Abstract
Bisphenol A (BPA) safety aspects on human health are debated extensively for long time. In the present study, we have studied the toxicity induced by BPA at no observed adverse effect level (NOAEL) using HepG2 cells. We report that BPA at 100 nM induced cytotoxicity to HepG2 cells as determined by MTT assay at 0–72 h. The toxicity was result of reduced oxygen consumption and reduced mitochondrial membrane potential associated with decreased ATP production. The BPA treatment resulted in increase of malondialdehyde (MDA) content with decreased glutathione and other antioxidant enzymes. BPA derived toxicity is a concern to human health and alternative non-toxic natural products/derivatives or adjuvants that serve as antidote will be relevant. In this context, Ashwagandha (Withania somnifera) a widely used herb to treat arthritis, rheumatism and to improve longevity for time immemorial is investigated for its antidote effect. Ashwagandha supercritical CO2 extract derived Withanolides (ADW) at 100 μg/ml protect HepG2 cells from BPA induced toxicity by suppressing mitochondrial damage and increased ATP production. Further, cellular MDA content was significantly suppressed with increased non-enzymic and antioxidant enzyme activities. These findings derived from the present study suggest the beneficial effect of ADW in mitigating BPA induced mitochondrial toxicity in HepG2 cells.
Abbreviations: GSH, reduced glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; LPO, lipid peroxidation; ADW, Ashwagandha supercritical CO2 extract derived Withanolides; ΔΨM, mitochondrial membrane potential
Keywords: Bisphenol A, HepG2 cells, Ashwagandha, Mitochondrial toxicity, Glutathione, Antioxidant enzymes
1. Introduction
Bisphenol A (BPA), is an industrial chemical that has been present in many hard plastic bottles, including baby bottles, food storage containers and dental sealants [1], [2]. Trace amount of BPA released from these products gets into food and consumed by humans. Thus, in humans, BPA is detected not only in serum and urine but also in the placenta and amniotic fluid [3], [4].
Studies employing standardized toxicity have thus far supported the safety of current low levels of human exposure to BPA [5], [6], [7]. However, considering that human exposure is abundant and prolonged, there are controversies about this criteria based on single dose exposure in animal studies. Recently, several studies have been being carried and found that a low dose of BPA below the no observed adverse effect level (NOAEL) have significant effects [8], [9].
The adverse effects of BPA are largely related to its estrogenic activity [10], [11] and result in disturbances to reproductive function [12], steroidogenesis [13] and adipogenesis [14]. However, BPA is reported to induce inflammatory cytokines [14] associated with increased oxidative stress which is detrimental to cell viability [15], [16].
The liver is the major organ for the metabolism and detoxification of xenobiotics, including BPA [17]. Therefore, the liver could be largely exposed to BPA, and could be susceptible to regular doses, than other organs. In humans, the urinary concentration of BPA was associated with abnormal liver function [18]. There are some reports that high doses of BPA altered liver weights in mice or rats [6], [7] and decreased the viability of rat hepatocytes [15]. Human hepatocarcinoma HepG2 cells are widely studied cell lines to understand the xenobiotic metabolism. It contains the entire battery of detoxification enzymes to metabolize BPA to sulfate and glucuronide conjugates [19], [20] and certainly qualifies as an in vitro model to study the BPA toxicity and serves as a platform to identify pharmacologically active compounds which acts as an antidote.
Ashwagandha (Withania somnifera) is a popular herb used in traditional medicine and remedies that have been in practice in India from time immemorial. Although trusted for its wide health benefits, the active principles of Ashwagandha for its pharmacological effects have not been understood to large extent. Recently, few studies using cell and animal models have demonstrated anti-inflammatory, anti-cancer, anti-diabetic, anti-stress, anti-oxidant, neuroprotective and immune-modulatory potentials of Ashwagandha and its derivatives [21], [22].
Thus, it is postulated that supercritical CO2 extract (SCFE) of Ashwagandha principally containing withanolides may rescue liver from BPA induced toxicity. Hence, we evaluated hepatoprotective effect of Ashwagandha derived withanolides against BPA induced toxicity in vitro using HepG2 cells.
2. Materials and methods
2.1. Chemicals
Bradford reagent, Bisphenol-A, cytochrome-C, 2,2-diphenyl-1-picryl hydrazyl (DPPH), diphenylamine (DPA), Dulbecco's minimum essential medium (DMEM), ferric chloride (anhydrous), Fetal bovine serum (FBS), glutathione (GSH), hydrogen peroxide, 3(4,5-dimethyl thiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT), β-nicotinamide adenine dinucleotide phosphate (β-NADPH), perchloric acid, thiobarbituric acid, xanthine and xanthine oxidase were purchased from Sigma–Aldrich (St. Louis, MO, USA). Oxygen Consumption Rate Assay Kit, (Cayman Chemical Company, 1180 E. Ellsworth Rd. Ann Arbor, MI 48108) ATP Colorimetric/Fluorometric Assay Kit, Bio Vision, Inc., 980 Linda Vista Avenue, Mountain View, CA 94043 All other reagents were of analytical grade.
2.2. Ashwagandha supercritical CO2 extract
Standardized Ashwagandha supercritical fluid (CO2) extract (ADW) was procured from Department of Phytochemistry – R&D centre, The Himalaya Drug Company, Bangalore, India. Briefly, 25 kg of the roots of Ashwagandha (Withania sominifera) was pulverized to fine powder and loaded with extractor. Super critical CO2 was pumped into the extractor at a pressure of 300 bar and 39 °C temperature for 2–3 h. Extract was separated into the container at pressure of 40 bar and 20 °C. The CO2 super critical liquid was recycled from the extraction vessel. The good agricultural and collection practices (GACP) were employed during farming, harvesting and collection of plant. The plant W. sominifera was identified and certified by Botanist and a voucher specimen of the same has been archived in the herbarium of R&D, The Himalaya Drug Company, Bangalore, India.
2.2.1. ADW marker compound analysis by mass spectrometry
The API 2000 (Applied biosystem/MDS SCIEX, Canada) mass spectrometer coupled with ESI (Electron spray ionization) source as an ionization interface and a chromatographic system. Batch acquisition and data processing was controlled by Analyst 1.5 version software. The MS parameters optimization was carried out with 1 mg/ml concentration of working solution of withania CO2 extract prepared in methanol (J.T. Baker brand). Molecular ionization intensity response was checked in both positive and negative ionization mode. It was found good intense response in the positive mode and other parameters like declustering potential (DP) 20 V, ion source gas (GS1 and GS2)55 and 65 psi, curtain gas (CUR) 30 psi, focusing potential (FP) 400 V, source temperature (TEM) 400 °C and ion spray voltage (IS) 5500 V and entrance potential (EP) 10 V were optimized to provide best sensitivity by multiple run through liquid chromatographic system. The compounds identified by mass spectrometry (Fig. 1) were characterized and given in Table 1.
Fig. 1.
Mass chromatogram showing compounds of Ashwagandha supercritical fluid (CO2) extract (ADW). The mass spectrometric conditions are as described in the text.
Table 1.
Compounds identified in Ashwagandha super-critical CO2 extract by mass spectrometry analysis.
| Sl. no. | Name of the compounds | Molecular mass (Da) | Obtained molecular mass [M+H] |
|---|---|---|---|
| 1 | Withaferin A, Withanolide F, Withanolide H, Withanolide J, Withanolide K | 470.60 | 471.25 m/z |
| 2 | Withanolide G, Withanolide I, Withanolide P | 454.60 | 455.25 m/z |
| 3 | Withanolide L | 452.60 | 453.20 m/z |
| 4 | Dihydrowithaferin A | 456.61 | 457.20 m/z |
2.3. Cell culture
All the experiments were performed using HepG2 cells on 10 passages after thawing. The HepG2 cells (Hepatocellular carcinoma cell line) was obtained from the National Center for Cell Science (NCCS) Pune, India, were maintained in culture using 25 cm2 polystyrene flasks (Tarsons) with DMEM containing 10% FBS, 1% antibiotic–antimycotic solution, and 3.7 g/L sodium bicarbonate under an atmosphere of 5% CO2 at 37 °C with 95% humidity. Continuous cultures were maintained by sub-culturing cells every 4 days at 2.2 × 106 cells/25 cm2 flasks by trypsination.
2.4. BPA induced cytotoxicity and protection by ADW
HepG2 cells were plated in 96-multiwell culture plates at 1 × 105 cells per well. To study BPA induced cytotoxicity, 24 h after plating, the medium was discarded and fresh medium containing BPA at various concentrations (10–100 nM) was added. At different time points (0–72 h), cellular viability was determined by the MTT assay [23]. In order to determine the effective concentration of ADW that protects 50% (EC50) of the cells from damage induced by the toxicant, cells were incubated with BPA for 0–72 h to induce significant cell death. Based on the dose–response curves of cell death protection by ADW against the BPA induced toxicity in HepG2 cells, the EC50 concentration was determined and used in the experiments to evaluate the protective potential of the ADW on several cellular parameters.
2.5. Oxygen consumption rate assay
Oxygen consumption rate assay kit was used to measure the oxygen consumption rate of the mitochondria in HepG2 cells according to manufacturer's instruction (Cayman). Briefly, HepG2 cells were plated in 96-multiwell black culture plates at 1 × 105 cells per well and incubated overnight. The spent culture medium was removed from all wells and replaced with 150 μl of fresh medium with or without test compound along with experimental controls. The readings were recorded using (BioTek, KC-4) plate on fluorometric mode by following the kinetics of the reaction at excitation 380 nm and emission 650 nm for 200 min with 1 min interval time.
2.6. ATP assay
The cellular ATP concentration was measured using an ATP Colorimetric/Fluorometric Assay Kit (BioVi-sion). Cells (106) were lysed in 100 μl of ATP assay buffer, homogenized, and centrifuged (13,000 × g, 2 min, 4 °C) to pellet insoluble materials. The supernatants were collected and added to 96-well plates (50 μl per well) along with 50 μl/well of the reaction mixture (ATP probe, ATP Converter, Developer Mix in ATP assay buffer). The plates were incubated at room temperature for 30 min, while being protected from light and absorbance in the wells was measured at 570 nm using a micro-plate reader (BioTek–KC-4). The absorbance of the no-ATP control was subtracted from each reading.
2.7. Mitochondrial membrane potential assay
Mitochondrial membrane potential (ΔΨM) was assessed using the fluorescent potentiometric dye JC-1 as described previously [24], [25]. Briefly, at 24 h after the BPA treatment with or without ADW extract HepG2 cells were harvested, washed twice with PBS, and centrifuged for 8 min at 4500 rpm at room temperature. Then the cells were suspended with JC-1 (5 μg/ml) in serum-free RPMI-1640 and incubated for 15 min at 37 °C. After staining, the cells were collected at room temperature and washed thrice with pre-warmed PBS. The cell pellet was then re-suspended in 1 ml of PBS. JC-1 fluorescence was quantitated using a fluorescence plate reader (BioTek, KC-4) at 37 °C. The fluorescence of the JC-1 monomer was measured at 485 nm (excitation) and 590 nm (emission). For each experiment, the ratios of J-1 aggregate to JC-1 monomer were normalized to untreated controls; values reported, therefore, represent a percentage of mitochondrial function in untreated cells.
2.8. Lipid peroxidation, glutathione levels and antioxidant enzyme activities in HepG2 cells
HepG2 cells were grown in 24 well plates until 70% confluence. Further cells were treated with BPA with or without ADW extract along with experimental controls. Twenty-four hours later, cell culture medium and cell scrapings were harvested and kept at −80 °C for following quantification of several parameters. Cell scrapings were harvested in lysis buffer (25 mM KH2PO4, 2 mM MgCl2, 5 mM KCl, 1 mM EDTA, 1 mM EGTA, 100 μM PMSF, pH 7.5) after rinsing the cells with PBS, (pH 7.4).
2.9. Biochemical analysis
2.9.1. Lipid peroxidation
The extent of lipid peroxidation was estimated by the levels of malondialdehyde measured using the thiobarbituric acid reactive substances (TBARS) assay at 535 nm [26]. The results are expressed as nmol/mg of protein using a molar extinction coefficient of 1.56 × 105 M Cm−1.
2.9.2. Measurement of nonenzymic antioxidants
Cells were homogenized in trichloroacetic acid (5%, w/v), and deproteinized supernatant was used for GSH assay. The glutathione content in the cell homogenate was determined by the DTNB-GSSG reductase recycling assay as previously described [27]. The results are expressed as nmol GSH/mg of protein.
2.9.3. Measurement of enzymic antioxidants
The antioxidant enzymes superoxide dismutase (SOD), catalase and glutathione peroxidase, (GPx) activities were analyzed using cytosolic fraction. Total SOD activity was determined by monitoring the inhibition of the reduction of ferricytochrome C at 550 nm, using the xanthine–xanthine oxidase system as the source of superoxide. One unit of the SOD is defined as the amount of the enzyme required to inhibit 50% of the rate of cytochrome C reduction [28]. Catalase activity was measured by following the rate of H2O2 consumption spectrophotometrically at 240 nm and expressed as μmol H2O2 oxidized/min/mg protein [29]. Glutathione peroxidase activity was determined by following the enzymatic NADPH oxidation at 340 nm [30].
2.10. Statistical analysis
Statistical analysis was carried out using Graph Pad Prism statistical software (Graph Pad Prism, San Diego, CA, USA). Results are analyzed by one-way analysis of variance (ANOVA) and the significance was calculated using the Tukey–Kramer multiple comparison test and results are considered as significant at P < 0.05.
3. Results
3.1. Cytotoxicity
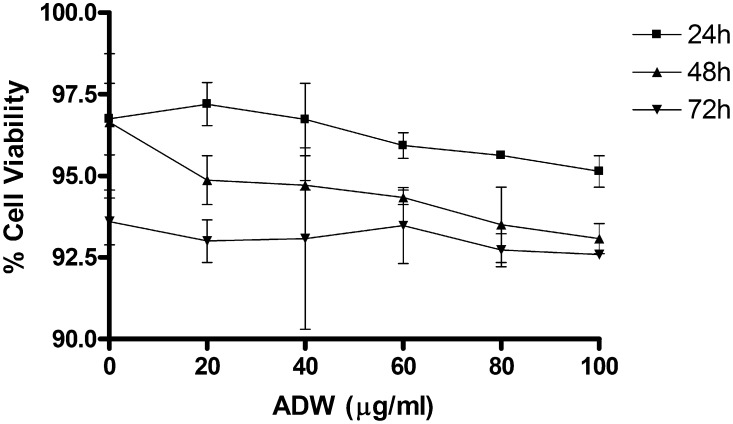
Cytotoxicity of BPA and ADW in HepG2 cells was evaluated using MTT assay (Fig. 2, Fig. 3). ADW did not present any cytotoxic effect at concentration ranging from 0 to 100 μg/ml (when tested for 0–72 h). On the other hand BPA was tested for its cytotoxicity with wide range of concentration for 0–72 h and the results are given in Fig. 2. The results showed that BPA at (10–200 nM) caused cytotoxicity to HepG2 cells. The CTC50 of BPA was determined to be 100 nM at 72 h. Hence, the cells were challenged against CTC50 in all the subsequent experiments for testing protective effect of ADW.
Fig. 2.
Effect of various concentrations of Bisphenol A on HepG2 cell cytotoxicity at 0–72 h. The cells were incubated with incremental concentrations of BPA and incubated for 24, 48 and 72 h and the cytotoxicity was determined as described in Section 2. Values are Mean ± SEM of three independent experiments carried out in triplicates.
Fig. 3.
Effect of incremental concentrations of Ashwagandha derived Withanolides (ADW) on HepG2 cell cytotoxicity at 0–72 h. The cells were incubated with various concentrations of ADW and incubated for 24, 48 and 72 h and the cytotoxicity was determined as described in Section 2. Values are Mean ± SEM of three independent experiments carried out in triplicates.
3.2. Effect of ADW on BPA induced toxicity on HepG2 cell viability
The ADW protection against BPA induced cytotoxicity was evaluated by MTT assay (Fig. 4). The cells were incubated with ADW (100 μg/ml) and BPA (100 nM) for 0–72 h and the cell viability was measured. BPA induced 6%, 35% and 56% cytotoxicity in HepG2 cells at 24, 48 and 72 h. The mitochondrial respiration inhibitor Antimycin A was used as negative control was very effective and caused 57%, 65% and 84% cytotoxicity to cells at 24, 48 and 72 h respectively. When ADW (100 μg/ml) was co-incubated with BPA, cell viability was significantly increased from 45% to 78% compared to BPA treated group and showed rescue effect of ADW against BPA induced toxicity.
Fig. 4.
Effect ADW on cell viability during BPA induced toxicity at 0–72 h. HepG2 cells were co-incubated with BPA (100 nM) in the presence or absence of ADW (100 μg/ml) for 0–72 h and the cytotoxicity was determined at 24, 48 and 72 h as described in Section 2. Antimycin A (10 μM) was used as positive control. Values are Mean ± SEM of three independent experiments carried out in triplicates. **Statistically significant at P < 0.05 compared to control. *Statistically significant at P < 0.05 compared to BPA.
3.3. Effect of ADW during BPA induced toxicity on mitochondrial functions
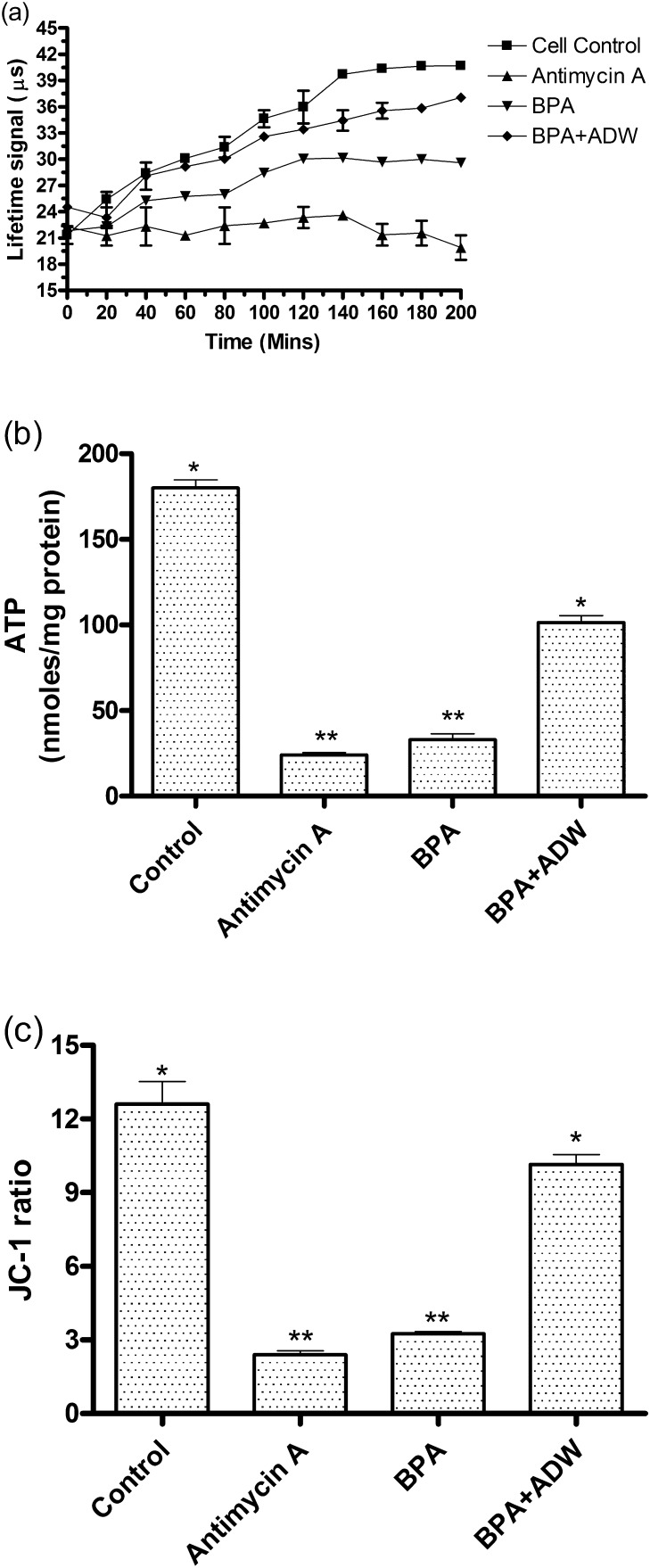
The oxygen consumption rate in the mitochondria of HepG2 cells treated with BPA was evaluated and the results are given in Fig. 5(a). The results show that BPA and antimycin A treated cells showed to decreased oxygen consumption compared to control which was measured as fluorescent life time signal (μs) over a period of 0–200 min. When the cells were treated with ADW along with BPA the oxygen consumption was increased significantly over 0–200 min and the oxygen consumption pattern was comparable to control cells.
Fig. 5.
Effect ADW on mitochondrial function during BPA induced toxicity. HepG2 cells were incubated with BPA (100 nM) in the presence or absence of ADW (100 μg/ml) for 72 h and the (a) oxygen consumption rate, (b) ATP content and (c) mitochondrial membrane potential was determined as described in Section 2. Antimycin A (10 μM) was used as positive control. Values are Mean ± SEM of three independent experiments carried out in triplicates. **Statistically significant at P < 0.05 compared to control. *Statistically significant at P < 0.05 compared to BPA.
The ATP concentration was measured in the HepG2 cells treated with BPA and the results are presented in Fig. 5(b). The results show that ATP level in the cells treated with BPA and antimycin A was significantly reduced by 7.5 folds and 5.45 folds compared to control at 24 h incubation time. While cells treated with ADW along with BPA could withstand the ATP depletion in a significant manner.
The mitochondrial membrane potential (ΔΨM) using JC-1 stain was measured in HepG2 cells treated with BPA and the results are given in Fig. 5(c). At 24 h the ΔΨM was increased significantly by 3.9 and 5.25 folds in cells treated with BPA and antimycin A. Whereas, the cells treated with ADW along with BPA significantly inhibited the increase in ΔΨM and inhibited mitochondrial membrane damage.
3.4. Effect of ADW on lipid peroxidation and non-enzymic antioxidants
The lipid peroxidation was significantly increased by 2.4 folds upon addition of BPA in HepG2 cells as shown in Fig. 6. The cells treated with antioxidants such as vitamin E and BHA could significantly inhibit the lipid peroxidation induced by BPA. In similar lines, ADW addition was very effective and significantly reduced the lipid peroxidation by 63.16% compared to BPA treated cells.
Fig. 6.
Effect of ADW on lipid peroxidation during BPA induced toxicity in HepG2 cells. HepG2 cells were co-incubated with BPA (100 nM) in presence or absence of ADW (100 μg/ml), vitamin E (25 μM) or BHA (10 μM) and the lipid peroxidation was determined at 24 h as described in Section 2. Vitamin E (25 μM) and BHA (10 μM) was used as negative controls. Values are Mean ± SEM of three independent experiments carried out in triplicates. **Statistically significant at P < 0.05 compared to control. *Statistically significant at P < 0.05 compared to BPA.
The effect of BPA treatment on GSH and GSSG levels in the HepG2 cells was evaluated and the results are given in Table 2. The results showed that non-enzymic antioxidant glutathione content was significantly reduced by 2.94 folds upon BPA treatment compared to control cells. The antimycin A treated group showed 4.29 folds reduction in GSH content. While, addition of ADW and vitamin E to cells treated with BPA showed to inhibit GSH depletion significantly. The BPA and antimycin A treatment significantly reduced the GSH/GSSG ratio compared to control cells. But ADW and vitamin E significantly increased the GSH/GSSG ratio. However increase in GSSG content is not proportional to depleted GSH in BPA and antimycin A treated cells.
Table 2.
Effect of ADW on non-enzymic antioxidants during BPA induced toxicity in HepG2 cells. Values are Mean ± SEM of three independent experiments carried out in triplicates.
| Groups | GSH (nmol/mg protein) | GSSG (nmol GSH equiv/mg protein) | GSH/GSSG ratio |
|---|---|---|---|
| Control | 78.45 ± 2.22* | 9.58 ± 1.35* | 8.18 |
| Antimycin A (10 μM) | 18.28 ± 3.15** | 33.97 ± 0.98** | 0.53 |
| BPA (100 nM) | 26.64 ± 4.12** | 14.68 ± 2.32 | 1.81 |
| BPA (100 nM) + vitamin E (25 μM) | 64.45 ± 2.22* | 14.58 ± 1.35 | 4.42 |
| BPA (100 nM) + ADW (100 μg/ml) | 68.89 ± 5.19* | 11.23 ± 3.68* | 5.94 |
Statistically significant at P < 0.05 compared to control.
Statistically significant at P < 0.05 compared to BPA.
3.5. Effect of ADW on antioxidant enzyme activity in BPA induced toxicity
The antioxidant enzymes catalase, glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities were evaluated and the results (Table 3) showed that BPA and Antimycin A inhibited the catalase activity by 66 and 61% respectively. The GPx activity was inhibited by 42 and 59% and SOD activity was inhibited by 38 and 54% respectively in BPA and antimycin A induced toxic conditions. Upon addition of ADW to cells treated with BPA the catalase activity was doubled, whereas GPX and SOD activity were increased by 25 and 3% respectively compared to BPA treated group. The antioxidant enzyme activities were increased in vitamin E treated groups challenged with BPA and the results are comparable with normal control cells.
Table 3.
Effect of ADW on antioxidant enzymes catalase, glutathione peroxidase and superoxide dismutase activity during BPA induced toxicity in HepG2 cells. Values are Mean ± SEM of three independent experiments carried out in triplicates.
| Groups | Catalasea | Glutathione peroxidiseb | Superoxide dismutasec |
|---|---|---|---|
| Control | 1.45 ± 0.18* | 28.68 ± 1.35* | 45.58 ± 1.56* |
| Antimycin A (10 μM) | 0.58 ± 0.05** | 11.57 ± 0.98** | 20.97 ± 1.51** |
| BPA (100 nM) | 0.48 ± 0.10** | 16.58 ± 1.68** | 28.28 ± 1.98** |
| BPA (100 nM) + vitamin E (25 μM) | 0.84 ± 0.12* | 23.68 ± 1.32* | 35.64 ± 3.11* |
| BPA (100 nM) + ADW (100 μg/ml) | 0.96 ± 0.09* | 20.67 ± 1.12* | 29.15 ± 2.61 |
Statistically significant at P < 0.05 compared to control.
Statistically significant at P < 0.05 compared to BPA.
μmol of H2O2 decomposed/min/mg protein.
μmol of NADPH oxidized/min/mg protein.
Units/mg protein.
4. Discussion
BPA is one of the major chemical contaminants produced worldwide and reported to have adverse effects on human health [10], [11], [12], [13], [14]. We report even below its NOAEL levels, it is shown to exert deleterious effects against human hepatocarcinoma HepG2 cells in vitro. Bisphenol A at 100 nM induced cytotoxicity in HepG2 cells in a time dependent manner. It is observed that at 24 h BPA induced 6% cytotoxicity to cells, whereas after 48 h it was 35% followed by 56% at the end of 72 h incubation. The mitochondrial respiratory inhibitor antimycin A (10 μM) induced toxicity over a period of 0–72 h in similar lines with BPA. Thus, demonstrating BPA was detrimental to cell viability and indicated as a potent mitochondrial respiratory inhibitor during 72 h incubation. Addition of ADW obtained through SCFE at 100 μg/ml to cells treated with BPA significantly increased the cell viability from 45 to 78% showing that herbal extract exerts cytoprotection by inhibition mitochondrial toxicity.
Taking a cue from the above observation it was experimentally shown that BPA disrupts mitochondrial homeostasis and induced superoxide anions production leading to excessive lipid peroxidation and increased mitochondrial membrane potential which is in agreement with earlier reports [31]. The susceptibility of HepG2 cells toward BPA induced cytotoxicity showed good co-relation between initial cell viability and lipid peroxidation compared to control in the present study (P < 0.05). While addition of ADW significantly increased the cell viability with decreased lipid peroxidation showing that herbal extract exerts cytoprotection by preventing excessive lipid peroxidation at first instance.
Majority of the studies till date have shown that BPA induced oxidative stress mediated mitochondrial dysfunction is the major cause for cytotoxicity [31]. The mitochondria are vital cellular machines for maintaining cellular energy and use oxygen to produce ATP through a process known as oxidative phosphorylation [32]. The inner mitochondrial membrane contains a respiratory chain of four multi-subunit protein complexes that release energy used to pump protons across this membrane. The created electrochemical gradient of protons and resulting mitochondria membrane potential (ΔΨM), drives ATP formation from ADP and phosphate [32]. Thus, any damage to mitochondria plays an important role in a wide range of human diseases [33], [34]. Cell death will be mediated by series of events like loss of ΔΨM, release of cytochome c, and depletion of ATP [35].
In normal physiologically active cells electrons provided to the respiratory chain by the oxidation of NADH and FADH2 are transferred from complex to complex and generate an electrochemical potential ΔΨM across the inner membrane. When protons accumulate in metabolically altered mitochondria, the ΔΨM increases and the mitochondria are hyperpolarized. This state is usually associated with ROS generation, due to poor electron flux leading to a direct reaction with oxygen [36]. If detoxification systems like manganese superoxide dismutase (MnSOD), mitochondrial glutathione peroxidase or GSH are overwhelmed, the ROS levels are increased, mitochondrial functions are impaired and cellular reactions can also be disturbed [37], [38].
Our results are in agreement with earlier reports and it showed that mitochondrial oxygen consumption pattern in the cells treated with BPA was significantly reduced compared to control with substantial decrease in the ATP content and increased mitochondrial membrane potential (ΔΨM). On contrary, cytotoxic effect mediated by increased lipid peroxidation and mitochondrial dysfunction due to BPA was negated by treatment with ADW in HepG2 cells. It was clearly shown that oxygen consumption pattern, ATP production was significantly increased, while ΔΨM was decreased thus facilitating the increased survival of HepG2 cells. But similar results were not observed with natural antioxidant vitamin E (results not shown) thus indicating that compounds present in ADW exerted cellular protection by novel mechanism not in lines with natural antioxidant compounds.
During mitochondrial toxicity due to impaired oxygen consumption and ATP production cellular antioxidant system plays a significant role in restoring the normal function of hepatocytes. Beside reversal of mitochondrial associated toxicity by ADW, we report significant decrease in lipid peroxides (MDA) with increased enzymic and non-enzymic antioxidant levels in HepG2 cells which is detrimental for maintaining cellular homeostasis. It is known that, GSH a non-enzymic antioxidant plays an important role in hepatocyte defense against ROS, free radicals and electrophilic metabolites [39], [40]. Hence, severe GSH depletion leaves cells more vulnerable to oxidative damage by radicals and increases protein thiolation or oxidation of SH groups that may lead to alterations in cellular calcium homeostasis [40]. A sustained increase in cytosolic calcium levels results in activation of enzymes (phospholipases, non-lysomal proteases, endonucleases) and cytoskeletal damage, which ultimately causes cell death [40]. The potential of ADW to maintain GSH at reasonably high levels is of importance against BPA induced toxicity. Therefore, the ability of ADW in preventing BPA induced GSH depletion by about 80% is very significant in restoring the cell viability. The GSSG formation was inhibited by ADW and this may be attributed to the formation of GSH conjugates rather than oxidation to GSSG in BPA induced toxic conditions. Earlier it was shown that Ashwagandha leaf extract and withanone, a major constituent in the leaf was beneficial to normal human fibroblasts and it showed, it was helpful to increase life span of fibroblasts by the reducing molecular damage and rendered protection against oxidative stress [41], [42]. In yet another study, it was clearly demonstrated that withanone significantly rescued the damages caused by methoxyacetic acid mediated mitochondrial dysfunction through inhibition of excessive reactive oxygen species which were detrimental to mitochondrial function [43].
Beside these, cells secrete strong antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase to combat severe oxidative stress during diseased/toxic conditions. Earlier it is reported that BPA exposure results severe ROS production and results in the inhibition of antioxidant enzymes [44]. In agreement with earlier reports the antioxidant enzymes viz.; superoxide dismutase, catalase and glutathione peroxidase activities were severely diminished with addition of BPA. While addition of ADW to cells treated with BPA showed increased antioxidant enzyme activities along with increased mitochondrial functions. The increase in the antioxidant enzyme activities adds to the fact that ADW restores and replenishes the antioxidant system and aids to restore normal mitochondrial functions in BPA intoxicated cells. Thus it is concluded that ADW exerts a strong cellular rejuvenation and acts as an antidote against NOAEL concentrations of BPA in HepG2 cells.
Conflict of interest
None.
Footnotes
Available online 3 July 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.008.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Brotons J.A., Olea-Serrano M.F., Villalobos M., Pedraza V., Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olea N., Pulgar R., Pérez P., Olea-Serrano F., Rivas A., Novillo-Fertrell A., Pedraza V., Soto A.M., Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikezuki Y., Tsutsumi O., Takai Y., Kamei Y., Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 4.Calafat A.M., Kuklenyik Z., Reidy J.A., Caudill S.P., Ekong J., Needham L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority (EFSA) Statement of EFSA on a study associating bisphenol A with medical disorders. Eur. Food Saf. Auth. J. 2008;838:1–3. [Google Scholar]
- 6.Tyl R.W., Myers C.B., Marr M.C., Thomas B.F., Keimowitz A.R., Brine D.R., Veselica M.M., Fail P.A., Chang T.Y., Seely J.C. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002;68:121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 7.Tyl W., Myers C.B., Marr M.C., Sloan C.S., Castillo N.P., Veselica M.M., Seely J.C., Dimond S.S., Van Miller J.P., Shiotsuka R.N. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 8.vom Saal F.S., Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welshons W.V., Nagel S.C., vom Saal F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 10.Kurosawa T., Hiroi H., Tsutsumi O., Ishikawa T., Osuga Y., Fujiwara T., Inoue S., Muramatsu M., Momoeda M., Taketani Y. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocrinol. J. 2002;49:465–471. doi: 10.1507/endocrj.49.465. [DOI] [PubMed] [Google Scholar]
- 11.Hiroi H., Tsutsumi O., Momoeda M., Takai Y., Osuga Y., Taketani Y. Differential interactions of bisphenol A and 17beta-estradiol with estrogen receptor alpha (ERalpha) and ERbeta. Endocrionl. J. 1999;46:773–778. doi: 10.1507/endocrj.46.773. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi T., Tsutsumi O., Ikezuki Y., Takai Y., Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocrinol. J. 2004;51:165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X., Chang H., Wiseman S., He Y., Higley E., Jones P., Wong C.K., Al-Khedhairy A., Giesy J.P., Hecker M. Bisphenol A disrupts steroidogenesis in human H295R cells. Toxicol. Sci. 2011;121:320–327. doi: 10.1093/toxsci/kfr061. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Jonathan N., Hugo E.R., Brandebourg T.D. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol. Cell. Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa Y., Tayama S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch. Toxicol. 2000;74:99–105. doi: 10.1007/s002040050659. [DOI] [PubMed] [Google Scholar]
- 16.Asahi J., Kamo H., Baba R., Doi Y., Yamashita A., Murakami D., Hanada A., Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010;87:431–438. doi: 10.1016/j.lfs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Knaak J.B., Sullivan L.J. Metabolism of bisphenol A in the rat. Toxicol. Appl. Pharmacol. 1966;8:175–184. doi: 10.1016/s0041-008x(66)80001-7. [DOI] [PubMed] [Google Scholar]
- 18.Lang I.A., Galloway T.S., Scarlett A., Henley W.E., Depledge M., Wallace R.B., Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 19.Audebert M., Dolo L., Perdu E., Cravedi J.P., Zalko D. Use of the gamma H2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch. Toxicol. 2011;85(11):1463–1473. doi: 10.1007/s00204-011-0721-2. [DOI] [PubMed] [Google Scholar]
- 20.Bursztyka J., Perdu E., Pettersson K., Pongratz I., Fernandez-Cabrera M., Olea N., Debrauwer L., Zalko D., Cravedi J.P. Biotransformation of genistein and bisphenol A in cell lines used for screening endocrine disruptors. Toxicol. In Vitro. 2008;22:1595–1604. doi: 10.1016/j.tiv.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Aalinkeel R., Hu Z., Nair B.B., Sykes D.E., Reynolds J.L. Genomic analysis highlights the role of the JAK-STAT signaling in the antiproliferative effects of dietary flavonoid – ‘Ashwagandha’ in prostate cancer cells. Evid. Based Complement. Altern. Med. 2010;7:177–187. doi: 10.1093/ecam/nem184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah N., Kataria H., Kaul S.C., Ishii T., Kaur G. Effect of the alcoholic extract of Ashwagandha leaves and its components on proliferation, migration, and differentiation of glioblastoma cells: combinational approach for enhanced differentiation. Cancer Sci. 2009;100:1740–1747. doi: 10.1111/j.1349-7006.2009.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Desai B.N., Myers B.R., Schreiber S.L. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2002;99(7):4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang T., Joseph J., Hillard C.J., Kalyanaraman B. Death-associated protein kinase as a sensor of mitochondrial membrane potential: role of lysosome in mitochondrial toxin-induced cell death. J. Biol. Chem. 2005;280(41):34644–34653. doi: 10.1074/jbc.M506466200. [DOI] [PubMed] [Google Scholar]
- 26.Ohakawa H., Ohishi U., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal. Biochem. 1979;95:145–149. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Anderson M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 28.Flohe L., Otting O. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 29.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 30.Flohe L., Gunzler W.A. Assays of glutathione peroxidise. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 31.Huc L., Lemarie A., Gueraud F., Helier-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation medicated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol. In Vitro. 2012;26:709–717. doi: 10.1016/j.tiv.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Castro I.P., Martins L.M., Tufi R. Mitochondrial quality control and neurological disease: an emerging connection. Expert Rev. Mol. Med. 2010;12:e12. doi: 10.1017/S1462399410001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valmas N., Zuryn S., Ebert P.R. Mitochondrial uncouplers act synergistically with the fumigant phosphine to disrupt mitochondrial membrane potential and cause cell death. Toxicology. 2008;252:33–39. doi: 10.1016/j.tox.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 35.Kuczynski B., Reo N.V. Evidence that plasmalogen is protective against oxidative stress in the rat brain. Neurochem. Res. 2006;31:639–656. doi: 10.1007/s11064-006-9061-7. [DOI] [PubMed] [Google Scholar]
- 36.Machida K., Tanaka T. Farnesol-induced generation of reactive oxygen species dependent on mitochondrial transmembrane potential hyperpolarization medicated by F(0)F(1)-ATPase in yeast. FEBS Lett. 1999;462:108–112. doi: 10.1016/s0014-5793(99)01506-9. [DOI] [PubMed] [Google Scholar]
- 37.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Pessayre D., Fromenty B. NASH: a mitochondrial disease. J. Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Kedderis G.L. Biochemical basis of hepatocellular injury. Toxicol. Pathol. 1996;24:77–83. doi: 10.1177/019262339602400111. [DOI] [PubMed] [Google Scholar]
- 40.Castell J.V., Gomez-Lechon M.J., Ponsoda X., Bort R. In vitro investigation of the molecular mechanisms of hepatotoxicity. In: Castell J.V., Gomez-Lechon M.J., editors. In Vitro Methods in Pharmaceutical Research. Academic Press; London: 1997. pp. 375–410. [Google Scholar]
- 41.Widodo N., Shah N., Priyandoko D., Ishii T., Kaul S.C. Deceleration of senescence in normal human fibroblasts by withanone extracted from Ashwagandha leaves. J. Gerontol. A: Biol. Sci. Med. Sci. 2009;64:1031–1038. doi: 10.1093/gerona/glp088. [DOI] [PubMed] [Google Scholar]
- 42.Widodo N., Priyandoko D., Shan N., Wadhwa R., Kaul S.C. Selective killing of cancer cells by Ashwagandha leaf extract and its component withanone involves ROS signaling. PLoS ONE. 2010;5:e13536. doi: 10.1371/journal.pone.0013536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priyandoko D., Ishii T., Kaul S.C., Wadhwa R. Ashwagandha leaf derived withanone protects normal human cells against the toxicity of methoxyacetic acid a major industrial metabolite. PLoS ONE. 2011;6:e19552. doi: 10.1371/journal.pone.0019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anjum S., Rahman S., Kaur M., Ahmad F., Rashid H., Ansari R.A., Raisuddin S. Melatonin ameliorates bisphenol-A induced biochemical toxicity in testicular mitochondria of mouse. Food Chem. Toxicol. 2011;49:2849–2854. doi: 10.1016/j.fct.2011.07.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.