Abstract
Background:
The high failure rate of rotator cuff repairs requires the development of methods to enhance healing at the tendon-bone junction of the repair site.
Purpose:
To assess functional recovery and structural outcomes in detail after implanting recombinant human bone morphogenetic protein–12 (rhBMP-12)/absorbable collagen sponge (ACS) as adjuvant treatment during open rotator cuff repair in patients over a 1-year postoperative follow-up.
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
A total of 20 patients were randomized into 2 groups, rhBMP-12/ACS and standard-of-care (SOC) control, with 16 and 4 patients, respectively. The patients underwent open repair of a rotator cuff tear at least 2 to 4 cm wide; in the rhBMP-12/ACS group, this was augmented with a bioscaffold containing rhBMP-12. Follow-up assessments were conducted with a 100-mm visual analog scale (VAS) for pain and active and passive ranges of motion (ROMs) including forward flexion, elevation in the scapular plane, abduction, and external rotation at 12, 16, 26, 39, and 52 weeks after surgery; isometric strength in scapular abduction and external rotation at 16, 26, 39, and 52 weeks; and magnetic resonance imaging (MRI) at 12 and 52 weeks.
Results:
The mean VAS score decreased from 37.9 mm preoperatively to 13.8 mm at week 52, and ROM and isometric strength recovered at week 52 in the rhBMP-12/ACS group. The mean VAS score decreased from 48.3 mm preoperatively to 1.5 mm at week 52, and ROM (excluding external rotation) and isometric strength recovered by week 52 in the SOC control group. Of the 16 patients in the rhBMP-12/ACS group, 14 showed an intact repair at week 12; the MRI scans of the other 2 patients could not be evaluated because of artifacts. In the SOC control group, 1 patient showed repair failure. At week 52, 14 repairs in the rhBMP-12/ACS group and 2 repairs with available MRI scans in the SOC control group remained intact.
Conclusion:
Functional recovery and structural outcomes in patients in whom rhBMP-12/ACS was used as adjuvant therapy in rotator cuff repair justify conducting future, larger, multicenter, prospective studies.
Registration:
NCT00936559, NCT01122498 (ClinicalTrials.gov identifier).
Keywords: rotator cuff repair, biological augmentation, tendon-to-bone healing, rhBMP-12, growth factor
Rotator cuff tears are common shoulder injuries in those who engage in frequent and repetitive overhead motion. The supraspinatus tendon is most often affected. These injuries frequently result in substantial pain and disability. Full-thickness tears of the rotator cuff commonly result from detachment of the tendon from the greater tuberosity, requiring tendon-to-bone repair. Because tissue healing between the repaired tendon and bone progresses slowly,12,14 prolonged periods of relative immobility are required after surgery to protect the intact repair. Despite careful postoperative management after rotator cuff repair, a high rate of incomplete healing and gap formation between the tendon and bone has been reported.2,5,6,9–11 Importantly, the functional results after rotator cuff repair, and particularly strength, are superior when complete structural recovery of the repair can be achieved.2,6,9–11 Thus, developing methods to enhance healing at the tendon-to-bone junction is imperative after rotator cuff repair.
Tissue engineering using an extracellular matrix scaffold combined with growth factors to promote tendon-to-bone healing may provide a viable solution.1,12,22,23 Recombinant human bone morphogenetic protein–12 (rhBMP-12) has been shown to induce tendon and ligament formation in animals and to improve tendon healing.13,15,21 Our study consisted of 2 parts. We provided evidence of the feasibility and safety of using a concentration of 0.015 mg/mL rhBMP-12/absorbable collagen sponge (ACS) as adjuvant therapy in open rotator cuff repair in patients in part 1 of our study.8 We evaluated pharmacokinetics, immunogenicity, and local and systemic adverse events including heterotopic ossification on computed tomography and repair integrity on magnetic resonance imaging (MRI). The purpose of part 2 of this study was to assess functional recovery and provide structural outcomes in detail after implanting rhBMP-12/ACS as adjuvant treatment during open rotator cuff repair in patients over a 1-year postoperative follow-up.
Methods
The study was conducted in accordance with applicable laws and regulations including the guidelines of the Declaration of Helsinki on human experimentation and the International Conference on Harmonisation’s guidelines for good clinical practice. The protocol and informed consent forms were approved by local institutional review boards in Europe and Japan and by local and national ethics committees in Europe, as appropriate, before any patients were enrolled. All study participants provided written informed consent.
Study Design
Because of regional considerations, 2 nearly identical studies were conducted both in Europe and Japan, involving 4 European sites and 9 Japanese sites. A total of 8 sites enrolled patients in this study. This study was a phase 1, multicenter, randomized study with standard-of-care (SOC) control, evaluating the clinical outcomes of using 0.015 mg/mL rhBMP-12/ACS as adjuvant therapy during open rotator cuff repair in patients with full-thickness rotator cuff tears 2 to 4 cm in size. The evaluation of outcomes included a serial assessment of rotator cuff integrity by MRI, a functional assessment of active and passive ranges of motion (ROMs), a visual analog scale (VAS) for pain, and isometric strength measurements.
Statistical Considerations
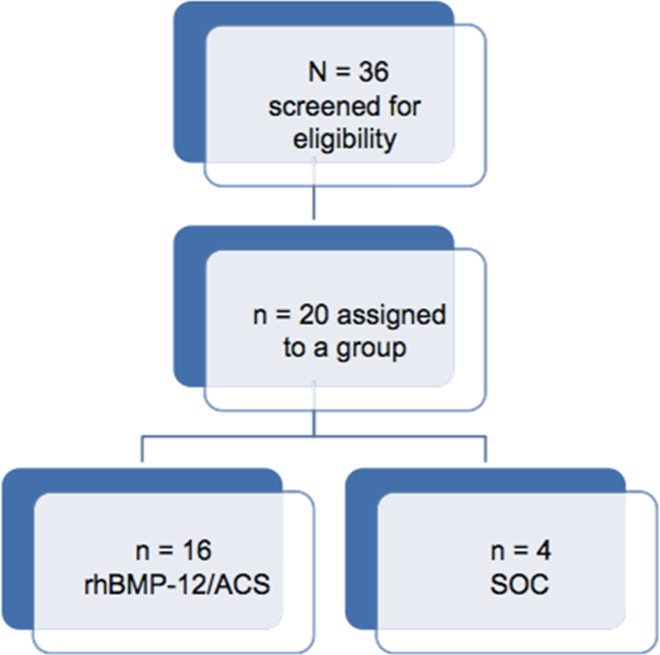
The sample size for the study was determined by clinical rather than statistical considerations. This study was designed to provide safety, particularly regarding heterotopic ossification formation, similar to that in a preclinical animal study,19 wherein bone formation developed in 11 of 16 cases and 7 of 12 cases for 2 different types of collagen sponge used. Given the animal data, a minimum number of 10 participants was considered sufficient to provide initial safety data. We decided to enroll 16 patients in the randomization process to account for screen failures or dropouts. We enrolled 4 control participants to provide a reference for the SOC alone (Figure 1). For the same reason, functional assessments were summarized with descriptive statistics by treatment group at each visit. No further statistical analysis was conducted.
Figure 1.
CONSORT diagram of patient assignment. ACS, absorbable collagen sponge; rhBMP-12, recombinant human bone morphogenetic protein–12; SOC, standard of care.
Randomization
After the verification of participants’ eligibility, the patients were enrolled in the study and randomly assigned to be administered either SOC with 0.015 mg/mL rhBMP-12/ACS or SOC alone at a ratio of 1:4, SOC:rhBMP-12/ACS via an automated enrollment system. A web-based enrollment system based on a predefined algorithm for randomized allocation was used. The investigators accessed this system from a computer to obtain randomized treatment allocation. Randomization was performed separately in Europe and Japan.
Inclusion and Exclusion Criteria
Patients were screened prospectively based on the following criteria: male patients committed to using a reliable method of birth control for the entire duration of the study and surgically sterile or postmenopausal female patients, between 25 and 75 years old, with a full-thickness supraspinatus and/or infraspinatus tendon tear 2 to 4 cm in size that was identified on closed MRI within 3 months before surgery. Minimal passive ROM of the affected shoulder of at least 150° in flexion and elevation in the scapular plane and 40° in external rotation was required.
The exclusion criteria were described in detail in part 1 of our study8 and are briefly described here: previous surgical intervention to the shoulder joint under study; presence of a subscapularis tear or labral abnormality requiring surgical repair; history of dislocations of the affected shoulder or instability identified on physical examination; inability to complete functional evaluations because of concurrent injuries or neurovascular impairment either in the arm under study (excluding the rotator cuff tear) or in the contralateral arm; stage 3 or 4 fatty infiltration of the rotator cuff muscles4,7 on MRI obtained within 3 months before surgical repair; moderate or severe degenerative glenohumeral arthritis (based on the arthrosis grading scale of Samilson and Prieto18), avascular necrosis, calcifying tendinitis, chondrocalcinosis, hypertrophic osteoarthropathy, Paget disease, or any other bone abnormalities (eg, heterotopic ossification, previous fracture) of the shoulder confirmed by radiography within 3 months before surgical repair; rheumatological conditions affecting the shoulder joints or autoimmune disorders; more than 3 corticosteroid injections in the affected shoulder within 1 year of the planned surgical procedure or a corticosteroid injection in the shoulder under study within 3 months of the planned surgical procedure; use of oral corticosteroid medication within 3 months before surgery; documented history or diagnosis of diabetes mellitus or fasting blood glucose level of >125 mg/dL (6.94 mmol/L); and local or systemic infections that would preclude rotator cuff repair.
Patient Demographics
Patients were the same as those in part 1 of the study8; detailed demographic characteristics were provided in part 1, with relevant characteristics summarized here. We screened 36 patients for eligibility and enrolled 20 patients in the study, 12 male and 8 female, with a mean age of 61 years (range, 48-71 years). Sixteen and 4 patients were randomized to the rhBMP-12/ACS and SOC control groups, respectively. Of the 16 patients in the rhBMP-12/ACS group, 6 were smokers, and none of the patients was a smoker in the SOC control group.
Surgical Procedure
A surgical manual was written, and standardized training for the surgical procedure and test article administration and placement were conducted in a cadaveric laboratory. This was mandatory for each participating surgeon. Fourteen qualified surgeons participated in this study, and 8 of them enrolled patients. Surgical procedure details were described in part 1 of this study.8 Briefly, full open surgical repair was performed, which represented the SOC. The tear size was measured after exposing the footprint and any necessary debridement (Table 1). Based on the investigators’ preference, 7 repairs were performed in a double-row suture-bridge fashion (type 1 repair) using 2 medial-row anchors and 2 transosseous-equivalent anchors laterally, 7 repairs were performed in a conventional double-row fashion with a medial and a lateral row (type 2 repair), and 6 repairs were performed using bone tunnels (type 3 repair) (see Table 1). If suture anchors were used, they were metal or PEEK anchors.
TABLE 1.
Tear Size, Repair Type, and MRI Results (Sugaya Classification) According to Treatment Groupa
| Tear Size, cm | Repair Typeb | Postoperative Sugaya Classificationc | ||
|---|---|---|---|---|
| Treatment Group | 12 wk | 52 wk | ||
| rhBMP-12/ACS | 2.7 | 1 | III | I |
| rhBMP-12/ACS | 2.5 | 1 | III | I |
| rhBMP-12/ACS | 2.7 | 1 | III | I |
| rhBMP-12/ACS | 2.7 | 1 | I | I |
| rhBMP-12/ACS | 2.5 | 2 | III | II |
| rhBMP-12/ACS | 3.0 | 2 | II | I |
| rhBMP-12/ACS | 4.0 | 2 | II | I |
| rhBMP-12/ACS | 3.0 | 2 | II | I |
| rhBMP-12/ACS | 2.5 | 2 | II | I |
| rhBMP-12/ACS | 2.0 | 3 | III | II |
| rhBMP-12/ACS | 2.0 | 3 | III | III |
| rhBMP-12/ACS | 2.0 | 3 | III | III |
| rhBMP-12/ACS | 2.0 | 3 | III | III |
| rhBMP-12/ACS | 3.0 | 3 | MA | NP |
| rhBMP-12/ACS | 2.5 | 1 | II | II |
| rhBMP-12/ACS | 3.0 | 1 | MA | MA |
| Standard of care | 4.0 | 1 | V | NP |
| Standard of care | 3.0 | 3 | NP | NP |
| Standard of care | 3.0 | 2 | I | I |
| Standard of care | 2.5 | 2 | III | II |
aACS, absorbable collagen sponge; MA, metal artifacts; MRI, magnetic resonance imaging; NP, not performed; rhBMP-12, recombinant human bone morphogenetic protein–12.
bRepair types: 1, double-row suture-bridge repair using 2 medial-row anchors and 2 transosseous-equivalent anchors laterally; 2, conventional double-row repair with 2 medial-row and 2 lateral-row anchors; 3, bone tunnel repair.
cSugaya classification types: I, sufficient thickness with homogenously low intensity; II, sufficient thickness with partial high intensity; III, insufficient thickness without discontinuity; IV, presence of a minor discontinuity; V, presence of a major discontinuity.
Test Article Preparation and Administration
Lyophilized rhBMP-12 and buffer were reconstituted with sterile water for injections and prepared to provide a 0.015-mg/mL concentration based on the sponsor’s instructions before surgery in patients with SOC and 0.015 mg/mL rhBMP-12/ACS (rhBMP-12/ACS group). The dry ACS was cut under sterile conditions to a 2.5 × 2.5–cm sponge. rhBMP-12/ACS was applied to the prepared footprint, with the aforementioned cuts that allowed ACS placement around the medial-row anchors or the medial entrance of the transosseous tunnels. The rotator cuff tear was repaired over the footprint and the ACS, and knots of medial- and lateral-row anchors or transosseous sutures were tied.
Postoperative Rehabilitation
The arm was immobilized with a shoulder brace or sling after surgical repair, allowing only passive mobilization. Active ROM and strengthening exercises were not allowed before postoperative weeks 6 and 12, respectively.
Functional Assessment
Postoperative follow-up assessments were conducted with a VAS for pain and ROM recorded at 12, 16, 26, 39, and 52 weeks after surgery; isometric strength at 16, 26, 39, and 52 weeks; and MRI at 12 and 52 weeks.
VAS for Pain
Pain of the affected shoulder was evaluated using a 100-mm VAS, with “0” and “100” representing “no pain” and the “worst pain possible,” respectively. The VAS was administered independent of the investigators’ assessment to maintain an unbiased assessment.
ROM Measurements
The standardized procedure for performing ROM testing was provided in the study’s reference manual. Active and passive ROM of the affected shoulder were assessed by the investigators using a standard 12-inch round goniometer in the following planes: forward flexion, elevation in the scapular plane, abduction in the supine position, and external rotation in the sitting position.
Isometric Strength Measurements
Isometric strength in scapular abduction and external rotation in the sitting position was measured by using a manual muscle testing system (Lafayette Instrument Co). Each strength measurement was obtained 3 times, with the maximal strength recorded.
MRI Assessment
The structural integrity of the repaired rotator cuff was evaluated from closed MRI scans. A full-thickness tear or retear was identified by the appearance of a fluid-equivalent signal or when the tendon could not be visualized in at least 1 section of a fluid-sensitive sequence. The classification system of Sugaya et al20 was used to quantify rotator cuff integrity postoperatively. The MRI findings were independently evaluated by 2 blinded orthopaedic shoulder surgeons (J.I., S.G.). The interobserver reliability in diagnostic agreement was significant (kappa statistic = 0.62-0.78). In addition, the MRI scans were reviewed twice by an orthopaedic shoulder surgeon (J.I.) at different time points with excellent intraobserver reliability, demonstrated by high intraclass correlation (0.95-0.96).
Results
All VAS, ROM, and strength data are presented in the Appendix.
VAS Scores
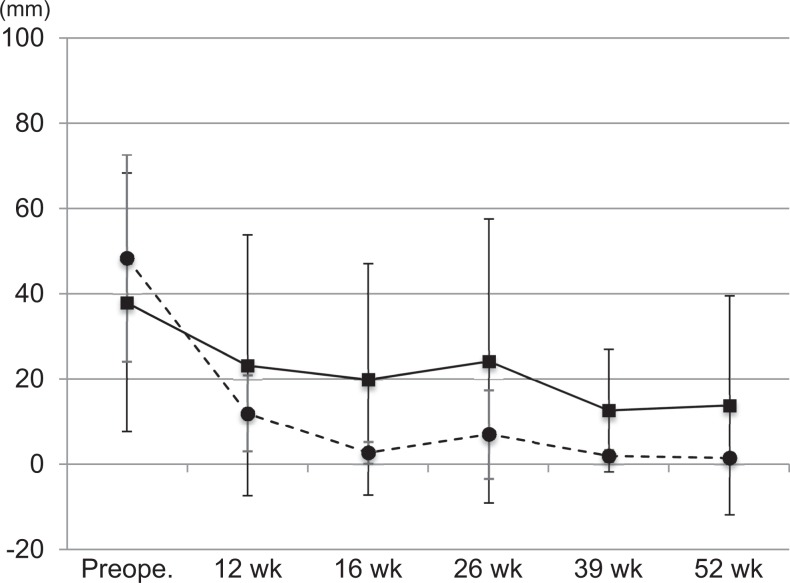
The VAS scores for pain are shown in Figure 2. The mean VAS score in the rhBMP-12/ACS group decreased from 37.9 ± 30.3 mm preoperatively to 13.8 ± 25.6 mm at week 52. The mean VAS score in the SOC control group decreased from 48.3 ± 24.2 mm preoperatively to 1.5 ± 0.7 mm at week 52.
Figure 2.
Serial visual analog scale scores for pain in the rhBMP-12/ACS (solid line) and SOC control (broken line) groups. Values are presented as mean ± SD. ACS, absorbable collagen sponge; rhBMP-12, recombinant human bone morphogenetic protein–12; SOC, standard of care.
ROM Measurements
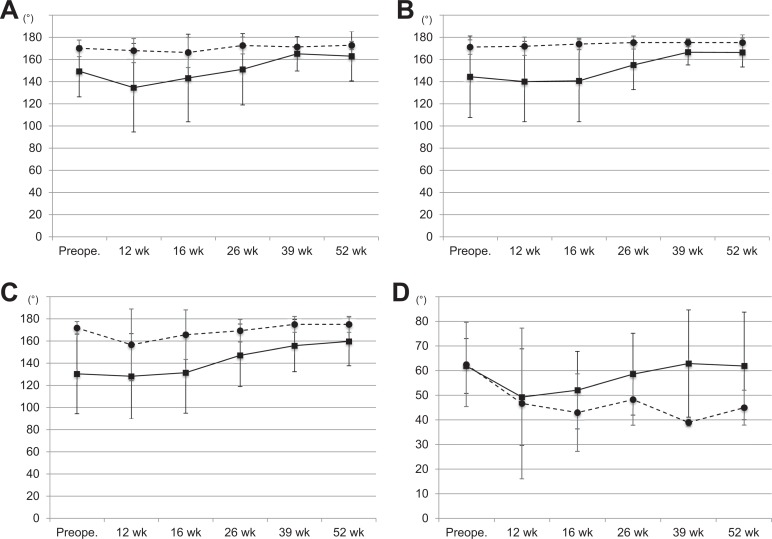
The values for active forward flexion are shown in Figure 3A. The mean forward flexion in the rhBMP-12/ACS group increased from 149.2° ± 23.1° preoperatively to 162.7° ± 22.2° at week 52. The mean forward flexion in the SOC control group remained essentially unchanged, from 169.8° ± 7.3° preoperatively to 172.5° ± 3.5° at week 52.
Figure 3.
Serial active range of motion (ROM) measurements: (A) forward flexion, (B) elevation in the scapular plane, (C) abduction, and (D) external rotation. Solid line, rhBMP-12/ACS group; broken line, SOC control group. Values are presented as mean ± SD. ACS, absorbable collagen sponge; rhBMP-12, recombinant human bone morphogenetic protein–12; SOC, standard of care.
The values of active elevation in the scapular plane are shown in Figure 3B. The mean elevation in the rhBMP-12/ACS group increased from 144.3° ± 36.6° preoperatively to 166.1° ± 13.0° at week 52. The mean elevation in the SOC control group remained unchanged, from 171.0° ± 7.0° preoperatively to 175.0° ± 7.1° at week 52.
The values of active abduction are shown in Figure 3C. The mean abduction in the rhBMP-12/ACS group increased from 130.6° ± 35.9° preoperatively to 159.7° ± 22.0° at week 52. The mean abduction in the SOC control group remained unchanged, from 171.8° ± 5.7° preoperatively to 175.0° ± 7.1° at week 52.
The values of active external rotation are shown in Figure 3D. The mean external rotation in the rhBMP-12/ACS group remained unchanged, from 61.9° ± 11.1° preoperatively to 61.9° ± 21.8° at week 52. The mean external rotation in the SOC control group increased from 62.5° ± 17.1° preoperatively to 45.0° ± 7.1° at week 52.
Isometric Strength Measurements
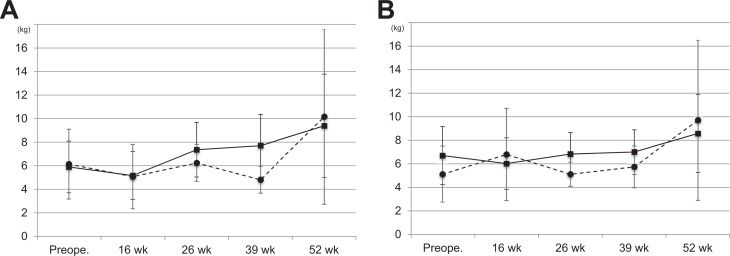
The values of isometric scapular abduction strength are shown in Figure 4A. The mean isometric abduction strength in the rhBMP-12/ACS group increased from 5.9 ± 2.2 kg preoperatively to 9.4 ± 4.4 kg at week 52. The mean isometric abduction strength in the SOC control group increased from 6.1 ± 3.0 kg to 10.2 ± 7.4 kg at week 52.
Figure 4.
Serial isometric strength measurements: (A) scapular abduction and (B) external rotation. Solid line, rhBMP-12/ACS group; broken line, SOC control group. Values are presented as mean ± SD. ACS, absorbable collagen sponge; rhBMP-12, recombinant human bone morphogenetic protein–12; SOC, standard of care.
The values of isometric external rotation strength are shown in Figure 4B. The mean isometric external rotation strength in the rhBMP-12/ACS group increased from 6.7 ± 2.5 kg preoperatively to 8.6 ± 3.3 kg at week 52. The mean isometric external rotation strength in the SOC control group increased from 5.1 ± 2.4 kg to 9.7 ± 6.8 kg at week 52.
MRI Findings
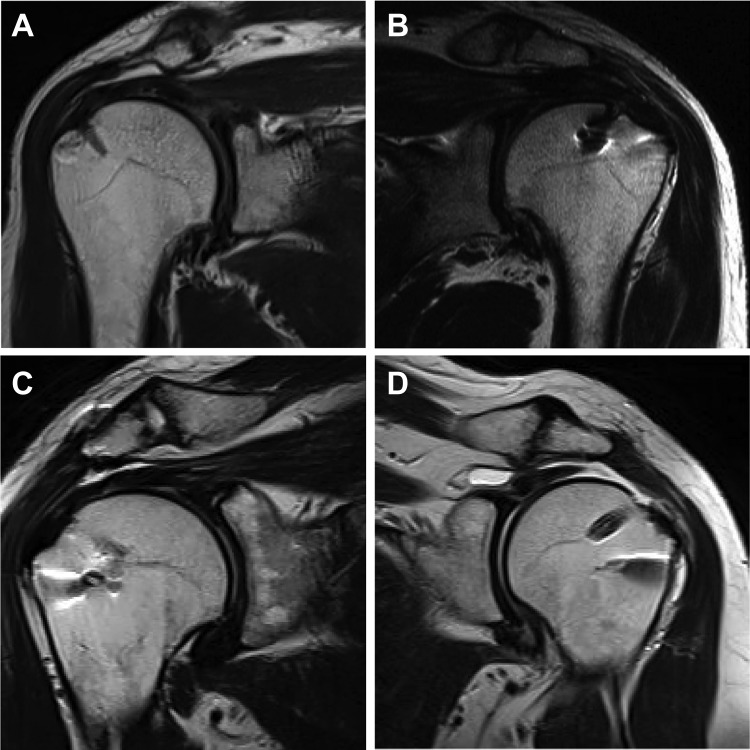
The estimated size of the rotator cuff defect, measured on closed MRI, ranged between 2 and 4 cm (mean, 2.5 cm). Using the Goutallier classification,4,7 fatty infiltration across the 20 patients was rated as “0,” “1,” and “2” in 7, 8, and 5 patients, respectively. Rotator cuff integrity was quantified based on the Sugaya classification20 and is reported in Table 1. At 12 weeks after surgery, MRIs in 14 of the 16 patients in the rhBMP-12/ACS group showed an intact repair (Sugaya type I, II, or III) (Figure 5A-C); the imaging results for the other 2 patients in this group could not be analyzed because of artifacts. Of the 4 patients in the SOC control group, 2 and 1 showed an intact repair and a failed repair (Sugaya type V), respectively (Figure 5D); the remaining patient did not undergo MRI at this time point. At 52 weeks after surgery, 15 of the 16 patients in the rhBMP-12/ACS group underwent MRI, with 14 showing an intact repair 52 weeks after surgery; the MRI scan for the remaining patient could not be analyzed because of artifacts. Of the 4 patients in the SOC control group, 2 showed an intact repair, with the other 2 patients in this group not undergoing an MRI assessment at 52 weeks after surgery.
Figure 5.
Postoperative magnetic resonance imaging (MRI) of (A) Sugaya type I: sufficient thickness with homogenously low intensity, (B) Sugaya type II: sufficient thickness with partial high intensity, (C) Sugaya type III: insufficient thickness without discontinuity, and (D) Sugaya type V: presence of major discontinuity.
Repair Failure
Retearing of the repaired supraspinatus tendon was identified on MRI 12 weeks after surgery in a 67-year-old female patient (height, 167 cm; weight, 84 kg; nonsmoker) in the SOC control group (see Figure 5D). She was assessed with a 3.0-cm tear and stage 1 fatty infiltration before surgery. The tear size was measured intraoperatively as 4.0 cm with a U shape. A suture bridge with a transosseous-equivalent technique, combined with acromioplasty, was used as the primary repair. The patient had moderate recovery at postoperative week 12. No further treatment was administered after the adverse event of repair failure at week 12, and the patient withdrew her participation in the study at postoperative week 38.
Discussion
To the best of our knowledge, this study is the first to evaluate functional recovery and structural outcomes on the rotator cuff tendon after implanting rhBMP-12/ACS as adjuvant treatment during open rotator cuff repair in humans. Functional recovery in the VAS score for pain, ROM, and isometric strength was similar in both groups. Postoperative structural integrity of the rotator cuff tendon was intact in 14 of 16 patients in the rhBMP-12/ACS group. An intact repair and repair failure were identified in 2 patients and 1 patient, respectively, of the 4 patients in the SOC control group.
To date, functional recovery after rotator cuff repair has been quantified using pain scales and shoulder motion in daily activities, with an absence of recovery data for quantitative muscle strength. Charousset et al3 reported that functional recovery was achieved as early as 3 months after surgery in their case series of 114 rotator cuff repairs, with the muscle strength component of the Constant score showing improvement from 6 to 12 months after surgery. Failure of the repair in their study was negatively associated with functional outcomes. In their series of 201 patients undergoing rotator cuff repair, Manaka et al16 reported that 63 patients (31%) achieved functional recovery (scores >80% on each component of the shoulder assessment) within 3 months, 81 patients (40%) within 3 to 6 months, and 57 patients (28%) after longer than 6 months. They indicated that the functional recovery period was shorter in younger patients without shoulder stiffness and with smaller rotator cuff tears. Functional recovery in pain scores, ROM, and strength in our study was achieved within 6 to 12 months after surgery in both groups, excluding 1 patient with a retear. Patients with shoulder stiffness and a small-sized tear were excluded from our study.
Our study was based on strong evidence of the effectiveness of rhBMP-12, applied on an ACS, in augmenting rotator cuff repair in animal studies and in determining possible beneficial concentrations.17,19 Greiner et al8 reported that the local application of 0.015 mg/mL rhBMP-12/ACS in open rotator cuff repair was feasible and likely to be safe for humans. Our study contributes important information to this body of knowledge, with possible evidence of implanting rhBMP-12/ACS as adjuvant therapy to obtain good functional recovery and tendon-to-bone healing after open rotator cuff repair.
The limitations of our study need to be acknowledged. Owing to the small sample size, there was no statistical analysis of the results, and the follow-up period of 12 months was relatively short. Although we used the VAS for pain, ROM, and isometric strength to inform our assessment of functional outcomes, future studies should include standardized shoulder-specific outcome measures. The different operative methods may have influenced clinical and structural outcomes. A positive feature of our study was the use of serial postoperative MRI to confirm rotator cuff integrity after repair. Furthermore, our study was a prospective multicenter study, and the evaluators were not the primary surgeons.
In conclusion, functional recovery and structural outcomes in patients in whom rhBMP-12/ACS was used as adjuvant therapy in rotator cuff repair justify conducting future, larger, prospective, multicenter studies.
Acknowledgment
The principal investigators for this study were as follows:
Europe: Stefan Greiner (Klinik für Orthopaedie, Charité–Universitaetsmedizin Berlin, Berlin, Germany), Rainer Boeger (Universitaetsklinikum Hamburg-Eppendorf and Clinical Trial Center North, MediGate GmbH, Hamburg, Germany), Markus Schofer (Klinik fuer Orthopaedie und Rheumatologie, Universitätsklinikum Giessen und Marburg, Marburg, Germany), and Arthur van Noort (Spaarne Hospital, Hoofddorp/Heemstede, the Netherlands).
Japan: Junji Ide (Kumamoto University Hospital, Kumamoto), Yu Mochizuki (Hiroshima Prefectural Hospital, Hiroshima), Kenji Kashiwagi (Chugoku Rosai Hospital, Kure), Katsuya Iwamoto (Kumamoto Saishunso National Hospital, Koshi), Yukihiko Hata (Shinshu University Hospital, Matsumoto), Satoshi Maeda (National Hospital Organization Kumamoto Medical Center, Kumamoto), Takayuki Kamiishi (Yokohama City University Hospital, Yokohama), Kazutaka Izawa (National Hospital Organization Toneyama National Hospital, Toyonaka), and Yozo Shibata (Fukuoka University Chikushi Hospital, Chikushino).
Appendix
TABLE A1.
Visual Analog Scale Scores for Pain (in mm)
| rhBMP-12/ACS | Standard of Care | |
|---|---|---|
| Before surgery | ||
| No. of patients | 16 | 4 |
| Mean | 37.9 | 48.3 |
| Median | 22.5 | 50.0 |
| SD | 30.3 | 24.2 |
| Range | 7-97 | 17-76 |
| 12 wk after surgery | ||
| No. of patients | 16 | 3 |
| Mean | 23.2 | 12.0 |
| Median | 10.5 | 12.0 |
| SD | 30.5 | 9.0 |
| Range | 0-100 | 3-21 |
| 16 wk after surgery | ||
| No. of patients | 16 | 3 |
| Mean | 19.9 | 2.7 |
| Median | 7.5 | 3.0 |
| SD | 27.1 | 2.5 |
| Range | 0-85 | 0-5 |
| 26 wk after surgery | ||
| No. of patients | 16 | 3 |
| Mean | 24.2 | 7.0 |
| Median | 8.5 | 2.0 |
| SD | 33.2 | 10.4 |
| Range | 0-93 | 0-19 |
| 39 wk after surgery | ||
| No. of patients | 15 | 2 |
| Mean | 12.6 | 2.0 |
| Median | 10.0 | 2.0 |
| SD | 14.3 | 1.4 |
| Range | 0-50 | 1-3 |
| 52 wk after surgery | ||
| No. of patients | 15 | 2 |
| Mean | 13.8 | 1.5 |
| Median | 1.0 | 1.5 |
| SD | 25.6 | 0.7 |
| Range | 0-80 | 1-2 |
TABLE A2.
Forward Flexion (in Degrees)
| rhBMP-12/ACS | Standard of Care | |||
|---|---|---|---|---|
| Active | Passive | Active | Passive | |
| Before surgery | ||||
| No. of patients | 16 | 16 | 4 | 4 |
| Mean | 149.2 | 163.3 | 169.8 | 171.0 |
| Median | 155.0 | 167.5 | 167.5 | 169.0 |
| SD | 23.1 | 10.4 | 7.3 | 6.2 |
| Range | 95-177 | 150-180 | 164-180 | 166-180 |
| 12 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 134.4 | 142.7 | 167.7 | 170.0 |
| Median | 140.0 | 142.5 | 163.0 | 165.0 |
| SD | 39.8 | 32.1 | 10.8 | 8.7 |
| Range | 35-180 | 76-180 | 160-180 | 165-180 |
| 16 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 143.1 | 152.3 | 166.0 | 168.7 |
| Median | 150.0 | 154.5 | 165.0 | 170.0 |
| SD | 39.3 | 25.4 | 13.5 | 12.1 |
| Range | 30-180 | 95-180 | 153-180 | 156-180 |
| 26 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 150.9 | 159.6 | 172.3 | 174.0 |
| Median | 160.0 | 170.0 | 172.0 | 172.0 |
| SD | 32.0 | 22.0 | 7.5 | 5.3 |
| Range | 65-180 | 110-180 | 165-180 | 170-180 |
| 39 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 164.8 | 169.1 | 171.0 | 175.5 |
| Median | 166.0 | 170.0 | 171.0 | 175.5 |
| SD | 15.5 | 12.2 | 1.4 | 2.1 |
| Range | 120-180 | 135-180 | 170-172 | 170-175 |
| 52 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 162.7 | 168.1 | 172.5 | 172.5 |
| Median | 168.0 | 170.0 | 172.5 | 172.5 |
| SD | 22.2 | 13.0 | 3.5 | 3.5 |
| Range | 90-180 | 130-180 | 170-175 | 170-175 |
TABLE A3.
Elevation in Scapular Plane (in Degrees)
| rhBMP-12/ACS | Standard of Care | |||
|---|---|---|---|---|
| Active | Passive | Active | Passive | |
| Before surgery | ||||
| No. of patients | 16 | 16 | 4 | 4 |
| Mean | 144.3 | 165.1 | 171.0 | 173.3 |
| Median | 157.5 | 170.0 | 170.5 | 173.5 |
| SD | 36.6 | 11.9 | 7.0 | 6.4 |
| Range | 70-180 | 150-180 | 163-180 | 166-180 |
| 12 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 140.0 | 147.9 | 171.7 | 174.3 |
| Median | 145.0 | 147.5 | 175.0 | 178.0 |
| SD | 36.1 | 24.5 | 10.4 | 8.1 |
| Range | 40-180 | 105-180 | 160-180 | 165-180 |
| 16 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 140.7 | 151.1 | 173.7 | 175.3 |
| Median | 147.5 | 155.0 | 176.0 | 176.0 |
| SD | 36.8 | 21.8 | 7.8 | 5.0 |
| Range | 30-180 | 110-180 | 165-180 | 170-180 |
| 26 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 154.9 | 160.2 | 175.0 | 176.7 |
| Median | 162.5 | 167.5 | 180.0 | 180.0 |
| SD | 22.1 | 21.5 | 8.7 | 5.8 |
| Range | 110-180 | 110-180 | 165-180 | 170-180 |
| 39 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 166.3 | 170.1 | 175.0 | 177.5 |
| Median | 167.0 | 170.0 | 175.0 | 177.5 |
| SD | 11.4 | 9.9 | 7.1 | 3.5 |
| Range | 140-180 | 145-180 | 170-180 | 175-180 |
| 52 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 166.1 | 170.9 | 175.0 | 175.0 |
| Median | 170.0 | 171.0 | 175.0 | 175.0 |
| SD | 13.0 | 10.8 | 7.1 | 7.1 |
| Range | 130-180 | 140-180 | 170-180 | 170-180 |
TABLE A4.
Abduction (in Degrees)
| rhBMP-12/ACS | Standard of Care | |||
|---|---|---|---|---|
| Active | Passive | Active | Passive | |
| Before surgery | ||||
| No. of patients | 16 | 16 | 4 | 4 |
| Mean | 130.6 | 160.8 | 171.8 | 173.3 |
| Median | 135.0 | 160.0 | 170.0 | 171.5 |
| SD | 35.9 | 10.1 | 5.7 | 4.7 |
| Range | 60-171 | 150-180 | 167-180 | 170-180 |
| 12 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 128.4 | 137.0 | 156.7 | 159.0 |
| Median | 130.0 | 135.0 | 170.0 | 172.0 |
| SD | 38.3 | 36.1 | 32.2 | 29.7 |
| Range | 40-180 | 50-180 | 120-180 | 125-180 |
| 16 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 131.6 | 140.6 | 165.7 | 167.7 |
| Median | 143.5 | 143.5 | 177.0 | 178.0 |
| SD | 36.4 | 29.6 | 22.3 | 19.7 |
| Range | 40-180 | 90-180 | 140-180 | 145-180 |
| 26 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 147.2 | 155.8 | 169.3 | 173.3 |
| Median | 152.5 | 162.5 | 168.0 | 175.0 |
| SD | 28.1 | 24.9 | 10.1 | 7.6 |
| Range | 80-180 | 100-180 | 160-180 | 165-180 |
| 39 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 155.9 | 163.1 | 175.0 | 177.5 |
| Median | 163.0 | 167.0 | 175.0 | 177.5 |
| SD | 23.4 | 16.6 | 7.1 | 3.5 |
| Range | 110-180 | 125-180 | 170-180 | 175-180 |
| 52 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 159.7 | 165.0 | 175.0 | 175.0 |
| Median | 165.0 | 166.0 | 175.0 | 175.0 |
| SD | 22.0 | 13.2 | 7.1 | 7.1 |
| Range | 90-180 | 130-180 | 170-180 | 170-180 |
TABLE A5.
External Rotation (in Degrees)
| rhBMP-12/ACS | Standard of Care | |||
|---|---|---|---|---|
| Active | Passive | Active | Passive | |
| Before surgery | ||||
| No. of patients | 16 | 16 | 4 | 4 |
| Mean | 61.9 | 70.8 | 62.5 | 72.0 |
| Median | 62.5 | 70.0 | 65.0 | 77.5 |
| SD | 11.1 | 10.5 | 17.1 | 22.6 |
| Range | 30-75 | 55-90 | 40-80 | 43-90 |
| 12 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 49.3 | 55.6 | 46.7 | 55.7 |
| Median | 50.0 | 62.5 | 40.0 | 45.0 |
| SD | 19.5 | 20.5 | 30.6 | 30.4 |
| Range | 10-75 | 15-85 | 20-80 | 32-90 |
| 16 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 52.1 | 59.7 | 43.0 | 53.0 |
| Median | 56.0 | 62.5 | 40.0 | 45.0 |
| SD | 15.7 | 17.5 | 15.7 | 14.7 |
| Range | 30-76 | 30-82 | 29-60 | 44-70 |
| 26 wk after surgery | ||||
| No. of patients | 16 | 16 | 3 | 3 |
| Mean | 58.6 | 65.6 | 48.3 | 55.0 |
| Median | 64.0 | 65.0 | 45.0 | 50.0 |
| SD | 16.6 | 16.2 | 10.4 | 13.2 |
| Range | 30-85 | 30-90 | 40-60 | 45-70 |
| 39 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 62.9 | 66.7 | 39.0 | 45.5 |
| Median | 66.0 | 72.0 | 39.0 | 45.5 |
| SD | 21.7 | 21.4 | 1.4 | 0.7 |
| Range | 20-90 | 30-90 | 38-40 | 45-46 |
| 52 wk after surgery | ||||
| No. of patients | 15 | 15 | 2 | 2 |
| Mean | 61.9 | 67.3 | 45.0 | 50.0 |
| Median | 70.0 | 72.0 | 45.0 | 50.0 |
| SD | 21.8 | 20.2 | 7.1 | 0.0 |
| Range | 10-90 | 20-90 | 40-50 | 50-50 |
TABLE A6.
Isometric Strength in Abduction (in kg)
| rhBMP-12/ACS | Standard of Care | |
|---|---|---|
| Before surgery | ||
| No. of patients | 16 | 4 |
| Mean | 5.9 | 6.1 |
| Median | 6.0 | 6.2 |
| SD | 2.2 | 3.0 |
| Range | 1.7-9.6 | 2.6-9.5 |
| 16 wk after surgery | ||
| No. of patients | 15 | 3 |
| Mean | 5.2 | 5.1 |
| Median | 4.8 | 3.7 |
| SD | 2.0 | 2.7 |
| Range | 2.5-10.3 | 3.3-8.2 |
| 52 wk after surgery | ||
| No. of patients | 15 | 2 |
| Mean | 9.4 | 10.2 |
| Median | 8.6 | 10.2 |
| SD | 4.4 | 7.4 |
| Range | 4.3-22.9 | 4.9-15.4 |
TABLE A7.
Isometric Strength in External Rotation (in kg)
| rhBMP-12/ACS | Standard of Care | |
|---|---|---|
| Before surgery | ||
| No. of patients | 16 | 4 |
| Mean | 6.7 | 5.1 |
| Median | 6.5 | 5.0 |
| SD | 2.5 | 2.4 |
| Range | 3.1-12.3 | 2.6-8.0 |
| 16 wk after surgery | ||
| No. of patients | 15 | 3 |
| Mean | 6.0 | 6.8 |
| Median | 5.8 | 4.8 |
| SD | 2.2 | 3.9 |
| Range | 2.5-9.8 | 4.3-11.3 |
| 52 wk after surgery | ||
| No. of patients | 15 | 2 |
| Mean | 8.6 | 9.7 |
| Median | 9.4 | 9.7 |
| SD | 3.3 | 6.8 |
| Range | 3.2-16.4 | 4.9-14.5 |
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by Pfizer Inc. H.O. and S.S. are employed by Pfizer.
Ethical approval for this study was obtained from the State of Berlin (protocol No. 3202V1-1001-WW) and the institutional review boards of the Japanese sites (protocol No. 3202V1-1003-JA).
References
- 1. Angeline ME, Rodeo SA. Biologics in the management of rotator cuff surgery. Clin Sports Med. 2012;31:645–663. [DOI] [PubMed] [Google Scholar]
- 2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. [DOI] [PubMed] [Google Scholar]
- 3. Charousset C, Grimberg J, Duranthon LD, Bellaïche L, Petrover D, Kalra K. The time for functional recovery after arthroscopic rotator cuff repair: correlation with tendon healing controlled by computed tomography arthrography. Arthroscopy. 2008;24:25–33. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. [DOI] [PubMed] [Google Scholar]
- 5. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. [DOI] [PubMed] [Google Scholar]
- 6. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. [DOI] [PubMed] [Google Scholar]
- 7. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 8. Greiner S, Ide J, Van Noort A, et al. Local rhBMP-12 on an absorbable collagen sponge as an adjuvant therapy for rotator cuff repair: a phase 1, randomized, standard of care control, multicenter study. Part 1: safety and feasibility. Am J Sports Med. 2015;43:1994–2004. [DOI] [PubMed] [Google Scholar]
- 9. Harryman DT, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA. Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed] [Google Scholar]
- 10. Huijsmans PE, Pritchard MP, Berghs BM, Van Rooyen KS, Wallace AL, De Beer JF. Arthroscopic rotator cuff repair with double row fixation. J Bone Joint Surg Am. 2007;89:1248–1257. [DOI] [PubMed] [Google Scholar]
- 11. Ide J, Tokiyoshi A, Hirose J, Mizuta H. Arthroscopic repair of traumatic combined rotator cuff tears involving the subscapularis tendon. J Bone Joint Surg Am. 2007;89:2378–2388. [DOI] [PubMed] [Google Scholar]
- 12. Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21:181–190. [DOI] [PubMed] [Google Scholar]
- 13. Jelinsky SA, Li L, Ellis D, et al. Treatment with rhBMP12 or rhBMP13 increase the rate and the quality of rat Achilles tendon repair. J Orthop Res. 2011;29:1604–1612. [DOI] [PubMed] [Google Scholar]
- 14. Koike Y, Trudel G, Uhthoff HK. Formation of a new enthesis after attachment of the supraspinatus tendon: a quantitative histologic study in rabbits. J Orthop Res. 2005;23:1433–1440. [DOI] [PubMed] [Google Scholar]
- 15. Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. [DOI] [PubMed] [Google Scholar]
- 16. Manaka T, Ito Y, Matsumoto I, Takaoka K, Nakamura H. Functional recovery period after arthroscopic rotator cuff repair: is it predictable before surgery? Clin Orthop Relat Res. 2011;469:1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16:S191–S197. [DOI] [PubMed] [Google Scholar]
- 18. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65:456–460. [PubMed] [Google Scholar]
- 19. Seeherman HJ, Archambault JM, Rodeo SA, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206–2219. [DOI] [PubMed] [Google Scholar]
- 20. Sugaya H, Maeda K, Matsuki K, Moriishi J. Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: single-row versus dual-row fixation. Arthroscopy. 2005;21:1307–1316. [DOI] [PubMed] [Google Scholar]
- 21. Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418–422. [DOI] [PubMed] [Google Scholar]
- 23. Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]