Abstract
Purpose
Understanding the apparent paradoxical role of zinc in the pathogenesis and prevention of age-related macular degeneration (AMD) has been limited by the lack of animal models for its detection in sub-retinal epithelial deposits (drusen), a definitive early hallmark of AMD. In-vitro studies using Zinpyr-1 showed drusen contained high levels of zinc, but the probe was not suitable for in-vivo studies. This study compares Zinpyr-1 to ZPP1, a new fluorescein-based probe for zinc, to assess the potential of ZPP1 for in-vivo detection of zinc in drusen.
Methods
Flat mounts of human sub-RPE tissue using the probes were analyzed by fluorescence and confocal microscopy. Flat mounts of sub-RPE tissue from mice deficient in superoxide dismutase isoform-1 (CuZn-SOD-KO) or isoform-2 (Mn-SOD-RPE-KO) were analyzed with sub-RPE deposits confirmed by histology.
Results
Drusen are detected in greater numbers and intensity with ZPP1 compared to Zinpyr-1. Using ZPP1, drusen was detected in a sample from a 46-year old human donor without ocular history, suggesting that ZPP1 might be sensitive enough to detect drusen at an early stage. With CuZn-SOD KO mice, ZPP1 detected sub-RPE deposits at 10 months of age, whereas Zinpyr-1 required 14 months.
Conclusion
Detection of sub-RPE deposits by ZPP1 was greatly enhanced compared to Zinpyr-1. This enhanced sensitivity will allow for more insightful analysis of zinc in AMD using human specimens and mouse models. This could result in the development of a sensitive in-vivo probe to enhance research on the role zinc in drusen formation and the early clinical diagnosis of AMD.
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of blindness in the developed world.1,2 The prevalence of AMD is expected to rise along with the aging demographics in these nations, with the United States alone experiencing a 50% increase in AMD cases between 2004 and 2020.3 Some epidemiological studies put the prevalence of AMD as high as 30% in individuals over the age of 75 years.4 The etiology of AMD is complex with both genetic and environmental risk factors associated with its development.5–7 Among the genetic factors gender, race (Caucasian), iris color, and polymorphisms within complement system genes8,9 are highly associated with AMD, while the leading environmental factors are age, tobacco smoking, diet, and light exposure.10–13
Clinically, AMD is generally observed to have stages14–16 with the severity of the disease in each stage graded according to criteria described by the Age-Related Eye Disease Studies (AREDS)17–21 and the Minnesota Grading System for post-mortem eyes.22,23 Asymptomatic early stage and intermediate AMD (AREDS grade 1 and 2) is characterized by a limited number and/or area of small (≤ 63 μm) or intermediate (63–124 μm) sized drusen and the presence retinal pigmentation changes (grade 2), especially in and around the macula. Drusen16 are extracellular deposits composed primarily of aggregated proteins and oxidized lipids and are found in the sub-retinal space between Bruch’s membrane (inner most layer of the choroid) and the retinal pigment epithelial (RPE) cell layer (outer most layer of the retina). Drusen tend to be seen in individuals over forty years of age and, while not directly associated with visual impairment, its presences is considered to be among the earliest hallmarks and a predictive indicator for further development of AMD.24 There is a direct correlation between the size and location of drusen at first diagnosis and further development of AMD.4 AREDS grade 3 AMD is characterized by a greater number or a greater area of intermediate to large (≥ 125 μm) sized drusen, and/or geographic atrophy (GA) not involving the central macula. Advanced AMD (AREDS grade 4) has two distinct subtypes, dry (GA involving the central macula) and wet (exudative, choroidal neovascularization, CNV) AMD. Both subtypes lead to visual impairment, but development of wet AMD results in a more rapid and severe loss of vision. While sharing the common early and intermediate phases, the exact signals and mechanisms that lead to dry or wet AMD are not completely understood. Although wet AMD is seen in only 10%–15% of AMD cases25 it is usually proceeded by the dry form suggesting that wet AMD is one potential sequela to dry AMD. Recent studies have suggested that dry AMD is associated with Alu RNA accumulation resulting from DICER1 deficiency26,27 while other studies have implicated certain genetic risk variants with the particular subtype of late AMD.28–31 Enhanced expression of vascular endothelial growth factor (VEGF) is thought to be associated with wet AMD, as anti-VEGF therapies have proven to be effective in limiting disease progression in some patients.32,33
Because of constant light exposure, high levels of polyunsaturated fatty acids, and the exceptionally high metabolic rate of retinal tissue, especially at the macula, oxidative stress and the production of reactive oxygen species are also thought to have important roles in the development of AMD.34–36 Trace metals, specifically iron, copper, and zinc, are important for the structure and function of many proteins and enzymes necessary for cellular homeostasis, including control and defense against reactive oxygen species. Dysregulation of these metals is associated with conditions that can lead to loss of visual function.37 Although iron is the most common metal in the body, zinc is the most prevalent metal in the retina and has been shown to be involved with many proteins necessary for both the general health and specific function (visual transduction) of the retina, clearly indicating a crucial role for zinc in retinal homeostasis.38 Unlike calcium or iron, there is no cellular storage of zinc. It must be obtained by diet, then delivered and regulated to precise levels in ocular tissues. Zinc is found throughout the retina, particularly at the inner nuclear layer (INL), outer plexiform layer (OPL) and the rod inner segments (RIS).37,38 There are also high concentrations of zinc in the RPE and choroid.39,40 Visual dysfunctions resulting from zinc deficiency (poor dark adaptation) and zinc excess (RPE and retinal cell toxicity) have been described.37,38
The exact role of zinc in the both pathogenesis and prevention of AMD is not well understood and in some instances, appears paradoxical. Zinc, and enzymes whose function is dependent on zinc, are known to have functional antioxidant properties within ocular tissue.41–45 As such, zinc has long been included in many dietary supplement regimes designed to limit AMD. The first controlled study examining the potential therapeutic benefit of zinc for AMD suggested that it could limit vision loss in patients already having drusen formation and pigmentary changes.46 However, several other early studies concluded that zinc had very limited47 or even no therapeutic value for AMD.48–50 In order to overcome the small size and inconsistent findings of these early studies, and because many patients were taking zinc supplements without a proven benefit, a large, randomized, double blind, controlled study was undertaken. This initial AREDS I21 study, plus a follow up study,51 showed that zinc or zinc plus other antioxidants given to individuals with intermediate stage AMD (grade 3) could reduce the odds of progression to advanced AMD, especially towards the exudative form of AMD. Subsequent studies suggested that efficacy of zinc in preventing advanced AMD might depend upon the genetic risk factors within the individual.52–54 Specifically, these studies found that individuals carrying complement factor H (CFH) alleles highly associated with AMD55–61, such as the rs1061170 (Y402H) polymorphism, did not benefit from zinc supplementation, but for individuals with low-risk CFH alleles or risk alleles in other genes (Age-Related Maculopathy Sensitivity 2 gene, ARMS2)62 zinc was beneficial. However, a more recent analysis using a larger cohort of patients from the AREDS studies suggested that the benefits from zinc and other antioxidants were not enhanced by any particular CFH or ARMS2 genotype.63 Other studies have shown that only individuals with dry AMD benefited from zinc supplements, whereas patients with wet AMD did not.46,64 A recent review65 of studies on zinc therapies and AMD concluded that it may be effective in limiting the progression to advanced AMD in certain individuals, but there was no conclusive evidence that zinc supplementation enhanced visual function or prevented early AMD.
In contrast to the reports about the clinical findings of zinc supplementation, fewer studies have concentrated on the mechanism(s) on how zinc might be efficacious in treating AMD and whether zinc, and/or its dysregulation, could be an initiating factor in AMD. Zinc levels increase in the choroid with age40 but are reduced in the choroid and RPE of individuals with AMD.39 Interestingly, zinc levels are increased in Bruch’s membrane of individuals with AMD especially at the macula.66 These changes in zinc localization seen with AMD led to speculation that zinc could be an early and crucial marker for drusen and indeed it was found using a fluorescein-base probe for zinc (Zinpyr-1) that sub-retinal pigment epithelial deposits in human donor tissue contain a high level of zinc.66 However, whether this translocation of zinc to Bruch’s membrane/drusen actually induces AMD or is secondary to some other initiating factor(s) is unresolved. The observations about efficacy of zinc supplements and CFH risk alleles for AMD lead to biophysical studies on the interaction between zinc and CFH. It was found that zinc at concentrations that could be reached under certain conditions in ocular tissue induced the polymerization of CFH thus inhibiting its ability to regulate complement activation.67 Inflammation due to excessive complement activation is thought to be a major mechanism for the tissue damage associated with AMD.68,69 Interestingly, there was no difference in zinc-induced polymerization between the AMD low-risk (402Y) and AMD high-risk (402H) alleles of CHF.70 Conversely, zinc has also been found to polymerize and limit the activity of pro-inflammatory complement factors, such as C3b, which could account for the therapeutic effect of zinc observed in some AMD patients.71,72 As with zinc translocation, the findings about interaction of zinc with proteins associated with AMD have resulted in much speculation on whether zinc is a causative factor, enhancing factor, or limiting factor in AMD.
To date, the relationship between zinc and AMD has only been explored with epidemiological and cell culture studies. Determining the cause/effect nature of zinc in AMD will require moving beyond such studies and into defined animal models. Given the complex etiology of AMD and the unique anatomy of the human retina, such as the presence of a fovea which is found only in primates, there is no animal model that completely replicates all aspects of AMD.73,74 Mice have proven useful in determining the precise mechanisms of many pathogenic conditions by the ability to generate defined genotypes. Although they lack a defined macula, there are numerous mouse models that reproduce important features of AMD such as the formation of sub-retinal epithelial deposits.73,75 Mice that are deficient for super oxide dismutase (SOD), a critical enzyme for the control of superoxide radicals generated during oxygen metabolism76,77, are of particular interest as an AMD model system. SOD exists as three isoforms, a cytosolic form (sod1, CuZn-SOD), a mitochondrial form (sod2, Mn-SOD), and a secreted form (sod3). Although all SOD isoforms are present in the retina, CuZn-SOD is the most prevalent.78 Mice lacking CuZn-SOD and mice whose expression of Mn-SOD has been reduced or eliminated from the retina have been reported to reproduce key features of human AMD including the formation of drusen.79–81
Since drusen appear to be both a precursor and early biomarker of AMD, we believe the ability to detect it using sensitive probes for zinc will allow us to start to understand the relationship of zinc in AMD to a particular genotype or environmental risk factor. Although Zinpyr-1 was used to identify zinc as a component of human sub-retinal epithelial deposits in ex-vivo assays, its chemical nature does limit its sensitivity for ex-vivo analysis as well as its ability to be useful in animal models and in vivo assays. We hypothesize that new and enhanced fluorescein-based probes for zinc, such as ZPP-1, will be more effective for the detection of zinc in sub-retinal epithelial deposits. Only with an enhanced probe can we begin to determine the cause/effect aspects of zinc in AMD. In this study, we assess the ability and sensitivity of fluorescein-based probes for zinc to detect drusen in human tissue and to apply those findings to mouse models to determine their suitability as models for understanding the mechanisms of zinc in the initiation and/or inhibition of AMD pathology.
METHODS
HUMAN TISSUE
Human donor eyes were obtained from the Montana Eye Bank Foundation (Missoula, Montana) and were used in accordance with the tenets of the Declaration of Helsinki for research involving human tissue and as approved by the Institutional Review Boards of the Montana State University, the University of Texas Medical Branch (Galveston), and the University of Minnesota. After enucleation the anterior segments of the eye plus the vitreous were removed and the eyecups were processed as previously described.82 Briefly, the eyecups were placed upside down on a perforated Teflon liner on cotton in a jar. The jars were purged with ultra-high purity argon gas, sealed, and stored at −80°C. For use, the eyecups were thawed at room temperature and, if necessary, the neural retina was gently removed from underlying tissue by forceps. Using 3.5–5.0 mm dermal biopsy punches (Miltex, Bethpage, New York) the tissue was trephined through the RPE to the sclera. The RPE/Bruch’s membrane/choroid complex was removed from the sclera with forceps, placed on a glass slide with the RPE layer facing up. Just enough zinc-free phosphate buffered saline (PBS, Mediatech, Manassas, Virginia) was placed on the tissue to cover it and then it was gently manipulated until flat. The RPE layer was then partially removed using a fine camel haired brush. RPE debris was further removed by washing with PBS three to six times with excess liquid blotted away after each wash. The dissection process was aided by the use of a Wild Heerbrugg M3 stereomicroscope (Leica, Gallen, Switzerland) equipped with a fiber optic illuminator (Cole-Parmer, Vernon Hills, Illinois, model 41500-55).
ANIMALS AND ANIMAL TISSUE PREPERATION
All mice were handled in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and the University of Minnesota Institutional Animal Care and Use Committee guidelines. Mice were housed under specific pathogen-free conditions. Mice deficient (knock-out mice, KO) in superoxide dismutase isoforms SOD1 (CuZn-SOD KO) and SOD2 (Mn-SOD-RPE KO) were used in this study. CuZn-SOD KO mice result from a global genetic knock-out of the sod1 gene.83 Global genetic knock-out of the sod2 gene results in perinatal mortality. However, mice whose expression of SOD2 is reduced conditionally and specifically within RPE cells (Mn-SOD-RPE KO) were created by crossing floxed-sod284 mice with mice that have doxycycline induced expression of Cre recombinase under control of the RPE-specific vitelliform macular dystrophy-2 gene promoter.85 CuZn-SOD KO and Mn-SOD-RPE KO mice were kindly provided by Dr. Deborah Ferrington, University of Minnesota.
After enucleation, eyes were placed in PBS and dissected free of remaining connective and extra ocular muscle tissue. The optic nerve was cut at the globe and a hole was made at the scleral-limbal junction using a 30-gauge needle. Starting at this hole the eye was cut circumferentially along the scleral-limbal junction. The anterior structures of the eye and the vitreous were discarded and the eyecup washed in PBS. Beginning at the outer margin, four equidistant radial cuts were made to flatten the eyecup onto a glass slide with the RPE side facing up. The retina was gently teased from the RPE with forceps with its final removal facilitated by cutting the optic nerve at the RPE. The radial cuts were extended and/or new cuts made to flatten the eyecup as much as possible. The RPE layer was partially removed by brushing and gentle agitation on an orbital shaker with debris removed by washing with PBS as described above. For histological examination, the eyes were fixed in 10% buffered formalin, paraffin embedded, sectioned (5 μm), and stained with hematoxylin and eosin (H&E).
FLUORESCENCE AND CONFOCAL MICROSCOPY
Following the dissection and preparation of the human and murine tissue, the presence of drusen was assayed for by labeling zinc with fluorescein-based compounds. This analysis was performed using a Leica DM 4000B microscope equipped with filters for detecting green light (blue light excitation at 460–500 nm with emission detection at 512–542 nm), red light (green light excitation at 540–552 nm with emission detection at 580–620 nm), and blue light (UV light excitation at 340–380 nm with emission detection at 450–490 nm). In human samples, large and/or numerous sub-retinal epithelial deposits could be visualized by the green autofluorescence induced with blue light.86 In murine tissue, green autofluorescence from the remaining optic nerve tissue served to orient the sample as the small size of the sub-retinal epithelial deposits prevented their visualization by autofluorescence only. Human and murine RPE cells (and RPE cellular debris) could be visualized by the red autofluorescence associated with lipofuscin. For both human and murine samples, exposure settings were then adjusted to the point where the green autofluorescence was no longer visible and those setting were then used to image drusen after zinc labeling. Zinc detection was done directly on the slides. The samples were treated for five minutes at room temperature, in dark conditions, with just enough volume to cover the tissue, with the indicated concentration of 4′,5′–bis[[bis(2-pyridinylmethyl)amino]methyl]-2′,7′-dichloro-3′,6′-dihydroxy-spiro[isobenzofuran-1(3H,9′-[9H]xanthen]-3-one (ZP-1, Zinpyr-1, Caymen Chemicals, Ann Arbor, Michigan)87 or 9-(2-carboxyphenyl)-2,7-dichloro-4,5bis[(2-picolyl)(pyrazin-2-ymethyl)aminomethyl]-6-hydroxy-3-xanthanone (ZPP1, Strem Chemicals, Newburyport, Massachusetts).88 The samples were then washed three times with PBS, cover slipped, and then examined for drusen. In order to confirm the specificity of the zinc labeling, some samples were treated for five minutes at room temperature with a zinc-specific chelating solution containing 50 mM N,N,N,N-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN, Tocris, Bristol, United Kingdom) and 10 mM diethylenetriaminepentaacetic acid (DTPA, Sigma, St. Louis, Missouri) prior to zinc detection. Where indicated, samples were also labeled with 4′,6-diamidino-2-phenylindole (DAPI, #H-1200, Vector Laboratories, Burlington, California) to visualize cell nuclei just prior to cover slipping.
Confocal images were obtained with a FluoView FV1000 confocal imaging system using an IX81 inverted microscope (Olympus, Tokyo, Japan). Z-stacks and three-dimensional renderings of the confocal images were constructed and rendered using Imaris (Bitplane, Belfast, United Kingdom) and Image J (National Institutes of Health, Bethesda, Maryland) software.
TITRATION OF ZINC PROBES
Starting with a 10 mM solution of zinc sulfate (ZnSO4), the indicated final concentration of Zn2+ was made to a final volume of 20 μL in H2O or in a solution of 10 mM TPEN/1 mM DPTA in a black wall, clear bottom, 96 well assay plate (Corning #3603, Corning, New York). 20 μL of the indicated concentration of Zinpyr-1 or ZPP1 was added to each well and the fluorescence emission measured with Synergy-HTX plate reader (Biotek, Winooski, Vermont) equipped with a 465/20 nm band pass excitation filter and 528/20 nm band pass emission filter.
RESULTS
DETECTION OF DRUSEN IN MOUSE MODELS OF AMD WITH ZINPYR-1
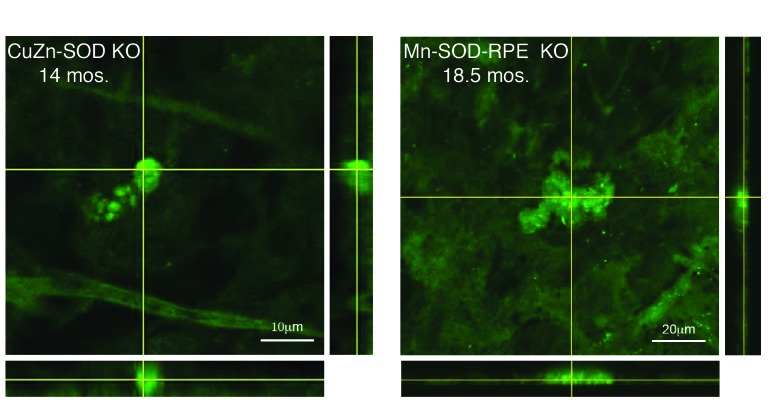
To advance our understanding about the role of zinc in drusen formation and AMD pathogenesis, and as a first step towards developing an in vivo drusen detection system, we asked if sub-retinal epithelial deposits could be detected in mice deficient in CuZn-SOD and Mn-SOD with Zinpyr-1. Immunofluorescence with confocal analysis of eyecup flat mounts prepared from aged CuZn-SOD KO (Figure 1, left) and Mn-SOD-RPE KO (Figure 1, right) mice were indicative for drusen. Analysis of samples from aged-matched wild-type control mice showed they were negative for such sub-retinal epithelial deposits and this was also confirmed by histological examination (as shown in Results, Analysis of the CuZn-SOD KO Mouse with ZPP1).
FIGURE 1.
Confocal images of eyecup flat mounts from CuZn-SOD KO and Mn-SOD-RPE KO mice labeled with 10 μM Zinpyr-1. Crosshairs are focused on sub-retinal epithelial drusen-like deposits. The depth of the deposits is visualized by reconstructed optical cross sections (confocal z-stacks) that are displayed along the bottom and right side of each image.
RATIONALE FOR USE OF ZPP1
While the above results suggest that the CuZn-SOD KO and Mn-SOD-RPE KO mouse models develop zinc-containing, sub-retinal epithelial deposits similar to human drusen, we observed that the light emitted by Zinpyr-1 significantly overlapped into the emission spectrum of other fluorophore channels. This precluded a clear and distinct co-visualization of RPE cells, thus preventing us from generating supporting evidence that the observed deposits were indeed located sub-RPE. It has also been reported that Zinpyr-1 has a relatively high background and thus a low zinc turn-on (dynamic range of light emission between probe that is bound or unbound with zinc) at physiological pH89,90 which precluded the use of Zinpyr-1 for in vivo detection of drusen in our mouse models of AMD. However, the importance of zinc in normal physiology and disease pathology, including neurobiology,91 has led to the development of an array of fluorescent probes for zinc with different solubilities and fluorescence properties.92 One such probe, ZPP1,88 which has a pyrazine for pyridine substitution compared to Zinpyr-1 (Figure 2),87 has been shown to have significantly lower background and a higher zinc turn-on rate than similar zinc probes.88–90 ZPP1 has been used to detect and quantify zinc in many cells types93 and was recently used in vivo in a mouse model of prostate cancer.94 Therefore, we analyzed the usefulness of ZPP1 to detect zinc-containing, sub-retinal epithelial deposits. Because of the prevalence of drusen in aged human donor tissue, we chose to optimize ZPP1 zinc labeling and compare it to Zinpyr-1 using human tissue followed by analysis of ZPP1 in a mouse model of AMD.
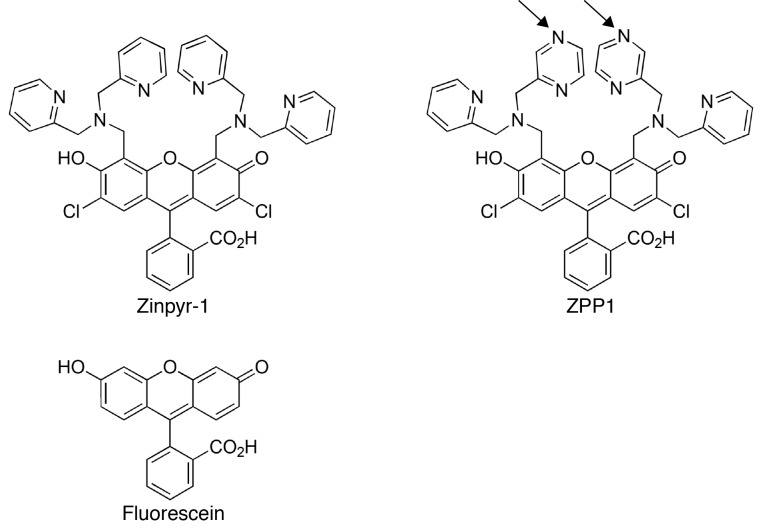
FIGURE 2.
Chemical structure of Zinpyr-1 and ZPP1. Arrows indicate changes within one pyridine arm of each dipicolylamine moiety resulting in a pyrazine (ZPP1) for pyridine (Zinpyr-1) functional group substitution. The bi-chlorinated fluorescein moiety in each compound is identical (lower part of Zinpyr-1 and ZPP1). For reference, the structure of unmodified fluorescein is shown (lower left).
ENHANCED DETECTION OF DRUSEN WITH ZPP1
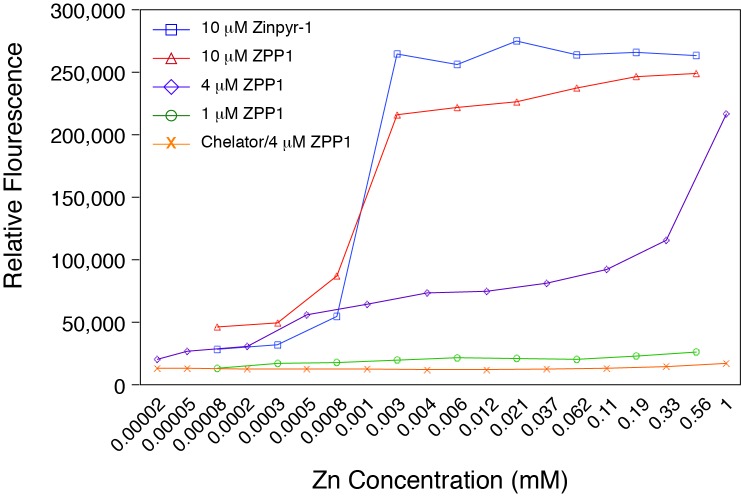
The initial concentration of 10 μM Zinpyr-1 for detection of zinc in the drusen of human tissue66 and in the analysis of our mouse models (above) had been determined empirically. In order to better determine the optimal concentration of the zinc probes we performed an in vitro titration of several dilutions of ZPP1 and 10 μM Zinpyr-1 against a large range of zinc concentrations. The analysis showed that a probe concentration in the range of 3–5 μM might be optimal for zinc detection (Figure 3). ZPP1 at 1 μM showed poor detection of zinc while 10 μM of both Zinpyr-1 and ZPP1 exhibited saturation kinetics. Dilution of zinc in a chelating solution of 10 mM TPEN/1 mM DPTA significantly reduced the light emission at all zinc concentrations with 4 μM ZPP1, indicative that the fluorescence from the probe resulted from its binding of zinc.
FIGURE 3.
Fluorescence emission of Zinpyr-1 and ZPP1. The low concentration of ZPP1 resulted in poor fluorescence emission while the high concentration of either probe exhibited saturation kinetics. Optimal fluorescence emission was noted with ZPP1 concentrations at 3–5 μM. The presence of the zinc-specific chelating agents TPEN/DPTA ablated the fluorescence for all concentrations of zinc analyzed with 4 μM ZPP1.
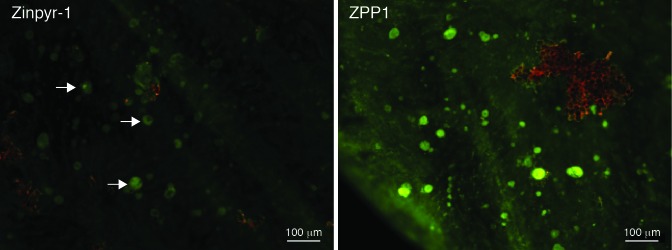
We then compared the sensitivity of Zinpyr-1 and ZPP1 for the detection of zinc in sub-retinal epithelial deposits. Adjacent punches were taken from the eyecup of an 80-year old human donor that had been clinically diagnosed with dry AMD with evidence of drusen, but was free of other ocular pathology. After adjustment of the exposure settings to minimize the green autofluorescence, the punches were labeled with 3 μM Zinpyr-1 or 3 μM ZPP1. Examination by fluorescence microscopy showed that samples labeled with ZPP1 were significantly brighter and revealed more drusen than the sample labeled with Zinpyr-1, which was only slightly brighter than the background autofluorescence (Figure 4). The fact that 3 μM ZPP1 was optimal for zinc detection in drusen was also confirmed empirically as samples from this donor labeled with 10 μM ZPP1 exhibited high background staining and spectral overlap into the red fluorescence channel.
FIGURE 4.
Comparison of drusen detection with Zinpyr-1 and ZPP1. Adjacent 3.5 mm tissue punches from an 80-year old human donor eye with a clinical diagnosis of dry AMD were labeled with 3 μM of each zinc probe and imaged at the same exposure. Arrows indicate faintly labeled drusen deposits (green) in the Zinpyr-1 sample compared to the numerous, easily visualized deposits in the ZPP1 sample. Only a small amount of RPE cellular debris (red, upper right) remained in the ZPP1 sample.
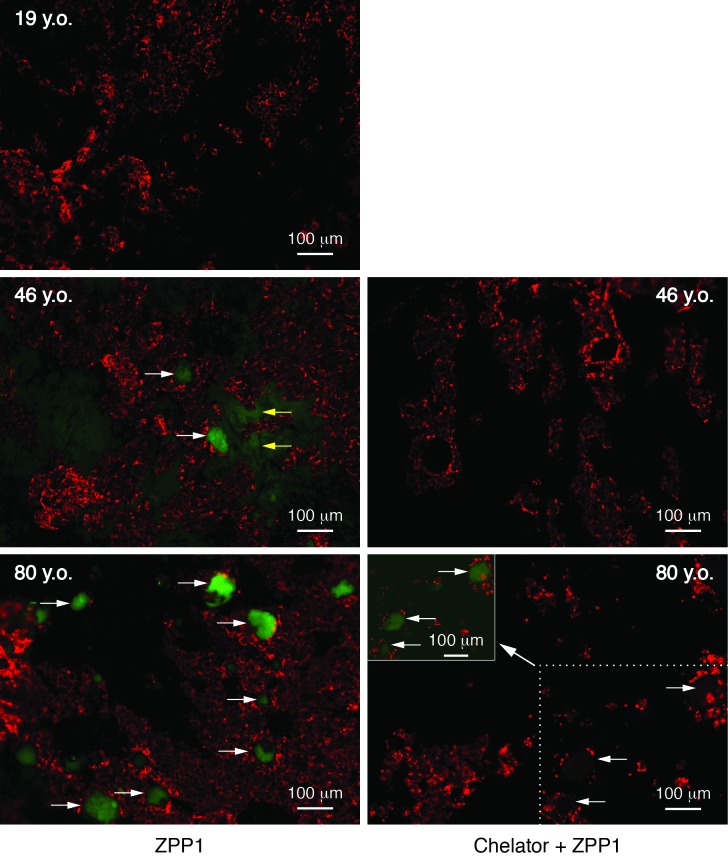
Since the use of ZPP1 resulted in significantly increased sensitivity for drusen, we asked if ZPP1 could detect drusen in samples where the deposits would be expected to be smaller and less frequent. We prepared tissue punches from the eyes of a 19-year old donor, a 46-year old donor, and the above 80-year old donor. The 19- and 46-year old donors had no history of ocular pathology. Immunofluorescence analysis with 3 μM ZPP1 (Figure 5, left panels) showed many sub-retinal epithelial deposits in the tissue from the 80-year old donor while the 19-year old donor tissue was devoid of deposits. Drusen were also detected in the sample from the 46-year old donor, however the deposits were smaller and less numerous than the 80-year old donor. Zinc could be detected faintly (Figure 5, middle left panel, yellow arrows) in the underlying sub RPE tissue of the 46-year sample but not in the 80-year old sample.
FIGURE 5.
Comparison of drusen detection in human donor tissue of various ages. Tissue punches from each donor were labeled with 3 μM ZPP1 (right) or with 50 mM TPEN/10 mM DTPA and then 3 μM ZPP1 (chelator + ZPP1, left) and then imaged at the same magnification and exposure. White arrows indicated drusen. Yellow arrows indicate the faintly staining Bruch’s membrane of the 46-year old donor sample. To confirm the presence of sub-retinal epithelial deposits in samples treated with the chelator, the chelator + ZPP1 sample from the 80-year old donor was also deliberately overexposed (a portion with now faintly visible drusen is presented as the inset at the upper left of the 80 y.o. chelator + ZPP1 fluorescence micrograph). RPE and RPE cellular debris is present (red).
To verify that we had successfully visualized drusen in the samples, we sought supporting evidence from zinc chelation controls and confocal microscopy analysis. Although the exposure settings are adjusted to minimize autofluorescence of drusen, application of zinc-specific chelators to samples prior to addition of ZPP1 provides additional evidence that the emitted fluorescence from samples assayed with only the probe is specifically due to its binding of zinc. TPEN is widely used in zinc imaging studies due its high cellular permeability.95,96 Previous studies demonstrated that treatment with only TPEN does not diminish the autofluorescence of sub-retinal epithelial deposits in human post mortem samples.66
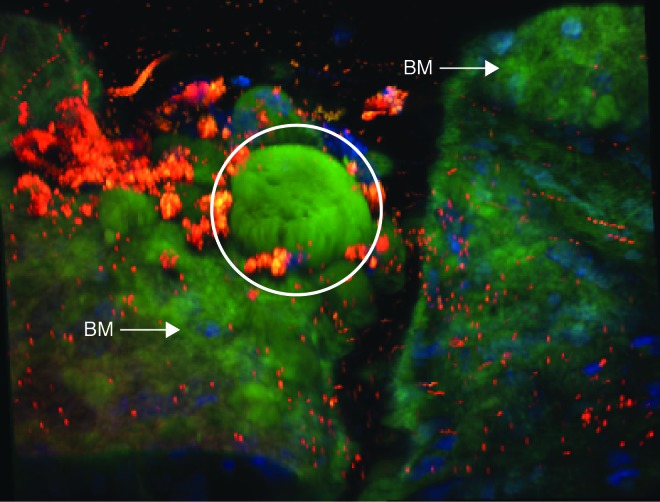
Additional samples adjacent to those analyzed with ZPP1 only from the 46- and 80-year old donors were prepared and then treated with TPEN/DPTA then ZPP1. Analysis of the chelator treated samples using the same exposure settings as the ZPP1 only samples showed a near total elimination of immunofluorescence from the deposits (Figure 5, right panels), suggesting that their visualization (Figure 5, left panels) was due to having significant amounts of zinc. That the chelator treated samples actually contained drusen was confirmed by a deliberate overexposure of the image from the 80-year old chelator plus ZPP1 sample (Figure 5, inset of lower right panel). That a faint fluorescence could be detected suggested that some zinc in drusen could not be chelated, a distinct possibility given that zinc concentrations in drusen can be over 500 parts per million and that it can be complexed to proteins with very high affinity.66,97 Examination of ZPP1 labeled samples by confocal microscopy (Figure 6) confirmed that the deposits were located at or below the RPE. In comparing the 46- and 80-year old samples it was of interest to note the increased size and more extensive protrusion of the drusen through the RPE in the older sample. Since it is known that Bruch’s membrane also contains zinc, we analyzed a sample from a 78-year old donor with a clinical diagnosis dry AMD with drusen (with no other ocular pathology) using ZPP1 plus DAPI (to visualize cell nuclei in sub-RPE tissue) to confirm the location of drusen relative to Bruch’s membrane and to show the enhanced concentration of zinc within the deposit. A three-dimensional confocal image of the sample clearly shows a large, intensely staining deposit (Figure 7, arrow) protruding from the less intensely staining Bruch’s membrane through the remaining RPE debris (red). This finding also confirms the faint zinc staining of sub-RPE tissue we observed in the 46-year old donor sample (Figure 5, middle right panel). Further examination of the fluorescence micrographs revealed a heterogeneous pattern of zinc staining within individual druse (Figure 5, lowest left druse with arrow in the lower left panel; Figure 6, several drusen in the upper right of 80-year old sample; and Figure 7). Hypo- and hyper- staining of spherules and other internal structures is a characteristic feature of drusen.66,86,98 Together, these results show that ZPP1 is a highly specific and sensitive probe for zinc and as such, could be useful in the detection of human drusen even in cases where the deposits are small and infrequent.
FIGURE 6.

Confocal microscopy analysis of human donor tissue. Samples from the 80-year old (top) and the 46-year old (bottom) donors were labeled with 3 μM ZPP1. Drusen (green) and RPE (red) are present in both samples with the size and number of drusen greater in the 80-year old sample. Hypo-staining spherules are present in some druse in the 80-year old sample (arrows). Z-stack images (right and bottom of each image) show the more prominent protrusion above and through the RPE of the drusen deposits from the 80-year old sample.
FIGURE 7.
Three-dimensional confocal image of human sub-RPE tissue from the 78-year old donor with AMD labeled with ZPP1. A large, intensely stained drusen deposit (green) is indicated by the white circle. Zinc in the underlying Bruch’s membrane (BM) is also visualized and indicate with arrows. A small amount of RPE cell debris (lipofuscin autofluorescence, red) is present and cell nuclei (DAPI, blue) from Bruch’s membrane and the underlying sub-RPE tissue are visible.
ANALYSIS OF THE CUZN-SOD KO MOUSE WITH ZPP1
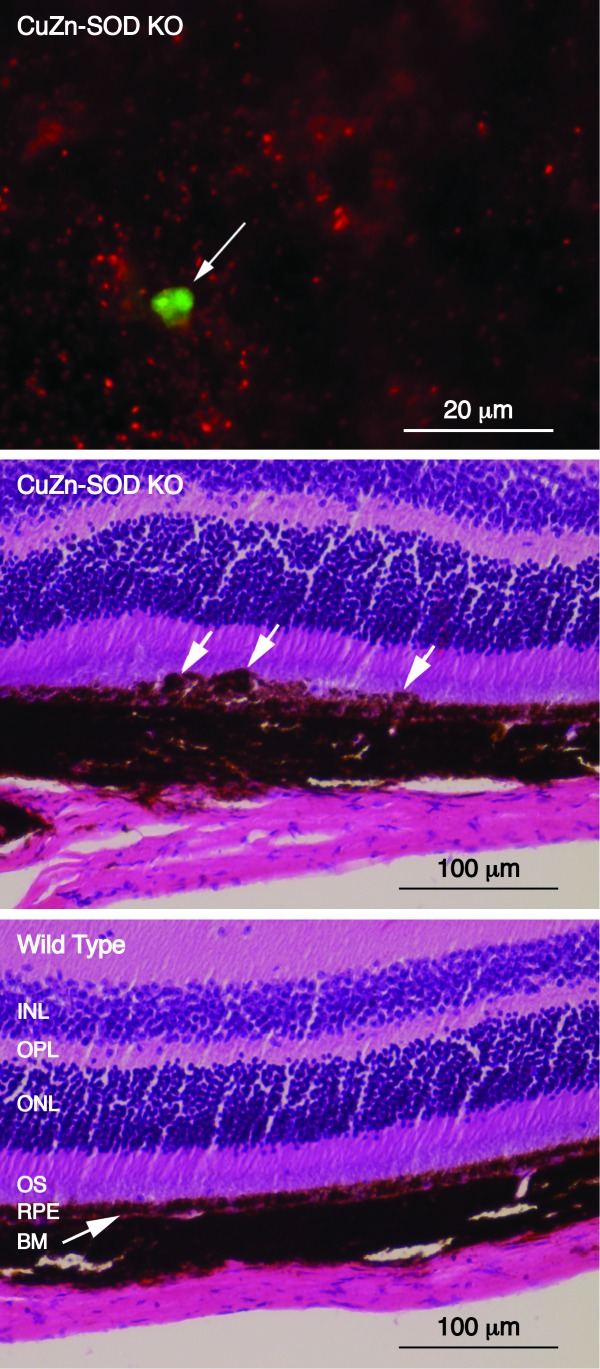
Comparison of Zinpyr-1 and ZPP1 using human donor tissue suggested that ZPP1 could be useful in detecting drusen at earlier ages. To confirm this and to test the suitability of ZPP1 for detection of sub-retinal epithelial deposits in mouse models of AMD, we analyzed ocular tissue from CuZn-SOD KO mice with ZPP1. Analysis of samples from 10-month old CuZn-SOD KO mice revealed zinc-containing sub-retinal epithelial deposits from that mouse (Figure 8, top). Histological analysis of the opposite eye from the same mouse also showed irregularities suggestive of sub-retinal epithelial deposits in the area between the RPE and Bruch’s membrane (Figure 8, middle, arrows) compared to an age matched wild type mouse (Figure 8, bottom). In addition, there appear to by slight morphological changes in the photoreceptor layer of the CuZn-SOD KO mouse. This detection of drusen at just 10 months with ZPP1 might represent a significant enhancement in sensitivity compared to Zinpyr-1, which had an earliest detection of deposits at 14 months (Figure 1). The use of ZPP1 at 3 μM in this analysis also resulted in a much lower background level of staining (compare Figure 1 and Figure 8). Together, these observations allow for the possibility that ZPP1 could be used for in vivo detection of sub-retinal epithelial deposits in mouse models of AMD.
FIGURE 8.
Analysis of CuZn-SOD KO mice. Top; fluorescence micrograph of an eyecup flat mount showing a sub-retinal epithelial deposit stained with ZPP1 (arrow, green) with RPE cell debris (red). Middle; photomicrograph showing the histological analysis of the opposite eye of the same animal. Sub-retinal epithelial deposits are indicated with white arrows. Bottom; histological analysis of an age-matched, wild type control mouse showing normal morphology. INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; OS, outer segments of photoreceptor cells; RPE, retinal pigment epithelial cells; BM, Bruch’s membrane.
DISCUSSION
Our understanding of the many pathogenic and preventative pathways associated AMD continues to evolve. Zinc has long been thought to be associated with limiting the progression of exudative AMD, perhaps through its function as a necessary antioxidant and an enzyme cofactor.21,46 Conversely, it has been recently suggested that zinc might contribute to AMD pathogenesis as evidenced by its high concentration in drusen66 and its association with certain complement system factors.67 However, if and how zinc contributes to AMD initiation and progression, whether it is actually beneficial for limiting AMD, and under what conditions it is beneficial, are unsettled matters that need to be more fully investigated. A more definitive understanding of the relationship between zinc and AMD will only come by having sensitive probes for zinc that can be used in a variety of model systems. This report documents the greatly enhanced sensitivity of a new fluorescein-based probe (ZPP1) for the visualization of zinc associated with drusen in human tissue and its subsequent application for detection of sub-retinal epithelial deposits in murine models of AMD. While the studies presented here were too limited to fully determine the roles of zinc in AMD, we believe our findings provide a way forward in elucidating how zinc can be preventative and pathogenic in AMD.
Confounding the analysis of the role of zinc in drusen formation or AMD prevention is the fact that drusen are actually highly heterogeneous in location, form, and composition16,99,100 suggesting that drusen formation could result from multiple independent pathways. Although it has been well established that zinc is a component of human drusen, the lack of sensitive probes for zinc has limited rigorous analysis of its presence in the various forms and locations of drusen. Thus, a clear association between zinc and any particular type of drusen or pathway of drusen formation remains to be established. Although we could not correlate the presence of zinc in drusen in our human samples with any particular genetic or environmental risk factors for AMD due to lack of information about the donors, our analysis of samples taken from both the peripheral retina and the macular area showed zinc in sub-retinal epithelial deposits that had a wide range of size and distribution patterns, confirming the observations made in earlier studies.66 Given the limited information about our samples, even with the use of a new probe (ZPP1) with greatly enhanced sensitivity, our data could possibly suggest that zinc in drusen is more the result of the mechanism(s) of deposit formation rather than the cause. However, the increased sensitivity of ZPP1 for zinc would make it useful in larger scale studies on human donor samples particularly when combined with information about genotype and environmental risk exposure(s) of the samples. This would allow us to start to develop correlations between zinc content and the type, location, and size of drusen, AMD risk factors, and AMD disease stage and form (GA or CNV). In addition, we believe ZPP1 or an analog(s) can also be developed into a probe for in vivo detection of zinc in sub-retinal epithelial deposits, allowing us to employ more specific tools for determining the role of zinc in drusen formation.
For this reason, the use of murine models would aid greatly in understanding of the relationship between zinc, drusen formation, and AMD risk factors. While mice lack a true macula, their importance as models for AMD cannot be understated as they allow correlations to be made about zinc and sub-retinal epithelial deposit formation to genotype and/or risk factor exposure. A recent and comprehensive review of murine AMD models detailed the presence of sub-retinal epithelial deposit in nineteen of the twenty-seven models described.73 Deposit formation was noted in a diversity of mice having deficiencies in chemokines, complement factors, glucose/lipid metabolism genes, and genes associated with oxidative damage and repair. However, as evidence for the heterogeneous character of sub-retinal epithelial deposits, the exact origin and composition of the deposits in some of these mouse models is still unsettled.101,102
Our initial analysis of zinc in sub-retinal epithelial deposits in murine AMD models was done on mice deficient in two isoforms of SOD, a zinc-containing enzyme critical for defense against oxidative damage. While in humans a clear association between genetic polymorphisms in SOD isoforms and AMD is tenuous,103–107 these murine models were chosen because they develop sub-retinal epithelial deposits that resemble certain aspects of human drusen. While not fully characterized in terms of a particular ocular location or being hard or soft drusen, the drusen found in CuZn-SOD KO mice has been described as domed-shape deposits located between Bruch’s membrane and the RPE.79 Further resemblance to human drusen is also evidenced by the fact that CuZn-SOD KO mouse drusen develop with age, can be hastened by light exposure, and contain some of the same biochemical markers such as vitronectin, carboxymethyl lysine, and tissue inhibitor of metalloproteinases 3 (TIMP3).79,108
Using two different fluorescein based probes for zinc we were able to detect drusen in samples taken from mice lacking CuZn-SOD (sod1) and Mn-SOD (sod2). Using Zinpyr-1, the earliest age for deposit detection was 14 months with the CuZn-SOD KO mice and 18 months with the Mn-SOD KO mice. However, the increased sensitivity of ZPP1 allowed us to detect drusen at 10 months of age in the CuZn-SOD KO mouse. In the initial study describing the AMD phenotype of CuZn-SOD KO mice79 it is of interest to note that very few drusen (< 10 per eye) were found by fundus examination until the mice were at least 15 months of age. This could be due to the low magnification of the ophthalmoscope combined with the poor color contrast between drusen and background tissue. Further, in vitro analysis, such as immunofluorescence, required the CuZn-SOD mice to be at least 12 months old for reasonable visualization of the drusen. We believe that our detection of small deposits (< 5 μm by immunofluorescence) at a younger time point of 10 months with ZPP1 will greatly enhance the efficaciousness of the CuZn-SOD KO model for in vitro studies examining the role of zinc in drusen formation. In addition, the fact that deposits in CuZn-SOD KO mice are generally visible by current retinal imaging systems, combined with the enhanced sensitivity and contrast associated with ZPP1, make it a very good in vivo model for determining if imaging zinc in sub-retinal epithelial deposits can be used for the early diagnosis of AMD.
While zinc appears to be a common component of murine sub-retinal epithelial deposits, analysis of other murine models whose deposits are induced or enhanced by other genetic mechanisms (and thus likely to differ in form, composition, and location) is necessary to confirm and fully elucidate the role of zinc in their formation. The association of CFH polymorphisms with AMD has led to the analysis of CFH deficient mice as a model of AMD.109 Sub-retinal epithelial deposits in CFH KO mice have been described as primarily basal laminar thus more characteristic of soft drusen,110,111 which is found exclusively in and around the macula and associated with advanced AMD.16 A preliminary analysis of CFH KO mouse sub-RPE tissue with ZPP1 was indicative for zinc-containing sub-retinal epithelial deposits. This is consistent with the finding that macular deposits from human donor samples contained the highest amounts of zinc.66
Just as the ability to detect small deposit in mouse models would provide a significant advantage to studies about the role of zinc in their formation, the ability to detect small drusen would greatly aid research done with human donor tissue and clinical analysis to establish zinc as a credible biomarker for early AMD. Using ZPP1 in fluorescence microscopy to detect zinc, we routinely detected human sub-retinal epithelial deposits in the range of 5–10 μm, considerably less than 25–30 μm sized deposits that currently represents the lower limit of detection in clinical examinations of the fundus. While the magnification and resolving power of microscopes used for immunofluorescence is greater than clinical ophthalmoscopes, we believe that light-emitting probes in conjunction with optical and technological enhancements to ophthalmoscopes will allow for routine detection of very small drusen. Under certain conditions we were also able to detect zinc in Bruch’s membrane or other sub-RPE tissue with ZPP1. This suggests that ZPP1 might be useful in tracking age-related changes in zinc levels in sub-retinal tissues. This could help elucidate the mechanisms and role of zinc not only in drusen formation, but also in AMD associated tissue damage.
As with anything that has potential clinical application, concerns about safety and toxicity should be considered even before any in vivo testing. Fluorescein itself is considered safe and is used in numerous human applications including ophthalmological imaging.112 Fluorescein is readily soluble in water, however the addition of zinc sensing moieties such as dipicolylamines requires an initial dilution in an organic solvent such as dimethyl sulfoxide (DMSO) before further dilution in aqueous solutions. There have been very few in vivo studies of any kind with fluorescein-based zinc probes94,113,114 and they are not currently used in any human application. While we ourselves have not done, or are aware of, any formal safety/toxicity studies for Zinpyr-1 or ZPP1 in humans, during our preliminary in vivo experiments we did not observe any mortality or ocular pathology following intravenous or intraocular injection of the probes into mice. While there are a number of technical issues to be resolved such as probe solubility, dosage, and route of administration, we believe our findings provide a starting point towards the development of a sensitive in vivo or clinical assay for drusen that is no more complex or time consuming than current ophthalmological imaging procedures involving fluorescein.
The aging demographics in developed nations and the economic and quality of life issues associated with visual disability makes AMD a significant and costly public health issue. In just the United States, billions of dollars are spent annually on anti-VEGF therapies for wet AMD. In 2010, ranibizumab (Lucentis) alone was the single largest drug expense for Medicare Part B, accounting for 10% of the budget.115 AMD is generally undiagnosed until the affected individual has visual impairment (late stage AMD), necessitating expensive medication (such as anti-VEGF therapies) and/or the expenses associated with accommodating visual impairment. There is a clear need for a simple, clinical test that can detect the initial ocular changes associated with early AMD. As drusen are considered the earliest indicator for AMD and contains high levels of zinc, we believe that a clinical assay for sub-retinal epithelial deposits based on zinc could be a highly credible and sensitive screen for earliest manifestations of AMD. In this way prevention and treatment strategies having both health and economic benefits can be implemented as soon as possible. We believe that our in vitro analysis showing ZPP1 to be a very sensitive probe for sub-retinal epithelial deposits in human and animal model samples to be important and necessary first steps towards in vivo detection assays that can advance basic research and early clinical detection of AMD as well as improve our understanding the role of zinc in drusen formation.
CONCLUSION
In this study, we compared the ability of Zinpyr-1 and ZPP1 to detect sub-retinal epithelial deposit by imaging the zinc content of the deposits. We conclude that ZPP1 is a superior probe for the detection of zinc in sub-retinal epithelial deposits in human and murine tissue. In human tissue ZPP1 can detect smaller drusen, determine them at a younger age, and demonstrate variability of zinc distribution within drusen much better. In murine models ZPP1 can detect sub-retinal epithelial deposits at a younger age and has not shown any toxicity when used intraocularly or intravenously. ZPP1 shows great promise for the in vivo detection of zinc in sub-retinal epithelial deposits in these models for AMD, which can greatly enhance our understanding of the role of zinc in AMD pathogenesis.
ACKNOWLEDGEMENTS
Funding Support: Beckman Initiative for Macular Research and a donation (Anonymous) for AMD research to the University of Minnesota, Department of Ophthalmology and Visual Neuroscience.
Financial Disclosures: None.
Author Contributions: Conception and Design (F.J.G.M.v K.); Funding (F.J.G.M.v K.); Experiments and Data collection (S.W.M., H.R.); Analysis of Data (F.J.G.M.v K., S.W.M., H.R.); Writing, Review, and Editing of Thesis (S.W.M., F.J.G.M.v K., H.R.); Final Approval of Thesis (F.J.G.M.v K., S.W.M., H.R.).
Other Acknowledgements: The authors thank Dr. Deborah Ferrington, PhD, University of Minnesota for helpful discussions and critical review; Daniel Schuster, PhD, and Rebecca Kapphahn, BS, University of Minnesota for help with initial animal experiments.
REFERENCES
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sergejeva O, Botov R, Liutkeviciene R, Kriauciuniene L. Genetic factors associated with the development of age-related macular degeneration. Medicina (Kaunas) 2016;52(2):79–88. doi: 10.1016/j.medici.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Patel N, Adewoyin T, Chong NV. Age-related macular degeneration: a perspective on genetic studies. Eye (London, England) 2008;22(6):768–776. doi: 10.1038/sj.eye.6702844. [DOI] [PubMed] [Google Scholar]
- 8.Khandhadia S, Cipriani V, Yates JR, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2012;217(2):127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61(2):118–125. doi: 10.1016/j.molimm.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong RA, Mousavi M. Overview of Risk Factors for Age-Related Macular Degeneration (AMD) J Stem Cells. 2015;10(3):171–191. [PubMed] [Google Scholar]
- 12.Lambert NG, ElShelmani H, Singh MK, et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016;54:64–102. doi: 10.1016/j.preteyeres.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon JM. Genetic and environmental underpinnings to age-related ocular diseases. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF28–30. doi: 10.1167/iovs.13-13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120(9):3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7(11):860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 16.Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49(3):1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Age-Related Eye Disease Study Research G. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132(5):668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 20.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen TW. The Minnesota Grading System using fundus autofluorescence of eye bank eyes: a correlation to age-related macular degeneration (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:383–401. [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen TW, Feng X. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(12):4484–4490. doi: 10.1167/iovs.04-0342. [DOI] [PubMed] [Google Scholar]
- 24.Williams MA, Craig D, Passmore P, Silvestri G. Retinal drusen: harbingers of age, safe havens for trouble. Age Ageing. 2009;38(6):648–654. doi: 10.1093/ageing/afp136. [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115(1):116–126. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471(7338):325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Tarallo V, Kerur N, et al. DICER1/Alu RNA dysmetabolism induces Caspase-8-mediated cell death in age-related macular degeneration. Proc Nat Acad Sci U S A. 2014;111(45):16082–16087. doi: 10.1073/pnas.1403814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Nat Acad Sci U S A. 2010;107(16):7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–439. 439e431–432. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobrin L, Reynolds R, Yu Y, et al. ARMS2/HTRA1 locus can confer differential susceptibility to the advanced subtypes of agerelated macular degeneration. Am J Ophthalmol. 2011;151(2):345–352 e343. doi: 10.1016/j.ajo.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein ML, Schultz DW, Edwards A, et al. Age-related macular degeneration. Clinical features in a large family and linkage to chromosome 1q. Arch Ophthalmol. 1998;116(8):1082–1088. doi: 10.1001/archopht.116.8.1082. [DOI] [PubMed] [Google Scholar]
- 32.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 34.Blasiak J, Petrovski G, Vereb Z, Facsko A, Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res Int. 2014;2014:768026. doi: 10.1155/2014/768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 36.Gerster H. Review: antioxidant protection of the ageing macula. Age Ageing. 1991;20(1):60–69. doi: 10.1093/ageing/20.1.60. [DOI] [PubMed] [Google Scholar]
- 37.Ugarte M, Osborne NN, Brown LA, Bishop PN. Iron, zinc, and copper in retinal physiology and disease. Surv Ophthalmol. 2013;58(6):585–609. doi: 10.1016/j.survophthal.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Ugarte M, Osborne NN. Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics. 2014;6(2):189–200. doi: 10.1039/c3mt00291h. [DOI] [PubMed] [Google Scholar]
- 39.Erie JC, Good JA, Butz JA, Pulido JS. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am J Ophthalmol. 2009;147(2):276–282 e271. doi: 10.1016/j.ajo.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Wills NK, Ramanujam VM, Kalariya N, Lewis JR, van Kuijk FJ. Copper and zinc distribution in the human retina: relationship to cadmium accumulation, age, and gender. Exp Eye Res. 2008;87(2):80–88. doi: 10.1016/j.exer.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Miceli MV, Tate DJ, Jr, Alcock NW, Newsome DA. Zinc deficiency and oxidative stress in the retina of pigmented rats. Invest Ophthalmol Vis Sci. 1999;40(6):1238–1244. [PubMed] [Google Scholar]
- 42.Osborne NN, Wood JP. The beta-adrenergic receptor antagonist metipranolol blunts zinc-induced photoreceptor and RPE apoptosis. Invest Ophthalmol Vis Sci. 2006;47(7):3178–3186. doi: 10.1167/iovs.05-1370. [DOI] [PubMed] [Google Scholar]
- 43.Henderson LM, Chappell JB, Jones OT. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988;255(1):285–290. [PMC free article] [PubMed] [Google Scholar]
- 44.Tate DJ, Jr, Miceli MV, Newsome DA. Zinc protects against oxidative damage in cultured human retinal pigment epithelial cells. Free Radic Biol Med. 1999;26(5–6):704–713. doi: 10.1016/s0891-5849(98)00253-6. [DOI] [PubMed] [Google Scholar]
- 45.O’Dell BL. Role of zinc in plasma membrane function. J Nutr. 2000;130(5S Suppl):1432S–1436S. doi: 10.1093/jn/130.5.1432S. [DOI] [PubMed] [Google Scholar]
- 46.Newsome DA, Swartz M, Leone NC, Elston RC, Miller E. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106(2):192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- 47.VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998;148(2):204–214. doi: 10.1093/oxfordjournals.aje.a009625. [DOI] [PubMed] [Google Scholar]
- 48.Cho E, Stampfer MJ, Seddon JM, et al. Prospective study of zinc intake and the risk of age-related macular degeneration. Ann Epidemiol. 2001;11(5):328–336. doi: 10.1016/s1047-2797(01)00217-4. [DOI] [PubMed] [Google Scholar]
- 49.Smith W, Mitchell P, Webb K, Leeder SR. Dietary antioxidants and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1999;106(4):761–767. doi: 10.1016/S0161-6420(99)90164-1. [DOI] [PubMed] [Google Scholar]
- 50.Stur M, Tittl M, Reitner A, Meisinger V. Oral zinc and the second eye in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(7):1225–1235. [PubMed] [Google Scholar]
- 51.Chew EY, Clemons TE, Agron E, et al. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013;120(8):1604–1611 e1604. doi: 10.1016/j.ophtha.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awh CC, Lane AM, Hawken S, Zanke B, Kim IK. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317–2323. doi: 10.1016/j.ophtha.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 53.Klein ML, Francis PJ, Rosner B, et al. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology. 2008;115(6):1019–1025. doi: 10.1016/j.ophtha.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 54.Lee AY, Brantley MA., Jr CFH and LOC387715/ARMS2 genotypes and antioxidants and zinc therapy for age-related macular degeneration. Pharmacogenomics. 2008;9(10):1547–1550. doi: 10.2217/14622416.9.10.1547. [DOI] [PubMed] [Google Scholar]
- 55.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 56.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Nat Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 58.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet. 2006;38(10):1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 59.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M, Atmaca-Sonmez P, Othman M, et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet. 2006;38(9):1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38(9):1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 62.Fritsche LG, Loenhardt T, Janssen A, et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40(7):892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- 63.Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS report number 38. Ophthalmology. 2014;121(11):2173–2180. doi: 10.1016/j.ophtha.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newsome DA. A randomized, prospective, placebo-controlled clinical trial of a novel zinc-monocysteine compound in agerelated macular degeneration. Curr Eye Res. 2008;33(7):591–598. doi: 10.1080/02713680802178437. [DOI] [PubMed] [Google Scholar]
- 65.Vishwanathan R, Chung M, Johnson EJ. A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(6):3985–3998. doi: 10.1167/iovs.12-11552. [DOI] [PubMed] [Google Scholar]
- 66.Lengyel I, Flinn JM, Peto T, et al. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp Eye Res. 2007;84(4):772–780. doi: 10.1016/j.exer.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Nan R, Gor J, Lengyel I, Perkins SJ. Uncontrolled zinc- and copper-induced oligomerisation of the human complement regulator factor H and its possible implications for function and disease. J Mol Biol. 2008;384(5):1341–1352. doi: 10.1016/j.jmb.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 68.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 69.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 70.Nan R, Farabella I, Schumacher FF, et al. Zinc binding to the Tyr402 and His402 allotypes of complement factor H: possible implications for age-related macular degeneration. J Mol Biol. 2011;408(4):714–735. doi: 10.1016/j.jmb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nan R, Tetchner S, Rodriguez E, et al. Zinc-induced self-association of complement C3b and Factor H: implications for inflammation and age-related macular degeneration. J Biol Chem. 2013;288(26):19197–19210. doi: 10.1074/jbc.M113.476143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smailhodzic D, van Asten F, Blom AM, et al. Zinc supplementation inhibits complement activation in age-related macular degeneration. PLoS One. 2014;9(11):e112682. doi: 10.1371/journal.pone.0112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Aspects Med. 2012;33(4):487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010;29(3):169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding JD, Kelly U, Groelle M, Christenbury JG, Zhang W, Bowes Rickman C. The role of complement dysregulation in AMD mouse models. Adv Exp Med Biol. 2014;801:213–219. doi: 10.1007/978-1-4614-3209-8_28. [DOI] [PubMed] [Google Scholar]
- 76.McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968–1988) Free Radic Biol Med. 1988;5(5–6):363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 77.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 78.Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39(3):471–475. [PubMed] [Google Scholar]
- 79.Imamura Y, Noda S, Hashizume K, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Nat Acad Sci U S A. 2006;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hashizume K, Hirasawa M, Imamura Y, et al. Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am J Pathol. 2008;172(5):1325–1331. doi: 10.2353/ajpath.2008.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Justilien V, Pang JJ, Renganathan K, et al. SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci. 2007;48(10):4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Kuijk FJ, Lewis JW, Buck P, Parker KR, Kliger DS. Spectrophotometric quantitation of rhodopsin in the human retina. Invest Ophthalmol Vis Sci. 1991;32(7):1962–1967. [PubMed] [Google Scholar]
- 83.Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139(9):4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 84.Ikegami T, Suzuki Y, Shimizu T, Isono K, Koseki H, Shirasawa T. Model mice for tissue-specific deletion of the manganese superoxide dismutase (MnSOD) gene. Biochem Biophys Res Commun. 2002;296(3):729–736. doi: 10.1016/s0006-291x(02)00933-6. [DOI] [PubMed] [Google Scholar]
- 85.Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112(6):1584–1592. doi: 10.1111/j.1471-4159.2010.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lengyel I, Tufail A, Hosaini HA, Luthert P, Bird AC, Jeffery G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45(9):2886–2892. doi: 10.1167/iovs.03-1083. [DOI] [PubMed] [Google Scholar]
- 87.Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ. Fluorescent sensors for Zn(2+) based on a fluorescein platform: synthesis, properties and intracellular distribution. J Am Chem Soc. 2001;123(32):7831–7841. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 88.Zhang XA, Hayes D, Smith SJ, Friedle S, Lippard SJ. New strategy for quantifying biological zinc by a modified zinpyr fluorescence sensor. J Am Chem Soc. 2008;130(47):15788–15789. doi: 10.1021/ja807156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong BA, Friedle S, Lippard SJ. Subtle modification of 2,2-dipicolylamine lowers the affinity and improves the turn-on of Zn(II)-selective fluorescent sensors. Inorg Chem. 2009;48(15):7009–7011. doi: 10.1021/ic900990w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong BA, Friedle S, Lippard SJ. Solution and fluorescence properties of symmetric dipicolylamine-containing dichlorofluorescein-based Zn2+ sensors. J Am Chem Soc. 2009;131(20):7142–7152. doi: 10.1021/ja900980u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 92.Huang Z, Lippard SJ. Illuminating mobile zinc with fluorescence from cuvettes to live cells and tissues. Methods Enzymol. 2012;505:445–468. doi: 10.1016/B978-0-12-388448-0.00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buccella D, Horowitz JA, Lippard SJ. Understanding zinc quantification with existing and advanced ditopic fluorescent Zinpyr sensors. J Am Chem Soc. 2011;133(11):4101–4114. doi: 10.1021/ja110907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghosh SK, Kim P, Zhang XA, et al. A novel imaging approach for early detection of prostate cancer based on endogenous zinc sensing. Cancer Res. 2010;70(15):6119–6127. doi: 10.1158/0008-5472.CAN-10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matias CM, Sousa JM, Quinta-Ferreira ME, Arif M, Burrows HD. Validation of TPEN as a zinc chelator in fluorescence probing of calcium in cells with the indicator Fura-2. J Fluoresc. 2010;20(1):377–380. doi: 10.1007/s10895-009-0539-y. [DOI] [PubMed] [Google Scholar]
- 96.Radford RJ, Lippard SJ. Chelators for investigating zinc metalloneurochemistry. Curr Opin Chem Biol. 2013;17(2):129–136. doi: 10.1016/j.cbpa.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flinn JM, Kakalec P, Tappero R, Jones B, Lengyel I. Correlations in distribution and concentration of calcium, copper and iron with zinc in isolated extracellular deposits associated with age-related macular degeneration. Metallomics. 2014;6(7):1223–1228. doi: 10.1039/c4mt00058g. [DOI] [PubMed] [Google Scholar]
- 98.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78(2):243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 99.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95(12):1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008;87(5):402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2- deficient mice. Nat Med. 2003;9(11):1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 102.Luhmann UF, Robbie S, Munro PM, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009;50(12):5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Esfandiary H, Chakravarthy U, Patterson C, Young I, Hughes AE. Association study of detoxification genes in age related macular degeneration. Br J Ophthalmol. 2005;89(4):470–474. doi: 10.1136/bjo.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gotoh N, Yamada R, Matsuda F, Yoshimura N, Iida T. Manganese superoxide dismutase gene (SOD2) polymorphism and exudative age-related macular degeneration in the Japanese population. Am J Ophthalmol. 2008;146(1):146. doi: 10.1016/j.ajo.2008.03.017. author reply 146–147. [DOI] [PubMed] [Google Scholar]
- 105.Kimura K, Isashiki Y, Sonoda S, Kakiuchi-Matsumoto T, Ohba N. Genetic association of manganese superoxide dismutase with exudative age-related macular degeneration. Am J Ophthalmol. 2000;130(6):769–773. doi: 10.1016/s0002-9394(00)00552-3. [DOI] [PubMed] [Google Scholar]
- 106.Kondo N, Bessho H, Honda S, Negi A. SOD2 gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Molecular Vision. 2009;15:1819–1826. [PMC free article] [PubMed] [Google Scholar]
- 107.Mrowicka M, Mrowicki J, Szaflik JP, et al. Analysis of antioxidative factors related to AMD risk development in the polish patients. Acta Ophthalmol. 2016 doi: 10.1111/aos.13289. [DOI] [PubMed] [Google Scholar]
- 108.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Nat Acad Sci U S A. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31(4):424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 110.Ding JD, Kelly U, Landowski M, et al. Expression of human complement factor H prevents age-related macular degenerationlike retina damage and kidney abnormalities in aged Cfh knockout mice. Am J Pathol. 2015;185(1):29–42. doi: 10.1016/j.ajpath.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Nat Acad Sci U S A. 2015;112(23):E3040–3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patel M, Kiss S. Ultra-wide-field fluorescein angiography in retinal disease. Curr Opin Ophthalmol. 2014;25(3):213–220. doi: 10.1097/ICU.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 113.De Leon-Rodriguez L, Lubag AJ, Jr, Sherry AD. Imaging free zinc levels in vivo - what can be learned? Inorganica Chim Acta. 2012;393:12–23. doi: 10.1016/j.ica.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Woodroofe CC, Masalha R, Barnes KR, Frederickson CJ, Lippard SJ. Membrane-permeable and -impermeable sensors of the Zinpyr family and their application to imaging of hippocampal zinc in vivo. Chem Biol. 2004;11(12):1659–1666. doi: 10.1016/j.chembiol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 115.Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2013;111:56–69. [PMC free article] [PubMed] [Google Scholar]