Abstract
Background
In sub-Saharan Africa, rates of sustained HIV virologic suppression remain below international goals. HIV resistance testing, while common in resource-rich settings, has not gained traction due to concerns about cost and sustainability.
Objective
We designed a randomized clinical trial (REVAMP) to determine the feasibility, effectiveness, and cost-effectiveness of routine HIV resistance testing in sub-Saharan Africa.
Approach
We describe challenges common to intervention studies in resource-limited settings, and strategies used to address them, including: 1) optimizing generalizability and cost-effectiveness estimates to promote transition from study results to policy; 2) minimizing bias due to patient attrition; and 3) addressing ethical issues related to enrollment of pregnant women.
Methods
The REVAMP study randomizes people in Uganda and South Africa with virologic failure on first-line therapy to standard of care virologic monitoring or immediate resistance testing. To strengthen external validity study procedures are conducted within publicly-supported laboratory and clinical facilities using local staff. To optimize cost estimates, we collect primary data on quality of life and medical resource utilization. To minimize losses from observation, we collect locally-relevant contact information, including Whatsapp account details, for field-based tracking of missing participants. Finally, pregnant women are followed with increase visit frequency to minimize risk to them and their fetuses.
Conclusions
REVAMP is a pragammatic randomized clinical trial designed to test the effectiveness and cost-effectiveness of HIV resistance testing versus standard of care in sub-Saharan Africa. We anticipate the results will directly inform HIV polity in sub-Saharan to optimize care for HIV-infected patients.
Keywords: HIV/AIDS, sub-Saharan Africa, antiretroviral therapy, randomized controlled trial, treatment failure, cost-effectiveness, genotypic resistance testing
Introduction
To achieve sustained control of the HIV epidemic, the Joint United Nations Programme on HIV/AIDS (UNAIDS) has set a “90-90-90” treatment target for 2020.1 Accomplishing this goal would mean that 90% of those receiving antiretroviral therapy (ART) will sustain virologic suppression. In sub-Saharan Africa, however, rates of virologic suppression remain well below that goal, with up to 1 in 3 experiencing virologic failure within two years of ART initiation.2,3
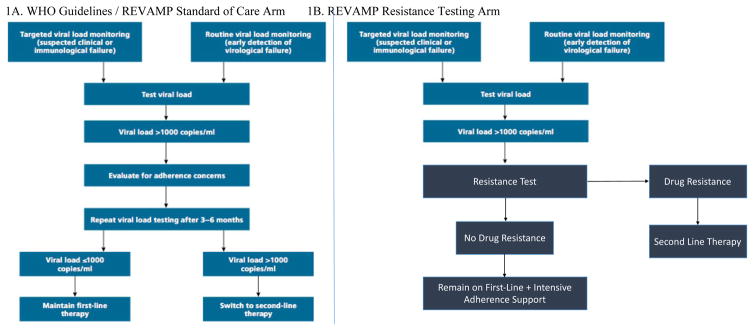
The optimal strategy for management of virologic failure in sub-Saharan Africa, in terms of both efficacy and cost, is unknown. The 2016 World Health Organization (WHO) HIV treatment guidelines recommend use of repeated viral load testing along with adherence support to guide treatment decisions (Figure 1A).4 Whereas increasing availability of viral load testing represents an important advance for HIV care in the region5, it also raises questions about the optimal management of patients with treatment failure. In particular, it is not known whether the use of viral load measurements alone may result in unnecessary switches to second-line therapy, which is more costly,6 is associated with increased toxicity, and requires an increased pill burden, which can challenge treatment adherence.7
Figure 1.
Figures 1A–B. World Health Organization (1A) and proposed REVAMP study resistance testing arm (1B) algorithms for management of virologic failure in HIV infection
An alternate approach to the WHO guidelines, which is employed in the North America and Europe, would include addition of HIV genotypic resistance testing at the time of virologic failure8. Studies in resource rich settings have demonstrated that the addition of resistance testing results in improved immunologic and virologic outcomes.9–12 Similar studies investigating the efficacy of resistance testing to support clinical care in sub-Saharan Africa are lacking. Moreover, although cost effectiveness studies have identified value of resistance testing in the United States,13 whether these findings translate to resource-limited settings remains unclear, due to contrasting results of modeling studies (Table 1). The main difference between these studies involves assumptions about the efficacy of first-line therapy in patients without significant drug resistance. For example, Levison et al posit that delivery of drug resistance results to patients could result in improved adherence, and cost-savings through salvage cheaper first-line therapy regimens. In contrast, Phillips et al predicted the opposite – that those with poor adherence and wild-type virus would remain poorly adherent – which mitigates the benefits of resistance testing. Consequently, there is an important need for studies to provide primary efficacy and resource data on the role for HIV resistance testing in sub-Saharan Africa.
Table 1.
Published studies of the cost-effectiveness of HIV-1 RNA resistance testing for management of virologic failure in sub-Saharan Africa
| Analysis Model | Country | Perspective | Time Horizon | Outcome | Sensitivity Analysis | Primary Result |
|---|---|---|---|---|---|---|
| Cost-Effectiveness of Preventing AIDS Complications state-transition model24 | South Africa | Modified societal | Lifetime | Cost/year of life saved | Univariate and multiway | Very cost effective (dominated) |
| Cost minimization model27 | South Africa | Presumed Payer | 5 years | Cost/strategy | Deterministic/probabilistic | Cost neutral |
| HIV synthesis transmission individual-based stochastic model26 | Zimbabwe | Unstated | 10 years | Cost/disability adjusted life year (DALY) averted | Several one way sensitivity analyses | Not cost-effective |
The goal of the REVAMP study (NCT 02787499) is to evaluate a resistance testing-based algorithm for management of HIV virologic failure (Figure 1B), and ultimately to determine whether resistance testing 1) improves clinical outcomes after virologic failure, 2) is feasible in the public healthcare sector in sub-Saharan Africa, and 3) if so, at what cost to payers. We designed the study with attention to these goals. We were particularly interested in providing results that were generalizable to people living with HIV in care at publically funded programs in the region, and enabling translation of our results into HIV program policy. Here we describe how the study was designed to accomplish these goals, with a specific focus on: 1) study design with a public health focus on evaluating effectiveness and cost-effectiveness; 2) strategies to minimize attrition of patients at high risk of death and loss from clinical care in resource limited settings; and 3) ethical issues surrounding enrollment (or exclusion) of pregnant women, a patient population for which the intervention of interest has particular relevance in order to prevent of mother to child transmission of HIV.
Study Design Overview
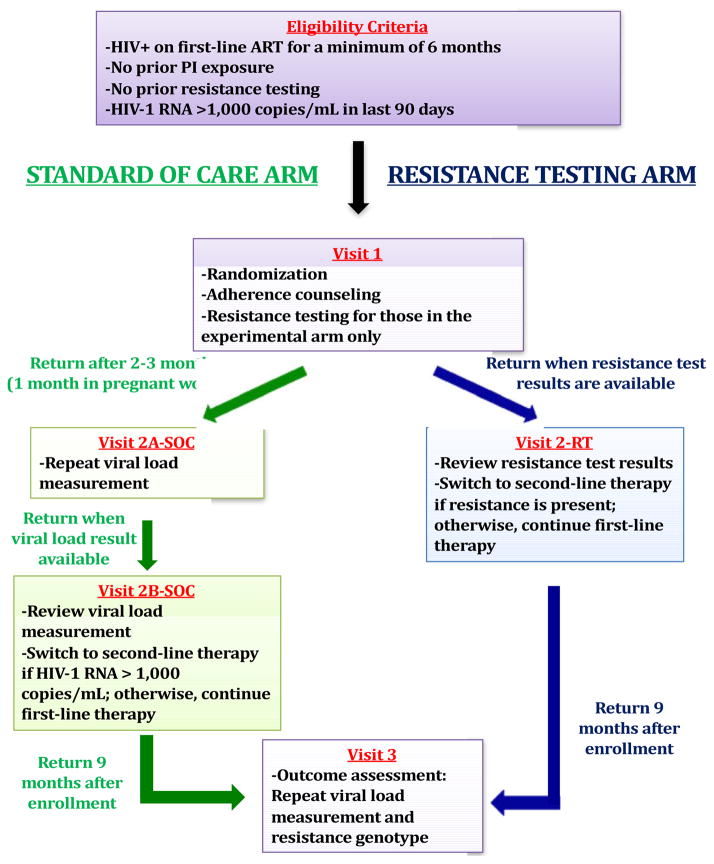
The REVAMP study is an open-label, randomized controlled trial designed to test the hypothesis that resistance testing improves rates of virologic re-suppression after virologic failure among patients in public HIV care programs in sub-Saharan Africa. The study is being conducted at study sites in both Uganda and South Africa. The study began enrolling participants in December 2016, and is slated to complete enrollment by the end of 2019. The study population includes HIV-infected patients on first-line antiretroviral therapy with a recent viral load >1,000 copies/milliliter (mL). Enrolled participants (eligibility criteria are listed in Table 2) are randomized using block randomization (stratified by clinic, pregnancy status, and duration on ART) into the WHO-based standard of care (SOC) arm, or to the resistance testing (RT) arm (Figure 2).
Table 2.
Enrollment Criteria
| Inclusion Criteria |
| 1. In care at a public HIV clinic within a PEPFAR-focus sub-Saharan African country (South Africa or Uganda) and living within 100 kilometers of the clinic |
| 2. Age ≥ 18 years at the time of enrollment |
| 3. Currently prescribed first-line (non-nucleoside reverse transcriptase inhibitor-based) ART for at least 6 months. Switches within first line regimens, including NNRTI and nucleos(t)ide backbone changes are allowed |
| 4. Detectable plasma viral load > 1,000 copies/mL and/or dried blood spot viral load >1,000 copies/mL within 90 days of enrollment |
| Exclusion Criteria |
| 1. Known prior drug resistance |
| 2. Prior exposure to PI-based ART |
| 3. Current clinical indication to start PI-based ART |
| 4. Not planning to remain in the clinic catchment area for the next nine months |
Figure 2.
Study Schema
Participants in the RT arm have blood collected for HIV-1 genotypic antiretroviral resistance testing on the day of enrollment, with results used to determine regimen selection. In general, participants without significant drug resistance will continue their first-line (non-nucleoside reverse transcriptase-based) ART regimen, whereas those with resistance will be changed to a second-line (protease inhibitor-based) regimen. Participants randomized into the SOC arm return three months after enrollment for repeat viral load testing per WHO guidelines. Those with a viral load ≤ 1,000 copies/mL will continue their first-line (NNRTI-based) ART regimen. Participants with a viral load >1,000 copies/mL initiate a second-line regimen. Aside from resistance testing in the RT arm at baseline, all other clinical care, including provision of ART and adherence support by clinic counselors, is supported by the partner clinic infrastructure and conducted in keeping with WHO and national HIV guidelines.
Participants return for the outcome assessment approximately nine months after enrollment. The primary outcome of interest is viral suppression (<200 copies/mL). We selected 9 months as the study endpoint to ensure a minimum of 6 months of observation after final regimen selection in both arms; in the SOC arm, regimen changes might occur at the 3-month follow-up viral load assessment. Outcomes are assessed using an intention-to-treat analysis, where missing or absent results are considered failures. Secondary outcomes of interest are viral suppression below the limit of assay detection, viral suppression on first-line therapy, drug resistance at study conclusion, and mortality, among others.
We aim to enroll a sample of 840 participants, with approximately 420 randomized to each arm. This sample will enable >80% power to detect a clinically significant, 10% or greater difference in the rate of our primary outcome (viral load <200 copies/mL) in the RT arm (superiority design), based on prior data demonstrating that approximately 70% of patients will achieve suppression after virologic failure under standard of care conditions.14–18 We believe provision of resistance results will promote improved adherence, both for those without significant drug resistance who are presented with information that their virus remains fully susceptible to first line regimens, and for those with drug resistance who are transitioned to more potent protease inhibitor-based regimens.19,20 For our primary outcome analysis, we will use a two-sample test of binomial proportions to compare the proportions of participants in each arm who achieve virologic suppression at study conclusion.
Promoting a Public Health Focus on Feasibility and Effectiveness
Because HIV resistance is currently limited in practice in sub-Saharan Africa by concerns about complexity and cost, an over-arching goal of the REVAMP study is to guide ministries of health and donor organizations regarding the feasibility and impact of resistance testing on clinical care within the existing framework of publically-funded and operated HIV clinics in the region. Although clinical trials network infrastructures provide data monitoring, laboratory quality control, and internal validity enhancement, they can challenge the external validity of public health interventions in low-resource settings.21,22 In contrast, our study design attempts to enhance effectiveness estimation through leveraging established partnerships with, and conduct within, existing ministry of health clinical and laboratory infrastructures. For example, an inclusion criterion of study participation is active enrollment at one of five publically-funded HIV clinics in Uganda and South Africa, and all study activities will take place within these clinics. To standardize regimen allocation in the RT arm and foster implementation of resistance testing in a real-world scenario, study clinicians developed an HIV-1 RNA resistance interpretation guide (Supplemental Appendix) to assist with treatment decision-making. Moreover, and perhaps most importantly, all study viral load and HIV resistance testing is conducted within existing laboratory facilities and by health workers currently used by the public health sector (the National Health Laboratory Service in South Africa, or the Joint Clinical Research Centre, in Uganda). To accomplish these goals, we developed relationships, certified through multilateral Memorandums of Understanding between US collaborating institutions, foreign collaborating institutions, clinical sites, and the partner laboratories during the proposal development stage. These agreements help ensure that study results are representative of the existing public HIV health care infrastructure.
Promoting Cost Effectiveness Estimation
Because resistance testing use in the sub-Saharan African region is partially limited by concerns about costs, another principle aim of the REVAMP study is to assess the value of HIV resistance testing in the region. Our goal is to estimate both short term costs and long term cost effectiveness from a national payer perspective to guide ministries of health and donor partners, who are currently the principal financiers of HIV care in sub-Saharan Africa. To do so, we will conduct both a short-term budget impact analysis of incorporating resistance testing into routine care and model the long-term cost effectiveness of adopting such an approach. We designed data collection tools to record resource utilization in “real time”.23 To enable this, costs are collected at each study visit on human resource use, HIV diagnostics including both viral load and resistance testing, therapeutic costs, including ART use, as well as costs for additional clinical visits and hospitalizations accrued during the study period. Human resource costs will be collected and summarized as hours/per patient spent by nurses, clinicians, counselors, phlebotomists, registrars, pharmacists, and laboratory personnel. We will also collect data on duration of time from laboratory testing to delivery of results to participants, to assess if and how delays affect the cost-effectiveness of resistance testing, as previously reported.24
First, we will conduct a budget impact analysis to estimate the short-term costs to ministries of health of implementing routine resistance testing. We will follow International Society of Pharmacoeconomics Outcomes Research guidelines to develop models to estimate the total costs of adopting resistance testing in South Africa and Uganda.25 We will populate these models with data provided by the clinical trial on costs of care, and add country-specific HIV epidemiology and expenditure data to estimate the budget impact of adopting resistance testing over five years for each strategy. We will build the model such that country-specific fields will be flexible to enable analysis for other countries, to facilitate policy planning and decision-making. In contrast to the future cost-effectiveness analysis, which will estimate incremental cost effectiveness in terms of QALYs per dollars spent between resistance testing and standard of care, the budget impact analysis will give payers an estimate of direct total programmatic costs for initiation of resistance testing country-wide in the public healthcare system.
If the resistance testing intervention is superior or similar to the standard of care strategy, we will proceed with a full cost-effectiveness analysis. This analysis seeks to build upon prior analyses that have considered the economics of resistance testing in sub-Saharan Africa.24,26,27 We will achieve this by incorporating primary efficacy, quality of life, and cost data from the clinical trial, and by estimating the cost utility of resistance testing versus standard of care. To optimize validity, we will follow best practice recommendations for modeling published by the International Society of Pharmacoeconomics Outcomes Research Good Research Practice Task Force28 and guidelines of the National Institute for Health and Clinical Excellence Reference Case.29 We have included the EuroQOL EQ-5D questionnaire, a generic (i.e. not disease specific) health-related quality of life questionnaire that has been validated in people with HIV in sub-Saharan Africa,30–33 with data collection at study start and conclusion. For this analysis, our outcome of interest will be the cost per quality adjusted life year ($/QALY), presented as an incremental cost-effectiveness ratio. We will populate the model for the first 9 months with data derived directly from the clinical trial. Thereafter, we plan to use published data on the natural history of HIV disease to model clinical outcomes every 6 months after study conclusion over a patient’s lifetime, including risk of opportunistic infections, hospitalization, quality of life, and death.30,34–37 To characterize uncertainty in the model, we will conduct a probabilistic sensitivity analysis, and present data as cost-effectiveness acceptability curves and probabilistic sensitivity analysis plots. Convergent validity will be undertaken to compare the model to similarly published models on the cost effectiveness of resistance testing. Whereas results will be immediately relevant for the South African and Ugandan populations, the proposed structure of the model will be designed such that it may be used for adaptation to countries with similar health care contexts.
Minimizing Losses from Observation in a Population at High Risk for Treatment Default and Mortality
Losses from observation are a well-described challenge to clinical trial design and analysis.38,39 Loss from observation can be a particularly vexing issue in studies targeting high-risk populations, such as HIV-infected populations experiencing virologic failure, who have high rates of loss from care and mortality.40,41 Whereas many studies include additional resources to monitor participants and enhance retention, this strategy has the potential to mitigate external validity by providing participants support systems that are not available outside of study settings. The REVAMP study includes a monitoring plan designed to balance a strategy of retention for outcome assessment with a desire to minimize contamination of results through provision of unsustainable participant support mechanisms. To do so, comprehensive, locally-relevant contact information is collected at each visit, including village leader contact information (if applicable), landlord contact information, and “Whatsapp” usernames. At the final outcome visit, we call participants who do not return within seven days of their scheduled visit. For those unreachable by phone, a study staff member will attempt to track them at home. Professional trackers have been for the purpose of locating missing study participants using the data collected at enrollment, adopting methods previously employed in Uganda with >90% success.42,43 Study staff will encourage participants to return to the clinic to complete procedures. If not possible, we will conduct the final blood draw and questionnaire in the field. As described previously, we will use an intention-to-treat analysis, allocating any participants without a confirmed viral load result as having a detectable viral load (failure).
Enrollment of Pregnant Women to Optimize Generalizability
Pregnant women are often excluded from clinical trials due to concerns about safety or feasibility.44,45 We sought to enhance generalizability of the study to pregnant women, for whom optimal management of virologic failure has particular relevance in the prevention of mother to child transmission of HIV infection. However, standard WHO guidelines do not describe management of virologic failure among pregnant women. If standard guidelines are followed, a three-month delay between identification of virologic failure and repeat laboratory evaluation might put their fetuses at undue risk of HIV transmission. Consequently, we altered study procedures to ensure safeguarding of pregnant women and their fetuses. To confirm pregnancy status, women under 50 years of age undergo urine β-human chorionic gonadotropin testing at the enrollment visit. Those who are confirmed to be pregnant, are referred for antenatal care in addition to completing study enrollment procedures. Pregnant women in the SOC arm return one month after study enrollment for a repeat viral load test (as opposed to three months for others in the SOC arm). This recommendation is in keeping with South African Prevention of Mother-to-Child Transmission Guidelines,46 and is intended to enable inclusion of pregnant women for generalizability purposes, while simultaneously maximizing chances of viral suppression prior to delivery. This adapted protocol was agreed upon by clinic staff at all study sites, and approved by ethical review committees in all three collaborating countries. Because of the unique characteristics of this population and group-specific protocol, randomization will be stratified by pregnancy status to minimize bias that could result from unbalanced inclusion across study arms. Although the study will not be powered to independently demonstrate benefit of resistance testing among pregnant women, their inclusion will provide an effect size in this sub-population, and determine the value of a dedicated study among them.
Conclusions
Over 12 million people in sub-Saharan Africa have gained access to ART over the past decade, but up to 1 in 3 of those individuals will develop virologic failure within two years of treatment initiation.2,3 Identifying the optimally effective and cost-effective method of managing virologic failure will be critical to ensure the long-term sustainability of HIV programs in the region. The REVAMP study is a pragmatic, randomized controlled trial that aims to provide valid and generalizable data on the feasibility, effectiveness, and cost effectiveness of HIV resistance testing care in sub-Saharan Africa through partnerships with the public health sector and simultaneous collection of quality of life and resource allocation data. We intend to use results from the REVAMP study to inform ministries of health and multinational donors on the optimal approach to management of virologic failure in sub-Saharan Africa.
Supplementary Material
Acknowledgments
FUNDING: This study is funded by the National Institute of Allergy and Infectious Diseases with support from the President’s Emergency Plain for AIDS Relief (NIH R01 AI124718). MJS receives additional support from the National Institutes of Mental Health (K23 MH099916). VCM and RTG receive additional support from NIH/NIAID
Footnotes
Trial registered at clinicaltrials.gov: NCT0278749
Conflicts of Interest
All authors report that there is no conflict of interest.
References
- 1.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 2.McMahon JH, Elliott JH, Bertagnolio S, Kubiak R, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low- and middle-income countries: a systematic review. Bull World Health Organ. 2013;91(5):377–385E. doi: 10.2471/BLT.12.112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet Infectious diseases. 2010;10(3):155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Recommendations for a public health approach. 2. 2016. Consolidated guidelines on the use of antiretroviral drugs for treatment and preventing HIV infection. [PubMed] [Google Scholar]
- 5.Mermin J, Ekwaru JP, Were W, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. Bmj. 2011;343:d6792. doi: 10.1136/bmj.d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 7.Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297–1307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2016. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 9.Cohen CJ, Hunt S, Sension M, et al. A randomized trial assessing the impact of phenotypic resistance testing on antiretroviral therapy. AIDS. 2002;16(4):579–588. doi: 10.1097/00002030-200203080-00009. [DOI] [PubMed] [Google Scholar]
- 10.Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet. 1999;353(9171):2195–2199. doi: 10.1016/s0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 11.Haubrich RH, Kemper CA, Hellmann NS, et al. A randomized, prospective study of phenotype susceptibility testing versus standard of care to manage antiretroviral therapy: CCTG 575. AIDS. 2005;19(3):295–302. [PubMed] [Google Scholar]
- 12.Tural C, Ruiz L, Holtzer C, et al. Clinical utility of HIV-1 genotyping and expert advice: the Havana trial. AIDS. 2002;16(2):209–218. doi: 10.1097/00002030-200201250-00010. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide hiv therapy: clinical impact and cost-effectiveness. Annals of internal medicine. 2001;134(6):440–450. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 14.El-Khatib Z, Delong AK, Katzenstein D, et al. Drug resistance patterns and virus re-suppression among HIV-1 subtype C infected patients receiving non-nucleoside reverse transcriptase inhibitors in South Africa. Journal of AIDS & clinical research. 2011;2(117) doi: 10.4172/2155-6113.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilks CF, Walker AS, Dunn DT, et al. Lopinavir/ritonavir monotherapy after 24 weeks of second-line antiretroviral therapy in Africa: a randomized controlled trial (SARA) Antiviral therapy. 2012;17(7):1363–1373. doi: 10.3851/IMP2253. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RK, Goodall RL, Ranopa M, et al. High Rate of HIV Resuppression After Viral Failure on First-line Antiretroviral Therapy in the Absence of Switch to Second-line Therapy. Clin Infect Dis. 2014;58(7):1023–1026. doi: 10.1093/cid/cit933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann CJ, Charalambous S, Sim J, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49(12):1928–1935. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobanputra K, Parker LA, Azih C, et al. Factors Associated with Virological Failure and Suppression after Enhanced Adherence Counselling, in Children, Adolescents and Adults on Antiretroviral Therapy for HIV in Swaziland. PloS one. 2015;10(2):e0116144. doi: 10.1371/journal.pone.0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciaffi L, Koulla-Shiro S, Sawadogo A, et al. Efficacy and safety of three second-line antiretroviral regimens in HIV-infected patients in Africa. AIDS. 2015;29:1473–1481. doi: 10.1097/QAD.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371(3):234–247. doi: 10.1056/NEJMoa1311274. [DOI] [PubMed] [Google Scholar]
- 21.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. Am J Epidemiol. 2010;172(1):107–115. doi: 10.1093/aje/kwq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Victora CG, Habicht JP, Bryce J. Evidence-based public health: moving beyond randomized trials. Am J Public Health. 2004;94(3):400–405. doi: 10.2105/ajph.94.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hlatky MA, Owens DK, Sanders GD. Cost-effectiveness as an outcome in randomized clinical trials. Clin Trials. 2006;3(6):543–551. doi: 10.1177/1740774506073105. [DOI] [PubMed] [Google Scholar]
- 24.Levison JH, Wood R, Scott CA, et al. The clinical and economic impact of genotype testing at first-line antiretroviral therapy failure for HIV-infected patients in South Africa. Clin Infect Dis. 2013;56(4):587–597. doi: 10.1093/cid/cis887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2007;10(5):336–347. doi: 10.1111/j.1524-4733.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 26.Phillips A, Cambiano V, Nakagawa F, et al. Cost-effectiveness of HIV drug resistance testing to inform switching to second line antiretroviral therapy in low income settings. PloS one. 2014;9(10):e109148. doi: 10.1371/journal.pone.0109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen S, Long L, Sanne I, Stevens WS, Fox MP. The net cost of incorporating resistance testing into HIV/AIDS treatment in South Africa: a Markov model with primary data. Journal of the International AIDS Society. 2011;14:24. doi: 10.1186/1758-2652-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caro JJ, Briggs AH, Siebert U, Kuntz KM Force I-SMGRPT. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--1. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15(6):796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Gepner AD, Korcarz CE, Aeschlimann SE, et al. Validation of a carotid intima-media thickness border detection program for use in an office setting. J Am Soc Echocardiogr. 2006;19(2):223–228. doi: 10.1016/j.echo.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Robberstad B, Olsen JA. The health related quality of life of people living with HIV/AIDS in sub-Saharan Africa - a literature review and focus group study. Cost effectiveness and resource allocation : C/E. 2010;8:5. doi: 10.1186/1478-7547-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelsma J, Maclean E, Hughes J, Tinise X, Darder M. An investigation into the health-related quality of life of individuals living with HIV who are receiving HAART. AIDS care. 2005;17(5):579–588. doi: 10.1080/09540120412331319714. [DOI] [PubMed] [Google Scholar]
- 32.Hughes J, Jelsma J, Maclean E, Darder M, Tinise X. The health-related quality of life of people living with HIV/AIDS. Disability and rehabilitation. 2004;26(6):371–376. doi: 10.1080/09638280410001662932. [DOI] [PubMed] [Google Scholar]
- 33.Louwagie GM, Bachmann MO, Meyer K, Booysen Fle R, Fairall LR, Heunis C. Highly active antiretroviral treatment and health related quality of life in South African adults with human immunodeficiency virus infection: A cross-sectional analytical study. BMC public health. 2007;7:244. doi: 10.1186/1471-2458-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;16(1):54–62. doi: 10.1097/00042560-199709010-00009. [DOI] [PubMed] [Google Scholar]
- 35.Luz PM, Morris BL, Grinsztejn B, et al. Cost-effectiveness of genotype testing for primary resistance in Brazil. Journal of acquired immune deficiency syndromes. 2015;68(2):152–161. doi: 10.1097/QAI.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 37.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Medical decision making : an international journal of the Society for Medical Decision Making. 2002;22(1):27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 38.Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clin Trials. 2004;1(4):368–376. doi: 10.1191/1740774504cn032oa. [DOI] [PubMed] [Google Scholar]
- 39.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabillard D, Lewden C, Ndoye I, et al. Mortality, AIDS-morbidity, and loss to follow-up by current CD4 cell count among HIV-1-infected adults receiving antiretroviral therapy in Africa and Asia: data from the ANRS 12222 collaboration. Journal of acquired immune deficiency syndromes. 2013;62(5):555–561. doi: 10.1097/QAI.0b013e3182821821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. Journal of acquired immune deficiency syndromes. 2010;53(3):405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300(5):506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foulkes MA, Grady C, Spong CY, Bates A, Clayton JA. Clinical research enrolling pregnant women: a workshop summary. J Womens Health (Larchmt) 2011;20(10):1429–1432. doi: 10.1089/jwh.2011.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mastroianni A, Faden R, Federman D. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. Institute of Medicine; Washington DC: 1994. [PubMed] [Google Scholar]
- 46.National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. National Department of Health; South Africa: 2015. Available at: http://www.sahivsoc.org/upload/documents/ART Guidelines 15052015.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.