Abstract
Background:
Diarrhea-predominant irritable bowel syndrome (IBS-D) impairs patient quality of life (QOL). Rifaximin is an oral, nonsystemic antibiotic indicated for IBS-D. The objective of this secondary analysis was to evaluate rifaximin retreatment on IBS-related QOL in patients with IBS-D.
Methods:
Patients received open-label rifaximin 550 mg three times daily for 2 weeks. Clinical responders [simultaneously meeting weekly response criteria for abdominal pain (⩾30% improvement from baseline in mean weekly pain score) and stool consistency (⩾50% decrease from baseline in number of days/week with Bristol Stool Scale (BSS) type 6 or 7 stools) during ⩾2 of first 4 weeks posttreatment] who relapsed during an up to 18-week treatment-free observation phase were randomly assigned to receive two 2-week courses of double-blind rifaximin or placebo, separated by 10 weeks. A validated 34-item IBS-QOL questionnaire examined patient responses in 8 domains.
Results:
The 2579 patients receiving open-label rifaximin experienced a mean improvement from baseline in IBS-QOL overall score of 54.9%. Responders to open-label rifaximin (n = 1074 of 2438 evaluable; 44.1%) had significantly greater improvement from baseline in IBS-QOL overall and all eight subdomain scores, including dysphoria, food avoidance, interference with activity, body image, and sexual function versus nonresponders at 4 weeks posttreatment (n = 1364; p < 0.001 for all comparisons). A significantly greater percentage of responders to open-label rifaximin achieved the minimally clinically important difference (MCID; ⩾14-point improvement from baseline) in the overall IBS-QOL score versus nonresponders [n = 561 (52.2%) versus n = 287 (21.0%); p < 0.0001]. Among 636 patients with IBS-D relapse, the MCID in the overall IBS-QOL score was achieved by a significantly greater percentage of patients receiving double-blind rifaximin versus placebo (38.6% versus 29.6%, respectively; p = 0.009).
Conclusions:
Open-label and blinded retreatment with a short course (2 weeks) of rifaximin improved IBS-QOL in patients with IBS-D [ClinicalTrials.gov identifier: NCT01543178].
Keywords: diarrhea, quality of life, irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by recurrent symptoms of abdominal pain related to defecation along with altered bowel function (i.e. changes in the form or frequency of stool1) and effects on health-related quality of life (QOL).2,3 IBS can further be subdivided by stool consistency, namely, constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), or a combination of both (IBS-M).4 Patients with IBS with moderate-to-severe symptoms often require more intensive management with prescription therapies.5 IBS negatively impacts aspects of patients’ daily activity and social wellbeing, including work productivity, social activities, and travel.6 Patients with IBS participating in a web-based survey indicated a restriction of daily activities on approximately 73 days/year (20% annually); 13% of respondents reported not working because of health-related reasons.7 Patients with IBS cited a number of factors they believed to be associated with severity of IBS symptoms [e.g. pain (80%), bowel difficulties (74%), bloating (69%), dietary limitations (69%)], with the number of factors cited directly correlating with an increase in IBS symptom severity.
Health-related QOL was shown to be impaired in patients with IBS, as demonstrated by a pooled analysis of 14 studies in which scores for all domains of the health-related QOL instrument (i.e. social function, physical role, emotional role, bodily pain, mental health, vitality, general health, and physical function) were decreased (i.e. indicating poorer QOL) in patients with IBS compared with individuals without IBS.2 Thus, it is apparent that QOL is substantially impacted in patients with IBS, but whether treatment of IBS improves QOL is not fully understood.
The gut microbiota may be altered in IBS, although variations are possibly species- and patient-specific, and additional research is needed to firmly establish a causative effect.8–12 Nonetheless, treatments aimed at modulating the gut microbiota have been shown to improve symptoms in patients with IBS.13,14 Rifaximin, an oral, nonsystemic, minimally absorbed antibiotic, significantly improved global and individual IBS-D symptoms in two randomized, placebo-controlled, phase III studies of single, short-course (2-week) therapy.15 A third phase III study [Trial 3 (TARGET 3)] examined the efficacy of rifaximin repeat treatment in patients with IBS-D.16 A significantly greater percentage of patients receiving rifaximin compared with placebo achieved the primary efficacy endpoint of response to repeat treatment with rifaximin compared with placebo (38.1% versus 31.5%, respectively; p = 0.03).
Based on these data, rifaximin is an effective option for the treatment of IBS-D,15,16 with a number needed to treat of 10.6,17 a number needed to harm of 846,17 and a low risk of bacterial antibiotic resistance.18,19 However, the impact of repeat treatment with rifaximin on patient QOL has not been previously reported. Many believe that QOL should be a major component of clinical studies and treatment trials in functional gastrointestinal disorders.3,20,21 The aim of this study was to evaluate the effect of repeat treatment with rifaximin on IBS-related QOL in patients with IBS-D.
Methods
Study design and patient population
This was a secondary analysis of a randomized, double-blind, placebo-controlled, multicenter, phase III trial [ClinicalTrials.gov identifier: NCT01543178] and the patient population and study design have been previously described.16 Briefly, individuals ⩾18 years of age with a diagnosis of IBS (based on Rome III criteria) with average symptom severity scores of ⩾3 for IBS-related abdominal pain (range, 0 = no pain to 10 = worst possible pain you can imagine) and bloating (range, 0 = not at all to 6 = a very great deal) during the screening phase, and with stools for ⩾2 days per week meeting Bristol Stool Scale (BSS) criteria for type 6 or type 7 consistency, were eligible for inclusion in the study.
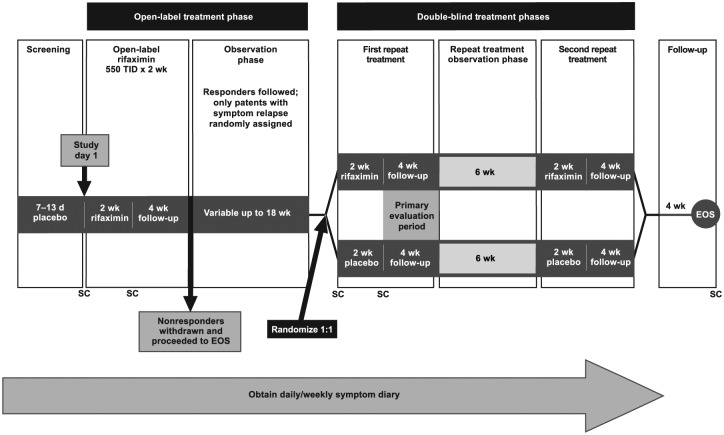
During the screening phase (10 ± 3 days), all patients received single-blind placebo three times a day and reported daily IBS-related symptoms (Figure 1). In the open-label treatment phase, patients received rifaximin 550 mg three times a day for 2 weeks, followed by a 4-week treatment-free follow-up period during which response [defined as meeting weekly response criteria for both abdominal pain (⩾30% improvement from baseline in mean weekly pain score) and stool consistency (⩾50% decrease from baseline in number of days/week with BSS type 6 or 7 stools) during ⩾2 of the 4 weeks] was evaluated. Nonresponders to open-label rifaximin were withdrawn from the study. Responders were subsequently followed, treatment-free, for up to 18 additional weeks (observation phase), or until relapse. Relapse after response to open-label rifaximin was defined as <30% improvement from baseline in mean weekly abdominal pain score or <50% reduction from baseline in number of days/week with BSS type 6 or 7 stools for ⩾3 weeks of a consecutive, rolling 4-week period during the treatment-free observation phase. Patients who relapsed were randomly assigned 1:1 to receive two 2-week repeat courses of rifaximin 550 mg three times a day or placebo, with repeat courses separated by 10 weeks (double-blind treatment phases). All patients provided written informed consent and the protocol was approved by all institutional review boards and ethics committees at all centers. All authors had full access to study data and reviewed and approved the final manuscript.
Figure 1.
Study design. Reprinted with permission from Lembo and colleagues16
EOS, end of study; SC, stool sample collection; TID, three times daily.
Quality-of-life assessments
Baseline data for the open-label and double-blind phases of the study were based on 7 days of patient diary data collected immediately preceding open-label and double-blind rifaximin repeat treatment, respectively. QOL was assessed using a validated 34-item IBS-QOL questionnaire,22 with each item scored on a 5-point Likert response scale (1 = ‘not at all,’ 2 = ‘slightly,’ 3 = ‘moderately,’ 4 = ‘quite a bit,’ or 5 = ‘extremely’ or ‘a great deal’). Data were converted to a summed score (range, 0–100) using the following formula: score = 100 × [(number of items × 5 − sum of all the items) / (5 × number of items − number of items)]. A higher score on the 0–100 scale indicated better QOL. IBS-QOL overall and eight subdomain scores (i.e. dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual function, and social relationships) were calculated. During the open-label treatment phase, the IBS-QOL questionnaire was administered in the clinic at open-label rifaximin baseline, by phone at end of the 4-week follow-up, and then by phone every 4 weeks for patients who continued to respond during the 18-week observation phase. During the double-blind treatment phases, the IBS-QOL questionnaire was administered in the clinic at double-blind baseline (randomization), by phone at the end of the 4-week follow-up (primary evaluation period), by phone at 4-week intervals of the repeat treatment observation phase, and in the clinic at the start of second repeat treatment and the end of the study. Scores were not calculated for patients with missing data for any item at a given assessment. An improvement in IBS-QOL overall score of ⩾14 points from baseline to a time point of interest was considered the minimally clinically important difference (MCID; i.e. the smallest difference perceived by patients to be beneficial).21
Statistical analyses
The change from open-label baseline in the IBS-QOL overall and subdomain scores to 4 weeks posttreatment was analyzed using descriptive statistics. Comparisons of IBS-QOL scores between groups (i.e. open-label responders versus nonresponders, open-label responders with and without relapse, rifaximin versus placebo) were analyzed using one-way analysis of variance (ANOVA). A two-way ANOVA was used to compare IBS-QOL scores in patients receiving one or two repeat treatments in the double-blind phase. An unstratified Cochran–Mantel–Haenszel general association test for categorical data was used to compare IBS-QOL scores in patients receiving open-label treatment who achieved improvement from baseline ⩾14 points. A Cochran–Mantel–Haenszel test stratified by analysis center, time to recurrence, and recurrence type was used to compare IBS-QOL scores in patients receiving double-blind treatment who achieved improvement from baseline ⩾14 points.
Results
Demographic and baseline disease characteristics
The mean baseline IBS-QOL overall score in the 2579 patients who were treated with open-label rifaximin was 48.3. A total of 1074 (44.1%) of the 2438 patients who received open-label rifaximin and completed the 4-week follow-up phase were responders (i.e. improvement in both abdominal pain and stool consistency for ⩾2 of 4 weeks following rifaximin treatment).16 A total of 1257 patients were nonresponders and were withdrawn from the study as prespecified in the protocol. During the observation phase, 692 patients experienced symptom relapse, and 636 of these patients were randomly assigned to receive repeat treatment with either rifaximin (n = 328) or placebo (n = 308). Demographic and baseline disease characteristics were generally comparable between both the open-label and double-blind populations and within the double-blind population (Table 1),16 with the exception that patients entering the double-blind phase had higher (i.e. improved) overall baseline IBS-QOL and subdomain scores compared with baseline scores of patients entering the open-label phase (i.e. prior to open-label rifaximin; Table 1).
Table 1.
Demographic and baseline characteristics.
| Characteristic | Open-label population |
Double-blind population |
|
|---|---|---|---|
| Rifaximin 550 mg (N = 2579) |
Rifaximin 550 mg (n = 328) |
Placebo (n = 308) |
|
| Age, y, mean (SD) | 46.4 (13.7) | 47.9 (14.2) | 45.6 (13.8) |
| Sex, male:female, % | 31.8:68.2 | 32.3:67.7 | 28.9:71.1 |
| Race, n (%) | |||
| White | 2155 (83.6) | 273 (83.2) | 262 (85.1) |
| Black | 289 (11.2) | 37 (11.3) | 31 (10.1) |
| Other | 135 (5.2) | 18 (5.5) | 15 (4.9) |
| Duration since first onset of IBS symptoms, y, mean (SD) | 10.9 (10.8) | 11.4 (11.0) | 11.2 (10.9) |
| Number of daily bowel movements, mean (SD) | 3.9 (2.2) | 3.8 (2.1) | 3.7 (2.1) |
| Average daily stool consistency score, mean (SD) | 5.6 (0.8) | 5.6 (0.8) | 5.6 (0.8) |
| Days with BSS stool type 6 or 7 in a week, mean (SD) | 4.9 (1.8) | 4.9 (1.8) | 5.0 (1.7) |
| Daily abdominal pain score, mean (SD) | 5.5 (1.7) | 5.7 (1.7) | 5.5 (1.6) |
| IBS-QOL overall score, n (%) | |||
| >40 (nonsevere) | 948 (36.8) | 133 (40.5) | 117 (38.0) |
| ⩽40 (severe) | 1611 (62.5) | 193 (58.8) | 190 (61.7) |
| Missing | 20 (0.8) | 2 (0.6) | 1 (0.3) |
| Baseline IBS-QOL domain scores, mean (SD) | |||
| Overall | 48.3 (21.2)a | 54.7 (23.5)h | 55.0 (24.2) |
| Dysphoria | 48.7 (25.5)b | 57.8 (26.6) | 57.8 (27.5) |
| Interference with activity | 39.5 (23.0)c | 46.3 (25.4) | 46.8 (26.6) |
| Body image | 47.9 (24.1)b | 52.2 (26.3) | 51.9 (26.3) |
| Health worry | 55.1 (21.9)d | 59.7 (23.3) | 60.7 (24.4) |
| Food avoidance | 34.0 (27.0)e | 39.6 (28.5) | 40.2 (29.0) |
| Social reaction | 52.6 (26.1)f | 58.1 (28.9)h | 59.5 (27.5) |
| Sexual | 65.2 (32.0)g | 69.7 (31.8) | 69.4 (33.5) |
| Relationships | 58.9 (26.5)f | 64.0 (27.2)h | 64.2 (28.6) |
Data missing for 20 patients.
Data missing for 14 patients.
Data missing for 19 patients.
Data missing for 15 patients.
Data missing for 13 patients.
Data missing for 17 patients.
Data missing for 12 patients.
Data missing for 1 patient.
BSS, Bristol Stool Scale; IBS, irritable bowel syndrome; IBS-QOL, irritable bowel syndrome quality of life questionnaire; SD, standard deviation.
Adapted with permission from Lembo and colleagues.16
Open-label phase
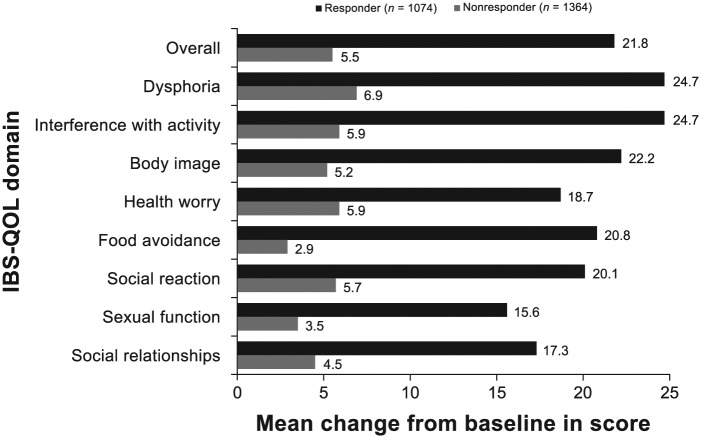
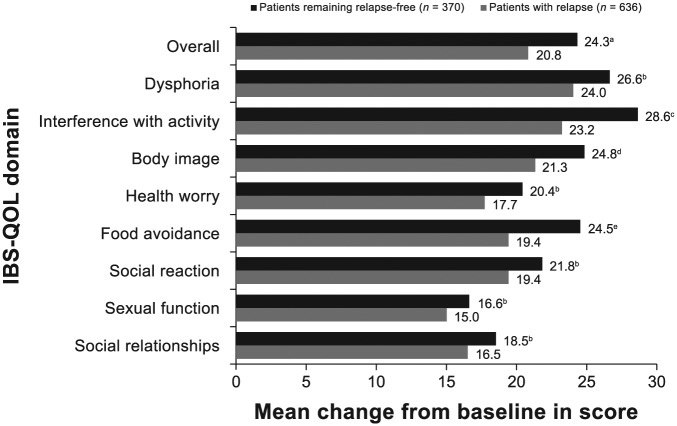
Patients receiving open-label rifaximin treatment reported improvements from baseline in IBS-QOL overall and eight subdomain scores (see Figure S1, published online) at 4 weeks posttreatment, with a mean improvement from baseline of 54.9% in the IBS-QOL overall score [95% confidence interval, 48.4–61.4%]. Responders to open-label treatment with rifaximin (n = 1074) had statistically significantly greater improvement from baseline in IBS-QOL overall and subdomain scores compared with nonresponders at 4 weeks posttreatment (n = 1364; Figure 2). The MCID in the IBS-QOL overall score from open-label baseline to 4 weeks posttreatment was achieved by 52.2% (n = 561) and 21.0% (n = 287) of responders and nonresponders, respectively (p < 0.0001). The mean change from baseline in overall IBS-QOL score and IBS-QOL subdomain scores for interference with activity, body image, and food avoidance at the 4-week posttreatment follow-up was statistically significantly greater in open-label responders to rifaximin remaining relapse-free during the open-label observation phase (n = 370), compared with responders who relapsed during the maintenance phase (n = 636; Figure 3). Open-label responders to rifaximin remaining relapse-free had numerically greater mean changes from baseline in IBS-QOL subdomain scores for dysphoria, health worry, social reaction, sexual function, and social relationships domain scores at the 4-week posttreatment follow-up compared with responders who relapsed during the maintenance phase, although between-group differences did not achieve statistical significance.
Figure 2.
Change from open-label baseline in IBS-QOL overall and subdomain scores for 1074 responders and 1364 nonresponders to open-label rifaximin at end of 4-week posttreatment follow-up. Positive numbers indicate improvement from baseline in IBS-QOL score. p < 0.001 for all comparisons (one-way ANOVA, with a factor of responder status). Number of patients with missing data: responder group (n = 61, overall and interference with activities; n = 57, sexual function; n = 60, dysphoria, body image, health worry, food avoidance, social reaction, and social relationship) and nonresponder group (n = 233, overall; n = 230, dysphoria, body image, food avoidance, health worry, and sexual function; n = 232, interference with activities, social reaction, and social relationship).
ANOVA, analysis of variance; IBS-QOL, irritable bowel syndrome quality of life questionnaire.
Figure 3.
Change from open-label baseline in IBS-QOL overall and subdomain scores at the 4-week posttreatment follow-up for responders who relapsed and were included in double-blind treatment phases (n = 636) compared with open-label responders who did not relapse during the open-label observation phase (n = 370). The p-value was based on a one-way ANOVA with a factor of evaluation-period status. Positive numbers indicate an improvement from baseline in IBS-QOL score.
Number of patients with missing data: patients remaining relapse-free (n = 40, overall score, interference with activity, health worry, social reaction, and social relationships; n = 39, dysphoria, body image, and food avoidance; n = 37, sexual function) and patients with relapse (n = 18, overall score, dysphoria, interference with activity, body image, and food avoidance; n = 17, health worry, social reaction, sexual function, and social relationships).
a, p = 0.02; b, NS; c, p = 0.002; d, p = 0.047; e, p = 0.01.
ANOVA, analysis of variance; IBS-QOL, irritable bowel syndrome quality of life questionnaire; NS, not significant.
Double-blind phase
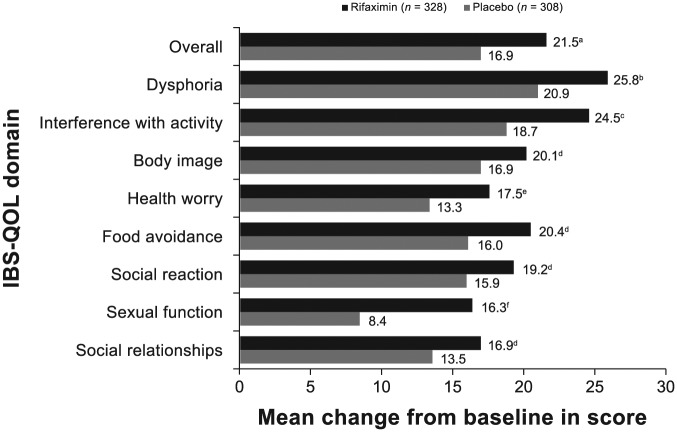
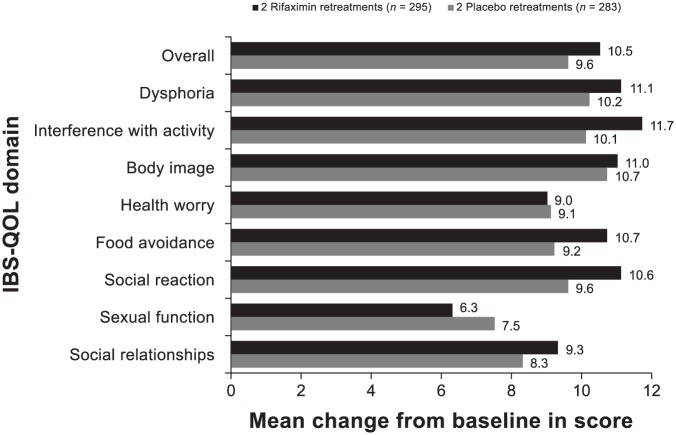
The mean change from open-label baseline to end of study (i.e. after double-blind rifaximin retreatment) in IBS-QOL overall and subdomain scores for dysphoria, interference with activity, health worry, and sexual function domains were significantly greater for patients receiving repeat treatment with rifaximin versus placebo in the double-blind phase (p ⩽ 0.05; Figure 4). Patients receiving double-blind rifaximin had greater mean improvement from open-label baseline to end of study (i.e. after double-blind rifaximin retreatment) in IBS-QOL subdomain scores for body image, food avoidance, social reaction, and social relationships domains compared with double-blind placebo, but these differences did not achieve statistical significance.
Figure 4.
Change from open-label baseline in IBS-QOL overall and subdomain scores at last visit for patients randomly assigned to receive rifaximin (n = 328) or placebo (n = 308) in the double-blind treatment phases. The p-value was based on a one-way ANOVA, with a factor of treatment group, adjusted for analysis center, time to recurrence, and recurrence type. Positive numbers indicate an improvement from baseline in IBS-QOL score. Number of patients with missing data: rifaximin (n = 2, overall score, dysphoria, interference with activity, body image, and food avoidance; n = 1, health worry, social reaction, sexual function, and social relationships); placebo (n = 1, overall and all subdomains).
a, p = 0.01; b, p = 0.02; c, p = 0.006; d, NS; e, p = 0.03; f, p < 0.001.
ANOVA, analysis of variance; IBS-QOL, irritable bowel syndrome quality of life questionnaire; NS, not significant.
The majority of patients included in the double-blind phase received two repeat treatments (rifaximin, 89.9%; placebo, 91.9%). The MCID in the IBS-QOL overall score from double-blind baseline to the end of first retreatment 4-week follow-up was achieved by a significantly greater percentage of patients receiving rifaximin compared with placebo (38.6% versus 29.6%, respectively; p = 0.009). For patients who received open-label rifaximin and both double-blind treatment courses, the improvement from double-blind baseline to the end of first double-blind treatment follow-up in IBS-QOL overall and most subdomain scores (excepting IBS-QOL health worry and sexual function subdomain scores) was greater compared with patients who received open-label rifaximin and two courses of placebo treatment (Figure 5). However, in patients who received two repeat treatments, differences between treatment groups (i.e. rifaximin versus placebo) were not statistically significant when comparing mean improvement in IBS-QOL overall score from open-label baseline with the 4-week follow-up after open-label rifaximin (i.e. treatment #1) or the first double-blind treatment (i.e. treatment #2) (see Figure S2, published online). A statistical analysis could not be conducted because there was an insufficient number of patients receiving both double-blind treatments who had IBS-QOL data at the end of study; consequently, no comparison of change in overall score from open-label baseline to end of study was conducted. Mean improvement values for IBS-QOL overall score from open-label baseline to 4 weeks after the first double-blind retreatment with rifaximin or placebo were comparable with those of the improvement from open-label baseline to the second double-blind retreatment baseline (see Figure S2, published online). Patients who completed the study had improvement from open-label baseline in IBS-QOL overall scores for the duration of the study (i.e. all assessments) with both rifaximin and placebo (see Figure S3, published online).
Figure 5.
Change from double-blind baseline in IBS-QOL overall and subdomain scores 6 weeks after receiving the first double-blind repeat treatment for patients receiving two repeat treatments with rifaximin or placebo in the double-blind phase. Analysis was performed using per protocol population. Positive numbers indicate an improvement from baseline in IBS-QOL score. Number of patients with missing data: rifaximin (n = 3) and placebo (n = 2), overall and all subdomain scores.
IBS-QOL, irritable bowel syndrome quality of life questionnaire.
Discussion
The findings of this study indicate that repeat treatment with rifaximin favorably impacts IBS-QOL overall and all eight subdomain scores in patients with IBS-D. Moreover, IBS-QOL overall and all subdomain scores were improved from baseline for up to 4 weeks posttreatment in patients receiving 2-week open-label rifaximin treatment, with the greatest improvements from baseline observed for the IBS-QOL subdomains of dysphoria and interference with activity. Interestingly, IBS-QOL subdomain scores for food avoidance, interference with activity, and dysphoria have been shown to be low (i.e. poorer QOL) in patients with IBS-D,23,24 and the majority of patients with IBS have self-reported intolerances to a number of foods, which aberrantly affects QOL.25 Indeed, in the open-label population, baseline IBS-QOL subdomain scores for food avoidance, interference with activity, and dysphoria were among the lowest, compared with other IBS-QOL subdomain scores. All of these subdomain scores significantly improved in responders treated with rifaximin compared with nonresponders. It is valuable to note that the IBS-QOL is a derivative measure that reflects the perceptions of patients with IBS, including the amount of control they have over their symptoms, if they can socialize, and their sexual relationships, which can have lasting effects even if there is some degree of symptom change. It was not designed as a surrogate measure of symptoms or function.
Improvements from open-label baseline in IBS-QOL overall and subdomain scores for food avoidance, interference with activity, and body image were significantly greater in patients who responded to open-label rifaximin who did not relapse during the treatment-free observation phase, as compared with those who responded and subsequently relapsed during the observation phase. This finding suggests that greater improvement in QOL following treatment with rifaximin is associated with a lower chance of subsequent symptom relapse. Indeed, responders to open-label treatment with rifaximin had significantly greater improvement from baseline in the IBS-QOL overall score based on an MCID ⩾14 points compared with nonresponders at 4 weeks posttreatment. The subgroup of nonresponders who achieved the MCID in IBS-QOL may not have met the formal prespecified criteria for response, but it is possible that they may have experienced enough improvement in their clinical symptoms with rifaximin treatment that they deemed their QOL improved. While a direct association between MCID and the symptomatic response to rifaximin is not entirely expected, clinically meaningful improvement in IBS-QOL appears to be consistent with improvements in abdominal pain and stool consistency previously reported for these patients.16 These data demonstrate that a single course of short-term treatment with rifaximin is associated with clinically meaningful improvement in QOL in patients with IBS-D.
For patients with open-label response to rifaximin who relapsed during the observation phase and were randomly assigned to double-blind rifaximin or placebo, a statistically significantly greater percentage of patients receiving rifaximin achieved an MCID in IBS-QOL overall score from double-blind baseline to end of first retreatment 4-week follow-up compared with placebo, providing further evidence of the sustained beneficial and clinically meaningful impact of rifaximin on QOL in patients with IBS-D. These favorable findings regarding QOL appear to align with the significant improvement in efficacy outcomes observed with rifaximin compared with placebo during the first double-blind retreatment phase of the study, suggesting that improvement in IBS-QOL may, in part, be driven by relief of IBS symptoms following repeat treatment with rifaximin versus placebo.16 Interestingly, when patients had recurrent IBS symptoms after responding to open-label rifaximin, the baseline QOL immediately preceding double-blind, repeat treatment was improved compared with that reported at open-label baseline. This is consistent with data previously reported for this trial for IBS symptom scores (e.g. 20% improvement in abdominal pain at double-blind baseline relative to symptom severity prior to open-label rifaximin), which were also lower (improved) compared with open-label baseline data.16 The improvement in QOL at baseline of the double-blind, repeat treatment phase reduces the statistical power to detect measurable improvement related to a potential carryover effect.26
The improvement from both open-label and double-blind baseline to the end of study observed in IBS-QOL overall scores between rifaximin and placebo with short-term exposure (i.e. 2 weeks) was comparable with data reported for patients with IBS-D receiving the mixed µ-opioid receptor agonist/δ-opioid receptor antagonist eluxadoline 100 mg and 75 mg after 12 weeks of twice-daily therapy.27 However, it is unclear whether improvements in IBS-QOL overall scores are durable for eluxadoline following treatment discontinuation. The selective type 3 serotonin receptor antagonist alosetron 1 mg administered twice daily improved IBS-specific QOL subdomain scores for emotional, mental health, sleep, energy, food/diet, social function, and physical role from baseline after 12 weeks in women with severe IBS-D.28 Alosetron 1 mg twice daily also improved IBS-specific QOL subscale scores for physical role, food/diet, and social function and met or exceeded the MCID in two randomized, placebo-controlled studies of women with IBS-D or IBS-M after 12 weeks of therapy.29 Similar to eluxadoline, alosetron must be administered daily, long-term, so durability of QOL improvement following treatment discontinuation is unclear. In addition, direct comparisons of alosetron with rifaximin are limited because of a different disease-specific QOL instrument used in alosetron trials.28,29
A limitation of the current study is that patients who responded to open-label rifaximin, but who did not relapse during the 18-week, treatment-free observation phase, discontinued from the study per protocol. Therefore, further assessment of this nonrelapsing patient population was not conducted to evaluate the duration of improvement in IBS-QOL scores beyond a total of 18 weeks after response was assessed. Another limitation of the current study is that when data were missing for an item within the subdomains of the IBS-QOL instrument, scores were not calculated at a given time point, limiting the data available for some assessments during the study. Data from some assessments included a small number of patients (e.g. patients who only received one repeat treatment course). In addition, some components of the IBS-QOL instrument, although a valid instrument for IBS-D, have been reported not to be optimal for patients with IBS-D,30 and thus using this instrument may not have fully assessed the condition of IBS-D and potential impact of treatments. This study was not sufficiently powered to examine specific demographic and baseline disease characteristics previously shown to be associated with QOL in patients with IBS-D (e.g. sex), and these analyses were not conducted.24 Finally, this study did not compare improvement in QOL with patient daily activities, which are often impaired by IBS.6
In conclusion, patients with IBS-D receiving short-term (2-week) repeat treatment with rifaximin experienced clinically meaningful improvements in QOL. Furthermore, repeat treatment with rifaximin provided incremental overall and subdomain QOL improvement in addition to the enduring improvement observed following initial treatment with open-label rifaximin. The improvements in QOL reported in the current study further support the clinical usefulness of a 14-day course of rifaximin 550 mg three times a day as treatment and repeat treatment for the management of IBS-D.
Supplementary Material
Acknowledgments
Technical editorial assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, Pennsylvania, USA. Funding for this support was provided by Salix Pharmaceuticals, Bridgewater, NJ, USA.
Footnotes
Funding: This study was funded by Salix Pharmaceuticals, Bridgewater, NJ, USA.
Conflict of interest statement: Brooks Cash has served as a speaker, consultant, or as an advisory board member for Salix Pharmaceuticals and Valeant, Bridgewater, NJ; Takeda, Deerfield, IL; Ironwood, Boston, MA; AstraZeneca, Wilmington, DE; Allergan, Parsippany, NJ; and IM HealthSciences, LLC, Boca Raton, FL. Mark Pimentel has served as a consultant for and has received research funding from Salix Pharmaceuticals, Bridgewater, NJ. In addition, Cedars-Sinai Medical Center, Los Angeles, CA, has a licensing agreement with Salix Pharmaceuticals, Bridgewater, NJ. Satish Rao has received a research grant for rifaximin in IBS from Salix Pharmaceuticals, Bridgewater, NJ. Leonard Weinstock has served on the speakers’ bureau for Salix Pharmaceuticals, Bridgewater, NJ; Entera Health, Cary, NC; Allergan, Parsippany, NJ; and Romark Labs, Tampa, FL; and is a primary investigator on a rifaximin trial for IBS for Salix Pharmaceuticals, Bridgewater, NJ. Lin Chang has served on scientific advisory boards for Ironwood, Boston, MA; IM HealthSciences, LLC, Boca Raton, FL; BioAmerica, Miami, FL; Synthetics Biologics, Rockville, MD; and Synergy Pharmaceuticals Inc, New York, NY. She has served as a speaker for a Takeda (Deerfield, IL) CME conference and an Allergan (Parsippany, NJ) symposium. Zeev Heimanson is an employee of Salix Pharmaceuticals, Bridgewater, NJ. Anthony Lembo has served as a consultant and an advisory board member for Salix Pharmaceuticals and Valeant, Bridgewater, NJ; Ironwood, Boston, MA; Forest, New York, NY; Allergan, Parsippany, NJ; Prometheus, San Diego, CA; Alkermes, Waltham, MA; AstraZeneca, Wilmington, DE; and Ardelyx, Fremont, CA.
Contributor Information
Brooks D. Cash, University of South Alabama, Digestive Health Center, 75 S. University Blvd, Suite 6000-B, Mobile, AL 36608, USA.
Mark Pimentel, GI Motility Program, Division of Gastroenterology, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Satish S. C. Rao, Section of Gastroenterology/Hepatology, Digestive Health Center, Medical College of Georgia Augusta University, Augusta, GA, USA
Leonard Weinstock, Specialists in Gastroenterology, LLC, Washington University School of Medicine, St Louis, MO, USA.
Lin Chang, Division of Digestive Diseases/Gastroenterology, David Geffen School of Medicine at UCLA, UCLA, Los Angeles, CA, USA.
Zeev Heimanson, Salix Pharmaceuticals, Bridgewater, NJ, USA.
Anthony Lembo, Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
References
- 1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 2. Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013; 19: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000; 119: 654–660. [DOI] [PubMed] [Google Scholar]
- 4. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 5. Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol 2011; 106: 1749–1759. [DOI] [PubMed] [Google Scholar]
- 6. Hungin APS, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005; 21: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 7. Drossman DA, Morris CB, Schneck S, et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol 2009; 43: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carroll IM, Chang YH, Park J, et al. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog 2010; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll IM, Ringel-Kulka T, Siddle JP, et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2012; 24: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kassinen A, Krogius-Kurikka L, Mäkivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007; 133: 24–33. [DOI] [PubMed] [Google Scholar]
- 11. Casén C, Vebø HC, Sekelja M, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther 2015; 42: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tana C, Umesaki Y, Imaoka A, et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 2010; 22: 512–519. [DOI] [PubMed] [Google Scholar]
- 13. Hungin APS, Mulligan C, Pot B, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice – an evidence-based international guide. Aliment Pharmacol Ther 2013; 38: 864–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol 2003; 98: 412–419. [DOI] [PubMed] [Google Scholar]
- 15. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22–32. [DOI] [PubMed] [Google Scholar]
- 16. Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016; 151: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 17. Shah E, Kim S, Chong K, et al. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012; 125: 381–393. [DOI] [PubMed] [Google Scholar]
- 18. DuPont HL, Wolf RA, Israel RJ, et al. Antimicrobial susceptibility of Staphylococcus isolates from the skin of patients with diarrhea-predominant irritable bowel syndrome treated with repeat courses of rifaximin. Antimicrob Agents Chemother 2017; 61: pii, e02165-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pimentel M, Chang L, Lembo A, et al. Rifaximin repeat treatment in diarrhea-predominant irritable bowel syndrome (IBS-D) produced by no clinically significant changes in stool microbial antibiotic sensitivity. Amer J Gastroenterol 2015; 110: S761. [Google Scholar]
- 20. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut 2001; 48: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drossman D, Morris CB, Hu Y, et al. Characterization of health related quality of life (HRQOL) for patients with functional bowel disorder (FBD) and its response to treatment. Am J Gastroenterol 2007; 102: 1442–1453. [DOI] [PubMed] [Google Scholar]
- 22. Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998; 43: 400–411. [DOI] [PubMed] [Google Scholar]
- 23. Singh P, Staller K, Barshop K, et al. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J Gastroenterol 2015; 21: 8103–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu L, Huang D, Shi L, et al. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes 2015; 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Böhn L, Störsrud S, Törnblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013; 108: 634–641. [DOI] [PubMed] [Google Scholar]
- 26. Leber PD, Davis CS. Threats to the validity of clinical trials employing enrichment strategies for sample selection. Control Clin Trials 1998; 19: 178–187. [DOI] [PubMed] [Google Scholar]
- 27. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016; 374: 242–253. [DOI] [PubMed] [Google Scholar]
- 28. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther 2012; 36: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watson ME, Lacey L, Kong S, et al. Alosetron improves quality of life in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2001; 96: 455–459. [DOI] [PubMed] [Google Scholar]
- 30. Andrae DA, Patrick DL, Drossman DA, et al. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual Life Outcomes 2013; 11: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.