth promoter by in vitro methods. Hum. Reprod. 2001;16:1030–1036. doi: 10.1093/humrep/16.5.1030. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y., Yoshisawa T., Katayama T. Comparative study on the natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in corn and wheat from high- and low-risk areas for human esophageal cancer in China. Appl. Environ. Microbiol. 1990;56:3723–3726. doi: 10.1128/aem.56.12.3723-3726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maaroufi K., Chekir L., Creppy E.E., Ellouz F., Bacha H. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon. 1996;34:535–540. doi: 10.1016/0041-0101(96)00008-6. [DOI] [PubMed] [Google Scholar]
- 15.Malekinejad H., Mass-Bakker R., Fink-Gremmels J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006;172:96–102. doi: 10.1016/j.tvjl.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Minervini F., Giannoccaro A., Cavallini A., Visconti A. Investigations on cellular proliferation induced by zearalenone and its derivatives in relation to the estrogenic parameters. Toxicol. Lett. 2005;159:272–283. doi: 10.1016/j.toxlet.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Mirocha C.J., Pathre P.V., Robinson T.S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 1981;19:25–30. doi: 10.1016/0015-6264(81)90299-6. [DOI] [PubMed] [Google Scholar]
- 18.NTP . National Toxicology Program; Research Triangle Park, NC, USA: 1982. Carcinogenicity bioassay of zearalenone in F344/N rats and F6C3F1 mice. National Toxicology Program Technical Reports Series 235. [Google Scholar]
- 19.Olsen M., Malmlof K., Pettersson K., Sandholm K., Kiessling K.H. Plasma and urinary levels of zearalenone and α-zearalenone in a prepubertal gilts fed zearalenone. Acta Pharmacol. Toxicol. (Copenh.) 1985;56:239–243. doi: 10.1111/j.1600-0773.1985.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 20.Osselaere A., Devreese M., Goossens J., Vandenbroucke V., De Barer S., De Backer P., Croubels S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem. Toxicol. 2013;51:350–355. doi: 10.1016/j.fct.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Price W.D., Lovell R.A., McChesney D.G. Naturally occurring toxins in feedstuffs: Center for Veterinary Medicine Perspective. J. Anim. Sci. 1993;71:2556–2562. doi: 10.2527/1993.7192556x. [DOI] [PubMed] [Google Scholar]
- 22.Richardson K.E., Hagler W.M., Campbell C.L., Hamilton P.B. Production of zearalenone, T-2 toxin, and deoxynivalenol by Fusarium spp. isolated from plant materials grown in North Carolina. Mycopathologia. 1985;90:155–160. doi: 10.1007/BF00436731. [DOI] [PubMed] [Google Scholar]
- 23.Schollenberger M., Muller H.M., Rufle M., Suchy S., Plank S., Drochner W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia. 2006;161:43–52. doi: 10.1007/s11046-005-0199-7. [DOI] [PubMed] [Google Scholar]
- 24.Shin B.S., Hong S.H., Bulitta J.B., Hwang S.W., Kim H.J., Lee J.B., Yang S.D., Kim J.E., Yoon H.S., Kim D.J., Yoo S.D. Disposition, oral bioavailability, and tissue distribution of zearalenone in rats at various dose levels. J. Toxicol. Environ. Health A. 2009;72:1406–1411. doi: 10.1080/15287390903212774. [DOI] [PubMed] [Google Scholar]
- 25.Songsermsakul P., Sontag G., Cichna-Markl M., Zentek J., Razzazi-Fazeli E. Determination of zearalenone and its metabolites in urine, plasma and faeces of horses by HPLC–APCI-MS. J. Chromatogr. B. 2006;843:252–261. doi: 10.1016/j.jchromb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Weaver G.A., Kurtz H.J., Behrens J.C., Robinson T.S., Seguin B.E., Bates F.Y., Mirocha C.J. Effects of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 1986;47:1395–1397. [PubMed] [Google Scholar]
- 27.Wood G.E. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 1992;70:3941–3949. doi: 10.2527/1992.70123941x. [DOI] [PubMed] [Google Scholar]
- 28.Ueno Y., Tashiro F. Alpha-zearalenol, a major hepatic metabolite in rats of zearalenone, an estrogenic mycotoxin of Fusarium species. J. Biochem. (Tokyo) 1981;89:563–571. doi: 10.1093/oxfordjournals.jbchem.a133232. [DOI] [PubMed] [Google Scholar]
- 29.Zollner P., Jodlbauer J., Kleinova M., Kahlbacher H., Kuhn T., Hochsteiner W., Lindner W. Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin-contaminated oats. J. Agric. Food Chem. 2002;50:2494–2498. doi: 10.1021/jf0113631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.