Abstract
Purpose of review
This review summarizes studies into the permissive role of T cells in the bone catabolic effects of hyperparathyroidism and parathyroid hormone (PTH).
Recent findings
Work in animals combined with recent translation studies in humans now highlight the potent amplificatory action of T cells on PTH-induced bone resorption. Mechanistic animal studies reveal a complex pathway by which PTH exploits natural self-renewal functions of CD4+ T cells, to drive TNFα production that promotes formation of IL-17A secreting Th17 T cells. TNFα and IL-17 further amplify osteoblastic RANKL production and down-modulate OPG, establishing conditions propitious for osteoclastic bone resorption.
Summary
These findings are consistent with, and add to, the traditional view of PTH-induced bone loss involving only osteoblast-lineage cells. T cells potently amplify traditional pathways and provide permissive costimulatory signals to bone marrow stromal cells, facilitating the development of an increased RANKL/OPG ratio favourable to bone resorption and bone loss.
Keywords: Hyperparathyroidism, osteoimmunology, osteoporosis, parathyroid hormone, PTH, T cells
Introduction
Parathyroid hormone (PTH) plays a key regulatory role in calcium metabolism, defending the body against hypocalcemia. Serum calcium is regulated across a narrow range, typically between 2.1 to 2.7 mmol/L [1] and even small decrements in serum ionized calcium elicit a compensatory response in the form of PTH secretion. PTH acts to replenish serum calcium through mobilization of skeletal calcium stores by stimulating the differentiation of osteoclasts and thus promoting bone resorption. PTH further enhances the tubular reabsorption of calcium and stimulates the kidneys to produce 1,25-dihydroxyvitamin D3 (vitamin D) [2]. However, a sustained overabundance of PTH leads to persistent skeletal catabolism that ultimately depletes the skeleton of bone mineral density (BMD) setting the stage for the development of osteopenia and osteoporosis [3].
The mechanism by which PTH promotes osteoclast differentiation is complex and involves multiple cell types, including bone marrow stromal cells (BMSC) the osteoblast progenitors, osteoblasts and osteocytes, and multiple cytokine mediators [4–6]. Paradoxically, in contrast to continuous high dose PTH induced bone loss, daily injections in humans of an active fragment of human PTH, known as Teriparatide, stimulates bone formation in both the trabecular and cortical bone compartments, increasing bone volume and strength and reducing the risk of fractures in humans. Teriparatide is the only available anabolic agent for fracture prevention [7, 8]. The anabolic properties of PTH can be mimicked in rodent models by daily intermittent administration of PTH (iPTH) [9]. However, because this is a purely therapeutic modality and anabolic PTH has not been implicated as a driver of parathyroid bone disease, this aspect will not be further discussed in this review.
Interestingly a tremendous degree of integration has been found to exist between the immune system and the skeleton, an immuno-skeletal interface (ISI). The ISI comprises cells and cytokine effectors with functions, albeit different, shared between the immune and skeletal systems [10, 11]. Under baseline conditions B cell progenitors and mature B cells (professional antigen presenting cells (APC)) and key component of humoral immunity, secrete large concentrations of Osteoprotegerin (OPG) [12] a physiological decoy receptor and inhibitor of the key osteoclastogenic cytokine Receptor activator of NF-κB ligand (RANKL).
CD4+ T cell subsets are key regulators of other adaptive immune cells including B cells and function to regulate the production of OPG from B cells. This is achieved through cytokine production as well as costimulatory interactions between the T cells and B cells. One important costimulatory interaction by which T cells regulate B cell OPG production involves ligation of CD40 Ligand (CD40L) on the T cell with its receptor CD40 expressed on the B cell [13, 12]. T cells, by controlling B cell OPG production and hence the balance of active RANKL, consequently, regulate basal osteoclastogensis and bone resorption [12].
Because of the dependency of the skeleton on factors produced by immune cells, pathological immune imbalance has consequences for basal bone turnover. For example, in the case of T cell immunodeficiency caused by HIV infection, a decline in B cell OPG (and increase in RANKL) leads to an elevated RANKL/OPG ratio favourable for osteoclastic bone resorption and bone loss in HIV animal models [14] and in HIV-infected humans [15].
It is further recognized that disruption of the ISI plays a significant role in bone loss associated with models of common osteoporotic maladies including estrogen deficiency bone loss, rheumatoid arthritis, periodontal infection (a leading cause of tooth loss) and immune regeneration associated with antiretroviral therapy used in HIV treatment [10]. This is because activated T cells and B cells secrete RANKL and other inflammatory cytokines such as TNFα and IL-17A that upregulate RANKL on other cells, typically cells of the mesenchymal lineage including BMSC, osteoblasts, osteocytes and synovial fibroblasts [16–20].
Another pathological condition that has recently been recognized to involve a disturbance within the ISI is hyperparathyroidism (PHPT). Although a putative role for T cells in hyperparathyroid bone loss was recognized almost two decades ago [21], it is only relatively recently that these observations have been validated and the mechanisms involved investigated and elucidated. This review will examine historic and recent evidence for a role of T cells in the catabolic activity of parathyroid hormone (PTH) on the skeleton.
Primary hyperparathyroidism
Pathological over-secretion of PTH is typical of several conditions including primary hyperparathyroidism, the result of direct over secretion of PTH by the parathyroid glands. PHPT is the most common cause of hypercalcemia and affects at least 1 in 1000 persons [1]. Although hypercalcemia may not always be present, PHPT is typically detected by hypercalcemia with increased, or inappropriately normal, plasma PTH [22]. The incidence of PHPT is low in younger populations and similar in men and women prior to 45 years of age. PHPT peaks in the elderly with most cases (74%) occurring in women in their 70s [1]. In the majority (80 to 85%) of cases PHPT is associated with a benign single adenoma. More rarely (in 10 to 15% of cases) a four-gland hyperplasia underlies the disease [1]. Current guidelines for PHPT treatment recommend parathyroidectomy as the only curative approach to this disease [22]. Because most patients with primary hyperparathyroidism are asymptomatic at the time of diagnosis [1] there is a potential for significant skeletal damage to accrue if PHPT ensues for long periods of time prior to diagnosis and therapeutic resolution.
Secondary hyperparathyroidism
Another common cause of PTH overexpression that leads to bone damage is secondary hyperparathyroidism (SHPT), a compensatory response of the body to serum hypocalcemia. In generally healthy people hypocalcemia may result from inadequate dietary calcium, but in sick or elderly populations hypocalcemia is frequently the result of malabsorption of calcium in the small intestine. One common cause of compromised calcium absorption is insufficiency (21 to 29 ng per/mL), or deficiency (<20 ng per/mL) in vitamin D, a hormone that plays a vital role in enhancing the efficiency of calcium absorption in the gut [2]. Establishment of secondary hyperparathyroidism in both aged men and women, as a consequence of declining vitamin D levels and diminished calcium absorption in the gut associated with natural aging, may underlie in part, the development of senile osteoporosis which drives bone loss in the aged [23].
Mechanisms of hyperparathyroid bone loss
Hyperparathyroidism in humans is commonly mimicked in rodents, most often rats or mice by continuous infusion of PTH by means of implantable osmotic minipumps, a treatment modality referred to as continuous PTH (cPTH). In mice, cPTH leads to increased osteoclast formation and increased bone resorption that causes both cortical and trabecular bone loss [24]. PTH drives osteoclast formation by stimulating production of the key osteoclastogenic cytokine RANKL from cells of the mesenchymal-lineage, particularly osteoblasts and osteocytes, [4–6, 25]. PTH further augments the bioactivity of RANKL by decreasing the osteoblastic production of OPG, the physiological decoy receptor and antagonist of RANKL [4, 25].
The Role of T cells in PTH bone loss
Interestingly, almost 2 decades ago investigations into parathyroid bone loss involving transplantation of human parathyroid glands into immunocompromised mice, revealed that T cell deficient nude mice were spared from bone loss, suggesting a possible role of T cells in the mechanism of PTH action [21]. These data however, conflicted with a previous study in which transfected tumours that overexpressed a PTH receptor agonist, Parathyroid hormone-related protein (PTHrP), was found to induce hypercalcemia and bone resorption in nude mice [26]. These differences in outcome may have been a consequence of higher levels of PTHrP achieved in the transfected tumours, compared to human tumour explants, thus driving bone resorption independently of the amplificatory effect of T cells [3]. Another consideration is that nude mice have an incomplete T cell deficiency and recover T cells over time. Partial T cell repopulation may have further contributed to the discrepancies between studies.
To revisit the role of T cells in PTH-induced bone resorption, we made use of a more advanced animal model. T cell receptor β (TCRβ) knockout (KO) mice are a strain that completely lack αβ T cells, the major population of T cells in the bone marrow. To validate a requirement for T cells in PTH-induced bone resorption, we mimicked hyperparathyroidism using cPTH administration in mice. While cPTH induced osteoclast formation, bone resorption, and cortical bone loss, in wild type (WT) mice, TCRβ KO mice lacking T cells were protected from these events [27].
Mechanistically, cPTH was found to stimulate T cell production of TNFα, a potent inflammatory cytokine that amplifies the osteoclastogenic and bone resorptive activity of RANKL. TNFα synergizes with RANKL at the signal transduction level [19, 28–30] and further increases the production of RANKL by BMSC and osteoblasts [31]. The importance of TNFα to the bone loss associated with cPTH was demonstrated by studies involving TNFα KO mice, that were protected from bone resorption and bone loss following cPTH administration. A specific role for T cell derived TNFα was further demonstrated though adoptive transfer of WT or TNFα KO T cells into T cell deficient mice. While immunocompromised mice reconstituted with WT T cells underwent bone resorption and loss of trabecular bone volume and cortical bone volume and thickness, mice reconstituted with TNFα KO T cells were completely protected [24].
T cell expressed CD40 ligand transduces T cell signals to osteoblasts
The finding of a role for T cells in cPTH-induced bone resorption is not inconsistent with the traditional role of osteoblast-linage cells in PTH-induced bone loss because T cells provide proliferative and survival cues to BMSC thus sensitizing them to PTH. This process involves ligation of CD40L, a T cell expressed costimulatory molecule, with its receptor CD40 on BMSC. This intensifies expression of RANKL and diminishes expression of OPG. This sensitization of BMSC by T cell expressed CD40L was demonstrated by studies in which ablation of CD40L on T cells blunted the bone catabolic activity of cPTH, leading to diminished number of BMSC diminished RANKL to OPG ratio and diminished osteoclastogenic activity [27]. Importantly, a critical link between T cells and BMSC was localized to T cell TNFα secretion. In vitro, purified BMSC treated with TNFα showed a significant increase in expression of CD40 transcript. Consistent with an in vivo role of T cell TNFα production in cPTH bone loss, T cell conditional deletion of TNFα led to a decreased ability of cPTH to upregulate CD40 expression and a relatively lower RANKL/OPG ratio in BMSC exposed to cPTH [24].
Interestingly, it has been reported that in vitro activated Th2 (but not Th1) T cells are also a source of PTH that in coculture experiments can regulate the differentiation of osteoblasts and their expression of RANKL and OPG [32].
Taken together these studies suggest that T cells, T cell TNFα production and T cell expressed CD40L play an essential permissive role in cPTH-induced bone loss by a mechanism involving support of BMSC proliferation, life span, and function [27, 24].
cPTH bone loss involves direct PTH receptor signalling in T cells
PTH promotes bone resorption by binding of to its receptor PTH/PTHrP receptor (PPR or PTHR1). PPR is expressed on multiple cell types including osteoblast lineage cells BMSC, and osteoblasts [4] and terminally differentiated osteoblasts, the osteocytes [5, 6]. Interestingly T cells have also been shown to express PTH receptors [33, 34, 32].
To determine whether PTH directly regulates T cell TNFα production by binding to PPR on the T cell, PPR was conditionally deleted on T cells by crossing T cell specific Lck-Cre and PPR floxed mice [24]. Conditional ablation of PPR on T cells prevented cPTH-induced bone loss confirming a direct effect of PTH on the T cell.
Role of IL-17A in cPTH bone loss
Th17 cells are a specific CD4+ T helper subset that are characterized by high levels of IL-17A secretion [35]. IL-17A is an effector cytokine that mediates potent osteoclastogenic activity, and like TNFα, functions by stimulating the production of RANKL from osteoblast-lineage cells [16, 17]. IL-17A is not directly osteoclastogenic as direct interactions between osteoclast progenitors and RANKL producing cells such as osteoblasts is required for IL-17-induced osteoclastogenesis [16]. Furthermore, IL-17A promotes RANK expression on monocytes increasing the number of osteoclast precursors and thus of RANKL responsive cells capable of differentiating into osteoclasts [36].
Th17 and IL-17A has further been implicated in cPTH bone loss. Treatment of mice with a neutralizing anti-IL-17A antibody blocks cPTH-induced bone loss in mice and prevents RANKL production by osteoblast-lineage cells [39]. Additional evidence was provided by lack of osteoclastic bone resorption and bone loss in response to cPTH in IL-17A receptor KO mice [39]. These data are consistent with the role of TNFα production by T cells in the cPTH response as TNFα itself is an inducer of Th17 differentiation. Indeed, transplantation of TNFα null T cells into TCRβ KO mice prevents the expression of IL-17A in T cells in response to cPTH confirming that the production of TNF by T cells is required for cPTH to expand Th17 cells [39].
As cPTH enhances the sensitivity of naive CD4+ cells to TNFα via GαS/cAMP/Ca2+ signaling, conditional deletion of GαS in CD4+ cells as well as administration of diltiazem, a calcium channel blocker each prevent expansion of Th17 cells and prevent cPTH-induced bone loss [39].
Importantly, translational human studies have indeed confirmed that IL-17A is upregulated in human patients with PHPT and normalized by parathyroidectomy [39]. IL-17A production by Th17 cells is now also considered to play a central role in the bone resorption and bone loss associated with autoimmune arthritis, a model of rheumatoid arthritis [37] and estrogen deficiency induced by ovariectomy in mice, a model of postmenopausal osteoporosis [38, 17].
Role of antigen presentation in cPTH bone loss
The ability of T cells to sensitize BMSC to cPTH through CD40L begs an interesting question. As CD40L is a T cell surface receptor expressed by activated T cells, these findings suggest that PTH either promotes T cell activation or that PTH targets activated T cells. Briefly, T cells are physiologically activated though antigen presentation by a pathway often referred to as the “dual signal hypothesis”. This involves two signals exchanged between the T cell and the antigen presenting cell (APC). The first signal is antigen specific and involves engagement of the T cell receptor (TCR) with a peptide antigen which is presented by the APC as a major histocompatibility (MHC) complex. MHC class I (MHCI) bearing antigens present to CD8+ T cells while MHC class II (MHCII) complexes present to CD4+ T cells. TCR engagement with antigen alone does not drive activation but initiates an anergic signal that without ratification commits the T cell to an anergic or antigen-specific unresponsive state. T cell activation requires a second costimulatory signal delivered by the APC that that is antigen-nonspecific and directed via CD80/CD86 ligands to the CD28 receptor on the T cell. Upon delivery of the costimulatory signal T cell undergoes full activation, proliferation, and differentiation. CD4+ differentiate into T helper subsets (Th) subsets while CD8 T cells differentiate into active cytotoxic effectors. Interruption of the CD28 signal is accomplished physiologically though the costimulation inhibitor, Cytotoxic T-lymphocyte associated protein 4 (CTLA4), a competitive inhibitor of CD80/86 and a product of activated T cells and Regulatory T cells (Tregs). CTLA4 production is a necessary regulatory step involved in quieting and ultimately terminating T cell responses after resolution of the immune challenge. T cell anergy may also be achieved pharmacologically, though administration of CTLA4-immunoglobulin (Ig) a fusion protein between CTLA4 and a human Ig chain. CTLA4-Ig is FDA approved for the treatment of rheumatoid arthritis [40, 41, 10].
To investigate the need for antigen mediated T cell activation in the mechanism of cPTH-induced bone loss, we injected cPTH treated mice with CTLA4-Ig and reported that resorption and loss induced by cPTH treatment was prevented by CTLA4-Ig. Furthermore, silencing of either MHCI or MHCII mediated antigen presentation to the TCR using OT-I or OT-II TCR transgenic mice, strains bearing TCRs with reactivity to ovalbumin, an antigen not endogenously present in mice, likewise prevented bone resorption and cortical bone loss induced by cPTH treatment [42]. Because T cell activation is necessary for CD40L expression by T cells, and CD40L is required to prime BMSC to respond to PTH for full bone catabolism [27], these data support a role of T cell activation and of antigen-driven processes in the mechanism of action of PTH.
Interestingly, although antigen presentation is necessary for PTH induced bone catabolism, our studies show that PTH does not itself induce T cell activation or CD40L expression directly [27]. This suggests that PTH appropriates otherwise activated T cells to effect bone resorption. However, under normal healthy conditions, T cell activation state is generally low. In fact, T cell survival and renewal of naive and memory T cells involves “tickling” reactions with MHC bound self-antigens generating persistent weak antigenic responses. These events are necessary for maintaining a diverse population of T cells, but importantly are low grade and do not initiate robust immune reactions characteristic of pathological antigenic responses [40, 43]. We thus believe that these homeostatic T cell responses to weak physiological antigens are crucial to the ability of PTH to appropriate these weakly activated T cells to effect bone catabolic function.
Conclusions
A decade of mechanistic studies reveal interesting new pathways involving T cells that, while consent with the traditional view of PTH-induced bone loss, significantly expand the mechanisms involved. Accumulated evidence now suggests that T cells are permissive and necessary for cPTH-induced bone resorption as T cells provide costimulatory signals that condition osteoblast-lineage cells to respond aggressively to cPTH signaling. In response to cPTH, T cells further secrete inflammatory cytokines including TNFα and IL-17A, that drive up the RANKL/OPG ratio by stimulating RANKL production and suppressing that of OPG. Conditional ablation of T cell TNFα or of IL-17A prevents PTH bone loss in mice, as does disruption of T cell costimulation with osteoblast-lineage cells. Finally, ablation of PPR in T cells further mutes PTH-induced bone catabolism signifying that T cells are direct targets of PTH signaling This immunocentric model is represented diagrammatically in Figure 1.
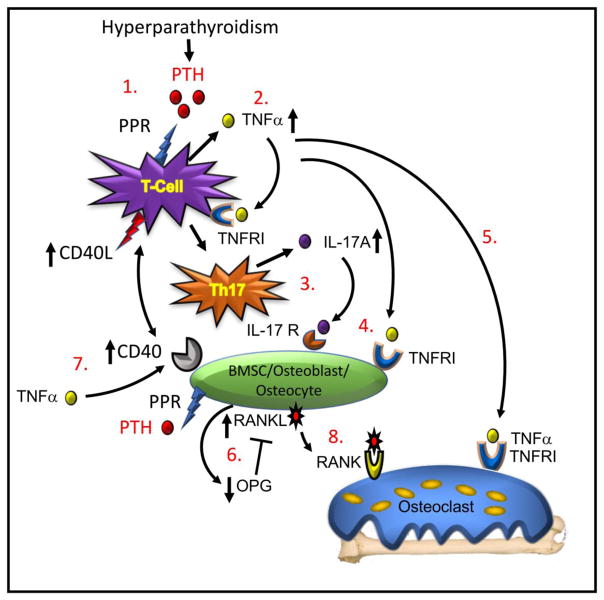
Figure 1. Model of T cell dependent mechanism of cPTH-induced bone loss.
(1.) Binding of PTH to its receptor (PPR) on the CD4+ T cell initiates (2.) production of TNFα, a cytokine that binds to a Type I receptor (TNFRI) and promotes differentiation of the T cell into (3.) a Th17 helper cell secreting IL-17A. (4.) Both of TNFα and IL-17A bind to receptors (TNFRI and IL-17R respectively) on BMSC, osteoblasts and osteocytes. (5.) TNFα further binds to osteoclastic cells amplifying RANKL activity on the differentiating osteoclast and stimulating bone resorption. (6.) TNFα and IL-17A promote production of RANKL and suppress production of OPG, the RANKL inhibitor, creating a balance of RANKL to OPG favourable to increased osteoclastic differentiation. (7.) TNFα further upregulates expression of CD40 on BMSC allowing a costimulatory signal to be transduced from the T cells though expression of CD40L. This signal primes the osteoblast to further respond to direct PTH stimulation to ensure robust RANKL production. (8) RANKL binds to its receptor RANK on osteoclast lineage cells causing osteoclast precursor differentiation and fusion into mature osteoclasts that resorb bone leading to bone loss. Model adapted from Li et al., [39].
Human translational studies have been lagging, however recent work has indeed validated elevated serum levels of IL-17A in human patients with PHPT and its normalization following parathyroidectomy. Additional human studies are urgently needed and once other aspects of the model are validated, there is significant potential for examination of new therapeutic targets for the treatment of bone loss secondary to hyperparathyroidism, including targeting of TNFα, IL-17A and of T cell costimulation pathways. Finally, these studies add to our knowledge of the ISI and the field of osteoimmunology that continues to evolve in unexpected directions.
Acknowledgments
The authors thank Dr. Daiana Weiss for critical reading of the manuscript.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
M. Neale Weitzmann is supported by a grant from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (5I01BX000105) and by National Institutes of Health (NIH) grants from NIAMS (AR056090, AR059364, AR068157, and AR070091) and NIA AG040013.
Roberto Pacifici is supported by NIH grants from NIDDK (DK108842), NIAMS (AR54625) and RR028009.
The contents of this manuscript do not represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the United States Government.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N Engl J Med. 2011;365(25):2389–97. doi: 10.1056/NEJMcp1106636. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Pacifici R. T cells: critical bone regulators in health and disease. Bone. 2010;47(3):461–71. doi: 10.1016/j.bone.2010.04.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277–86. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-awadh AN, Delgado-Calle J, Tu X, Kuhlenschmidt K, Allen MR, Plotkin LI, et al. Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology. 2014;155(8):2797–809. doi: 10.1210/en.2014-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong J, Piemontese M, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone. 2014;66:146–54. doi: 10.1016/j.bone.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11(7):418–28. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JW, Li JY, Walker LD, Tyagi AM, Reott MA, Yu M, et al. T cell-expressed CD40L potentiates the bone anabolic activity of intermittent PTH treatment. J Bone Miner Res. 2015;30(4):695–705. doi: 10.1002/jbmr.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover - role of the immune system. Nat Rev Endocrinol. 2016;12(9):518–32. doi: 10.1038/nrendo.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161(11):6113–21. [PubMed] [Google Scholar]
- 14.Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, Guidot DM, et al. Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A. 2010;107(31):13848–53. doi: 10.1073/pnas.1003020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titanji K, Vunnava A, Sheth AN, Delille C, Lennox JL, Sanford SE, et al. Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog. 2014;10(10):e1004497. doi: 10.1371/journal.ppat.1004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSelm CJ, Takahata Y, Warren J, Chappel JC, Khan T, Li X, et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J Cell Biochem. 2012;113(9):2895–902. doi: 10.1002/jcb.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111(8):1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–37. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- •21.Hory BG, Roussanne MC, Rostand S, Bourdeau A, Drueke TB, Gogusev J. Absence of response to human parathyroid hormone in athymic mice grafted with human parathyroid adenoma, hyperplasia or parathyroid cells maintained in culture. J Endocrinol Invest. 2000;23(5):273–9. doi: 10.1007/BF03343723. This was the first study to suggest a role for T cells in hyperparathyroid bone loss. [DOI] [PubMed] [Google Scholar]
- 22.Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JE, Rejnmark L, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int. 2017;28(1):1–19. doi: 10.1007/s00198-016-3716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggs BL, Melton LJ., 3rd Involutional osteoporosis. N Engl J Med. 1986;314(26):1676–86. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- •24.Tawfeek H, Bedi B, Li JY, Adams J, Kobayashi T, Weitzmann MN, et al. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. PLoS One. 2010;5(8):e12290. doi: 10.1371/journal.pone.0012290. This study demonstrated for the first time that direct PTH signaling in T cells is necessary for bone catabolism by conditional ablation of the T cell PPR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, et al. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–54. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 26.Guise TA, Chirgwin JM, Favarato G, Boyce BF, Mundy GR. Chinese hamster ovarian cells transfected with human parathyroid hormone-related protein cDNA cause hypercalcemia in nude mice. Lab Invest. 1992;67(4):477–85. [PubMed] [Google Scholar]
- •27.Gao Y, Wu X, Terauchi M, Li JY, Grassi F, Galley S, et al. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab. 2008;8(2):132–45. doi: 10.1016/j.cmet.2008.07.001. This study showed that a T cell costimulatory ligand (CD40L) interacts with BMSC expressed CD40 receptor to prime BMSC to respond to cPTH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106(12):1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. Tumor Necrosis Factor-alpha (TNF) Stimulates RANKL-induced Osteoclastogenesis via Coupling of TNF Type 1 Receptor and RANK Signaling Pathways. J Biol Chem. 2001;276(1):563–8. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 30.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143(3):1108–18. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 31.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25(3):255–9. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 32.Young N, Mikhalkevich N, Yan Y, Chen D, Zheng WP. Differential regulation of osteoblast activity by Th cell subsets mediated by parathyroid hormone and IFN-gamma. J Immunol. 2005;175(12):8287–95. doi: 10.4049/jimmunol.175.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCauley LK, Rosol TJ, Merryman JI, Capen CC. Parathyroid hormone-related protein binding to human T-cell lymphotropic virus type I-infected lymphocytes. Endocrinology. 1992;130(1):300–6. doi: 10.1210/endo.130.1.1309334. [DOI] [PubMed] [Google Scholar]
- 34.Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10(3):229–40. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 36.Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, et al. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther. 2010;12(1):R29. doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •37.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–82. doi: 10.1084/jem.20061775. The first major study showing the key role of Th17 T cells and IL-17A to RANKL production and osteoclastic bone resorption in autoimmune arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS One. 2012;7(9):e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Li JY, D’Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T, et al. IL-17A Is Increased in Humans with Primary Hyperparathyroidism and Mediates PTH-Induced Bone Loss in Mice. Cell Metab. 2015;22(5):799–810. doi: 10.1016/j.cmet.2015.09.012. This study demonstrates a role of Th17 and IL-17A in the osteoclastic bone resorption induced by cPTH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192(4):F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafian N, Sayegh MH. CTLA4-Ig: a novel immunosuppressive agent. Expert Opin Investig Drugs. 2000;9(9):2147–57. doi: 10.1517/13543784.9.9.2147. [DOI] [PubMed] [Google Scholar]
- •42.Bedi B, Li JY, Grassi F, Tawfeek H, Weitzmann MN, Pacifici R. Inhibition of antigen presentation and T cell costimulation blocks PTH-induced bone loss. Ann N Y Acad Sci. 2010;1192(1):215–21. doi: 10.1111/j.1749-6632.2009.05216.x. This study shows that endogenous basal antigen presentation to T cells is necessary for iPTH to promote catabolic effects though T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11(2):173–81. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]