Abstract
Background
In contemporary atrial fibrillation trials most deaths are cardiac related, whereas stroke and bleeding represent only a small subset of deaths. We aimed to evaluate the long-term risk of cardiac events and all-cause mortality in individuals with atrial fibrillation compared to no atrial fibrillation.
Design
A systematic review and meta-analysis of studies published between 1 January 2006 and 21 October 2016.
Methods
Four databases were searched. Studies had follow-up of at least 500 stable patients for either cardiac endpoints or all-cause mortality for 12 months or longer. Publication bias was evaluated and random effects models were used to synthesise the results. Heterogeneity between studies was examined by subgroup and meta-regression analyses.
Results
A total of 15 cohort studies was included. Analyses indicated that atrial fibrillation was associated with an increased risk of myocardial infarction (relative risk (RR) 1.54, 95% confidence interval (CI) 1.26–1.85), all-cause mortality (RR 1.95, 95% CI 1.50–2.54) and heart failure (RR 4.62, 95% CI 3.13–6.83). Coronary heart disease at baseline was associated with a reduced risk of myocardial infarction and explained 57% of the heterogeneity. A prospective cohort design accounted for 25% of all-cause mortality heterogeneity. Due to there being fewer than 10 studies, sources of heterogeneity were inconclusive for heart failure.
Conclusions
Atrial fibrillation seems to be associated with an increased risk of subsequent myocardial infarction in patients without coronary heart disease and an increased risk of, all-cause mortality and heart failure in patients with and without coronary heart disease.
Keywords: Atrial fibrillation, myocardial infarction, mortality, heart failure
Introduction
Current treatment of thromboembolic complications associated with atrial fibrillation (AF) has mainly focused on the prevention of stroke.1,2 Notwithstanding, patients with AF frequently develop coronary heart disease (CHD). Similar cardiovascular risk factors for CHD and AF have been suggested to reflect a common pathway of underlying vascular disease.3
In a recent meta-analysis of patients with AF, cardiac deaths accounted for 46% of all deaths during follow-up, whereas non-haemorrhagic stroke/systemic embolism and haemorrhage-related deaths represented only 5.7% and 5.6% of the total mortality, respectively.4 As part of the Atherosclerosis Risk in Communities (ARIC) study,5 including participants free of CHD at baseline, AF was associated with a 63% increased risk of incident myocardial infarction (MI). These studies limited inclusion to either AF-only or CHD-free patients.
The objective of the present systematic review was therefore to summarise the evidence from contemporary epidemiological studies, which included patients with predominantly sinus rhythm with or without CHD at baseline, examining the association between AF and the risk of major cardiac events (CEs) and mortality, followed by an evaluation of the influence of core patient-level characteristics such as age, gender and CHD at baseline.
Methods
The review protocol has been registered at http://www.crd.york.ac.uk/PROSPERO/ (reg. CRD42016033209). The manuscript was prepared according to the 2009 PRISMA checklist.6
Eligibility criteria
We selected randomised trials or observational studies of clinically stable patients that evaluated MI, heart failure (HF) and mortality as endpoints in AF patients versus patients without AF; both categories with or without preceding CHD.
To reflect contemporary management in view of the large differences in risk and outcome as compared to earlier management strategies7 we considered articles published between 1 January 2006 and 21 October 2016.
In order to minimise the problems associated with small-scale studies, at least 500 participants who were haemodynamically stable on inclusion and followed for 12 months or longer were required. As the unfavourable prognosis of AF complicating an acute MI8 and cardiac surgery9 is well established, studies selected had to have included patients who had sustained an MI or coronary artery bypass graft operation (CABG) more than 30 days prior to inclusion. Studies recruiting patients with acute AF at baseline were excluded, as were outcome studies comparing the prognostic impact of rate versus rhythm control in AF patients.
Literature search
We searched the databases Embase, Medline, Cochrane Library and Pubmed (including articles published ahead of print). Our search strategy combined text words and subject headings, with details presented in Supplementary Appendix 1.
Study selection
Two investigators independently evaluated studies for eligibility. Discrepancies were solved by repeated review and discussion. Reference lists were scrutinised to detect studies overlooked by the search.
Data abstraction
Data were extracted according to an a priori protocol registering the mean age of the cohort, frequency of men, CHD status at baseline, study design, median follow-up period and main results from multivariate analysis.
The study quality was assessed by evaluating design characteristics using the Newcastle–Ottawa scale (NOS) for cohort studies.10 The checklist includes core domains to assess: (a) methods for selecting study participant; (b) appropriate control for confounding (comparability); and (c) methods assessing outcome variables.
Assessment of exposure and outcome
The diagnosis of AF should have been based upon electrocardiographic criteria and supported by hospital discharge letters and other available medical records. Objective criteria for CHD were: a confirmed hospitalisation for MI, percutaneous coronary intervention (PCI) or CABG, a clinical diagnosis of angina pectoris verified by a positive stress test, or significant coronary artery stenosis on a coronary angiogram.
Outcomes should have been verified from hospital records. Endpoint MI was defined as pure MI according to current standards, although the composite endpoints CEs (MI, angina pectoris, PCI or CABG), major CEs (coronary death and non-fatal MI) and cardiovascular events (composite of CEs (MI, acute and subacute ischaemic heart disease, CABG or PCI), cerebrovascular events (occlusion/stenosis of the precerebral/cerebral arteries or subarachnoid haemorrhage) and peripheral events (vascular interventions)) were accepted for the studies by Ruigomez et al.,11 Marte et al.12 and Vermond et al.,13 respectively.
Statistical analysis
To calculate an overall relative risk (RR) we used the effect estimates and its 95% confidence interval (CI) given from multivariable analysis, and a standard random effects meta-analysis.14
The magnitude of between-study heterogeneity was evaluated by the I2 statistics. I2 values of 25%, 50% and 75% indicate low, moderate and high heterogeneity.15 To investigate possible sources of heterogeneity, we performed analyses stratified by the patient characteristic CHD at baseline, and the study characteristic prospective versus retrospective timing of the study. Univariable random effects meta-regression analyses were used to examine whether effect estimates were affected by these covariates as well as by the patient covariates mean age of the cohort and frequency of men. The proportion of heterogeneity explained by the covariates was calculated by comparing the estimated between-study variance, τ2, with its value when no covariates were fitted. For power consideration we needed a minimum of 10 studies per covariate in a single model of meta-regression.16 Sensitivity analyses were conducted by omitting one study in turn from the meta-analysis and assessing its effect on the overall results.17 Publication bias was assessed using Egger’s test, which can be used only when at least 10 studies are included in the meta-analysis.18
The analyses were performed using STATA 13.0 (StataCorp LP, College Station, TX, USA).
Results
Study selection
Our search identified 7927 references. Excluding duplicate publications and irrelevant content, 15 publications (18 studies) met our inclusion criteria.5,11–13,19–29 The flow of information is presented in Supplementary Appendix 2. Goto et al.26 reported results from a CHD population (Goto_RCHD) and a risk factor-only population (Goto_RRFO). Otterstad et al.27 reported results for patients with prevalent AF (Otterstad_APAF) and incident AF (Otterstad_AIAF). Andersson et al.29 presented results separately for men and women.
Characteristics of the studies included in the meta-analysis
The studies comprised a total of 220,928 participants, of whom 17.5% were exposed to AF (Table 1). The median age was 63 years (range 47–72), 59% were men (range 0–100), median follow-up was 4.9 years (range 1–21.6). CHD at baseline was present in eight studies (criteria for ruling CHD out are presented in Supplementary Appendix 3).
Table 1.
Characteristics of the 15 publications (18 studies) included in the meta-analysis.
| Study | Publication, year | Exposure status AFyes/AFno n/n | Endpoints considered | Cardiovascular diseases at baseline | Study design | Age, years, Mean | Men (%) | Follow-up, years Median | |
|---|---|---|---|---|---|---|---|---|---|
| Otterstad et al.27 | 2006 | 313/7352 | MI, HF, mortality | Yes | Cohort | Retrospective | 63.2 | 79 | 4.95 |
| 574/6778 | MI, HF, mortality | Yes | Subgroup | Retrospective | 63.3 | 79 | 4.95 | ||
| Ruigomez et al.11 | 2008 | 831/8226 | CE,a HF | No | Cohort | Retrospective | 63.5 | 46.5 | 3.6 |
| Goto et al.26 | 2008 | 713/10,850 | MI, HF, mortality | Yes | Cohort | Retrospective | 69.1 | 49.5 | 1 |
| 4725/32,999 | MI, HF, mortality | No | Subgroup | Retrospective | 68.3 | 69.9 | 1 | ||
| Marte et al.11 | 2009 | 37/576 | Major CE,b mortality | Yes | Cohort | Prospective | 62.8 | 67.5 | 4.0 |
| Bouzas-Mosquera et al.28 | 2010 | 619/16,481 | MI, mortality | Yes | Cohort | Retrospective | 64.3 | 59.1 | 6.5 |
| Conen et al.24 | 2011 | 1011/33,711 | MI, mortality | No | Cohort | Prospective | 53 | 0 | 15.4 |
| Jabre et al.25 | 2011 | 729/2187 | Mortality | Yes | Cohort | Prospective | 68 | 58 | 6.6 |
| Bramlage et al.23 | 2013 | 455/5317 | Mortality | Yes | Cohort | Retrospective | 65.2 | 74.6 | 1 |
| Soliman et al.22 | 2014 | 1631/22,297 | MI | No | Cohort | Prospective | 63.5 | 41.5 | 4.5 |
| O’Neal et al.20 | 2014 | 434/4174 | MI | No | Cohort | Prospective | 72.5 | 40 | 12.2 |
| Chao et al.21 | 2014 | 12,114/12,114 | MI | No | Cohort | Retrospective | 47.0 | 59.9 | 5.7 |
| Andersson et al.29 | 2014 | 9519/12,468 | MI, mortality, HF | No | Cohort – women | Retrospective | 67.7 | 0 | 6.5 |
| Cohort – men | Retrospective | 54.9 | 100 | ||||||
| Rohla et al.19 | 2015 | 146/1288 | Mortality | Yes | Cohort | Prospective | 65.9 | 72.1 | 4.7 |
| Soliman et al.5 | 2015 | 1545/12,917 | MI | No | Cohort | Prospective | 54 | 44 | 21.6 |
| Vermond et al.13 | 2015 | 265/8000 | CV,c HF, mortality | No | Cohort | Prospective | 49 | 49.8 | 9.7 |
AF: atrial fibrillation; MI: myocardial infarction; HF: heart failure; CEs: cardiac events; CV: cardiovascular event.
CE defined as myocardial infarction, angina pectoris, percutaneous coronary intervention or coronary artery bypass grafting.
Major CE defined as coronary death and nonfatal myocardial infarction.
CV defined as cardiac events (acute myocardial infarction, acute and subacute ischaemic heart disease, coronary artery bypass grafting or percutaneous transluminal coronary angioplasty), cerebrovascular events (occlusion or stenosis of the precerebral or cerebral arteries or subarachnoid haemorrhage) and peripheral events (other vascular interventions such as percutaneous transluminal angioplasty or bypass grafting of the aorta and peripheral vessels).
All studies were cohort designs, eight with prospective timing. Confounding by age, gender, baseline cardiovascular disease and cardiac risk factors was adjusted for in multivariate analysis (Supplementary Appendix 4). In general, the studies were judged as being of a good quality (Supplementary Appendix 5).
Table 2 presents inclusion criteria, study design, risk estimates and treatment with oral anticoagulants (OACs), which was reported in five studies of CHD patients, ranging from 25% to 57%, and in two for studies of patients without CHD range 1.3% to 19.9%. Comorbidity details are presented in Supplementary Appendix 6. In general, AF patients were older, had more cardiovascular risk factors and comorbidities, higher body mass index and worse kidney function than non-AF patients.
Table 2.
Inclusion criteria and results in the 15 studies included in the meta-analysis.
| Study | Inclusion criteria and design | Estimates (95% CI) % AF patients treated with OACs |
|---|---|---|
| Otterstad et al.27 | In 7665 patients with stable CHD the prognostic impact of baseline and new onset AF was assessed in comparison with non-AF patients in a randomised trial (ACTION) | HR (MI; HF; mortality): Prevalent AF: 1.2 (0.8–1.8); 1.7 (1.0–2.7); 1.4 (1.0–2.0) Incident AF: 1.0 (0.6–1.6); 5.4 (3.7–8.0); 3.0 (2.4–3.9) OAC: Prevalent AF: 25%. Incident AF: n.a. |
| Ruigomez et al.11 | In 9057 patients selected from general practice in the UK the incidence of MI was determined for patients with a first diagnosis of AF and a control group without AF | RR 2.1 (1.6–2.9) for MI, angina, PCI or CABG OAC: n.a. |
| Goto et al.26 | In 63,589 patients with atherothrombosis, of whom 37,724 had CHD, the prognostic impact of AF vs. non-AF on subsequent MI, heart failure and mortality was studied | Adjusted event rates among AF vs. non-AF: CHD: 1.6% vs. 1.4%. Total 1.4% vs. 1.1% for MI CHD: 9.4% vs. 3.8%. Total 8.3% vs. 2.7% for HF CHD: 4.4% vs. 2.3%. Total 4.3% vs. 2.3% for mortality OAC: Prevalent AF in the total study group of 63589 patients: 53% |
| Marte et al.12 | In 613 patients who underwent coronary angiography the risk of mortality and major coronary events (coronary death and non-fatal MI) was compared between patients with AF vs. SR | HR 4.8 (2.2–10.8) for mortality HR 3.6 (1.4–9.7) for major coronary events OAC: Prevalent AF: 57% |
| Bouzas-Mosquera et al.28 | In 17,100 patients with known or suspected CHD referred for exercise ECG the prognostic impact of prevalent AF was compared with non-AF | HR 0.77 (0.53–1.11) for non-fatal MI HR 1.45 (1.20–1.76) for mortality OAC: n.a. (not included in adjusted analyses) |
| Conen et al.24 | In 34,722 women (Women’s Health Study) free of AF and CV disease at baseline the association between AF and mortality and MI was evaluated | HR 3.1 (2.1–4.8) for MI HR 2.1 (1.6–2.8) for mortality OAC: At the time of incident AF diagnosis: 53% |
| Jabre et al.25 | In 3220 patients hospitalised with first-ever MI long-term mortality among patients with AF prior to MI and late AF was compared with those in SR | AF prior to MI: HR 1.5 (1.3–1.7) for mortality Late AF (>30 days after MI): HR 2.6 (2.2–3.0) for mortality OAC: n.a. |
| Bramlage et al.23 | In 5772 patients who underwent PCI the prognostic impact of AF was compared with patients in SR | OR 0.5 (0.2–1.1) for MI OR 1.8 (1.2–2.8) for mortality OAC: Prevalent AF: 26% |
| Soliman et al.22 | In 14,462 participants free from CHD on inclusion the risk of MI following AF at baseline and during follow-up was compared with non-AF participants | HR 1.6 (1.3–2.0) for MI OAC: Prevalent AF: 1.3%. Incident AF: n.a. |
| O’Neal et al.20 | In 4608 participants free from CHD at baseline the risk of MI following AF vs. non-AF was explored | HR 1.7 (1.4–2.2) for MI OAC: n.a. |
| Chao et al.21 | In 12,114 patients with AF with CHA2DS2-VASC score of 0 (men) or 1 (women) the risk of MI was compared with age, sex and CHA2DS2-VASC score-matched controls with SR (1:1) | HR 2.9 (2.2–3.9) for MI OAC: Prevalent AF: 0% |
| Andersson et al.29 | The clinical outcome of 9519 patients with incident AF and no other diseases was compared with 12,468 controls without AF, matched for age, sex and calendar year of the diagnosis of AF | HR for MI; HF; mortality Women: 1.6 (1.3–2.0; 4.8 (4.0–5.8); 1.4 (1.3–1.6) Men: 1.2 (1.0–1.4); 4.4 (3.7–5.3); 1.2 (1.0–1.3) OAC: n.a |
| Rohla et al.19 | In 1434 stable CHD patients, long-term mortality was compared between patients with AF vs. SR | HR 1.7 (1.13–2.5) for mortality OAC: Prevalent AF: 36% |
| Soliman et al.5 | In 23,928 participants without CHD at baseline, the prognostic impact of baseline AF vs. non-AF on subsequent MI was studied | HR 1.7 (1.3–2.3) for MI OAC: Prevalent AF: 19.9% |
| Vermond et al.13 | In 8265 participants without AF at baseline the association between subsequent AF and CV events and mortality was assessed | HR 2.2 (1.1–4.8) for CV events (cardiac, cerebrovascular and peripheral vascular) HR 3.0 (1.7–5.3) for mortality OAC: n.a. |
CI: confidence interval; OAC: oral anticoagulation; AF: atrial fibrillation; CHD: coronary heart disease; HR: hazard ratio; MI: myocardial infarction; HF: heart failure; n.a.: not available; UK: United Kingdom; RR: relative risk; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; SR: sinus rhythm; ECG: electrocardiogram; CV: cardiovascular; OR: odds ratio.
Endpoint MI
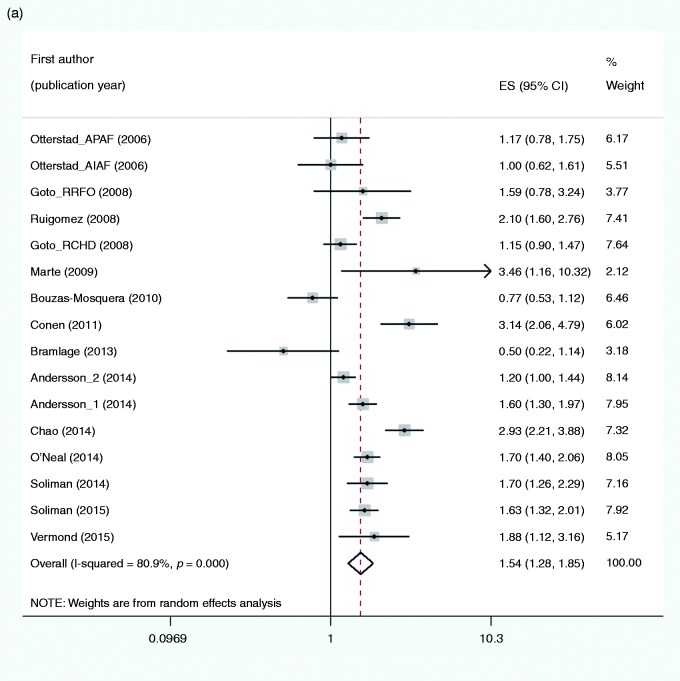
The pooled estimate including 16 studies (Figure 1(a)) showed an increased risk of MI in subjects with AF as compared to no AF (RR 1.54, 95% CI 1.28–1.85, I2 = 80.9%). CHD at baseline and the frequency of men were associated with a reduced risk of MI and explained 57% and 26% of heterogeneity, respectively (Tables 3 and 4). Furthermore, the timing of the study was borderline significant and explained 11% of heterogeneity. The effect of these three covariates was not investigated simultaneously in meta-regression both due to the correlation between CHD and gender (R = 0.5325) and few studies. In sensitivity analyses the results appeared to be robust to the influence of individual studies (Supplementary Appendix 7(a)). Publication bias was not indicated (Egger test, P = 0.967).
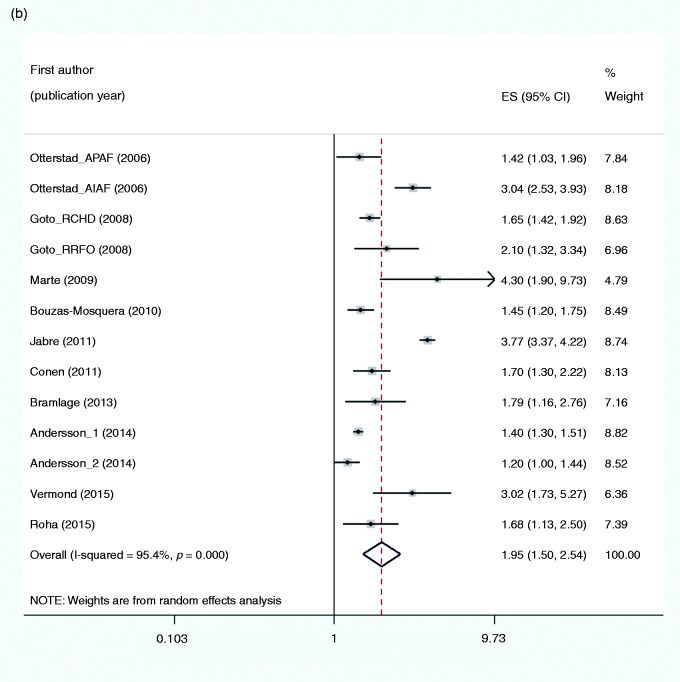
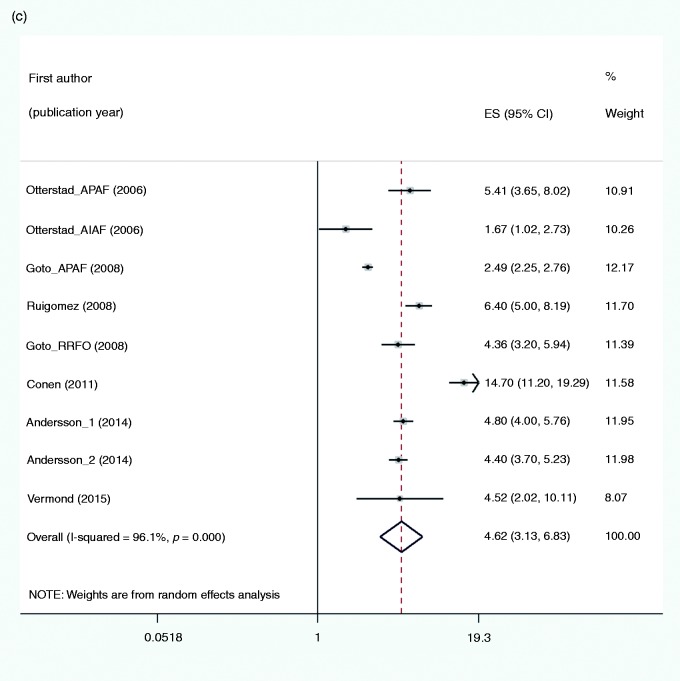
Figure 1.
Forest plots for the risk of: (a) myocardial infarction; (b) all-cause mortality; and (c) heart failure associated with atrial fibrillation. ES (95% CI): risk ratio (95% confidence interval).
Table 3.
Subgroup analysis performed with patient and study-level characteristics considered as potential sources of heterogeneity.
| N | Summary RR (95% CI)a | RR=1 (z) | P value | I2 (%) | |
|---|---|---|---|---|---|
| Endpoint myocardial infarction | |||||
| All studies | 16 | 1.54 (1.28–1.85) | 4.64 | <0.001 | 80.9 |
| Subdivision | |||||
| Coronary heart disease at baseline | |||||
| Yes | 6 | 1.02 (0.77–1.36) | 0.15 | 0.879 | 55.3 |
| No | 10 | 1.84 (1.53–2.21) | 6.53 | <0.001 | 77.2 |
| Composite endpoint | |||||
| Yes | 3 | 2.10 (1.66–2.66) | 6.19 | <0.001 | 0.0 |
| No | 13 | 1.45 (1.19–1.77) | 3.64 | <0.001 | 82.9 |
| Prospective cohort study | |||||
| Yes | 6 | 1.88 (1.56–2.26) | 6.61 | <0.001 | 46.8 |
| No | 10 | 1.32 (1.02–1.72) | 2.12 | 0.034 | 85.1 |
| Endpoint all-cause mortality | |||||
| All studies | 13 | 1.95 (1.50–2.54) | 4.98 | 0.001 | 95.4 |
| Subdivision | |||||
| Coronary heart disease at baseline | |||||
| Yes | 8 | 2.12 (1.48–3.04) | 4.10 | <0.001 | 94.8 |
| No | 5 | 1.58 (1.29–1.94) | 4.43 | <0.001 | 73.2 |
| Prospective cohort study | |||||
| Yes | 5 | 2.60 (1.64–4.13) | 4.04 | <0.001 | 90.1 |
| No | 8 | 1.64 (1.38–1.95) | 5.59 | <0.001 | 83.5 |
| Endpoint heart failure | |||||
| All studies | 9 | 4.62 (3.13–6.83) | 7.68 | <0.001 | 96.1 |
| Subdivision | |||||
| Coronary heart disease at baseline | |||||
| Yes | 3 | 2.84 (1.65–4.91) | 3.75 | <0.001 | 88.3 |
| No | 6 | 5.92 (4.08–8.58) | 9.38 | <0.001 | 92.0 |
| Prospective cohort study | |||||
| Yes | 2 | 8.68 (2.75–27.40) | 3.69 | <0.001 | 86.5 |
| No | 7 | 3.94 (2.84–5.48) | 8.16 | <0.001 | 94.0 |
RR (95% CI): relative risk with its 95% confidence interval.
Estimates from random effects model.
Table 4.
Results from the random effects meta-regression model considering the different patient and study-level variables.
| N | Level | β-coefficient | Standard error (β) | t | P value | τ 2a | Adj. R2 (%)b | |
|---|---|---|---|---|---|---|---|---|
| Endpoint myocardial infarction | ||||||||
| Covariates | ||||||||
| None | 16 | 0.4296 | 0.1133 | 3.79 | 0.002 | 0.1395 | − | |
| Coronary heart disease at baseline | 16 | yes/no | −0.5870 | 0.1847 | −3.18 | 0.007 | 0.0598 | 57.15 |
| Composite endpoint | 16 | yes/no | 0.4183 | 0.3063 | 1.37 | 0.194 | 0.1353 | 3.02 |
| Mean age of the cohort | 16 | years | −0.0245 | 0.0140 | −1.75 | 0.103 | 0.1162 | 16.71 |
| Male gender of the cohort | 16 | percentage | −0.0078 | 0.0037 | −2.12 | 0.053 | 0.1031 | 26.08 |
| Prospective cohort study | 16 | yes/no | 0.4027 | 0.2164 | 1.86 | 0.084 | 0.1236 | 11.42 |
| Publication year of studies | 16 | year | 0.0313 | 0.0356 | 0.88 | 0.394 | 0.1373 | 1.56 |
| Endpoint all-cause mortality | ||||||||
| Covariates | ||||||||
| None | 13 | 0.6601 | 0.1107 | 5.96 | <0.001 | 0.1301 | − | |
| Coronary heart disease at baseline | 13 | yes/no | 0.2188 | 0.2268 | 0.96 | 0.356 | 0.1270 | 2.38 |
| Mean age of the cohort | 13 | years | 0.0033 | 0.0192 | 0.17 | 0.866 | 0.1418 | −8.95 |
| Male gender of the cohort | 13 | percentage | 0.0006 | 0.0039 | 0.14 | 0.889 | 0.1448 | −11.28 |
| Prospective cohort study | 13 | yes/no | 0.4371 | 0.2072 | 2.11 | 0.059 | 0.0970 | 25.48 |
| Publication year of studies | 13 | year | −0.0253 | 0.0364 | −0.70 | 0.501 | 0.1352 | −3.89 |
| Endpoint heart failure | ||||||||
| Covariates | ||||||||
| None | 9 | 1.5302 | 0.2024 | 7.56 | <0.001 | 0.3349 | − | |
| Coronary heart disease at baseline | 9 | yes/no | −0.7321 | 0.3648 | −2.01 | 0.085 | 0.2335 | 30.28 |
| Mean age of the cohort | 9 | years | −0.0338 | 0.0321 | −1.05 | 0.328 | 0.3218 | 3.92 |
| Male gender of the cohort | 9 | percentage | −0.0102 | 0.0052 | −1.94 | 0.093 | 0.2436 | 27.27 |
| Prospective cohort study | 9 | yes/no | 0.8720 | 0.4398 | 1.98 | 0.088 | 0.2136 | 36.24 |
| Publication year of studies | 9 | year | 0.0517 | 0.0628 | 0.82 | 0.438 | 0.3490 | −4.19 |
τ2, between-study variance.
Proportion of between-study variance (heterogeneity) explained by the covariate.
Endpoint all-cause mortality
The pooled estimate of 13 studies (Figure 1(b)) showed an increased risk of all-cause mortality in subjects with AF as compared to no AF (RR 1.95, 95% CI 1.50–2.54, I2 = 95.4%). Only the study characteristic timing was borderline significant associated with the outcome and explained 25% of heterogeneity (Table 4). In sensitivity analyses the results appeared to be robust (Supplementary Appendix 7(b)). Publication bias was not indicated (Eggers test, P = 0.619).
Endpoint HF
The pooled estimate of nine studies (Figure 1(c)) showed an increased risk of HF in subjects with AF as compared to no AF (RR 4.62, 95% CI 3.13–6.83, I2 = 96.1%). The presence of CHD at baseline, the frequency of men and timing of the study explained 30%, 27% and 36% of heterogeneity, respectively (Table 4). The borderline significance might be due to power deficiency because of the scarce number of studies. In sensitivity analyses the results appeared robust (Supplementary Appendix 7(c)). Publication bias was not examined given the number of studies.
Discussion
Prevalent and incident AF appear to be significantly associated with MI, HF and all-cause mortality. Meta-regression analyses indicated that heterogeneity could be explained by patient and study characteristics related to the presence or absence of CHD, gender and study design being retrospective or prospective.
For the endpoint MI, heterogeneity was first of all explained by pre-existing CHD; AF patients without pre-existent CHD demonstrated a significantly higher risk of MI when compared to non-AF patients as opposed to stable CHD patients.
We judged all included studies to be of satisfactory methodological quality. A retrospective design was a major limitation and differences in data quality might explain the tendency towards a higher risk of all endpoints related to a prospective design, as seen in Table 3. This review is based on a comprehensive literature search designed to avoid a large heterogeneity related to comorbidities and acute stage scenarios of CHD manifestations and interventions, and the meta-analysis is based on comprehensively study-level adjusted estimates. However, the probability of unmeasured confounding cannot be excluded in observational studies.
In a very recent meta-analysis of cohort studies, Odutayo et al.30 reported an increased risk for a range of different outcomes when exposed to AF, with a high level of heterogeneity. In contrast to our study, not only stable individuals with or without CHD were included, but also a variety of study populations, such as post-acute MI and post-cardiac surgery. The present meta-analysis differs substantially, as we have narrowed the spectra of patient inclusions and performed a literature search which includes text words and subject headings. Odutayo et al. did not provide detailed information on OACs given to patients with versus without CHD, but sensitivity analysis revealed lower all-cause mortality in studies with a higher proportion of participants receiving OACs.
In a recent meta-analysis of 12 studies, Guo et al.31 reported the same increased risk of MI in AF patients as in the present study. The risk of HF and death was not studied. Interestingly, and in line with the present study, AF was associated with a significantly increased risk of MI in patients free from CHD at baseline. Stroke prevention is a major public health priority32 and it is of interest that a greater proportion of CHD patients were treated with an OAC than CHD-free patients, which might be explanatory for their reduced risk of MI as seen in both the study of Guo et al. and in the present analysis.
Supporting the trend towards a higher female risk of MI among AF patients in our study. Emdin et al.33 reported an association with a higher risk of fatal and non-fatal cardiovascular events in women.
This systematic review/meta-analysis demonstrates a relationship between AF and an increased risk of CEs and mortality, but causal evidence cannot be established. In a recent study, the presence of AF has been shown to be independently associated with a heightened risk of MI despite a lower baseline burden and progression rate of coronary atheroma.34 Similar cardiovascular risk factors of AF and MI may reflect a common pathway of underlying disease and act as confounders such that AF may not be regarded as a causal risk factor for MI, HF and death, but a surrogate of more severe disease. In view of this, Wijesurendra et al.35 performed magnetic resonance imaging before and after catheter ablation for AF, and stated that AF may be the consequence (rather than the cause) of an occult cardiomyopathy which persists despite a significant reduction in AF burden after ablation.
Conclusions
AF seems to be associated with an increased risk of subsequent MI in patients without CHD and with an increased risk of all-cause mortality and HF whatever the coronary status. However, AF should not be regarded as a causal risk factor for these conditions.
Acknowledgements
The authors would like to thank Matthew McGee, Morbid Obesity Center, Vestfold Hospital Trust, for proofreading the manuscript. Funding source: Vestfold Hospital Trust and University of Oslo, Norway.
Author contribution
VR, IS and JEO contributed to the conception and design of the work and drafted the manuscript. JM and TE contributed to the design. JS contributed to acquisition, JM and TE contributed to interpretation and VR, IS and JEO to both. All authors critically revised the manuscript, gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 2.EHRA Scientific Committee Task Force, Gorenek B, Pelliccia A, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace: European pacing, arrhythmias, and cardiac electrophysiology: Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2017; 19: 190–225. DOI: 10.1093/europace/euw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermond RA, Van Gelder IC, Crijns HJ, et al. Does myocardial infarction beget atrial fibrillation and atrial fibrillation beget myocardial infarction? Circulation 2015; 131: 1824–1826. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Outes A, Lagunar-Ruiz J, Terleira-Fernandez AI, et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016; 68: 2508–2521. [DOI] [PubMed] [Google Scholar]
- 5.Soliman EZ, Lopez F, O’Neal WT, et al. Atrial fibrillation and risk of ST-segment elevation versus non-ST-segment elevation myocardial infarction: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2015; 131: 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 7.Aune E, Endresen K, Fox KA, et al. Effect of implementing routine early invasive strategy on one-year mortality in patients with acute myocardial infarction. Am J Cardiol 2010; 105: 36–42. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt J, Duray G, Gersh BJ, et al. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 2009; 30: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 9.Kaw R, Hernandez AV, Masood I, et al. Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2011; 141: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality in non-randomized studies in meta-analysis. Ottawa, Canada: Ottawa Health Research Institute, 1999. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 29 May 2014).
- 11.Ruigomez A, Johansson S, Wallander MA, et al. Risk of cardiovascular and cerebrovascular events after atrial fibrillation diagnosis. Int J Cardiol 2009; 136: 186–192. [DOI] [PubMed] [Google Scholar]
- 12.Marte T, Saely CH, Schmid F, et al. Effectiveness of atrial fibrillation as an independent predictor of death and coronary events in patients having coronary angiography. Am J Cardiol 2009; 103: 36–40. [DOI] [PubMed] [Google Scholar]
- 13.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol 2015; 66: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 17.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010; 1: 112–125. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002–d4002. [DOI] [PubMed] [Google Scholar]
- 19.Rohla M, Vennekate CK, Tentzeris I, et al. Long-term mortality of patients with atrial fibrillation undergoing percutaneous coronary intervention with stent implantation for acute and stable coronary artery disease. Int J Cardiol 2015; 184: 108–114. [DOI] [PubMed] [Google Scholar]
- 20.O’Neal WT, Sangal K, Zhang ZM, et al. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol 2014; 37: 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao TF, Huang YC, Liu CJ, et al. Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2-VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm 2014; 11: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 22.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Int Med 2014; 174: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bramlage P, Cuneo A, Zeymer U, et al. Prognosis of patients with atrial fibrillation undergoing percutaneous coronary intervention receiving drug eluting stents. Clin Res Cardiol: Official journal of the German Cardiac Society 2013; 102: 289–297. [DOI] [PubMed] [Google Scholar]
- 24.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA: The journal of the American Medical Association 2011; 305: 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabre P, Jouven X, Adnet F, et al. Atrial fibrillation and death after myocardial infarction: a community study. Circulation 2011; 123: 2094–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J 2008; 156: 855–863. 863, e852. [DOI] [PubMed] [Google Scholar]
- 27.Otterstad JE, Kirwan BA, Lubsen J, et al. Incidence and outcome of atrial fibrillation in stable symptomatic coronary disease. Scand Cardiovasc J: SCJ 2006; 40: 152–159. [DOI] [PubMed] [Google Scholar]
- 28.Bouzas-Mosquera A, Peteiro J, Broullon FJ, et al. Effect of atrial fibrillation on outcome in patients with known or suspected coronary artery disease referred for exercise stress testing. Am J Cardiol 2010; 105: 1207–1211. [DOI] [PubMed] [Google Scholar]
- 29.Andersson T, Magnuson A, Bryngelsson IL, et al. Gender-related differences in risk of cardiovascular morbidity and all-cause mortality in patients hospitalized with incident atrial fibrillation without concomitant diseases: a nationwide cohort study of 9519 patients. Int J Cardiol 2014; 177: 91–99. [DOI] [PubMed] [Google Scholar]
- 30.Odutayo A, Wong CX, Hsiao AJ, et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016; 354: i4482–i4482. [DOI] [PubMed] [Google Scholar]
- 31.Guo XY, Li N, Du X, et al. Atrial fibrillation is associated with an increased risk of myocardial infarction: insights from a meta-analysis. Atherosclerosis 2016; 254: 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Hobbs FR, Taylor CJ, Jan Geersing G, et al. European Primary Care Cardiovascular Society (EPCCS) consensus guidance on stroke prevention in atrial fibrillation (SPAF) in primary care. Eur J Prev Cardiol 2016; 23: 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016; 532: h7013–h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayturan O, Puri R, Tuzcu EM, et al. Atrial fibrillation, progression of coronary atherosclerosis and myocardial infarction. Eur J Prev Cardiol 2017; 24: 373–381. [DOI] [PubMed] [Google Scholar]
- 35.Wijesurendra RS, Liu A, Eichhorn C, et al. Lone atrial fibrillation is associated with impaired left ventricular energetics that persists despite successful catheter ablation. Circulation 2016; 134: 1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]