Abstract
Crohn’s disease (CD) is a chronic inflammatory disease, in most patients involving the small and large bowel. In recent years, with the use of small bowel video capsule endoscopy (SBCE), it has become clear that in 50% or more of adults with established CD there is not only distal but also proximal small bowel involvement that suggests poor prognosis.
A great deal of effort has been put into early diagnosis and stratification of patients into low versus high risk, thus directing treatment from step-up, or accelerated step-up, to top-down therapies.
SBCE has been used for assessment of small bowel pathologies for over 15 years, mainly for occult gastrointestinal bleeding and suspected CD. In recent years, a colonic capsule, with cameras on both sides and a wider angle of view, has been developed and is used by some to survey both small and large bowel. Recently the same capsule, with adjustments, has been released in Europe, concentrating (with specialized software) on inflammatory bowel disease.
In this review I summarize the new data regarding the use of SBCE as well as the small bowel colon (SBC) versions of capsule endoscopy in established CD and the ways these could alter the management of such patients.
Keywords: colitis, Crohn’s disease, PillCam Colon2, PillCam Crohn’s, video capsule endoscopy
Introduction
Small bowel video capsule endoscopy (SBCE) has been on the market for more than 15 years and is widely used for two main indications: obscure gastrointestinal bleeding and suspected Crohn’s disease (CD).1
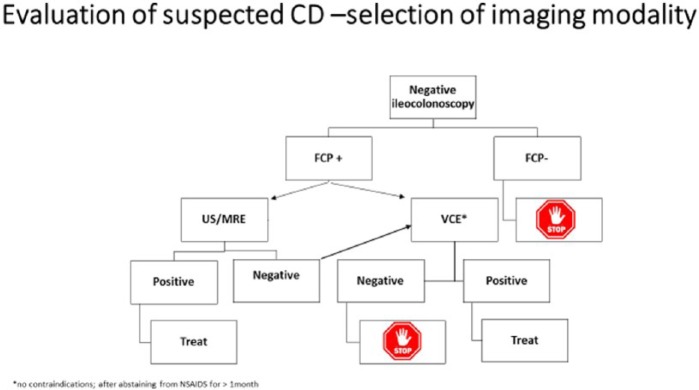
In the setting of suspected CD, it is widely accepted and used in many instances as the primary modality after a negative ileocolonoscopy when there are no obstructive symptoms.2,3 It may even be equivalent to ileocolonoscopy in the setting of suspected CD, though without the ability to take biopsies.4 As the findings captured on SBCE examination are nonspecific, there have been attempts to increase the pretest probability of the subjects that will swallow the capsule. These include withdrawal of nonsteroidal anti-inflammatory drugs (NSAIDs) at least 1 month prior to performing the test, including patients with extra-intestinal manifestations or abnormal laboratory tests (anemia, thrombocytosis, elevated CRP, etc.), as well as the use of fecal calprotectin as a selection tool.3,5–7 A fecal calprotectin level <50 ug/g dramatically reduces the chance of that person having CD; this chance is 1% if the level is 40 ug/g.6,7
We suggest the algorithm shown in Figure 1 for the evaluation of patients with suspected CD.
Figure 1.
Evaluation of suspected Crohn’s disease.
If the negative predictive value of the panenteric capsule in the small bowel and colon is as good as that of the SBCE (i.e. >95%), one might use it as a screening tool even before performing ileocolonoscopy.
Contrary to its pivotal role in suspected CD, the role of capsule endoscopy in established CD has been questioned, with existing guidelines being non-supportive of its use.2,3 The guidelines confine its use only to patients with unexplained bleeding/anemia, or discrepancies between symptoms and laboratory tests.2,3 Even then, they advise using either cross-sectional imaging or a patency capsule (PC) – with no indication that one is preferred – prior to the use of the regular capsule to assure patency and avoid capsule retention.2,3
The aim of this article is to review new data that have accumulated since these guidelines were written on SBCE in patients with established CD, as well as on the use of the PillCam Colon 2 capsule as a panenteric capsule and its use in CD patients, and on the new PillCam Crohn’s capsule with its recently released inflammatory bowel disease (IBD). software. This knowledge will allow us to assess the possible impact of a panenteric capsule endoscopy (CE) on the management of CD.
Established CD: potential roles of capsule endoscopy and safety issues
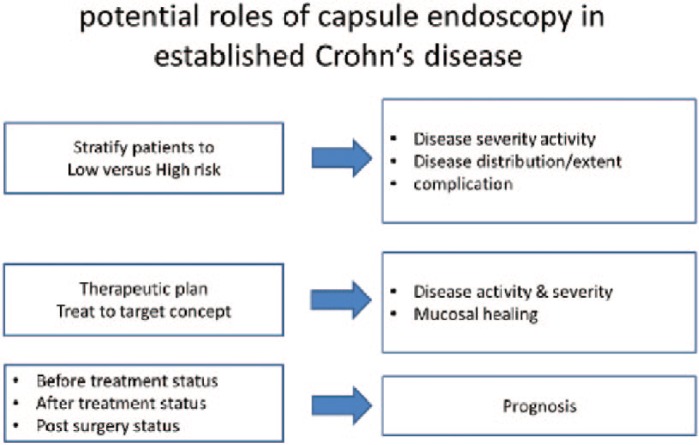
Potential roles of CE in established CD (Figure 2):
Figure 2.
Reasons to use capsule endoscopy in known Crohn’s disease.
knowledge of the extent of the disease both in small and large bowel (i.e. phenotype classification);
knowledge of severity of the disease (i.e. phenotype classification);
monitoring drug effects [mucosal healing (MH)];
treatment modifications according to new CE data;
prognostic tool before/after treatment;
prognostic tool post-surgery.
Safety issues regarding the use of CE:
Real-life retention rate;
the best mode to avoid retention.
Classification of established CD: new knowledge on the extent of disease
In recent years, a lot has been discussed on the mode and timing of treatments in established CD patients. The mode of treatment – step-up versus top-down – the timing of starting biologic therapy and the time to de-escalate treatment have all been extensively discussed. Many variables have been suggested over the years as being associated with aggressive disease. Among these are age <40 years, smoking, perianal disease, stenotic disease, upper gastrointestinal involvement, extensive disease and lack of MH after induction of clinical remission.8
To intelligently tackle these questions, one needs to objectively know the disease phenotype (extent and severity), preferably at diagnosis. While this is easily done in the colon, using colonoscopy, there is no simple, friendly endoscopic mode to evaluate the small bowel apart from SBCE. Greener and colleagues have shown that when one applies SBCE in conjunction with magnetic resonance enterography (MRE) to patients with established CD, the Montreal classification (location and behavior) changes in about two-thirds of patients.9 The number of patients having proximal small bowel disease (L4) increased from 14% prior to the SBCE study to 51% after its use.9 The number of patients with stricturing phenotype (B2) increased from 31% at entrance to 59% after they were subjected to both SBCE and MRE.9 SBCE was significantly better than MRE in identifying proximal L4 disease, while MRE was, of course, significantly better in diagnosing B2 disease.9,10 Many other retrospective studies using SBCE in established CD found proximal small bowel involvement in 50–65% of patients.11–13 Two such retrospective studies have shown that those patients with proximal L4b (jejunal disease) lesions had worse prognosis than those without such lesions in terms of shorter durations between exacerbation, more aggressive exacerbations necessitating hospitalization, as well as exacerbations needing surgery, adding another, potentially treatable, poor prognostic factor.14,15 Recently a Korean group published a novel predictive model for the clinical course of CD as part of a longitudinal study (CONNECT), in which proximal (jejunal) disease was one of the four bad predictors.16
Moreover, among our 52 known CD patients that were in clinical remission (CDAI <150) only 15% had endoscopic small bowel MH, the others having either mild (64%) or moderate-to-severe (21%) small bowel inflammation as assessed by the Lewis inflammatory score. In those patients that were in clinical and biomarker remission, 62% had mild endoscopic inflammation and 5% moderate-to-severe endoscopic disease.17 Deep remission (i.e. clinical, biomarker and endoscopic) was achieved in only 13.4% of patients, irrespective of which therapy they received.17 Thus, only when one applies a combined endoscopic modality (small and large bowel) as well as MRE, preferably at diagnosis of the disease, will one know the true phenotype and burden of disease, meaning a proper treatment strategy can be planned. Using a panenteric capsule will allow a friendlier endoscopic visualization of the whole bowel that will make it easier to monitor treatment effects later.
Monitoring drug effects: mucosal healing
Nowadays, the evolving concepts of treatment in established CD are moving toward reaching not only clinical but also biomarker and endoscopic remission (i.e. MH). So far, most of the data on MH come from ileocolonoscopy and were relevant to the colon and in part the terminal ileum. The data regarding small bowel MH were very limited.18
Recently, an Irish group published a prospective study in which patients with established CD underwent SBCE examinations prior to and following biological treatment with Humira. The follow-up SBCE exams were carried out 12 and 52 weeks after treatment was started.19,20 Complete MH was achieved in 50% of treated patients after 52 weeks.19 The time to maximal MH of the small bowel is longer than that to achieve MH in the colon (52 weeks).20
Kopylov and colleagues prospectively followed CD patients in clinical remission. They demonstrated that only 33% of patients that were in both clinical and biomarker remission had MH, and only 13% of the total group were in deep biological remission.17 Because these studies were completed using SBCE, there were no data reported on colonic MH.
A pilot study from Portugal using the PillCam Colon 2 as a panenteric capsule on 12 patients demonstrated that monitoring whole-gut mucosal inflammation is feasible, and was achieved in 3 (25%) of the 12 patients.21 Thus, a panenteric, relatively patient-friendly follow up of CD patients to monitor disease activity and effects of various treatment regimens is feasible using CE.
Treatment modifications
Over the years, a few studies have been published on the effect of CE results on treatment modifications and change in management. In a multicenter retrospective study of 187 patients with established CD, a change in treatment, based on CE results, was suggested for 52% of patients. Here, too, there was proximal small bowel involvement in 50% of patients.22 The modifications included initiation or intensification of anti-inflammatory therapy in most patients (82%), biologic therapy in 30% of patients, immune modulatory therapy in 36% and surgical intervention in 2%.22 In 2% of patients, biologic therapy was stopped. Smaller studies have demonstrated clinical improvement with directed medical therapy based on CE results in 24 out of 30 patients23 and in 10 out 41 in another study.24 Cotter and colleagues followed 50 consecutive patients. The proportion of patients on thiopurines or biologic therapy increased from 4% prior to the capsule study to 30% post-CE.25 Thus, CE findings impact our clinical decisions and upgrade our treatment to either adding immunomodulators or biologic therapy, or downgrade it by stopping biologic therapy or immunomodulators. A word of caution is that all of the big studies were retrospective in nature, and the aim of the prospective one was not a change of treatment.24
Capsule endoscopy as a prognostic tool in established CD and post-surgery
The main data on the impact of CE on prognosis (i.e. hospitalization, severity of relapse and surgery), in patients with established CD comes from two retrospective studies, mentioned earlier, from France and Korea, in both of which proximal disease (i.e. jejunal-L4b disease) was associated with worse outcome – more severe relapses, increased rate of surgery and hospitalizations.14,15 It is quite obvious that if the extent of disease and its severity are a poor prognostic factor, then the use of a panenteric capsule, be it the PillCam C2 or the new PillCam Crohn’s capsule, will give a better answer to both of these questions in the same examination and will allow a better decision.
We have examined whether the use of periodic CE examination of patients with established CD and in clinical remission may predict relapse and complication. In a preliminary report, as the follow up is not yet complete, we found that the initial CE results at inclusion can predict patient relapse. If the Lewis score of any tertile, and particularly the proximal ones, was >790 the odds ratio (OR) to relapse were very high [OR 10.9, 95% confidence interval (CI) 2.5–47.8; p = 0.002], significant proximal small bowel disease (Lewis score >790) (OR 14.3, 95% CI 1.4–154.3; p = 0.03).26 On the other hand, in a recent meta-analysis of studies that evaluated patients with established CD, MH was found to be significantly associated with improved outcome after a follow up of 12 weeks to 24 months (OR 11.06, 95% CI 3.74–32.73; p < 0.001).27
Three studies have been published on postoperative recurrence: two using the SBCE and a recent one using the PillCam C2 as a panenteric capsule.
The two using SBCE were compared to ileocolonoscopy and had conflicting/opposite results. The French study from the GETAID group by Bourreille and colleagues prospectively evaluated 32 patients who underwent both examinations. CE had a relatively low sensitivity of 76% but good specificity of 90%.28 The other, from Spain, included 24 postoperative patients. Recurrence was visualized with colonoscopy in six patients and with CE in five. Ten additional recurrences were visualized only with CE. Proximal involvement was detected in 13 patients. All patients preferred CE to ileocolonoscopy.29
A recent prospective German study used the PillCam C2 as a panenteric capsule for the evaluation of postoperative recurrence. Twenty-two patients were included. The capsule, given 4–8 weeks postoperatively, found relapse (Rutgeerts index ⩾2) in 3 of 16 (19%) of the patients. A second capsule given 4–8 months postoperatively detected active inflammation in 50% of patients, whereas ileocolonoscopy revealed significant inflammation in only 33%. PillCam C2 detected all the flares that were found on ileocolonoscopy.30
Safety issues regarding CE
Real-life retention rates
The greatest fear and main reason to restrict the use of CE in patients with established CD are the high reported rates of retention.31 Capsule retention is defined as a failure to excrete the capsule for 2 weeks or more, requiring either directed medical, endoscopic or surgical intervention.32 This risk of capsule retention was reported to be as high as 13% in older studies;23,33 however, these studies were completed without proper evaluation for patency. Current guidelines require that small bowel patency should be assessed using PC or cross-sectional imaging.2,3 In more recent series that adhered to these recommendations, the risk of retention was much lower (1.5–2.5%).34,35
In a study by Yadav and colleagues it was concluded that the PillCam PC and radiological examinations were equally reliable in their ability to exclude small bowel obstruction or stricture.36 In our prospective study of patients with established CD, patients were subjected to MRE followed by PC, and if patent by either SBCE or PillCam C2 depending on disease location.17 This enabled us to evaluate MRE versus PC in the prediction of patency. We found that if MRE was used, about 50% of patients would be denied the regular capsule as opposed to less than 25% if PC was used.37 The two factors that truly predicted retention using MRE were the stricture’s length (>10 cm) and the presence of two or more prestenotic dilatations.37 Moreover, there were three patients in which the MRE found no patency problem and the PC was retained. Thus, the positive predictive value of MRE for retention was relatively low, but the negative one was high (>92%).37 Many other prospective studies that used PC prior to the use of the regular CE had practically no or very minimal retention rate.19,20,38 Kopylov and colleagues examined the rate of symptomatic retention of the PC and found it to be very low at 22/1615 patients (1.2%).39 Out of the 22 patients, only one needed surgery, while the rest resolved spontaneously or with the use of steroids.39
Panenteric capsule endoscopy
In this review a lot of new data, reasons and logic for use of CE in the context of established CD have been given in detail. Most of the data were gained using SBCE, apart from a few already mentioned cases that used the PillCam C2 to examine both the small and large bowel.6,21,30 The concept of using CE for evaluation of the entire gut in a single noninvasive examination seems a very logical and patient-friendly approach. To date, a few proof-of-concept studies have been done using either the PillCam C2 CE or the newly introduced PillCam Crohn’s capsule. Two have already been presented – looking at whole-gut MH21 or at postoperative recurrence.30 D’Haens and colleagues assessed the safety and feasibility of using the PillCam C2 to assess active colonic CD versus ileocolonoscopy.40 Forty patients prospectively underwent PillCam C2 CE followed by ileocolonoscopy. The CD index of severity (CDEIS) was assessed by both techniques. They found good correlation between the two techniques in assessing the CDEIS in the terminal ileum and colon, though the capsule systematically underestimated the severity of disease compared to ileocolonoscopy. The sensitivity of CE to detect colonic ulcerations was 86%.40 They concluded that the capsule was safe (no retentions) and better tolerated than ileocolonoscopy for the assessment of mucosal activity in selected populations.40 A similar study evaluated the PillCam C2 capsule versus ileocolonoscopy in children. The authors prospectively enrolled 40 patients, mean age 13, that swallowed the capsule and did the ileocolonoscopy on the same day once the capsule was expelled. The patients also underwent an MRE and small-intestine contrast ultrasonography (SICUS).41 The investigators were blinded to the patient’s history or test results. The simple endoscopic score for CD, Lewis score and US/MRE parameters for activity were used to classify patients as active or inactive. Ileocolonoscopy was the comparator for the colon, while for the small bowel a consensus panel was used. The authors found that the sensitivity, specificity, positive and negative predictive values of the PillCam C2 to detect colonic inflammation were 89% and 100%, 100% and 91% respectively and 90%, 94%, 95% and 90% respectively for the small bowel.41 These results were better than those of SICUS or MRE. There were no serious adverse events. The authors concluded that this single noninvasive tool makes it possible to evaluate the entire gut with high diagnostic accuracy.41
Recently, a new SBC capsule has been introduced in Europe – PillCam Crohn’s (Medtronic, Dublin, Ireland). The pill is similar to PillCam C2 in its hardware components, but is designed to allow complete coverage of the gut from mouth to anus. The new capsule comes with an IBD dedicated software (Rapid 9), in which the small bowel is divided into three segments according to their true length (not time as in the PillCam SB3), and the colon divided into two parts (right and left). The descriptors used are most severe lesion (MSL), most common lesion (MCL) and the extent of involvement in the specific segment; they are also shown visually (GI map). One can also choose to use the Lewis score for the small bowel. Leighton and colleagues compared the diagnostic yield of the capsule (without the accompanying new software) with ileocolonoscopy in 66 patients with active, established CD.42 The diagnostic yield (per subject) for active CD was 83% with the new capsule versus 70% with ileocolonoscopy. Two-thirds of the patients had lesions on both modalities. Twelve patients were found to have active CD only by the capsule, 5 of which were in the terminal ileum.42 Leighton and colleagues concluded that these preliminary results suggest that the SBC capsule is at least as good as, if not better than ileocolonoscopy, but that further studies are needed. Recently, Eliakim and colleagues reported the first prospective, proof-of-concept, feasibility study using the new capsule along with the new software in patients with IBD.43 Sixty-six patients were enrolled, of which 49 ingested the capsule (14 were patency failure and 5 withdrew consent). Two-thirds of the patients had established CD (which was active in 62%), 21% had suspected CD and 10% had ulcerative colitis.43 The entire bowel cleanliness ranged from good to excellent in 96% of patients, and all videos met the primary endpoint with no serious adverse events (all capsules reached the rectum/toilet with no retention). The reading time of the entire film was reasonable (3.9 in a range of 1–7, very short to very long). They concluded that the new system was safe and allowed complete evaluation of the entire gut in CD patients.43
Thus, we now have a few possible options to evaluate relapse in a patient with established CD (Figure 3).
Figure 3.
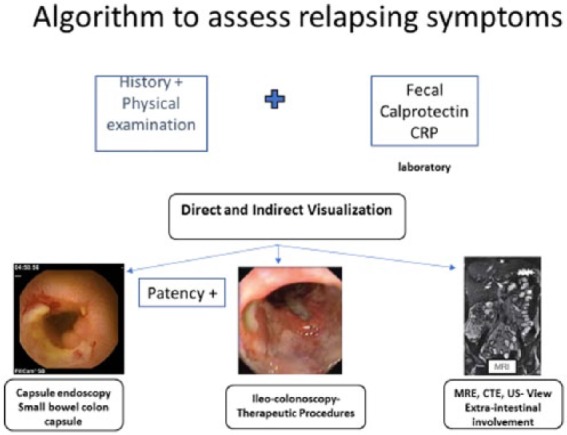
Proposed algorithm to assess relapse in patients with established Crohn’s disease.
We can probably choose the best modality according to our primary goal. If we want to classify the disease extent, the SBC capsule will give us the best information, although for the small bowel only one might use the PillCam SB3 with a lighter preparation; for the colon, ileocolonoscopy, MRE or US/SICUS. Last, for postoperative recurrence the use of fecal calprotectin along with either ileocolonoscopy, CE, MRE or US are all adequate alternatives.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The author received consultant/lecture payments from Given Imaging.
References
- 1. Carter D, Eliakim R. Current role of endoscopy in inflammatory bowel disease diagnosis and management. Curr Opin Gastroenterol 2014; 30: 370–377. [DOI] [PubMed] [Google Scholar]
- 2. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015; 47: 352–376. [DOI] [PubMed] [Google Scholar]
- 3. Annese V, Daperno M, Rutter MD, et al. ; European Crohn’s and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn’s Colitis 2013; 7: 982–1018. [DOI] [PubMed] [Google Scholar]
- 4. Leighton JA, Gralnek IM, Cohen SA, et al. Capsule endoscopy is superior to small bowel follow through and equivalent to ileocolonoscopy in suspected Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 600–615. [DOI] [PubMed] [Google Scholar]
- 5. Koulaouzidis A, Douglas S, Rogers MA, et al. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol 2011; 46: 561–566. [DOI] [PubMed] [Google Scholar]
- 6. Kopylov U, Yung DE, Engel T, et al. Fecal calprotectin for the prediction of small bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2016; 28: 11374–1144. [DOI] [PubMed] [Google Scholar]
- 7. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol 2015; 110: 444–454. [DOI] [PubMed] [Google Scholar]
- 8. Yarur AJ, Strobel SG, Deshpande AR, et al. Predictors of aggressive inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011; 7: 652–629. [PMC free article] [PubMed] [Google Scholar]
- 9. Greener T, Klang E, Yablecovitch D, et al. ; on behalf of the Israeli IBD Research Nucleus (IIRN). The impact of magnetic resonance enterography and capsule endoscopy on the re-classification of disease in patients with known Crohn’s disease: a prospective Israeli IBD research nucleus (IIRN) study. J Crohn’s Colitis 2016; 10: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen DM, Nathan T, Rafaelsen SR, et al. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of ME enterography or CT enterography. Clin Gastroenterol Hepatol 2011; 9: 124–129. [DOI] [PubMed] [Google Scholar]
- 11. Herrerías JM, Caunedo A, Rodríguez-Téllez M, et al. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy 2003; 35: 564–568. [DOI] [PubMed] [Google Scholar]
- 12. Petruzziello C, Onali S, Calabrese E, et al. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol 2010; 16: 3299–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehdizadeh S, Chen GC, Barkodar L, et al. Capsule endoscopy in patients with Crohn’s disease: diagnostic yield and safety. Gastrointest Endosc 2010; 71: 121–127. [DOI] [PubMed] [Google Scholar]
- 14. Flamant M, Trang C, Maillard O, et al. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1390–1396. [DOI] [PubMed] [Google Scholar]
- 15. Park SK, Yang SK, Park SH, et al. Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol 2013; 47: 400–408. [DOI] [PubMed] [Google Scholar]
- 16. Park Y, Cheon JH, Park YL, et al. Development of a novel predictive model for the clinical course of Crohn’s disease: results from the CONNECT study. Inflamm Bowel Dis 2017. (In Press). [DOI] [PubMed] [Google Scholar]
- 17. Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 18. Efthymiou A, Viazis N, Mantzaris G, et al. Does clinical response correlate with mucosal healing in patients with Crohn’s disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis 2008; 14: 1542–1547. [DOI] [PubMed] [Google Scholar]
- 19. Hall BJ, Holleran GE, Smith SM, et al. A prospective 12-week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. Eur J Gastroenterol Hepatol 2014; 26: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 20. Hall B, Holleran G, Chin JL, et al. A prospective 52-week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohn’s Colitis 2014; 8: 1601–1609. [DOI] [PubMed] [Google Scholar]
- 21. Boal Carvalho P, Rosa B, Dias de Castro F, et al. PillCam COLON 2 in Crohn’s disease: a new concept of pan-enteric mucosal healing assessment. World J Gastroenterol 2015; 21: 7233–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kopylov U, Nemeth A, Koulaouzidis A, et al. Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015; 21: 93–100. [DOI] [PubMed] [Google Scholar]
- 23. Mow WS, Lo SK, Targan SR, et al. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol 2004; 2: 31–40. [DOI] [PubMed] [Google Scholar]
- 24. Voderholzer WA, Beinhoelzl J, Rogalla P, et al. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut 54: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cotter J, Dias de Castro F, et al. Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohn’s Colitis 2014; 8: 1610–1615. [DOI] [PubMed] [Google Scholar]
- 26. P174. Capsule endoscopy findings and faecal calprotectin levels predict clinical relapse in patients with quiescent small bowel Crohn’s disease. J Crohn’s Colitis 2016; 10: S178–S179. [Google Scholar]
- 27. Niv Y. Small-bowel mucosal healing assessment by capsule endoscopy as a predictor of long-term clinical remission in patients with Crohn’s disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017; 29: 844–848. [DOI] [PubMed] [Google Scholar]
- 28. Bourreille A, Jarry M, D’Halluin PN, et al. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: a prospective study. Gut 2006; 55: 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pons Beltran V, Nos P, Bastida G, et al. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc 2007; 66: 533–540. [DOI] [PubMed] [Google Scholar]
- 30. Hausmann J, Schmelz R, Walldorf J, et al. Pan-intestinal capsule endoscopy in patients with postoperative Crohn’s disease: a pilot study. Scand J Gastroenterol 2017; 52: 840–845. [DOI] [PubMed] [Google Scholar]
- 31. Eliakim R. Video capsule endoscopy of the small bowel. Curr Opin Gastroenterol 2013; 29: 133–139. [DOI] [PubMed] [Google Scholar]
- 32. Cave D, Legnani P, de Franchis R, et al. ICCE consensus for capsule retention. Endoscopy. 2005; 37: 1065–1067. [DOI] [PubMed] [Google Scholar]
- 33. Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol 2006; 101: 2218–2222. [DOI] [PubMed] [Google Scholar]
- 34. Kopylov U, Nemeth A, Koulaouzidis A, et al. Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015; 21: 93–100. [DOI] [PubMed] [Google Scholar]
- 35. Nemeth A, Kopylov U, Koulaouzidis A, et al. Use of patency capsule in patients with established Crohn’s disease. Endoscopy 2016; 48: 373–379. [DOI] [PubMed] [Google Scholar]
- 36. Yadav A, Heigh RI, Hara AK, et al. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc 2011; 74:834–839. [DOI] [PubMed] [Google Scholar]
- 37. Rozendorn N, Klang E, Lahat A, et al. Prediction of patency capsule retention in known Crohn’s disease patients by using magnetic resonance imaging. Gastrointest Endosc 2016; 83: 182–187. [DOI] [PubMed] [Google Scholar]
- 38. Niv E, Fishman S, Kachman H, et al. Sequential capsule endoscopy of the small bowel for follow up of patients with known Crohn’s disease. J Crohn’s Colitis 2014; 8: 1616–1623. [DOI] [PubMed] [Google Scholar]
- 39. Kopylov U, Nemeth A, Cebrian A, et al. symptomatic retention of the patency capsule: a multicenter real life case series. Endosc Inter Open 2016; 4: E964–E969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D’ Haens G, Lowenberg M, Samaan MA, et al. Safety and feasibility of using second generation PillCam colon capsule to assess active Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1480–1486. [DOI] [PubMed] [Google Scholar]
- 41. Oliva S, Cucchiara S, Civitelli F, et al. Colon capsule endoscopy compared with other modalities in evaluation of pediatric Crohn’s disease of the small bowel and colon. Gastrointest Endosc 2016; 83: 975–983. [DOI] [PubMed] [Google Scholar]
- 42. Leighton JA, Helper DJ, Gralneck IM, et al. Comparing diagnostic yield of a novel pan-enteric capsule endoscope with ileocolonoscopy in patients with active Crohn’s disease: a feasibility study. Gastrointest Endosc 2017; 85; 196–205. [DOI] [PubMed] [Google Scholar]
- 43. Eliakim R, Spada C, Fernandez-Urien I, et al. Evaluation of a new panenteric capsule system (PillCam Crohn’s) in patients with suspected or established inflammatory bowel disease: assessing the system functionality to visualize and assess the small & large bowel (SBC). In: UEGW, Barcelona, 2017. [Google Scholar]