Abstract
Astronauts are exposed to charged particles during space travel, and charged particles are also used for cancer radiotherapy. Premature ovarian failure is a well-known side-effect of conventional, low linear energy transfer (LET) cancer radiotherapy, but little is known about the effects of high LET charged particles on the ovary. We hypothesized that lower LET (16.5 keV/µm) oxygen particles would be less damaging to the ovary than we previously found for iron (LET=179 keV/µm). Adult female mice were irradiated with 0, 5, 30, or 50 cGy oxygen ions or 50 cGy oxygen plus dietary supplementation with the antioxidant alpha lipoic acid (ALA). 6h after irradiation, percentages of ovarian follicles immunopositive for γH2AX, a marker of DNA double strand breaks, 4-HNE, a marker of oxidative lipid damage, and PUMA, a proapoptotic BCL-2 family protein, were dose-dependently increased in irradiated mice compared to controls. One week after irradiation, numbers of primordial, primary, and secondary follicles per ovary were dose-dependently decreased, with complete absence of follicles in the 50 cGy groups. The ED50 for primordial follicle destruction was 4.6 cGy for oxygen compared to 27.5 cGy for iron in our previous study. Serum FSH and LH concentrations were significantly elevated in 50 cGy groups at 8wk. Supplementation with ALA mitigated the early effects, but not the ultimate depletion of ovarian follicles. In conclusion, oxygen charged particles are even more potent inducers of ovarian follicle depletion than charged iron particles, raising concern for premature ovarian failure in astronauts exposed to both particles during space travel.
Keywords: charged particles, ionizing radiation, ovarian follicle, premature ovarian failure, apoptosis, oxidative stress, space radiation
Introduction
Temporary amenorrhea and premature ovarian failure are common side effects of therapeutic photon (x- or γ- radiation) radiation to the whole body or pelvis (Lo Presti, et al. 2004, Meirow and Nugent 2001, Wallace, et al. 1989). Photon radiation damages ovarian follicles at all stages of development, but the quiescent primordial follicles, are most sensitive to destruction (Kim and Lee 2000, Lee, et al. 2000, Lee and Yoon 2005). Because the pool of primordial follicles is believed to be finite, destruction of primordial follicles accelerates the onset of ovarian failure. Premature ovarian failure causes early loss of fertility, as well as increased risk of osteoporosis, cardiovascular disease, and Alzheimer’s disease (Dubey, et al. 2005, Molina, et al. 2005, Shuster, et al. 2008, Silva, et al. 2001, Svejme, et al. 2012).
Biological effects of ionizing radiation depend on dose, dose rate, and quality of the radiation (ICRP 2003). Linear energy transfer (LET) is an important element of radiation quality. Most prior studies have examined the effects of low LET photon radiation, while very few studies have examined the effects of high LET radiation, such as high charge and energy (HZE) particles, on the ovary. Low-LET radiation produces ionization sparsely inside cells, whereas high-LET radiation produces dense ionization and is thought to be more destructive (Sridharan, et al. 2015, Tokuyama, et al. 2015). Both low LET proton ions and high LET carbon ions have been increasingly used in cancer therapy due to the ability to deliver high doses to the tumor while relatively sparing surrounding tissues (Suit, et al. 2010). Exposure to charged particle radiation from solar particle events and galactic cosmic rays (GCR) will also be an important occupational hazard for astronauts on deep space missions, such as a mission to Mars. This is of increasing concern as women now make up half of recent National Aeronautics and Space Administration (NASA) astronaut classes (Ronca, et al. 2014). GCRs consist of 85% low LET protons, 14% helium ions, and 1% HZE particles (Bourdarie and Xapsos 2008). However, HZE particles such as oxygen and iron, will make up 21% of the estimated ionizing dose equivalent from GCR exposure during deep space travel (Slaba, et al. 2015). The estimated ionizing dose equivalent of a three-year Mars mission is 40 cGy (1.07 Sv absorbed dose) (Barcellus-Hoff, et al. 2015, Cucinotta and Durante 2006).
We recently reported that high LET charged iron particles (LET = 179 keV/µm) induce apoptosis, oxidative damage, and DNA double strand breaks in oocytes and granulosa cells of ovarian follicles, depleting nearly all of the primordial follicle pool at doses of 30 and 50 cGy (Mishra, et al. 2016). We also showed that dietary supplementation with the antioxidant alpha lipoic acid (ALA) was partially protective against the early ovarian effects of iron ions, but was not able to prevent premature ovarian failure. We hypothesized that oxygen charged particles, with LET of 16.5 keV/µm, would be less potent inducers of ovarian follicle destruction than iron charged particles. We established the dose-response relationships for induction of ovarian follicle DNA double strand breaks, oxidative damage and apoptosis and follicle depletion by charged oxygen particles and tested ALA supplementation as a potential protective countermeasure.
Materials and Methods
Animals
Twelve-week old female mice (C57BL/6J from Jackson Laboratories, N=8/experimental group) were randomly assigned to experimental group. Mice were irradiated with 0, 5, 30 or 50 cGy charged oxygen particles (LET = 16.5 keV/µm) at energy of 600 MeV/u and dose rates of 12.5–44.3 cGy/minute. Two groups were irradiated at the highest dose, one fed AIN-93M diet and the other fed the same diet supplemented with 150 mg/kg diet of the antioxidant ALA (ALA from Sigma Aldrich; diets formulated by Bio-Serv, Flemington, NJ), beginning one week before irradiation and continuing until euthanasia. Irradiations were performed at the NASA Space Radiation Laboratory, Brookhaven National Laboratory, NY (La Tessa, et al. 2016). Mice for the 0 cGy group were transported and restrained identically to the irradiated groups. All animal procedures were approved by the Institutional Animal Care and Use Committees at Brookhaven National Laboratory and the University of California Irvine. Mice were euthanized by CO2 inhalation at 6h, 1 wk and 8 wk after irradiation. Ovaries and blood were collected at the time of euthanasia.
Vaginal cytology
Estrous cycling was monitored every morning by microscopic examination of fresh vaginal lavage fluid obtained in 0.9% sodium chloride (Cooper, et al. 1993) beginning at 6 wk after irradiation and continuing for at least 3 estrous cycles or 14 days if the mice were not cycling. Mice were housed individually for one week before and while monitoring estrous cycles and were euthanized on the day of metestrus of the estrous cycle at approximately 8 wk after irradiation. Estrous cycles were not monitored in the mice euthanized at the acute, 6h and 1 wk time points because we wished to euthanize the mice as close to the designated acute time point as possible.
Ovarian histomorphometric analysis
Ovaries were fixed in Bouin's fixative (Fisher Scientific), washed in 50% ethanol, stored in 70% ethanol, embedded in paraffin, and serially sectioned at 5 µm thickness. Sections were stained with Gill's hematoxylin (Fisher Scientific) and eosin Y (Sigma Aldrich). Ovarian follicles were counted blind to treatment group using an Olympus BX60 light microscope equipped with 4× Plan Acromat, 10×, 20×, and 40× Plan Fluor objectives. Images were captured using a Retiga 2000R cooled CCD digital camera system and QCapture Pro software (QImaging). Ovarian follicles were classified as primordial (single layer of flattened granulosa cells), primary (single layer of cuboidal or mixed cuboidal/flattened granulosa cells), secondary (more than one layer of granulosa cells), or antral (multiple layers of granulosa cells and possessing an antral space or spaces) and were further classified as healthy or atretic as described previously (Lim, et al. 2013, Lopez and Luderer 2004). Atretic secondary and antral follicles were identified by the presence of 3 or more pyknotic granulosa cells per largest cross section and separation of the oocyte from the granulosa cells. Atretic primordial and primary follicles were identified by shrunken, eosinophilic oocytes. Primordial, primary, and secondary follicles were counted in every fifth serial section. The counts were multiplied times 5 to obtain estimates of the total number of follicles per ovary. To avoid over counting, primordial and small primary follicles were only counted if the oocyte nucleus was clearly visible; larger primary and secondary follicles were only counted if the oocyte nucleolus was clearly visible. Antral follicles were followed through every section taking care to count each antral follicle only once.
Immunohistochemistry
Ovaries were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) in PBS, cryoprotected in 15% sucrose (Fisher Scientific), embedded in optimal cutting temperature (OCT) embedding compound (Electron Microscopy Sciences), and stored at −80°C until sectioning. Ovaries were serially sectioned at 7 µm thickness and slides were stored at −80°C until staining. Slides were immunostained using primary antibodies to phosphorylated H2A histone family, member X (γH2AX), 4-hydroxynonenal (4-HNE), p53 up-regulated modulator of apoptosis (PUMA), and secondary antibody as detailed in Table 1. Diaminobenzidine substrate (Roche) was used for visualization of antibody binding, and sections were counterstained with Gill's hematoxylin. Scoring was done blind to treatment group as described previously (Mishra, et al. 2016). The percentages of follicles with positive granulosa cells or positive oocytes per section were calculated, and the percentages from the six scored sections per ovary were averaged for analysis.
Table 1.
Antibodies used for immunohistochemistry
| Protein target | Name of antibody |
Manufacturer/Catalog | Species raised in; monoclonal or polyclonal |
Dilution | Secondary antibody dilution |
|---|---|---|---|---|---|
| Phospho-Histone H2A.X | Anti-ϒH2AX | Cell Signaling, #9718 | Rabbit; monoclonal | 1:200 | 1:200 |
| p53 upregulated modulator of apoptosis | Anti-PUMA | Abcam, #ab9643 | Rabbit polyclonal | 1:200 | 1:400 |
| 4-hydroxynonenal adducts | Anti- 4-Hydroxy- 2-nonenal | αDiagnostics, #HNE11-S | Rabbit; Polyclonal | 1:700 | 1:200 |
Measurement of hormones
Serum concentrations of follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured 8 wk after irradiation using Millipore Pituitary Panel Multiplex kits at the Center for Research in Reproduction, University of Virginia, Charlottesville, VA. The intra- and inter-assay coefficients of variation were 5.5% and 11.5%, respectively.
Statistical analyses
All data are presented as the mean ± SEM in figures. Differences among treatment groups were analyzed by analysis of variance (ANOVA) followed by post hoc LSD test (for equal variances) or Dunnett T3 test (unequal variances) for follicle counts or hormone concentrations. Levene's test was used to check for equality of variances. Differences among groups for endpoints expressed as percentages were analyzed by non-parametric Kruskal Wallis test followed by Mann Whitney U test for intergroup comparisons. Statistical analyses were carried out using SPSS 20 (Windows) or SPSS 23 (Macintosh; IBM Corporation).
Results
Charged oxygen particles increase ovarian histone 2AFX phosphorylation
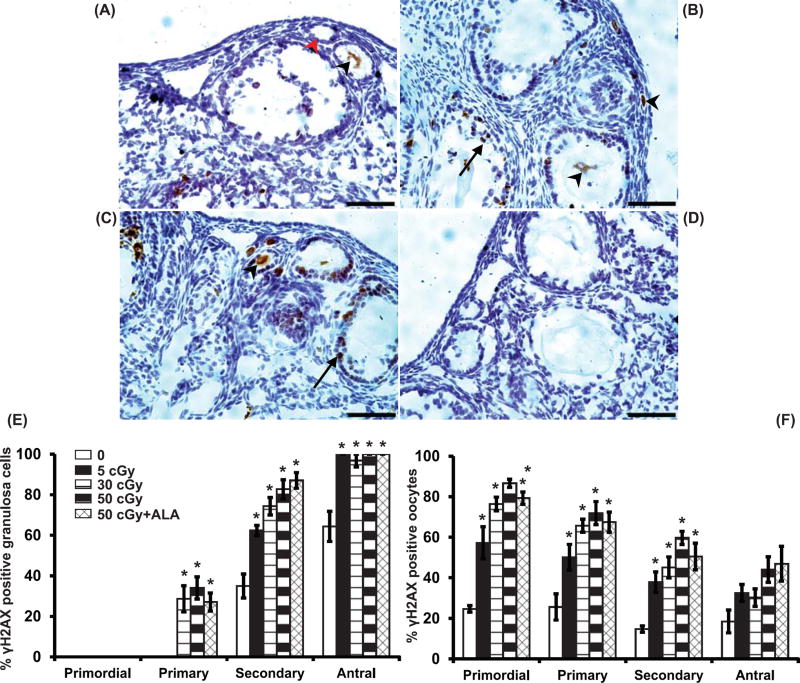
We assessed DNA double strand breaks using γH2AX immunostaining 6h after irradiation with charged oxygen particles. At 6h the percentages of primary, secondary, and antral follicles with γH2AX-positive granulosa cells increased in a dose-dependent manner (P<0.005, effect of group by Kruskal Wallis test; Figure 1A–C,E). The percentages of primordial, primary, and secondary, follicles with γH2AX-positive oocytes also increased in a dose-dependent manner (P<0.007, effect of group; Figure 1A–C,F; Figure 2 top). ALA-supplementation did not mitigate these increases in γH2AX immunostaining after 50 cGy oxygen irradiation.
Figure 1. Charged oxygen particles increase γH2AX immunostaining in ovarian follicles.
Mice in this and subsequent figures were fed AIN-93M diet or diet supplemented with 150 mg/kg ALA from one wk before irradiation with the indicated doses of charged oxygen particles until euthanasia. Double strand DNA damage was analyzed using γH2AX immunostaining in ovarian sections 6h after irradiation. Representative images of γH2AX immunostaining in 0 cGy (A), 5 cGy (B), 50 cGy (C) ovaries and lack of immunostaining in negative control with primary antibody replaced by nonimmune IgG (D). cells are indicated by black arrows (granulosa) and arrowheads (oocytes). Scale bars, 50 m. Graphs show the mean ± SEM percentage of follicles with γH2AX-positive granulosa cells or oocytes. (E) There were statistically significant differences in percentages of primary, secondary and antral follicles with γH2AX-positive granulosa cells among groups (P<0.005, Kruskal Wallis tests). (F) There were statistically significant differences in percentages of primordial, primary, and secondary follicles with γH2AX-positive oocytes (P<0.007, Kruskal Wallis tests). *P<0.05 versus 0 cGy control by Mann Whitney test. N=4 mice/group.
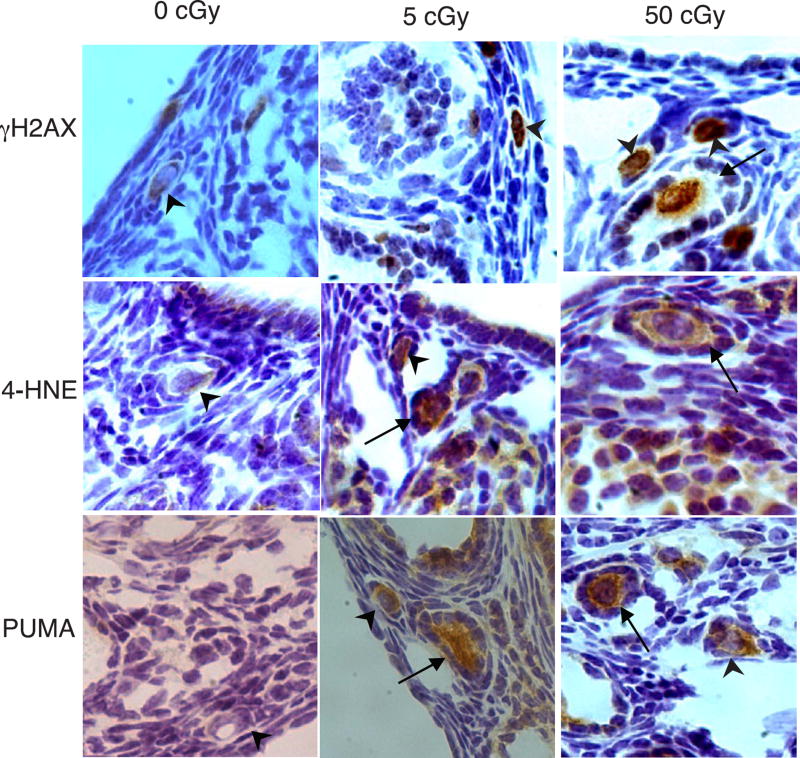
Figure 2. Representative γH2AX, 4-HNE, and PUMA immunostaining in primordial and primary follicles after irradiation with charged oxygen particles.
Processing of ovaries and immunostaining were performed as described in methods. Representative images of ovarian sections immunostained with antibodies to γH2AX (top row), 4-HNE (middle row), or PUMA (bottom row). Doses of charged oxygen particles are indicated at top. Arrowheads indicate primordial follicles and arrows indicate primary follicles Original magnification 400×.
Charged oxygen particles increase ovarian oxidative stress
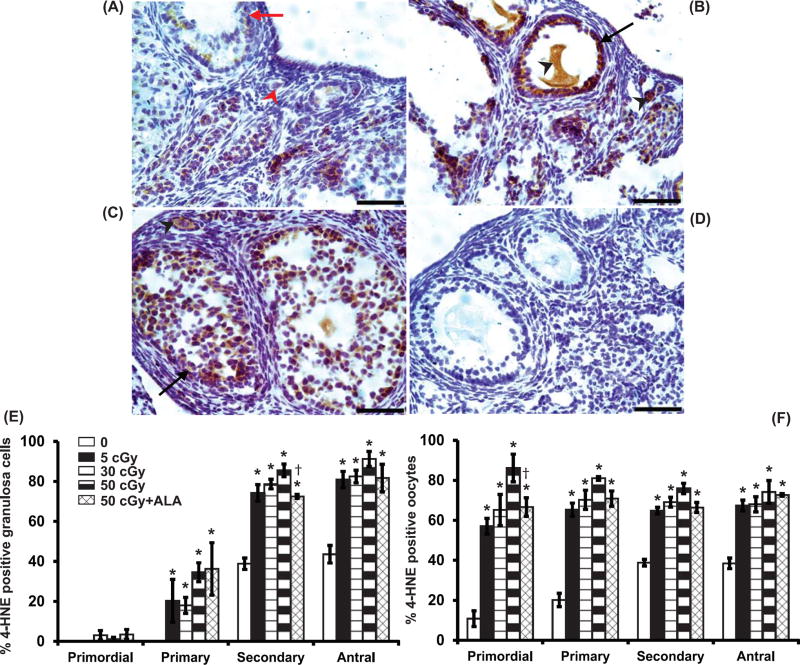
We assessed oxidative lipid damage 6h after irradiation with charged oxygen particles using immunostaining for 4-HNE (Mishra, et al. 2016). At 6h after irradiation with charged oxygen particles, the percentages of primary, secondary and antral follicles with 4-HNE positive granulosa cells were dose-dependently increased (P<0.03, effect of group by Kruskal Wallis test, Figure 3A–C,E). At 6h the percentages of primordial, primary, secondary and antral follicles with 4-HNE positive oocytes also increased in a dose-dependent manner (P<0.04, Figure 3A–C,F; Figure 2 middle). ALA-supplementation partially mitigated the effects of 50 cGy oxygen irradiation on lipid peroxidation in granulosa cells of secondary follicles and oocytes of primordial follicles (Fig. 3E and F).
Figure 3. Charged oxygen particles increase lipid peroxidation in ovarian follicles.
Lipid peroxidation was analyzed using 4-HNE immunostaining in ovarian sections 6h after irradiation. Representative images of 4-HNE immunostaining in 0 cGy (A), 5 cGy (B), 50 cGy (C) ovaries and lack of immunostaining in negative control ovary with primary antibody replaced by nonimmune IgG (D). Representative positively stained cells are indicated by black arrows (granulosa) and arrowheads (oocytes). Scale bars, 50 m. Graphs show the means ± SEM of percentages of follicles with 4-HNE positive granulosa cells or oocytes. (E) Percentages of follicles with 4-HNE positive granulosa cells varied significantly among groups for primary, secondary, and antral follicles (P<0.03, Kruskall Wallis test). (F) Percentages of follicles with 4-HNE positive oocytes varied significantly among groups for primordial, primary, secondary, and antral follicles (P<0.04, Kruskall Wallis test). *P<0.05 versus 0 cGy control by Mann Whitney test. †P<0.05 versus 50 cGy. N=4 mice/group.
Charged oxygen particles increase ovarian follicular apoptosis
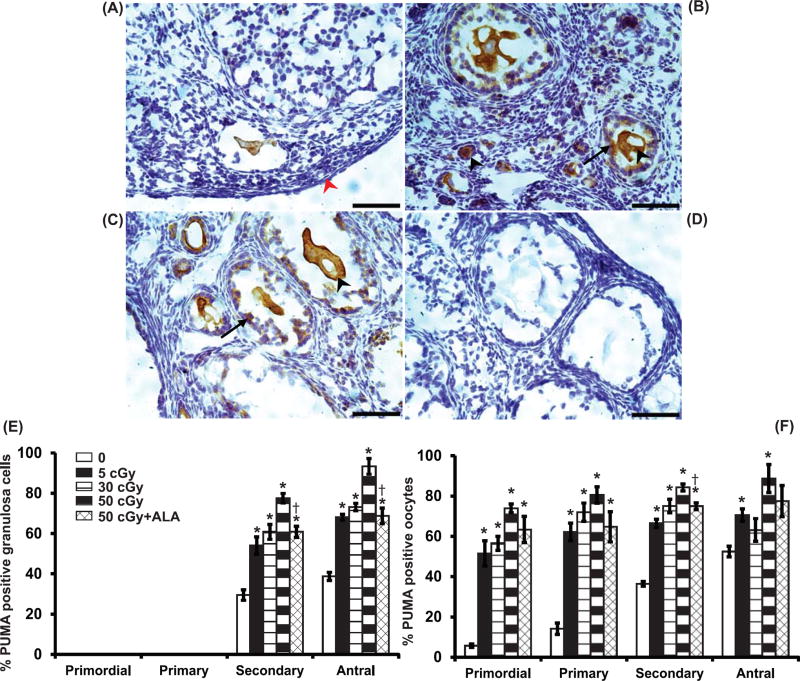
Because acute increases in PUMA (pro-apoptotic BCL-2 family member) are required for γ-radiation-induced germ cell death in neonatal ovaries (Kerr, et al. 2012), and we observed that PUMA increased in iron ion irradiated adult ovaries together with caspase 3 activation (Mishra, et al. 2016), we assessed follicle apoptosis at 6h post-irradiation using PUMA immunostaining. The percentages of secondary and antral follicles with PUMA-positive granulosa cells were dose-dependently elevated at 6 h after oxygen irradiation (P<0.02, effect of group by Kruskal Wallis test; Figure 4A–C,E). The percentages of primordial, primary, secondary, and antral follicles with PUMA-positive oocytes were also dose-dependently increased at 6h (P<0.005; Figure 4A–C,F; Figure 4A; Figure 2 bottom). The increases in PUMA levels after charged oxygen irradiation in secondary follicle oocytes and granulosa cells and antral follicle granulosa cells were mitigated by ALA supplementation (Figure 4E,F).
Figure 4. Induction of the proapoptotic protein PUMA in ovarian follicles by charged oxygen particles.
Representative images of PUMA immunostaining in 0 cGy (A), 5 cGy (B), 50 cGy (C) ovaries and lack of immunostaining in negative control ovary with primary antibody replaced by nonimmune IgG (D). Representative positively stained cells are indicated by black arrows (granulosa) and arrowheads (oocytes), while negatively stained are indicated by red arrows/arrowheads. Scale bars, 50 m. Graphs show the means ± SEM of percentages of follicles with PUMA-positive granulosa cells or oocytes at 6h after irradiation. (E) Percentages of secondary and antral follicles with PUMA-positive granulosa cells varied significantly among groups (P<0.05, Kruskal Wallis). (F) Percentages of primordial, primary, and secondary follicles with PUMA-positive oocytes varied significantly among groups (P<0.05, Kruskal Wallis). *P<0.05 versus 0 cGy control by Mann Whitney test. †P<0.05 versus 50 cGy. N=4 mice/group.
Charged oxygen particles disrupt estrous cycling
Estrous cycling was monitored by vaginal cytology from 6 to 8 wk after irradiation. 100% of mice in both 50 cGy exposure groups, 75% of mice in the 30 cGy group, and 37.5% of mice in the 5 and 0 cGy groups had irregular estrous cycles, defined as not having regular cycles of 4 to 5 day length (P=0.003, differences among groups by Fisher's exact test). 37% of the mice in the 50 cGy groups did not cycle at all, while all of the mice in the 0, 5, and 30 cGy groups cycled, whether regularly or irregularly (Table 2). For the mice that were cycling, cycle lengths tended to be longer in the mice in the 30 and 50 cGy groups compared to the 0 and 5 cGy groups (P<0.001, effect of group; Table 2), with the 30 cGy group having significantly longer cycles than the 0 cGy group. However, there were no statistically significant differences among groups in the percentages of days with predominantly cornified cytology, characteristic of estrus, or with predominantly leukocytic cytology, characteristic of metestrus and diestrus (Table 2).
Table 2.
Effects of charged oxygen particles on estrous cycle length
| Group | Number (%) cycling |
Cycle length ± SEM (days)* |
Cornified cytology (% of days) |
Leukocytic cytology (% of days) |
|---|---|---|---|---|
| 0 cGy | 8 (100) | 4.0±0.1 | 41.9±2.5 | 37.9±2.5 |
| 5 cGy | 8 (100) | 4.3±0.2 | 35.9±2.3 | 41.6±2.8 |
| 30 cGy | 8 (100) | 5.2±0.3** | 40.8±6.7 | 38.5±3.5 |
| 50 cGy | 5 (63) | 6.1±0.6 | 31.7±4.9 | 48.6±2.5 |
| 50 cGy plus ALA | 5 (63) | 5.5±0.5 | 31.5±7.1 | 42.8±6.7 |
Includes only mice that were cycling
P<0.05 versus 0 cGy by Dunnett T3 test
Charged oxygen particles deplete ovarian follicles
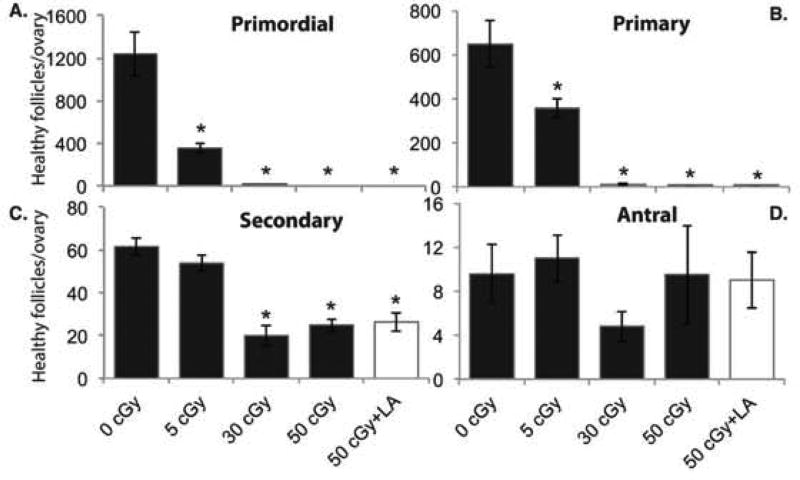
To examine the consequences of DNA damage, oxidative lipid damage, and apoptosis induction by oxygen charged particles in ovarian follicles, we counted healthy primordial, primary, secondary, and antral follicles at 1 wk after irradiation. The numbers of healthy primordial (P<0.001, effect of group by Kruskal Wallis test; Figure 5A), primary (P<0.001; Figure 5B), and secondary (P=0.002, Figure 5C) follicles per ovary were dose-dependently decreased in mice irradiated with oxygen. The numbers of healthy antral follicles were not affected by charged oxygen irradiation, but we had limited power to detect differences because of the large inter-mouse variability in this endpoint. ALA supplementation did not mitigate the depletion of ovarian follicles 1 wk after irradiation with 50 cGy charged oxygen particles.
Figure 5. Charged oxygen particles deplete ovarian follicles.
Ovaries were processed for histology, and follicles were classified and counted as described in Methods (N=4–5 mice/group). Graphs show the mean ± SEM of number healthy follicles of the indicated stages of development per ovary. (A) Primordial follicles, P<0.001, effect of group by Kruskal Wallis test. (B) Primary follicles, P<0.001, effect of group by Kruskal Wallis test. (C) Secondary follicles, P=0.002, effect of group by Kruskal Wallis test. (D) Antral follicles, effect of group not statistically significant. *P<0.05, compared to 0 cGy control by Mann Whitney test.
Charged oxygen particles are more potent destroyers of primordial follicles than charged iron particles
We analyzed the dose-response for primordial follicle depletion by oxygen ions (data from the present study) and iron ions (data from (Mishra, et al. 2016)) using linear regression on the log transformed follicle counts. We then calculated the ED50 for primordial follicle depletion at 1 wk after irradiation. The ED50 was 27.5 cGy for iron and 4.6 cGy for oxygen, indicating that charged oxygen particles with LET 16.5 keV/µm are more potent destroyers of primordial follicles than charged iron particles with LET of 179 keV/µm.
Charged oxygen particles alter reproductive hormone concentrations
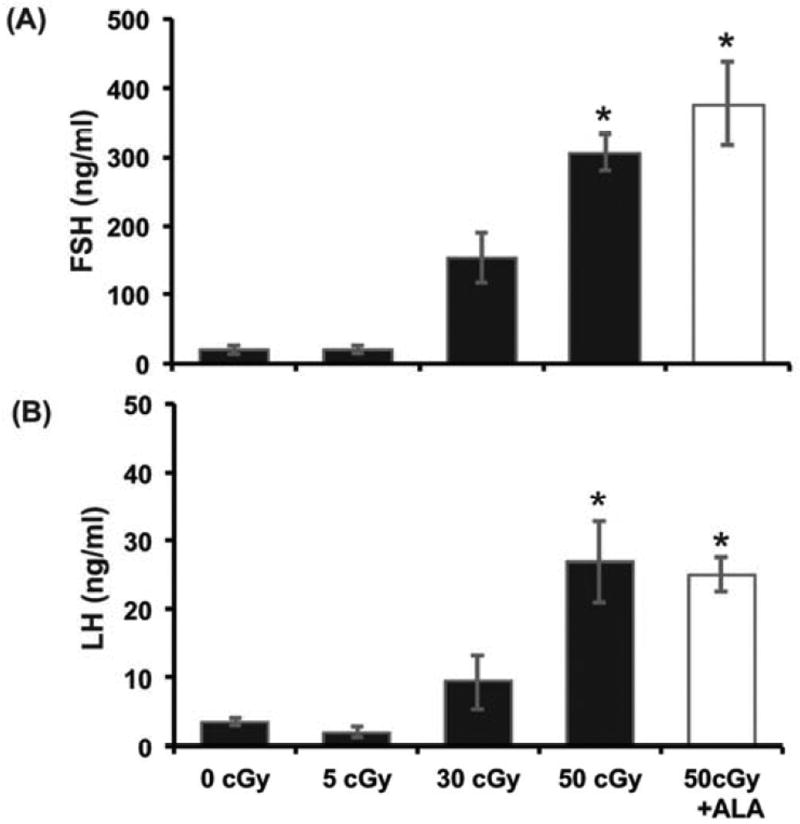
If the hypothalamic-pituitary-ovarian axis were functioning normally, one would expect the depletion of ovarian follicles to result in decreased negative feedback to the hypothalamus and pituitary, increasing the circulating levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) secreted from the anterior pituitary gland. Consistent with this, serum FSH and LH concentrations increased with charged oxygen particle dose 8 wk after irradiation (P<0.001, effects of treatment group; Figure 6 A,B). Mice irradiated with 50 cGy oxygen fed ALA supplemented diet had similar levels of FSH and LH as the 50 cGy iron group fed nonsupplemented diet (Figure 6 A,B).
Figure 6. Charged oxygen particles decrease ovarian negative feedback to the hypothalamus and pituitary.
Graphs show the mean ± SEM of serum FSH (A) or LH (B) levels at 8 wk after irradiation (N=6–8 mice/group). There were significant differences in FSH and LH concentrations among treatment groups (P<0.001). *P<0.05, compared to 0 cGy control.
Discussion
High LET radiation causes dense ionization, which is believed to be more damaging to DNA than low LET radiation. Women are exposed to high LET charged particle radiation during travel in deep space and ion beam radiotherapy; however, the ovarian effects of most charged particles have not been studied. Our results reveal that acute exposure to charged oxygen particles dose-dependently increased DNA damage (γH2AX immunostaining), oxidative lipid damage (4-HNE immunostaining) and apoptosis (PUMA immunostaining) in ovarian follicles at 6h, resulting in depletion of ovarian follicles at 1 wk, irregular estrous cycling, and elevated serum concentrations of LH and FSH due to loss of ovarian negative feedback. Ovarian follicle numbers were dose-dependently depleted at 1 wk post irradiation, with complete absence of primordial and near-complete absence of primary follicles after irradiation with 30 cGy and 50 cGy oxygen ions. Dietary supplementation with the antioxidant ALA was not protective against ovarian follicle depletion caused by exposure to 50 cGy charged oxygen particles. Our results also show that charged oxygen particles are more potent destroyers of primordial follicles than charged iron particles (Mishra, et al. 2016).
Depletion of ovarian follicles and resultant premature ovarian failure is a significant adverse side-effect of γ-irradiation for cancer treatment (Lee, et al. 2000, Lee and Yoon 2005, Lo Presti, et al. 2004, Wallace, et al. 1989, Wallace, et al. 2003). The ED50 for follicle depletion in humans by γ-radiation has been estimated to be less than 2 Gy (Wallace, et al. 2003). The ED50 for follicle destruction in 6 wk old ICR mice 1 wk after irradiation is less than 60 cGy (10% of total follicles remaining) (Mathur, et al. 1991) and in 6 wk old C57BL6/DBA F1 hybrid mice 24h after irradiation it is less than 50 cGy (40% of primordial follicles remaining) (Pesty, et al. 2009). These doses were the lowest tested in both studies, and the data do not permit accurate estimation of ED50. Using data from the current study and from our prior study (Mishra, et al. 2016), we estimated the ED50 for primordial follicle depletion at one week after irradiation as 27.5 cGy for charged iron particles and 4.6 cGy for charged oxygen particles. These results suggest that oxygen and perhaps iron ion radiation have greater relative biological effectiveness for ovarian follicle destruction than γ-radiation, but more detailed dose response data comparing γ-radiation and charged particle radiation side by side in mice of the same strain and age are necessary to confirm this. The greater potency of lower LET oxygen compared to higher LET iron was unexpected, but can perhaps be explained by the relationship between LET and fluence (number of particles received by a surface per unit area). At LET of 16.5 keV/µm, oxygen has a fluence of about 10−7 particles/cm2 second steradian MeV/nucleon, while iron at LET of 179 keV/µm iron has a fluence of 3×10−8 particles/cm2 second steradian MeV/nucleon (Bourdarie and Xapsos 2008, ICRP 2003). Therefore, at the same radiation dose more oxygen particles are expected to traverse a primordial follicle than iron particles, causing greater damage.
The molecular mechanisms underlying follicle destruction by radiation exposure are not completely understood. Studies in diverse cell types support that about one-third of the detrimental effects of ionizing radiation at the cellular level are due to direct DNA damage and two-thirds are due to generation of reactive oxygen species (ROS) from ionization of water (Dayal, et al. 2008, Spitz, et al. 2004). Increased ROS persist for hours, days, and even weeks post-irradiation, reflecting perturbations to the redox homeostasis of cells (Dayal, et al. 2008, Spitz, et al. 2004). We previously showed that rapid and sustained increases in ROS occurred in human COV434 granulosa cells within 30 min after 1 or 5 Gy γ-irradiation, followed by apoptotic death at 6 h, whereas overexpression of glutamate cysteine ligase subunit genes to enhance glutathione synthesis prevented the radiation-induced rise in ROS and prevented apoptotic death of the cells (Cortés-Wanstreet, et al. 2009). Consistent with involvement of ROS in follicle destruction, we observed significantly increased immunostaining for 4-HNE, a marker of oxidative lipid damage, in oocytes and/or granulosa cells of follicles at all stages of development 6h after exposure to even the lowest dose of charged oxygen particles. Moreover, oxidative DNA damage is a known cause of double strand DNA breaks (Cadet, et al. 2012), and we observed increased immunostaining for γH2AX in oocytes and granulosa cells at 6h after oxygen ion irradiation. In our prior work, we observed similar increases in 4-HNE and γH2AX, as well as nitrotyrosine, a marker of oxidative protein damage, in ovarian follicles after irradiation with 50 cGy charged iron particles (Mishra, et al. 2016). The thiol ALA acts as a direct antioxidant in vitro at concentrations not achievable in vivo (Petersen Shay, et al. 2008, Rochette, et al. 2013). The in vivo antioxidant activity of ALA is mediated largely by increased cellular uptake of ascorbate and cysteine and by increased translation of the transcription factor NRF2, a master regulator of the antioxidant response (Petersen Shay, et al. 2012, Rochette, et al. 2013, Suh, et al. 2004). The lack of a prominent protective effect of dietary supplementation with ALA against the ovarian effects of 50 cGy oxygen ions is most likely due to overwhelming ROS generation by this relatively high dose that could not be overcome with supplementation of a single antioxidant. Supporting this notion, we observed minor, but statistically significant protective effects of ALA against oxidative lipid damage in secondary and primordial follicles.
The pro-apoptotic BH3-only BCL-2 family protein PUMA is required for germ cell apoptosis induction by γ-radiation in neonatal ovaries (Kerr, et al. 2012). We previously showed that charged iron particles increase PUMA protein expression in oocytes and granulosa cells of follicles at 6 h after irradiation, while caspase 3 activation is only observed in granulosa cells of secondary follicles at that time point (Mishra, et al. 2016). Therefore, in this study we analyzed the expression of PUMA as a marker of follicular apoptosis induction. In the present study, we observed significant dose-dependent increases in PUMA immunostaining in oocytes of all follicle stages and in granulosa cells of secondary and antral follicles 6h after oxygen ion irradiation. Moreover, ALA supplementation decreased the magnitude of the effects of 50 cGy oxygen irradiation on PUMA expression to the levels observed with 30 cGy irradiation. In contrast, ALA supplementation decreased oocyte and granulosa cell PUMA expression in 50 cGy iron ion irradiated mice to levels observed in 0 cGy sham-irradiated mice (Mishra, et al. 2016).
Consistent with depletion of ovarian follicles resulting in loss of ovarian negative feedback to the hypothalamus and pituitary, we observed significantly increased serum LH and FSH concentrations in the 50 cGy irradiated groups 8 wk after irradiation. Support for loss of ovarian negative feedback as the cause of the increased LH and FSH concentrations comes from our observation of complete depletion of primordial follicles in the 30 and 50 cGy groups at one week after irradiation. By 8 wk after irradiation nearly all of the remaining primary, secondary, and antral follicles would have already matured and ovulated or undergone atresia because the estimated time required for maturation of rodent follicles from the primordial to the ovulatory stage is 7–8 weeks (Hirshfield 1997). Increased production of LH and FSH due to pituitary tumors or other causes is very rare, and we feel that it is unlikely for the aforementioned reasons. However, in future studies, it would be useful to confirm that serum concentrations of estradiol and progesterone are low at 8 wk after irradiation.
In conclusion, our results show that a 5 cGy dose of charged oxygen particle radiation, which is 8-fold lower than the estimated cumulative radiation dose during a Mars mission of 40 cGy, decreases the primordial follicle pool by 71.5%, while 30 cGy and 50 cGy doses destroy essentially all primordial follicles. We compared the dose-response for primordial follicle depletion by oxygen ions in the present study to the dose-response for iron ions in our prior study (Mishra, et al. 2016) and found that oxygen ions are more potent destroyers of primordial follicles than iron ions. Our results further show that follicle destruction by charged oxygen particles is preceded at 6h after irradiation by dose-dependent increases in DNA double strand breaks, oxidative lipid damage, and apoptosis in ovarian follicles. ALA supplementation was somewhat protective against induction of lipid peroxidation and apoptosis by 50 cGy charged oxygen particles, but this was insufficient to protect against follicle depletion. Our results raise concerns that exposure to space radiation may increase the risk for early ovarian senescence in female astronauts and that use of heavy ions in cancer radiotherapy may pose a greater risk for ovarian damage than conventional photon radiation.
Acknowledgments
The authors thank Drs. Peter Guida, Chiara La Tessa, Michael Sivertz, and Adam Rusek, and staff at the NASA Space Radiation Laboratory for their expertise and support in carrying out the irradiations.
Funding
Supported by National Aeronautics and Space Administration grant NNX14AC50G to UL; National Institutes of Health grant P30CA062203, the University of California Irvine (UC Irvine) Chao Family Comprehensive Cancer Center; the Center for Occupational and Environmental Health, UC Irvine; the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934. BM was partially supported by a First award fellowship from the National Space Biomedical Research Institute grant PF04302.
Footnotes
Declaration of interest
The authors declare they have nothing to disclose.
References
- Barcellus-Hoff MH, Blakely EA, Burma S, Fornace AJJ, Gerson S, Hlatky L, Kirsch DG, Luderer U, Shay J, Wang Y, Weil MM. Concepts and Challenges in Cancer Risk Prediction for the Space Radiation Environment. Life Sciences in Space Research. 2015;6:92–103. doi: 10.1016/j.lssr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Bourdarie S, Xapsos M. The Near-Earth Space Radiation Environment. IEEE Transactions on Nuclear Sciences. 2008;55:1810–1832. [Google Scholar]
- Cadet J, Ravanat J-L, TavernaPorro M, Menoni H, Angelov D. Oxidatively Generated Complex DNA Damage: Tandem and Clustered Lesions. Cancer Letters. 2012;327:5–15. doi: 10.1016/j.canlet.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Goldman JM, Vandenbergh JG. Monitoring of the Estrous Cycle in the Laboratory Rodent by Vaginal Lavage. In: Heindel JJ, Chapin RE, editors. Female Reproductive Toxicology. San Diego: Academic Press, Inc.; 1993. pp. 45–55. [Google Scholar]
- Cortés-Wanstreet MM, Giedzinski E, Limoli CL, Luderer U. Overexpression of Glutamate Cysteine Ligase Protects Human COV434 Granulosa Tumor Cells against Oxidative and γ-Radiation-Induced Cell Death. Mutagenesis. 2009;24:211–224. doi: 10.1093/mutage/gen073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Durante M. Cancer Risk from Exposure to Galactic Cosmic Rays: Implications for Space Exploration by Human Beings. Lancet Oncology. 2006;7:431–435. doi: 10.1016/S1470-2045(06)70695-7. [DOI] [PubMed] [Google Scholar]
- Dayal D, Martin SM, Limoli CL, Spitz DR. Hydrogen Peroxide Mediates the Radiation-Induced Mutator Phenotype in Mammalian Cells. Biochemical Journal. 2008;413:185–191. doi: 10.1042/BJ20071643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular Consequences of Menopause and Hormone Therapy: Importance of Timing of Treatment and Type of Estrogen. Cardiovascular Research. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Overview of Ovarian Follicular Development: Considerations for the Toxicologist. Environmental and Molecular Mutagenesis. 1997;29:10–15. [PubMed] [Google Scholar]
- ICRP. Relative Biological Effectiveness (RBE), Quality Factor (Q), and Radiation Weighting Factor (wR). ICRP Publication 92. Annals of the ICRP. 2003;33:1–121. doi: 10.1016/s0146-6453(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK, Strasser A. DNA Damage-Induced Primordial Follicle Oocyte Apoptosis and Loss of Fertility Require TAp63-Mediated Induction of Puma and Noxa. Molecular Cell. 2012;48:343–352. doi: 10.1016/j.molcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Lee CJ. Effect of Exogenous Melatonin on the Ovarian Follicles in ϒ-irradiated Mouse. Mutation Research. 2000;449:33–39. doi: 10.1016/s0027-5107(00)00027-0. [DOI] [PubMed] [Google Scholar]
- La Tessa C, Sivertz M, Chiang I-H, Lowenstein D, Rusek A. Overview of the NASA Space Radiation Laboratory. Life Sciences in Space Research. 2016;11:18–23. doi: 10.1016/j.lssr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Park HH, Do BR, Yoon YD, Kim JK. Natural and Radiation-Induced Degeneration of the Primordial and Primary Follicles in the Mouse Ovary. Animal Reproduction Science. 2000;59:109–117. doi: 10.1016/s0378-4320(00)00072-5. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Yoon Y-D. ϒ-Radiation-Induced Follicular Degeneration in the Prepubertal Mouse Ovary. Mutation Research. 2005;578:247–255. doi: 10.1016/j.mrfmmm.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Lim J, Lawson GW, Nakamura BN, Ortiz L, Hur JA, Kavanagh TJ, Luderer U. Glutathione-Deficient Mice Have Increased Sensitivity to Transplacental Benzo[a]pyrene-Induced Premature Ovarian Failure and Ovarian Tumorigenesis. Cancer Research. 2013;73:908–917. doi: 10.1158/0008-5472.CAN-12-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Presti A, Ruvulo G, Gancitano RA, Cittadini E. Ovarian Function Following Radiation and Chemotherapy for Cancer. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2004;113:S33–S40. doi: 10.1016/j.ejogrb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Lopez SG, Luderer U. Effects of Cyclophosphamide and Buthionine Sulfoximine on Ovarian Glutathione and Apoptosis. Free Radical Biology and Medicine. 2004;36:1366–1377. doi: 10.1016/j.freeradbiomed.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Mathur S, Nandchahal K, Bhartiya HC. Radioprotection by MPG of Mice Ovaries Exposed to Sublethal Gamma Radiation Doses at Different Postnatal Ages. Acta Oncologica. 1991;30:981–983. doi: 10.3109/02841869109088253. [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The Effects of Radiotherapy and Chemotherapy on Female Reproduction. Human Reproduction Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- Mishra B, Ortiz L, Luderer U. Charged Iron Particles, Typical of Space Radiation, Destroy Ovarian Follicles. Human Reproduction. 2016;31:1816–1826. doi: 10.1093/humrep/dew126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JR, Barton DL, Loprinzi CL. Chemotherapy-Induced Ovarian Failure. Manifestations and Management. Drug Safety. 2005;28:401–416. doi: 10.2165/00002018-200528050-00004. [DOI] [PubMed] [Google Scholar]
- Pesty A, Doussau M, Lahaye J-B, Lefèvre B. Whole-Body or Isolated Ovary 60Co Irradiation: Effects on in Vivo and in Vitro Folliculogenesis and Oocyte Maturation. Reproductive Toxicology. 2009;29:93–98. doi: 10.1016/j.reprotox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Petersen Shay K, Michels AJ, Li W, Kong A-NT, Hagen TM. Cap-Independent Nrf2 Translation Is Part of a Lipoic Acid-Stimulated Detoxification Stress Response. Biochimica et Biophysica Acta. 2012;1823:1102–1109. doi: 10.1016/j.bbamcr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen Shay K, Moreau RF, Smith EJ, Hagen TM. Is α-Lipoic Acid a Scavenger of Reactive Oxygen Species in vivo? Evidence for Its Initiation of Stress Signaling Pathways that Promote Endogenous Antioxidant Capacity. IUBMB Life. 2008;60:362–367. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- Rochette L, Ghibu S, Richard C, Zeller M, Cottin Y, Vergely C. Direct and Indirect Antioxidant Properties of α-Lipoic Acid and Therapeutic Potential. Molecular Nutrition and Food Research. 2013;57:114–125. doi: 10.1002/mnfr.201200608. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Baker ES, Bavendam TG, Beck KD, Miller VM, Tash JS, Jenkins M. Effects of Sex and Gender on Adaptations to Space: Reproductive Health. Journal of Womens Health. 2014;23:967–974. doi: 10.1089/jwh.2014.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic Oophorectomy in Premenopausal Women and Long-term Health. Menopause International. 2008;14:111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I, Mor G, Naftolin F. Estrogen and the Aging Brain. Maturitas. 2001;38:95–100. doi: 10.1016/s0378-5122(00)00195-x. [DOI] [PubMed] [Google Scholar]
- Slaba TC, Blattnig SR, Norbury JW, Rusek A, La Tessa C, Walker SA. GCR Simulator Reference Field and a Spectral Approach for Laboratory Simulation, NASA Technical Publication. Langley, VA: NASA; 2015. [Google Scholar]
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic Oxidation/Reduction Reactions and Cellular Response to Ionizing Radiation: A Unifying Concept in Stress Response Biology. Cancer and Metastasis Reviews. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- Sridharan DM, LJ C, Whalen MK, Cucinotta FA, Pluth JM. Defining the Biological Effectiveness of Components of High-LET Track Structure. Radiation Research. 2015;184:105–119. doi: 10.1667/RR13684.1. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu R-M, Hagen TM. Decline in Transcriptional Activity of Nrf2 Causes Age-Related Loss of Glutathione Synthesis, which Is Reversible with Lipoic Acid. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J, A T. Proton vs Carbon Ion Beams in the Definitive Radiation Treatment of Cancer Patients. Radiotherapy and Oncology. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Svejme O, Ahlborg HG, Nilsson J-Å, Karlsson MK. Early Menopause and Risk of Osteoporosis, Fracture and Mortality: A 34-Year Prospective Observational Study in 390 Women. BJOG. 2012;119:810–816. doi: 10.1111/j.1471-0528.2012.03324.x. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Furusawa Y, Ide H, Yasui A, Terato H. Role of Isolated and Clustered DNA Damage and the Post-Irradiating Repair Process in the Effects of Heavy Ion Beam Irradiation. Journal of Radiation Research. 2015;56:446–455. doi: 10.1093/jrr/rru122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WHB, Shalet SM, Hendry JH, Morris-Jone PH, Gattamaneni HR. Ovarian Failure Following Abdominal Irradiation in Childhood: The Radiosensitivity of the Human Oocyte. British Journal of Radiology. 1989;62:995–998. doi: 10.1259/0007-1285-62-743-995. [DOI] [PubMed] [Google Scholar]
- Wallace WHB, Thomson AB, Kelsey TW. The Radiosensitivity of the Human Oocyte. Human Reproduction. 2003;18:117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]