Highlights
-
•
Intraperitoneal administration of 4-NP induces hepatic steatosis in male Sprague-Dawley rats.
-
•
Hepatocytes apoptosis is highly implicated in the occurrence and development of NAFLD.
-
•
Hepatic mitochondrial disturbance promotes deleterious consequences, such as OS and accumulation of triglycerides (steatosis).
Abbreviations: APNEIs, alkylphenol polyethoxylates; 4-NP, 4-nonylphenol; FAO, fatty acid oxidation; ROS, reactive oxygen species; Cyt c, cytochrome c; NAFLD, nonalcoholic fatty liver disease; NASH, non-alcoholic hepatic steatosis; OS, oxidative stress; GOT, glutamic-oxalacetic transaminase; GPT, glutamate pyruvate transaminase; γ-GT, gamma glutamyltransferase; LDH, lactate dehydrogenase; AhR, aril hydrocarbon receptor; PPAR, peroxisome proliferation-activated receptor; TAG, triacylglycerol; FFA, free fatty acid; HSC, hepatic stellate cell; IR, insulin resistance
Chemical compounds studied in this article: 4-Nonylphenol (PubChem CID: 1752), Xylene (PubChem CID: 6850715), Aprotinin (PubChem CID: 22833874), Bouin's fluid (PubChem CID: 124013), Hematoxylin Eosin (PubChem CID: 86598188), Trizol (PubChem CID: 378478), Superoxide (PubChem CID: 5359597), Malondialdehyde (PubChem CID: 10964), Hydrogen peroxide (PubChem CID: 784), Diamninobenzidine Tetrahydrochloride (PubChem CID: 23892), Collagenase (PubChem CID: 5046512), Tromethamine (Tris) (PubChem CID: 6503), Sodium chloride (PubChem CID: 5234), Phenylmethylsulfonyl fluoride (PubChem CID: 4784), Nitrotetrazolium Blue chloride (PubChem CID: 9281), Thiobarbituric Acid (PubChem CID: 2723628)
Keywords: 4-Nonylphenol, Hepatic steatosis, Oxidative stress, Genes, Liver, Apoptosis
Abstract
An emerging literature suggests that early life exposure to 4-nonylphenol (4-NP), a widespread endocrine disrupting chemical, may increase the risk of metabolic syndrome. In this study, we investigated the hypothesis that intraperitoneal administration of 4-NP induces hepatic steatosis in rat. 24 male Sprague-Dawley rats were administered with 4-NP (0, 2, 10 and 50 mg/kg b.wt) in corn oil for 30 days. Liver histology, biochemical analysis and gene expression profiling were examined. After treatment, abnormal liver morphology and function were observed in the 4-NP-treated rat, and significant changes in gene expression an indicator of hepatic steatosis and apoptosis were observed compared with controls. Up-regulated genes involved in apoptosis, hepatotoxity and oxidative stress, increased ROS and decrease of antioxidant enzyme were observed in the 4-NP exposed rat. Extensive fatty accumulation in liver section and elevated serum GOT, GPT, LDH and γ-GT were also observed. Incidence and severity of liver steatosis was scored and taken into consideration (steatosis, ballooning and lobular inflammation). Hepatocytes apoptosis could promote NAFLD progression; Fas/FasL, TNF-α and Caspase-9 mRNA activation were important contributing factors to hepatic steatosis. These findings provide the first evidence that 4-NP affects the gene expression related to liver hepatotoxicity, which is correlated with hepatic steatosis.
1. Introduction
4-Nonylphenol (4-NP) is the final product of alkylphenol polyethoxylates (APNEIs), which is widely used in the preparation of lubricating oil additives, resins, plasticizers, surface-active agents, detergents, paints, cosmetics and can be found in almost all environmental water matrices [19]. 4-NP is persistent and more stable than Nonylphenol polyetholate (NPE) [41]. Its ability to accumulate in the organs of aquatic species is considered a potential hazard for humans and animals exposed within the food chain. This prevalent situation increases the risk of exposure for men who live in urban areas and have a fish based diet (probably from contaminated waters) [24].
To date, studies based on NP compounds toxicity focused mainly on estrogenic effects and related alteration to development of reproductive system in mammalian models (e.g., rat, mouse), and in aquatic organisms (e.g., fish, crustaceans) [42]. Hepatic tissue impairment was observed in different species exposed to NP [57]. In any event, the liver was proved the major organ of accumulation, biotransformation and degradation of environmental pollutants such as 4-NP [32], [10]. Specific estrogen receptor exists in the liver, and cellular response involving estrogen interactions have been identified. In rat, NP is extensively glucuronidated by liver microsomes and the glucuronidation is mediated by UGT2B1, an isoform of UDP-glucuronosyltransferase [59], [12] a rainbow trout liver after injection [49]. After glucuronidation, the resultant glucuronide is excreted mainly into the bile in rats. The major metabolites conjugate are NP-glucuronide and p-nonylcatechol glucuronide, these were detected in the rat liver and serum after oral administration [12]. Moreover, evaluation of blood biochemistry was considered a useful tool for the diagnosis of liver diseases and assessing the histopathological studies. Laurenzana et al. [60] have reported that NP decreased hepatic testosterone hydroxylation and CYP2C expression level after oral administration, and also inhibit in vitro CYP1A1 activity in rat liver microsomes [25]. Suggesting, potential inhibition of cyt P450s and ER functions by NP, which may delay excretion from the liver.
However, there is limited information concerning the effects of 4-NP based on the liver tissues damage. On the other hand, only a few studies reported the effects of 4-NP at low doses. Based on the above undertaken, there is an urgent need for quality toxicological studies to help understand the underlying molecular mechanism in order to identify biomarkers when 4-NP-induced hepatic steatosis. 4-NP inducing non-alcoholic fatty liver disease (NAFLD) in rat is not yet widely studied and elucidated. We recall that, the mechanisms underlying the development of this disease remain unclear; hepatic steatosis is considered as the manifestation of the metabolic syndrome. In view of this, hepatic steatosis can lead to development of obesity, insulin resistance and type 2 diabetes [36], [37]. In addition, excessive weight gain affects more than 60% of diabetic and obese patients [35]. Moreover, below disorders of IR, the ability of insulin to repress hormone sensitive lipase level is reduced, leading to an increased incidence of TAG lipolysis and release of increased FFA in the liver [33]. On the other hand, it has been demonstrated that mitochondrial dysfunction is a key mechanism of drug-induced liver damage, that involves the parent chemical or a reactive metabolite generated through cyt P450 (CYP450) enzymes. 4-NP has toxicological effects that can affect multiple pathways, such as aril hydrocarbon receptor (AhR) and peroxisome proliferation-activated receptor (PPAR) [10]. Previous studies demonstrated the involvement of PPAR isoforms α and β in the regulation of important biological processes, including lipidic and glucidic metabolism [54], [50]. Thus, several xenobiotics can induce mitochondrial dysfunction or reactive metabolite generation through cyt P450-mediated metabolism, also involved in the process of β-oxidation of the free fatty acids [30], [23]. These mitochondrial disturbances can lead to a variety of deleterious consequences such as oxidative stress, energy shortage, accumulation of triglycerides (steatosis), and cell death [4].
The liver lesion is commonly referred to as macrovacuolar steatosis (MS), which is also observed in large number of obese and diabetic patients, even in those that do not drink alcohol. MS is relatively a benign liver lesion in the short term. Furthermore, study has also confirmed lipid droplets in mouse fibroblasts (3T3-Li cells) exposed to 4-NP [31]. Liver injury progression leads to non-alcoholic hepatic steatosis (NASH) and apoptosis, which may end in hepatocellular carcinoma [13]. Bernabò et al. [6] study based on amphibian reported large lipid droplets in liver histology and ultrastructure after acute exposure to NPE. There is evidence that liver apoptosis, cell proliferation, and related genes, Fas/FasL, Bax/Bcl-2, and Caspase-8 play an important role in the genesis and development of NASH [26]. However, currently there is a paucity of information about the action and mechanism of 4-NP induced hepatic steatosis in rats. Consequently, in this original research, we aimed to profile the gene expression of liver tissues and also gain insight into the mechanisms responsible for 4-NP-induced hepatic steatosis after treatment with 4-NP at various doses during 30 days period in male rats.
2. Materials and methods
2.1. Chemicals and reagents
4-Nonylphenol (NP) was purchased from DR Co. (Augsburg, Germany, purity: 98%). Corn oil was obtained from Sigma–Aldrich (St. Louis, MO, USA). Sigma Chemical Co. (St Louis, MO) USA, Collagenase, Trypsin–EDTA were obtained from GIBCO (Grand Island, NY, USA). Sodium lauryl sulphate from SRL, Eosin stain and Hematoxylin stain were obtained from HiMedia (Mumbai). LDH, GOT, GPT, γ-GT, CAT, GSH-Px, SOD, H2O2, and MDA assay kit (Jiancheng Bioengineering Ltd., Nanjing, China).
2.2. Animals and experimental design
Twenty-four male Sprague-Dawley rats (70–80 g) were obtained from the Experimental Animal Center of Tongji Medical College Animal Laboratory (Wuhan, China). The rats were kept at a controlled temperature (24 ± 3 °C) under 12 h light–dark cycles, humidity (50 ± 5%) environment, and fed libitum. All protocols were approved by the institutional ethics committee for animal research of Ministry of Health, People's Republic of China and received the certificate number (2011-s2456). The rats were randomly divided into four groups, each group containing six rats. Each group (labeled as: control group, low dose group, middle dose group and high dose group) were fed different doses of 4-nonylphenol A 0, 2, 10, 50 mg/kg body weight (b. wt) respectively in corn oil every forty-eight hours by intra-peritoneal injection for 30 days. The doses and time used for the present study were derived from published data [56], [55] and the result of our preliminary experiment. After 30-days of treatment, the rats were sacrificed. Livers were excised, blotted and weighed, and either fixed for histopathological studies, or stored at −80 °C respectively.
2.3. Assessment of serum hepatic marker enzymes
Serum was separated from blood by centrifugation at 3000 rpm for 15 min at 4 °C and kept in a freezer. The activities of serum glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), γ-Glutamyl transferase (γ-GT) and lactate dehydrogenase (LDH) were measured using commercially assay kits (Jiancheng Bioengineering Ltd., Nanjing, China) according to the manufacturers' instructions.
2.4. Evaluation of hepatic antioxidant enzyme and non-enzymatic assays
2.4.1. Enzyme extraction and assay
The liver was homogenized using lysis buffer (containing 1 mM Na2EDTA, 150 mM NaCl, 10 mM PMSF, 10 mM Tris, 1 mM Aprotinin) to evaluate oxidative stress following the protocol of assay kit (Jiancheng Bioengineering Ltd., Nanjing, China). All operations were done at 4 °C. Protein concentrations were determined using a BCA kit (Beyotime Biotech Inc., China) that employed serum albumin as a standard.
2.4.2. Estimation of liver antioxidant parameters
Measurements of catalase (CAT), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) activities, as well as hydrogen peroxide (H2O2) and malondialdehyde (MDA) levels were performed.
CAT can decompose H2O2, the absorbance at 240 nm decreasing in time with advancing reaction, CAT activity was calculated according to the rate of change in absorbance: one unit is defined as the degradation of 1 nmol H2O2 in the reaction system per mg protein per minute.
GSH-Px activities were assayed by quantifying the rate of oxidation of reduced glutathione to glutathione disulfide by H2O2. One unit of GSH-Px was defined as the amount that reduced the level of GSH by 1 μM in 1 min/mg protein as determined spectrophotometrically at 412 nm.
SOD activity in supernatants was determined by measuring the reduction of nitro blue tetrazolium (NBT) by O2− produced from the xanthine–xanthine oxidase system. One unit of SOD was defined as the amount protein that inhibits the rate of NBT reduction by 50%. Results were defined as U/mg protein.
2.4.3. Reactive oxygen species
Hydrogen peroxide (H2O2) and titanium dioxide over sulfuric acid were used to form a yellow complex, which has a characteristic absorption in 415 nm. Results were defined as μmol/mg protein. The level were assessed to determine the concentration of H2O2 as a marker for reactive oxygen species (ROS).
2.4.4. Assessment of lipid peroxidation
The liver homogenates was assessed to determine the concentration of malondialdehyde (MDA) as a marker for lipid peroxidation (LPO) and measuring thiobarbituric-acid (TBA) reacting substances at 532 nm. The level of MDA was expressed as nmol MDA per milligram protein.
2.5. Histological and immunohistological examination
2.5.1. Hematoxylin eosin
The liver tissues fixed in Bouin's solution were transferred to 70% ethanol and embedded in paraffin. They were cut at 4 μm thickness and stained (hematoxylin and eosin (H&E)) based on standard procedures. Six slides were prepared from each liver. All sections were evaluated for the degree of liver injury. All specimens were examined using a light microscope (IX-71, Olympus, Tokyo, Japan) with high-power magnification 200×. The extent of hepatocytes steatosis was graded (grades 1–3) according to the Brunt et al. grading and staging system for NASH [7] see (Table 1). We considered only H & E necessary to perform the evaluation and the whole area of the section of each stained slides was scanned and analyzed in triplicate for each rat.
Table 1.
Grading and staging of histopathological of liver steatosis.
| Grade | Histological characteristics |
|---|---|
| 1-Mild | Steatosis: predominantly macrovesicular |
| Ballooning: occasionally observed | |
| Lobular inflammation: scattered and mild acute inflammation | |
| 2-Moderate | Steatosis: any degree usually mixed macrovesicular and microvesicula |
| Ballooning: obvious and present in centrilobular zone | |
| Lobular inflammation: associated with ballooned hepatocytes | |
| 3-Severe | Steatosis: typically involves >66% of lobules |
| Ballooning: predominantly, marked in centrilobular zone | |
| Lobular inflammation: scattered acute and chronic inflammation |
According to Brunt et al. grading and staging system for NASH [7].
2.5.2. In situ nick-end labeling (TUNEL)
To detect apoptotic cell death, paraffin embedded sections were stained by the TUNEL technique using an in situ apoptosis detection kit (Wuhan Boster Biological Technology, Ltd., Wuhan, China) according to the instructions provided by the manufacturer. The sections were deparaffinized with xylene, rehydrated and treated with 200 μg/ml proteinase K for 15 min at room temperature. Endogenous peroxidase was inactivated by covering the sections with 3% H2O2 in H2O for 5 min at room temperature. End-labeling was obtained through catalytically adding residues of digoxygenin-labeled 11-dUTP and dATP to the 3′-hydroxyl ends of DNA with the enzyme TDT. The reaction buffer containing: dATP, dUTP and TDT, was performed for 60 min at 37 °C in a humid atmosphere. The digoxygenin was identified immunohistochemically with a digoxygenin-specific peroxidase-conjugated antibody (30 min in a humid atmosphere at room temperature). For the color reaction, metal-enhanced diaminobenzidine was used as substrate. The sections were counterstained with hemato-xylin. Positive and negative control sections were included in each sample. The apoptotic cells were identified based on intense brown nuclear staining observed under light microscope (IX-71, Olympus, Tokyo, Japan). The data were expressed as the average of apoptotic cell numbers per sample. Apoptotic cells were identified by their brownish staining. The whole area of the section was scanned with the high-power magnification (400×). The apoptotic index was then calculated as follows: AI = (number of apoptotic cells per section/ total number of cells per section) x 100%.
2.5.3. PCNA immunohistology
Proliferating cell nuclear antigen (PCNA) immunohistochemical staining was achieved to evaluate hepatocytes proliferation. Liver tissues were fixed for 24 h in neutral buffered formalin, processed routinely and embedded in wax. Immunohistochemical staining was performed as previously described Kalinichenko et al., 2003. The liver tissues were sectioned and stained utilizing a mouse monoclonal antibody against PCNA and the SABC Staining Kit (Wuhan Boster Biological Technology, Wuhan, China) according to manufacturer's protocol, then subjected to photomicroscopic observation (IX-71, Olympus, Tokyo, Japan) with high-power magnification 200×. PCNA positive cells in the liver sections were measured. The tissue sections were counted for each rat. The data were expressed as the average of PCNA positive cell numbers per sample.
2.6. RNA extraction and RT-PCR
Total RNA was extracted with Trizol reagents (Invitrogen, Carlsbad, CA, USA) and the purity was determined by the Eppendorf BioPhotometer (Ependorf, Germany), which showed an optical density ratio (OD260/280) between 1.8 and 2.0. Total RNA of 2 μg was reversely transcribed to the complementary DNA by Revert Aid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). The quantitative RT-PCR was performed with an ABIPRISM® 7900HT Sequence Detection System (Applied Biosystems, USA) using Platinum® SYBR® Green qPCR SuperMix-UDG with ROX (Invitrogen, USA). The β-actin was used in parallel for each run as an internal control. A 10 μl PCR reaction system was used, including 2 μl cDNA, 3 μl primers, and 5 μl Super Mix-Rox. A 4-step experimental run protocol was carried out and the amplification conditions were as follows: 50 °C for 2 min (UDG incubation); 95 °C for 2 min (initial denaturation); 40 cycles of 15 s at 95 °C (denaturation), and 1 min at 60 °C (elongation). The relative expression of target genes was calculated using 2−ΔΔCt method [38]. The primer sequences were designed according to the cDNA sequences from the GenBank. All primers were synthesized by the Biosia Corp (Shanghai, China). The sequences of primers used as follows:
SOD 1 forward: 5′-ACACAAGGCTGTACCACTGC-3′;
reverse: 5′-CCACATTGCCCAGGTCTCC-3′
GPx forward: 5′-GTCCACCGTGTATGCCTTCTCC-3′;
reverse: 5′-TCTCCTGATGTCCGAACTGATTGC-3′
HSP 70 forward: 5′-ATCTCCTGGCTGGACTCTAACA-3′
reverse: 5′-CACCCATCTGTCTCCTAGATCA-3′
TNF-α forward: 5′-TATGGCCCAGACCCTCACA-3′
reverse: 5′-GGAGTAGACAAGGTACAACCCATC-3′
Fas forward: 5′-ACATGGACAAGAACCATTATGCTGA-3′
reverse: 5′-CTGGTTTGCACTTGCACTTGGTA-3′
FasL forward: 5′-CATGCAGCAGCCCATGAATTAC-3′
reverse: 5′-CTCTAGGCCCACAAGATGGACAG-3′
Bcl-2 forward: 5′-TGAAGCGGTCCGGTGGATA-3′
reverse: 5′-CAGCATTTGCAGAAGTCCTGTGA-3′
Bax forward: 5′-CAGGATGCGTCCACCAAGAA-3′
reverse: 5′-CGTGTCCACGTCAGCAATCA-3′
Caspase-9 forward: 5′-TGCACTTCCTCTCAAGGCAGGACC-3′
reverse: 5′-TCCAAGGTCTCCATGTACCAGGAGC-3′
β-actin forward: 5′-ACTATCGGCAATGAGCGGTTCC-3′
reverse: 5′-CTGTGTTGGCATAGAGGTCTTTACG-3′
2.7. Statistical analysis
All values expressed as (mean ± SD) were compared by one way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test. All statistical analyses were carried out using SPSS statistical package 12.0 (SPSS Inc, Chicago, IL, USA) to determine whether the treatments of results were significant (*p < 0.05) or extremely significant (**p < 0.01 and ***p < 0.001) different from the control groups without 4-NP.
3. Results
3.1. Body and liver weight
At the beginning of the experiment, rats from different groups showed approximately similar body weight values. The treatment with 4-NP promoted an increase in the body weight gained when compared to the initial weight of rats. The final body weight (b. wt) of animals from these groups was not statistically different in values from untreated animals (Table 2). However, significant changes were observed between absolute and relative weight of 4-NP -treated groups rat liver.
Table 2.
Body weight (g) and selected absolute (g) and relative organ weight (mg/g) of male rats in control and treatment groups. Doses of 4-NP (mg/kg b.wt.).
| Initial body weight (g) | Final body weight (g) | Liver weight |
||
|---|---|---|---|---|
| Absolute (g) | Relative-to-body (mg) | |||
| Control | 78.93 ± 5.96 | 246.63 ± 14.89 | 7.63 ± 1.54 | 30.82 ± 5.27 |
| 2 mg | 75.25 ± 4.72 | 243.40 ± 11.76 | 7.94 ± 1.13 | 32.72 ± 5.08 |
| 10 mg | 73.37 ± 6.61 | 260.30 ± 16.63 | 9.23 ± 0.78* | 35.69 ± 4.20 |
| 50 mg | 76.33 ± 5.97 | 267.91 ± 14.91 | 9.44 ± 0.67* | 35.26 ± 2.54* |
Values are expressed as mean ± SD, n = 6. The symbol represents statistical significant (ANOVA) from control.
p < 0.05.
3.2. Effect of treatments on liver function
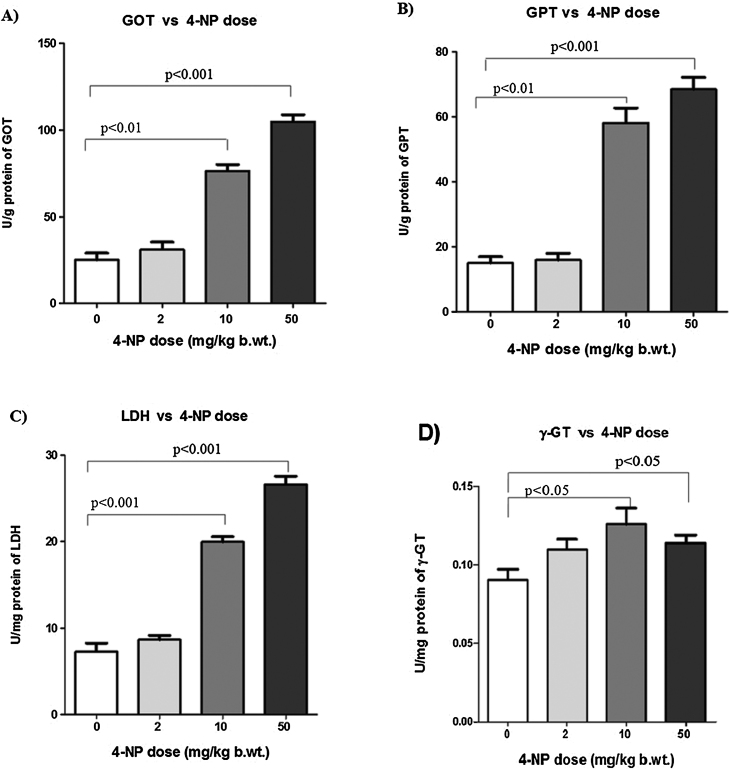
The activities of GOT, GPT, LDH and γ-GT in blood are commonly used to evaluate the liver function. Fig. 1 shows that the activities of GOT, GPT, LDH (p < 0.01, p < 0.001) and γ-GT (p < 0.05) were significantly increased in response to 4-NP-treated group compared to control.
Fig. 1.
Effect of 4-NP on liver biomarkers. (A) Effect of 4-NP on GOT activities (U/g of protein) in the liver of rats. (B) Effect of 4-NP on GPT activities (U/g of protein) in the liver of rats. (C) Effect of 4-NP on LDH activities in (U/mg of protein) the liver of rats. (D) Effect of 4-NP on γ-GT activities (U/mg of protein) in the liver of rats. Values are expressed as mean ± SD, n = 6. The symbol represents statistical significant (ANOVA) from control: *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. Effect of treatments on hepatic ROS and LPO
H2O2 and MDA assay were routinely used to measure the extent of lipid peroxidation. Results of Table 3 shows that the levels of H2O2 (p < 0.01) at 50 mg/kg b. wt and MDA (p < 0.01) at 10 and 50 mg/kg b. wt were significantly increased in response to 4-NP-treated group compared to control.
Table 3.
Effect of 4-NP on liver oxidative stress and antioxidant enzyme activity in all experimental groups. Doses of 4-NP (mg/kg b.wt.).
| CAT (U/mg prot) | GSH-Px (U/mg prot) | SOD (U/mg prot) | H2O2 (μmol/mg prot) | MDA (nmol/mg prot) | |
|---|---|---|---|---|---|
| Control | 14.045 ± 2.08 | 1.932 ± 0.15 | 14.502 ± 2.79 | 2.045 ± 0.41 | 1.461 ± 0.49 |
| 2 mg | 10.23 ± 1.91** | 1.280 ± 0.45** | 14.156 ± 2.18 | 2.428 ± 1.00 | 1.881 ± 0.55 |
| 10 mg | 5.833 ± 2.67*** | 0.632 ± 0.39*** | 12.359 ± 1.82 | 2.451 ± 0.28 | 3.013 ± 0.46** |
| 50 mg | 4.318 ± 2.28*** | 0.471 ± 0.29*** | 10.414 ± 2.20* | 3.066 ± 0.47** | 3.199 ± 0.81** |
Effect of 4-NP on liver antioxidant enzymes and reactive oxygen species. Values are expressed as mean ± SD, n = 6. The symbol represents statistical significant (ANOVA) from control.
p < 0.05.
p < 0.01.
p < 0.001.
3.4. Effect of treatments on antioxidant enzyme activity
Three antioxidant enzymes (SOD, CAT and GSH-Px) on rat liver were determined and the results are shown in Table 3. All the antioxidant enzyme activities were decreased significantly (p < 0.05, p < 0.01, p < 0.001) in response to 4-NP-treated group compared to control.
3.5. Effect on expression of oxidative stress-related genes in liver
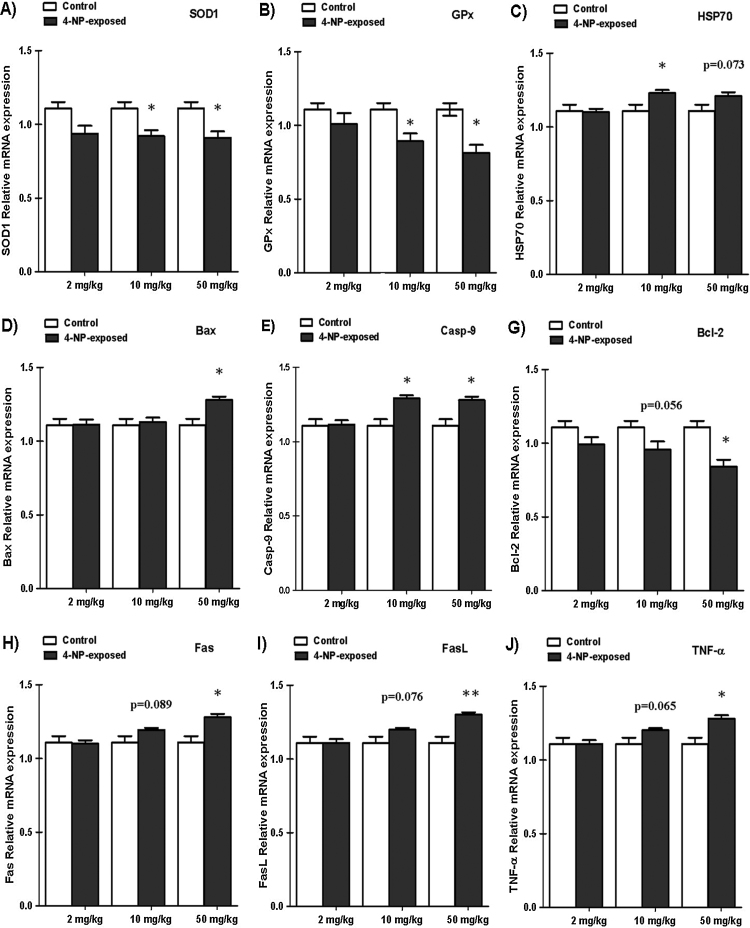
The levels of SOD1, GPx and HSP70 in the livers (Fig. 2A–C) showed significant decrease in 4-NP-treated group compared to control (p < 0.05, p < 0.01). While the level of HSP70 increased significantly (p < 0.05).
Fig. 2.
Effects of 4-NP (mg/k b. wt) on oxidative stress-related genes expression of experimental rat livers ((A) SOD1, (B) GPx, (C) HSP70). Apoptotic-related genes ((D) Bax, (E) Bcl-2, (F) Casp-9). Hepatotoxicity-related genes ((G) Fas, (H) FasL, (I) TNF-α). The housekeeping gene β-actin is used as an internal positive control. The relative expression of target genes is calculated using 2−ΔΔCt. Error bars represent the standard deviation. Significant difference:*p < 0.05, **p < 0.01 versus control group. n = 6.
3.6. Effect on expression of apoptosis-related genes in liver
The levels of Casp-9, Bax and Bcl-2 in the livers (Fig. 2DF) showed significant increases in 4-NP-treated group compared to control (p < 0.05). While the level of Bcl-2, which decreased significantly (p < 0.05) (Fig. 2E).
3.7. Effect on expression of hepatotoxicity-related genes in liver
The levels of Fas, FasL and TNF-α in the livers (Fig. 2H-I) showed significant increase in 4-NP-treated group compared to control (p < 0.05, p < 0.01).
3.8. Histological and immunohistological examination
3.8.1. Hematoxylin eosin
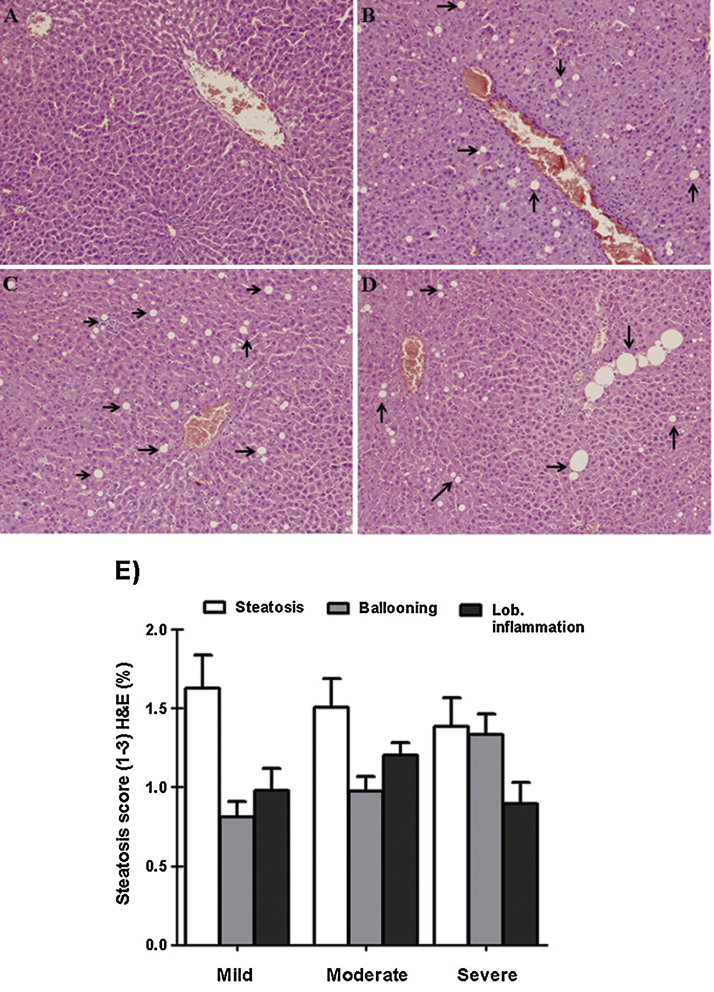
Taking into consideration the alterations observed in plasma biomarkers, oxidative stress markers, and the microscopic aspect of the liver, no change was observed in the rats given corn oil. Thus changes refer especially to the 4-NP treated group. The accumulation of lipids revealed by H&E technique (vacuoles in white) (Fig. 3B–D) was confirmed by TUNEL (lipids in gray) (Fig. 4B–D). Fig. 3E. Show the liver Steatosis, ballooning and lobular inflammation scores according to Brunt et al. [7] The liver ballooning scores of D group were higher than those of the B and C group at each point. While lobular inflammation scores were higher than those of the B and D group at each point.
Fig. 3.
Hepatic histology of rats livers sections, showing cellular changes, particularly vacuoles (arrows), indicating steatosis. (D) Show several balloon cells that are much larger than the surrounding steatotic hepatocytes but with the same cytoplasmic characteristic as more obvious balloons, such as those seen in (B and C). Observations done at 200× magnification (H&E). (E) Grading for steatohepatitis, three grades are summarized (steatosis, ballooning and lobular inflammation).
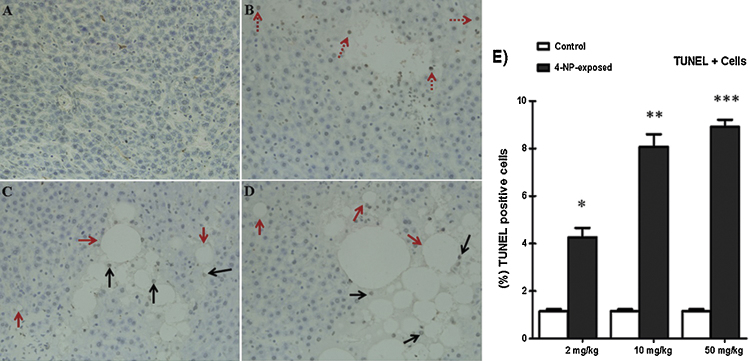
Fig. 4.
Detection of apoptosis TUNEL assay in the liver tissue of rats. The presence of apoptosis after treatment with (2, 10 and 50 mg/kg/ b.wt) of 4-NP by intraperitoneal injection for 30 days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
(B–D) DNA fragmentation, characteristic of apoptosis (blacks arrows), (red arrows) indicates ballooning. (E) The graph representing average number of TUNEL-positive cells in each group). Observations done at 200× magnification. Apoptotic index of liver (%). The tissue sections were counted for each rat. The symbol represents statistical significant (ANOVA) from control: *p < 0.05, **p < 0.01, ***p < 0.001.
3.8.2. In-situ nick-end labeling (TUNEL)
The TUNEL outcomes of all treatment groups are presented in (Fig. 3). After evaluation with the light microscopic has revealed a characteristic appearance of the normal hepatic tissue for the control group (Fig. 4A). However, TUNEL-positive cells increased significantly in response to 4-NP treatment at 10 and 50 mg/kg b. wt. In contrast, no TUNEL positive cells were seen in control group. Thus, despite similar metabolic abnormalities, an equivalent degree of steatosis has also been observed in 4-NP treated group. Observed histological changes in liver tissues have shown a dose-dependent increase (Fig. 4B–D).
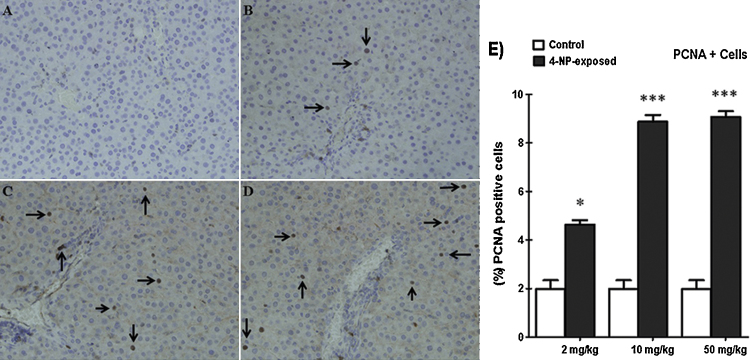
3.8.3. PCNA immunohistology
The PCNA outcomes of all treatment groups are presented in (Fig. 5). PCNA is an auxiliary protein of DNA polymerase-delta and higher level of its expression is correlated cell proliferation, suggesting PCNA is an excellent marker of cellular proliferation. After evaluation with the light microscopic has revealed characteristic appearance of normal hepatic tissue of control group (Fig. 2A). Conversely, in 4-NP-treated were markedly increased PCNA positive cell (4.64%, 8.87% and 9.07%) respectively in response to 4-NP (2, 10 and 50 mg/kg b. wt) in the liver tissues compared with corresponding control group. However, no significant PCNA positive cells were observed in corresponding control group. Whereas significant histological alterations in liver tissues have observed a dose-dependent manner (Fig. 5C,D).
Fig. 5.
4-NP promotes hepatic cell proliferation in acute liver injury. Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) was carried out as previously described. (A) PCNA staining liver sections in the rats without 4-NP administration. (B–D) Low middle and high dose respectively, arrows indicate PCNA+ cells in the liver sections. (E) Quantitative expression of PCNA was significantly higher in the liver cells at dose 10 and 50 mg/kg b. wt. The graph representing average number of PCNA+ cells in each group. Observations done at 200× magnification. PCNA+ index of liver (%). The tissue sections were counted for each rat. The symbol represents statistical significant (ANOVA) from control: *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
NAFLD has become the leading cause of chronic liver injury in developed countries. Although the exact cause of NAFLD is still unknown, there are emerging experimental and epidemiological studies proposing that some environmental contaminants may exert effects on promoting metabolic diseases and associated NAFLD [17]. In this study, we sought to investigate whether administration of 4-NP induces steatosis in rats. Plasma GOT, GPT, LHD and γ-GT are the most sensitive markers in the diagnosis of hepatic injury. The results showed that the levels of GOT, GPT, LHD and γ-GT were significantly increased in response to 4-NP-treated group compared to control. GOT and GPT are normally localized in the hepatic cytoplasm, while increase in serum GOT and GPT levels by 4-NP suggests hepatic damage with their subsequent release into circulation. This has also been confirmed by [39]. It is important to note that, some studies have shown no changes in the serum SGOT and SGPT even at high doses of 4-NP [11], [53]. This contradiction could be due to the degree of toxicity depending on the dose, time, frequency, and strains.
Through hepatic histopathological examination and serum assays has also confirmed, microvesicular steatosis was observed in 4-NP-treated group. As illustrated in result section the hepatocytes ballooning is the most characteristic feature of steatosis-hepatitis; which is typically associated with formation of Mallory's hyaline. The severity of steatosis appears to be important in determining the extent of apoptosis. For this purpose, we carried out the grading and staging system for NASH according to [7].
To investigate the mechanism of intraperitoneal exposure to 4-NP, which lead to increased susceptibility of the development of NAFLD in male rats, a massively parallel deep-sequencing was carried out to identify the genome-wide profiles of the liver tissues after treatment. We observed that the expression of Fas/FasL, TNF-α and Casp-9 mRNA activation in 4-NP-treated group increased with hepatic steatosis. The expression level increased gradually showing a positive correlation with NASH levels and the liver apoptosis percentage, suggesting that the incidence and development of up regulation of Fas/FasL, TNF-α and Casp-9 activation at dose 10 and 50 mg/kg b.wt. respectively thus indicating that 4-NP induces extrinsic apoptosis; as echoed by Jubendradass et al. [19]. In addition to this, TNF-α acts as a simulator of NADPH-dependent H2O2 generation [22]. Increase of TNF-α and lipid peroxidation were observed at 4-NP-treated group and particularly significant at dose 50 mg/kg b.wt. This is the most important factor causing and maintaining insulin resistance [48], and closely related to the development of NAFLD and other metabolic disorders [16]. Despite the existing correlation between steatosis and insulin resistance, below disorders of IR, the ability of insulin to repress hormone sensitive lipase level is reduced, leading to an increased incidence of TAG lipolysis and release of increased FFA into the liver [47]. This process is an independent hazard factor for NAFLD severity.
Several genes associated with liver function were significantly changed. Alterations in the expression of Bcl-2 and Bax genes indicate the involvement of an intrinsic apoptotic mechanism. Whereas caspases point towards the role of an alternative pathway [18]. In our present findings, dysregulation of Casp-9 and Bax mRNA (apoptotic), and also Bcl-2 expression (pro-survival) results in the release of cyt C; cyt C, a pro-apoptotic factor released from outer mitochondrial membrane to cyt, form a complex with Apaf-1 and procaspase-9, resulting in activation of Casp-9. Activated Casp-9 activates the effector Casp-3 leading to degradation of DNA and cellular constituents [1]. Nevertheless, in response to DNA damage, protein p53 will be activated [34]. This protein, directly or indirectly, modulates the expression of some proteins that control mitochondrial membrane permeability (MMP), resulting in the release of cyt C [14]. Mitochondrial cyt C together with the Apaf-1 (apoptotic protease activating factor 1) and dATP (nucleotide precursors) form apoptosome that activates Casp-9 (initiator caspase). Furthermore, this initiator caspase begin the apoptosis process through the activation of executor caspase [45]. DNA fragmentation was confirmed by TUNEL assay. These observations further validate the theory that mitochondria dysfunction induced by 4-NP can be a cause, effect or concurrent feature in the development of NAFLD. This assertion has been confirmed by [40]. In addition, mitochondrial dysfunction involved in the process of β-oxidation of FFA, then impairs fat homeostasis in the liver, but also leads to an overproduction of oxidative stress, resulting in the generation of ROS that trigger LPO, cytokine overproduction and cell death [46]. On the other hand, ROS produced mitochondria FAO in liver cells will further simultaneously release cyt C, which eventually triggers hepatic apoptosis through the mitochondria-dependent pathways [27].
A wide range of injurious stresses relevant to liver disease lead to MMP pore opening [29]. It can therefore be proved that, ROS overproduction or antioxidant reductions are the direct causes of oxidative stress. Previous research have reported that endocrine disruptor like bisphenol A exhibit adverse effects at very low doses. However, only a few studies have reported the effects of NP at low doses. Administration of NP at a dose level of 50 μg/kg b.wt/day for 30 days has been shown to increase oxidative stress in blood of adult male rats. Many factors may cause the production of ROS, including 4-NP exposure. The change in antioxidant enzyme activities is relevant to the ability of the liver to fight against oxidative stress during 4-NP exposure. This study showed that SOD, CAT, GSH-Px levels were significantly decreased in the liver after the treatment of 4-NP compared with the control group. In order to support this hypothesis we have considered in our work that enhance of H2O2 and MDA as marker for LPO and as indicator of oxidative liver injury [9]. All these indicated that 4-NP promote antioxidant activity to induce liver damage. SOD presents in three isoforms, copper-zinc-containing superoxide dismutase (Cu–Zn–SOD, SOD1), manganese containing superoxide dismutase (Mn–Zn–SOD, SOD2) and extracellular superoxide dismutase (Cu–Zn–SOD, SOD3). The SOD1 isoenzyme is the most abundant one in the cytoplasm [5]. Reduction of SOD1 activity in 4-NP-treated group in this study might be due to the enhanced production of superoxide radical anions. GPx is an antioxidant enzyme, which modifies the peroxide anion to a non-toxic hydroxyl compound in order to protect the membrane structure and function. The decrease of these antioxidants enzymes may be suggestive for the process of LPO. These processes are situated to be responsible for initiating necroinflammation, and ROS, which are generated during FFA metabolism in microsomes, peroxisomes and mitochondria. These cytotoxic ROS and LPO products are able to disperse within the extracellular interspaces worrying HSC and Kupffer cells [47], that may play substantial roles in the evolution of chronic liver inflammation and fibrosis development [43].
HSP70 is one of the most abundantly induced proteins under a variety of stress conditions and the extensive oxidative stress marker at present [51], and is often correlated with the emphasis on the process of apoptosis [3] by inhibiting the formation of apoptosome [8]. This can also suppress the apoptotic process through other pathway by blocking the activation of stress induced kinases such as apoptosis signal-regulating kinase 1 (ASK1) and c-Jun N-terminal kinases (JNK) [15]. The increase in the levels of HSP70 mRNA may be due to the presence of higher event of OS and cell damage. This can be confirmed by the increase in the number of positive PCNA cell staining of hepatocytes, which is the central molecule responsible for taking decisions of life and death of the cell. Its increase is associated with cell death [28]. This observation may be due to ROS induction and disruption of the balance between ROS and antioxidant defense system. This data concurs with previous findings [21]. On the other hand, Excess FA accumulation in hepatocytes induces oxidative stress in mitochondria but also microsomes and peroxisomes. This study also demonstrated increase in body weight gain and absolute and relative liver weight of 4-NP treatment rats. The augmentation of the size of the liver is commonly observed in patients with several Hepatomegaly [2] and also might be a crucial mechanism for its developmental toxicity in male rat hepatocytes.
In addition, FAO and oxidative stress occurs in the mitochondria, and are considered to play a critical role in the pathogenesis of NALFD [58]. Hepatocytes apoptosis is commonly manifested in several liver diseases. In previous years, large progress has been made with regard to elucidate the role of apoptosis in the occurrence and development of NAFLD (e.g., [44], [52] and its subsequent progression to NASH, liver fibrosis, cirrhosis and liver cancer.
5. Conclusion
Intraperitoneal administration of 4-NP promotes an increase in the susceptibility to hepatic steatosis, which is associated with apoptosis, positive proliferation of hepatocytes and subsequent oxidative stress prior to the development of NALD. However, this present study may merit discussion and it would be important to research new mechanism-specific biomarkers of hepatocellular injury, which would be a suitable opportunity to assess the impacts of variations in 4-NP concentrations on liver function and damages as well as protein measurements by immunoblot analysis to support this conclusion. However, our present research demonstrated the first evidence that 4-NP promote hepatic steatosis in male rats, disturbing hepatotoxicity-related genes expression in liver; as well as up-regulation of the expression of Fas/FasL ratio and TNF-α, which are a positive correlation of NALD and also have been associated with many pathologic states in humans.
Conflict of interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of People’s Republic of China (30972436 and 81172623).
References
- 1.Arya A.K., Pokharia D., Tripathi K. Relationship between oxidative stress and apoptotic markers in lymphocytes of diabetic patients with chronic non healing wound. Diabetes Res. Clin. Pract. 2011;94(December (3)):377–384. doi: 10.1016/j.diabres.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Aly H.A., Domenech O., Abdel-Naim A.B. Aroclor 1254 impairs spermatogenesis and induces oxidative stress in rat testicular mitochondria. Food Chem. Toxicol. 2009;47(8):1733–1738. doi: 10.1016/j.fct.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Beere Helen M. The stress of dying': the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 2004;117(13):2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 4.Begriche K., Massart J., Robin M.A., Borgne-Sanchez A., Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011;54(April (4)):773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Bera Asit K., Rana Tanmoy, Bhattacharya Debasis, Das Subhashree, Pan Diganta, Das Subrata K. Sodium arseniteinduced alteration in hepatocyte function of rat with special emphasis on superoxide dismutase expression pathway and its prevention by mushroom lectin. Basic Clin. Pharmacol. Toxicol. 2011;109(4):240–244. doi: 10.1111/j.1742-7843.2011.00718.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernabò Ilaria, Biasone Patrizia, Macirella Rachele, Tripepi Sandro, Brunelli Elvira, acirella S. Liver histology and ultrastructure of the italian newt (lissotriton italicus): normal structure and modifications after acute exposure to nonylphenol ethoxylates. Exp. Toxicol. Pathol. 2014;66(9):455–468. doi: 10.1016/j.etp.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94(September (9)):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 8.Obregón Eduardo Bustos, Esponda Pedro. El antiandrógeno flutamida uroduce un aumento de la apoptosis y de la proteína de stress Hsp70 en El epidídimo del ratón. Int. J. Morphol. 2009;27(2):463–468. [Google Scholar]
- 9.Cesaratto Laura, Vascotto Carlo, Calligaris Sebastian, Tell Gianluca. The importance of redox state in liver damage. Ann. Hepatol. 2004;3(3):86–92. [PubMed] [Google Scholar]
- 10.Cocci P., Mosconi G., Palermo F.A. Effects of 4-nonylphenol on hepatic gene expression of peroxisome proliferator-activated receptors and cytochrome P450 isoforms (Cyp1a1 and Cyp3a4) in juvenile sole (Solea solea) Chemosphere. 2013;93(October (6)):1176–1181. doi: 10.1016/j.chemosphere.2013.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Cunny H.C., Mayes B.A., Rosica K.A., Trutter J.A., Van Miller J.P. Subchronic toxicity (90-day) study with para-nonylphenol in rats. Regul. Toxicol. Pharmacol. 1997;26(October (2)):172–178. doi: 10.1006/rtph.1997.1154. [DOI] [PubMed] [Google Scholar]
- 12.Doerge D.R., Twaddle N.C., Churchwell M.I., Chang H.C., Newbold R.R., Delclos K.B. Mass spectrometric determination of p-nonylphenol metabolism and disposition following oral administration to Sprague-Dawley rats. Reprod. Toxicol. 2002;16(January–February (1)):45–56. doi: 10.1016/s0890-6238(01)00198-8. [DOI] [PubMed] [Google Scholar]
- 13.Ekstedt Mattias, Franzén Lennart E., Mathiesen Ulrik L., Thorelius Lars, Holmqvist Marika, Bodemar Göran, Kechagias Stergios. Long-term follow-up of patients with Nafld and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 14.Endo M., Mori M., Akira S., Gotoh T. C/Ebp homologous protein (Chop) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J. Immunol. 2006;176(May (10)):6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- 15.Gabai Vladimir L., Mabuchi Katsuhide, Mosser Dick D., Sherman Michael Y. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 2002;22(10):3415–3424. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenfield Jerry R., Campbell Lesley V. Relationship between inflammation, insulin resistance and type diabetes: ‘cause or effect’? Curr. Diabetes Rev. 2006;2(2):195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 17.Grun F., Blumberg B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009;304(May (1–2)):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haupt Susan, Berger Michael, Goldberg Zehavit, Haupt Ygal. Apoptosis-the P53 network. J. Cell Sci. 2003;116(20):4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 19.Jubendradass R., D'Cruz Shereen Cynthia, Rani S. Judith Amala, Mathur P.P. Nonylphenol induces apoptosis via mitochondria-and fas-l-mediated pathways in the liver of adult male rat. Regul.Toxicol. Pharmacol. 2012;62(3):405–411. doi: 10.1016/j.yrtph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Korkmaz A., Ahbab M.A., Kolankaya D., Barlas N. Influence of vitamin C on bisphenol a, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010;48(October (10)):2865–2871. doi: 10.1016/j.fct.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Krieger-Brauer H.I., Kather H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and nadph-dependent H2O2 generation in 3T3 L1-cells. Biochem. J. 1995;307(April (Pt. 2)):549–556. doi: 10.1042/bj3070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labbe Gilles, Pessayre Dominique, Fromenty Bernard. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 2008;22(4):335–353. doi: 10.1111/j.1472-8206.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 24.Lagos-Cabré Raúl, Moreno Ricardo D. Contribution of environmental pollutants to male infertily: a working model of germ cell apoptosis induced by plasticizers. Biol. Res. 2012;45(1):5–14. doi: 10.4067/S0716-97602012000100001. [DOI] [PubMed] [Google Scholar]
- 25.Lee Ping C., Patra Sharmistha Chakraborty, Stelloh Cary T., Lee Winnie, Struve Mark. Interaction of nonylphenol and hepatic Cyp1a in Rats. Biochem. Pharmacol. 1996;52(6):885–889. doi: 10.1016/0006-2952(96)00409-1. [DOI] [PubMed] [Google Scholar]
- 26.Li C.P., Li J.H., He S.Y., Li P., Zhong X.L. Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic fatty liver disease pathogenesis. Genet. Mol. Res. 2014;13(2):3991–3999. doi: 10.4238/2014.May.23.10. [DOI] [PubMed] [Google Scholar]
- 27.Li Z., Berk M., McIntyre T.M., Gores G.J., Feldstein A.E. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47(May (5)):1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maga G., Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J. Cell Sci. 2003;116(Pt. 15):3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 29.Malhi Harmeet, Gores Gregory J., Lemasters John J. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(S1):S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 30.Masubuchi Yasuhiro, Suda Chieko, Horie Toshiharu. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J. Hepatol. 2005;42(1):110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Masuno H., Okamoto S., Iwanami J., Honda K., Shiosaka T., Kidani T., Sakayama K., Yamamoto H. Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicol. Sci. 2003;75(October (2)):314–320. doi: 10.1093/toxsci/kfg203. [DOI] [PubMed] [Google Scholar]
- 32.Matos C.A., Perez R.M., Lemos L.B., Medina-Pestana J.O., Lanzoni V.P., Alberto F.L., Moreira E.S., Silva A.E., Ferraz M.L. Factors associated with the intensity of liver fibrosis in renal transplant patients with hepatitis B virus infection. Eur. J. Gastroenterol. Hepatol. 2007;19(August (8)):653–657. doi: 10.1097/MEG.0b013e328133f091. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaka Takashi, Shimano Hitoshi. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J. Diabetes Investig. 2011;2(3):170–175. doi: 10.1111/j.2040-1124.2011.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meek David W. Tumour suppression by P53: a role for the dna damage response? Nat. Rev. Cancer. 2009;9(10):714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 35.Musso Giovanni, Cassader Maurizio, Gambino Robert. Cholesterol-lowering therapy for the treatment of nonalcoholic fatty liver disease: an update. Curr. Opin. Lipidol. 2011;22(6):489–496. doi: 10.1097/MOL.0b013e32834c37ee. [DOI] [PubMed] [Google Scholar]
- 36.Nemoto Y., Saibara T., Ogawa Y., Zhang T., Xu N., Ono M., Akisawa N. Tamoxifen-induced nonalcoholic steatohepatitis in breast cancer patients treated with adjuvant tamoxifen. Intern. Med. 2002;41(May (5)):345–350. doi: 10.2169/internalmedicine.41.345. [DOI] [PubMed] [Google Scholar]
- 37.Osman K.A., Osman M.M., Ahmed M.H. Tamoxifen-induced non-alcoholic steatohepatitis: where are we now and where are we going? Expert Opin. Drug Safe. 2007;6(1):1–4. doi: 10.1517/14740338.6.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Pfaffl Michael W. Real-time PCR. In: Dorak T., editor. Vol. 63. International University Line; 2006. pp. 63–82. (Relative Quantification). [Google Scholar]
- 39.Recknagel Richard O., Glende Eric A., Jr., Dolak James A., Waller Robert L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989;43(1):139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 40.Rector R.S., Uptergrove G.M., Borengasser S.J., Mikus C.R., Morris E.M., Naples S.P., Laye M.J. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic oletf rats. Am. J. Physiol. Endocrinol. Metab. 2010;298(June (6)):E1179–E1187. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivero C.L., Barbosa A.C., Ferreira M.F., Dorea J.G., Grisolia C.K. Evaluation of genotoxicity and effects on reproduction of nonylphenol in Oreochromis niloticus (Pisces: Cichlidae) Ecotoxicology. 2008;17(November (8)):732–737. doi: 10.1007/s10646-008-0222-0. [DOI] [PubMed] [Google Scholar]
- 42.Roig B., Cadiere A., Bressieux S., Biau S., Faure S., de Santa Barbara P. Environmental concentration of nonylphenol alters the development of urogenital and visceral organs in avian model. Environ. Int. 2014;62(January):78–85. doi: 10.1016/j.envint.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Rolo Anabela P., Teodoro João S., Palmeira Carlos M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012;52(1):59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Schattenberg J.M., Singh R., Wang Y., Lefkowitch J.H., Rigoli R.M., Scherer P.E., Czaja M.J. Jnk1 but not Jnk2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43(January (1)):163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 45.Schuler M., Bossy-Wetzel E., Goldstein J.C., Fitzgerald P., Green D.R. P53 induces apoptosis by caspase activation through mitochondrial cytochrome C release. J. Biol. Chem. 2000;275(March (10)):7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 46.Serviddio G., Bellanti F., Tamborra R., Rollo T., Romano A.D., Giudetti A.M., Capitanio N. Alterations of hepatic Atp homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. Eur. J. Clin. Invest. 2008;38(4):245–252. doi: 10.1111/j.1365-2362.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 47.Takaki Akinobu, Kawai Daisuke, Yamamoto Kazuhide. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (Nash) Int. J. Mol. Sci. 2014;15(5):7352–7379. doi: 10.3390/ijms15057352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarantino Giovanni, Savastano Silvia, Colao Annamaria. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J. Gastroenterol. 2010;16(38):4773. doi: 10.3748/wjg.v16.i38.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thibaut R., Debrauwer L., Rao D., Cravedi J.-P. characterization of biliary metabolites of 4-n-nonylphenol in rainbow trout (Oncorhynchus mykiss) Xenobiotica. 1998;28(8):45–57. doi: 10.1080/004982598239164. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y., Feinstein S.I., Fisher A.B. Peroxiredoxin 6 as an Antioxidant enzyme: protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J. Cell. Biochem. 2008;104(July (4)):85. doi: 10.1002/jcb.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webb Diane, Gagnon Marthe Monique. The value of stress protein 70 as an environmental biomarker of fish health under field conditions. Environ. Toxicol. 2009;24(3):287–295. doi: 10.1002/tox.20432. [DOI] [PubMed] [Google Scholar]
- 52.Wieckowska A., Zein N.N., Yerian L.M., Lopez A.R., McCullough A.J., Feldstein A.E. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(July (1)):27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 53.Woo G.H., Shibutani M., Ichiki T., Hamamura M., Lee K.Y., Inoue K., Hirose M. A Repeated 28-day oral dose toxicity study of nonylphenol in rats, based on the ‘enhanced oecd test guideline 407’ for screening of endocrine-disrupting chemicals. Arch. Toxicol. 2007;81(February (2)):77–88. doi: 10.1007/s00204-006-0129-6. [DOI] [PubMed] [Google Scholar]
- 54.Wu X.X., Kakehi Y., Mizutani Y., Lu J., Terachi T., Ogawa O. Activation of caspase-3 in renal cell carcinoma cells by anthracyclines or 5-fluorouracil. Int. J. Oncol. 2001;19(July (1)):19–24. doi: 10.3892/ijo.19.1.19. [DOI] [PubMed] [Google Scholar]
- 55.Yamasaki K., Okuda H., Takeuchi T., Minobe Y. Effects of in utero through lactational exposure to dicyclohexyl phthalate and p,p′-Dde in Sprague-Dawley rats. Toxicol. Lett. 2009;189(August (1)):14–20. doi: 10.1016/j.toxlet.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 56.You L., Casanova M., Archibeque-Engle S., Sar M., Fan L.Q., Heck H.A. impaired male sexual development in perinatal sprague-dawley and long-evans hooded rats exposed in utero and lactationally to p,p′-Dde. Toxicol. Sci. 1998;45(October (2)):162–173. doi: 10.1093/toxsci/45.2.162. [DOI] [PubMed] [Google Scholar]
- 57.Zha Jinmiao, Wang Zijian, Wang Ning, Ingersoll Chris. Histological alternation and vitellogenin induction in adult rare minnow (Gobiocypris rarus) after exposure to ethynylestradiol and nonylphenol. Chemosphere. 2007;66(3):488–495. doi: 10.1016/j.chemosphere.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L., Yu X., Meng Q., Li H., Niu C., Jiang Y., Cai Y. Resistin reduces mitochondria and induces hepatic steatosis in mice by the protein kinase C/protein Kinase G/p65/Ppar gamma coactivator 1 alpha pathway. Hepatology. 2013;57(April (4)):1384–1393. doi: 10.1002/hep.26167. [DOI] [PubMed] [Google Scholar]
- 59.Moffatt J., Hashimoto M., Kojima A., Kennedy D.O., Murakami A., Koshimizu K., Ohigashi H., Matsui-Yuasa I. Apoptosis induced by 1′-acetoxychavicol acetate in Ehrlich ascites tumor cells is associated with modulation of polyamine metabolism and caspase-3 activation. Carcinogenesis. 2000;21(12):2151–2157. doi: 10.1093/carcin/21.12.2151. [DOI] [PubMed] [Google Scholar]
- 60.Laurenzana E.M., Balasubramanian G., Weis C. Effect of nonylphenol on serum testosterone levels and testicular steroidogenic enzyme activity in neonatal, pubertal, and adult rats. Chem. Biol. Interact. 2002;139(1):23–41. doi: 10.1016/s0009-2797(01)00291-5. [DOI] [PubMed] [Google Scholar]