Abstract
Background
Previous studies have shown a high prevalence of failure of passive transfer of immunity (FPT) in Swiss dairy calves.
Objectives
To investigate risk factors associated with poor colostrum quality and FPT on Swiss dairy farms.
Animals
Colostrum and serum samples from 373 dam‐calf pairs at 141 farms.
Methods
The gamma globulin (Gg) concentrations of the dams’ colostrum and the calves’ serum samples were determined by electrophoresis. Potential risk factors were assessed by logistic regression of questionnaire data.
Results
Prevalence values of 15.5% (95% confidence interval [CI], 12.0–19.6%) for low‐quality colostrum (<50 g Gg/L) in cows and 43.5% (95% CI, 38.4–48.8%) for FPT (serum Gg < 10 g/L) in calves were estimated. The main factors associated with low colostrum quality included colostrum leakage before or during parturition and a time lag > 6 hours between parturition and first milking. The results confirm that the occurrence of FPT in calves primarily was influenced by the quality of colostrum, the amount of ingested colostrum, and the time between birth and first feeding.
Conclusions and Clinical Importance
These results confirm a large potential for improvement in colostrum harvesting and colostrum feeding procedures in the study herds. Control for colostrum leaking intra‐partum, early colostrum milking, and ensuring that the calves ingest a sufficient volume of colostrum within the first hours of life are measures that can be readily implemented by farmers to decrease the incidence of FPT without additional workload.
Keywords: Failure of passive transfer, Immunoglobulins, Management, Risk factors
Abbreviations
- AP
ante‐partum
- CI
confidence interval
- FPT
failure of passive transfer of immunity
- Gg
gamma globulin
- Ig
immunoglobulin
- LRT
likelihood ratio test
- PP
post‐partum
- SD
standard deviation
Colostrum intake is of vital importance for the protection of newborn calves against infectious agents during their first days and weeks of life.1, 2 Besides the time until first feeding after birth and the volume of colostrum ingested,3, 4 the immunoglobulin (Ig) content of colostrum also plays an important role in the success (or failure) of transfer of passive immunity.2, 5, 6 A low colostral Ig concentration can lead to hypogammaglobulinemia or FPT, and consequently increased risk of infectious diseases for the calf even if the total amount of colostral Ig ingested (calculated from the colostrum Ig concentration in g/L and the volume ingested in L) appears to be sufficient.7 The quality of the dam's colostrum and the Ig concentration in her calf's serum have been compared pairwise in only a few studies.5, 6, 8 Although the Ig concentration in colostrum and in calf serum was compared in these 3 studies, the only risk factor for FPT assessed in 2 of the studies5, 8 was colostrum quality. In the third study,6 herd was identified as the most relevant risk factor for hypoproteinemia (as an indicator of hypogammaglobulinemia) in calves, but only a few other risk factors were assessed.
The objectives of the study here as follows: (1) to investigate the quality of the colostrum fed to dairy calves on Swiss farms and to identify risk factors associated with poor colostrum quality, (2) to describe the relationship between the gamma globulin (Gg) concentration in the colostrum fed and the serum Gg concentration of the calves, and (3) to identify additional risk factors (beside colostrum quality) associated with FPT in calves.
Materials and Methods
Colostrum and serum samples from dams and their calves, respectively, were collected from dairy farms in the region Emmental/Bernese Oberland of Switzerland by co‐workers of 3 private veterinary practices from August 2014 to May 2015. At each farm, 1 to 3 cow‐calf pairs were sampled. For each pair, a colostrum sample was collected by the farmer and stored at 4°C. The sample was taken from the first milking used for the first feeding of the calf. A blood sample was taken from the calf by jugular venipuncture at the age of 2–5 days by a veterinarian. The stored colostrum sample was collected at the same time. In case of a twin birth, a blood sample of each calf was collected. Blood samples were centrifuged (10 minutes, 10 × g) and the serum was separated. Colostrum and serum samples were kept frozen (−20°C) until analyzed in the laboratory.
A questionnaire was designed to assess risk factors potentially associated with colostrum quality and passive transfer of immunity to the calves. The questionnaire was filled out with the herd managers for each farm and each cow‐calf pair. The assessed risk factors are listed in Tables 1 and 2.
Table 1.
Univariable logistic regression for factors associated with insufficient Gg concentrations (<50 g/L) in colostrum samples
| Gg ≥ 50 g/L | Gg < 50 g/L | OR (95% CI) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Individual variables | |||||
| Duration of gestation | |||||
| ≥280 days | 277 | 84.5 | 51 | 15.5 | 1 |
| <280 days | 30 | 81.1 | 7 | 18.9 | 1.3 (0.5–3.0) |
| Lactation number | |||||
| 1 | 76 | 84.4 | 14 | 15.6 | 1 |
| 2 | 57 | 72.2 | 22 | 27.8 | 2.1 (1.0–4.4)# |
| 3 | 63 | 86.3 | 10 | 13.7 | 0.9 (0.4–2.1) |
| ≥4 | 117 | 90.7 | 12 | 9.3 | 0.6 (0.2–1.3) |
| Duration of dry period | |||||
| ≥6 weeks | 224 | 85.2 | 39 | 14.8 | 1 |
| <6 weeks | 12 | 75.0 | 4 | 25.0 | 1.9 (0.6–6.0) |
| Leakage of colostrum AP | |||||
| None | 179 | 90.4 | 19 | 9.6 | 1 |
| Weak | 112 | 81.2 | 26 | 18.8 | 2.2 (1.2–4.1)* |
| Intensive | 19 | 61.3 | 12 | 38.7 | 6.0 (2.5–14.1)*** |
| Time to first milking PP | |||||
| <2 hours | 177 | 88.1 | 24 | 11.9 | 1 |
| 2–≤6 hours | 117 | 83.0 | 24 | 17.0 | 1.5 (0.8–2.8) |
| >6 hours | 21 | 67.7 | 10 | 32.3 | 3.5 (1.5–8.3)** |
| Location of parturition | |||||
| Tied‐in stall | 267 | 84.8 | 48 | 15.2 | 1 |
| Calving box | 30 | 78.9 | 8 | 21.1 | 1.5 (0.6–3.4) |
| Free stall | 3 | 60.0 | 2 | 40.0 | 3.7 (0.6–22.8) |
| Outdoors | 15 | 100.0 | 0 | 0 | – |
| Course of parturition | |||||
| Normal | 262 | 86.2 | 42 | 13.8 | 1 |
| Assisted (farmer) | 38 | 79.2 | 10 | 20.8 | 1.6 (0.8–3.5) |
| Assisted (veterinarian) | 13 | 68.4 | 6 | 31.6 | 2.9 (1.0–8.0)* |
| Cesarean section | 2 | 100.0 | 0 | 0.0 | – |
| Puerperal disease (individual) | |||||
| No | 277 | 82.9 | 57 | 17.1 | 1 |
| Yes | 37 | 97.4 | 1 | 2.6 | 0.1 (0.02–1.0)* |
| Herd‐level variables | |||||
| Dairy farm only | |||||
| Yes | 248 | 85.5 | 42 | 14.5 | 1 |
| No | 65 | 81.2 | 15 | 18.8 | 1.4 (0.7–2.6) |
| Infertility (herd level) | |||||
| No | 225 | 84.3 | 42 | 15.7 | 1 |
| Yes | 86 | 85.1 | 15 | 14.9 | 0.9 (0.5–1.8) |
| Increased incidence of puerperal diseases | |||||
| No | 298 | 84.2 | 56 | 15.8 | 1 |
| Yes | 13 | 92.9 | 1 | 7.1 | 0.4 (0.1–3.2) |
| Increased incidence of lameness | |||||
| No | 303 | 85.1 | 53 | 14.9 | 1 |
| Yes | 8 | 66.7 | 4 | 33.3 | 2.9 (0.8–9.8) |
| Mastitis (herd level) | |||||
| No | 130 | 83.9 | 25 | 16.1 | 1 |
| Rarely | 101 | 87.1 | 15 | 12.9 | 0.8 (0.4–1.5) |
| Sometimes | 78 | 83.0 | 16 | 17.0 | 1.1 (0.5–2.1) |
| Often | 2 | 66.7 | 1 | 33.3 | 2.6 (0.2–29.8) |
Gg, gamma globulin; OR, odds ratio; CI, 95% confidence interval; AP, ante‐partum; PP, post‐partum.
*P ≤ 0.05; **P ≤ 0.01 and ***P ≤ 0.001; # P = 0.054.
Table 2.
Univariable logistic regression for factors associated with insufficient serum Gg concentrations (<10 g/L) in newborn dairy calves
| Gg ≥ 10 g/L | Gg < 10 g/L | OR (95% CI) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Individual variables | |||||
| Gg colostrum | |||||
| Gg ≥50 g/L | 200 | 63.7 | 114 | 36.3 | 1 |
| Gg <50 g/L | 10 | 17.2 | 48 | 82.8 | 8.4 (4.1–17.3)*** |
| Time of first feeding | |||||
| <2 hours | 121 | 64.0 | 68 | 36.0 | 1 |
| 2–≤6 hours | 77 | 51.7 | 72 | 48.3 | 1.7 (1.1–2.6)* |
| >6 hours | 11 | 34.4 | 21 | 65.6 | 3.4 (1.5–7.5)** |
| Time of second feeding | |||||
| <6 hours | 21 | 60.0 | 14 | 40.0 | 1 |
| 6–≤12 hours | 138 | 60.0 | 92 | 40.0 | 1.0 (0.5–2.1) |
| >12 hours | 47 | 46.5 | 54 | 53.5 | 1.7 (0.8–3.8) |
| Colostrum of first milking fed at second feeding | |||||
| No | 187 | 57.9 | 136 | 42.1 | 1 |
| Yes | 23 | 46.9 | 26 | 53.1 | 1.6 (0.9–2.8) |
| Colostrum volume at first feeding | |||||
| ≥2 L | 138 | 62.7 | 82 | 37.3 | 1 |
| <2 L | 70 | 47.0 | 79 | 53.0 | 1.9 (1.2–2.9)** |
| Colostrum volume at second feeding | |||||
| ≥2 L | 167 | 62.1 | 102 | 37.9 | 1 |
| <2 L | 39 | 40.6 | 57 | 59.4 | 2.4 (1.5–3.9)*** |
| Colostrum quality and volume at first feeding | |||||
| Good quality and volume | 129 | 71.7 | 51 | 28.3 | 1 |
| 1 insufficient parameter | 78 | 45.3 | 94 | 54.7 | 3.0 (2.0 ‐ 4.7)*** |
| Both insufficient | 1 | 5.9 | 16 | 94.1 | 40.5 (5.2–313.2)*** |
| Colostrum quality and volume at second feeding | |||||
| Good quality and volume | 159 | 69.4 | 70 | 30.6 | 1 |
| 1 insufficient parameter | 47 | 38.5 | 75 | 61.5 | 3.6 (2.3–5.7)*** |
| Both insufficient | 0 | 0 | 14 | 100.0 | ‐ |
| Herd‐level variables | |||||
| Dairy farm only | |||||
| Yes | 169 | 58.5 | 120 | 41.5 | 1 |
| No | 39 | 48.7 | 41 | 51.3 | 1.5 (0.9–2.4) |
| Increased incidence of calf pneumonia | |||||
| No | 166 | 55.7 | 132 | 44.3 | 1 |
| Yes | 42 | 60.9 | 27 | 39.1 | 0.8 (0.5–1.4) |
| Increased incidence of calf diarrhea | |||||
| No | 105 | 52.5 | 95 | 47.5 | 1 |
| Yes | 103 | 61.7 | 64 | 38.3 | 0.7 (0.5–1.0) |
| Increased incidence of umbilical disease | |||||
| No | 206 | 56.6 | 158 | 43.4 | 1 |
| Yes | 2 | 66.7 | 1 | 33.3 | 0.7 (0.1–7.3) |
| Increased incidence of otitis | |||||
| No | 206 | 56.6 | 158 | 43.4 | 1 |
| Yes | 2 | 66.7 | 1 | 33.3 | 0.7 (0.1–7.3) |
See abbreviations and footnotes in Table 1.
The farmers gave written informed consent to participate prior to the start of the study. All procedures were in conformity with the Swiss legislation.
For the assessment of colostrum quality and passive immunity, the gamma globulin (Gg) concentrations of colostrum and serum samples were measured by use of electrophoresis1 as in previous studies.9, 10 Colostrum samples with Gg concentration <50 g/L were considered as being of insufficient quality,4 and Gg concentrations <10 g/L in the serum of the calves were considered to be indicative of FPT.10
Data were entered in a Microsoft Excel2 spreadsheet. The cow‐calf data (entered as 1 dataset) and the herd data were analyzed in STATA/IC 14.0.3 Plausible factors that could explain insufficient colostrum and calf serum Gg concentrations at the individual and at the herd level were tested for statistically significant associations (P ≤ 0.05) in univariable and multivariable logistic regression models. Univariable analyses show the odds ratios (OR) with 95% confidence intervals (CI) for the different categories of a variable compared with the basis category. The investigated factors could be either risk factors (OR > 1) or protective factors (OR < 1) with regard to insufficient quality (colostrum Gg) or FTP (serum Gg). The results of the logistic regression models without random effects are shown because clustering at the herd level (estimated using a generalized estimating equation [GEE] model with farm as panel data) did not influence the 95% CIs of the estimates. Variables with a P‐value <0.2 in the univariable analysis as well as biologically meaningful variables (eg, duration of gestation) were included in the multivariable logistic regression models. In the multivariable analyses, the influence of a whole variable on the overall model (without backward or forward selection) was tested by use of the likelihood ratio test (LRT). The LRT value represents the difference of the deviation (−2 log likelihood) of the model with and without the variable. The P‐value of the LRT statistics was assessed with the chi‐square test using the corresponding degrees of freedom. The correlation between the Gg contents of colostrum and calf sera was estimated using the nonparametric Spearman rank correlation test because Bartlett's test showed that the variances of both variables were unequal.
Results
Animals
A total of 377 pairs of colostrum serum samples were collected from 141 dairy farms. Of these, 373 sample pairs were analyzed. Four sample pairs were excluded because of excessive hemolysis or inappropriate sampling time. Thirteen farms supplied 1 cow‐calf sample pair, 31 farms 2, 90 farms 3, and 7 farms 4 cow‐calf paired samples. The samples from 5 farms included samples from a twin birth. Because of a limited number of missing values in the questionnaires, the analyses were not all performed with the total number of 373 paired samples.
The cows included in the study belonged to the breeds Swiss Fleckvieh (n = 154, 42.0%), Red Holstein (n = 89, 24.3%), Simmentaler (n = 45, 12.3%), Holstein (n = 37, 10.0%), Brown Swiss (n = 15, 4.1%), Jersey (n = 11, 3.0%), and other breeds (n = 16, 4.3%, including Original Braunvieh, Hinterwäldler, and Normande).
Ninety colostrum samples were collected from primiparous cows (24.3%) and 281 from pluriparous cows (75.7%). Pluriparous cows were on average in their third lactation (standard deviation [SD], 1.9).
Colostrum gamma globulin concentrations and colostrum feeding
The mean Gg concentration in colostrum was 74.8 g/L (SD, 25.8; minimum, 5.4 g/L; maximum, 173.8 g/L; Fig 1). The mean Gg concentration in the colostrum of primiparous cows was 69.2 g/L (SD, 20.1), 65.2 g/L (SD, 19.8), and 70.5 g/L (SD, 23.2) in the colostrum of cows starting their second and third lactation, respectively, and 86.5 g/L (SD, 29.5) in cows in their fourth or higher lactation.
Figure 1.
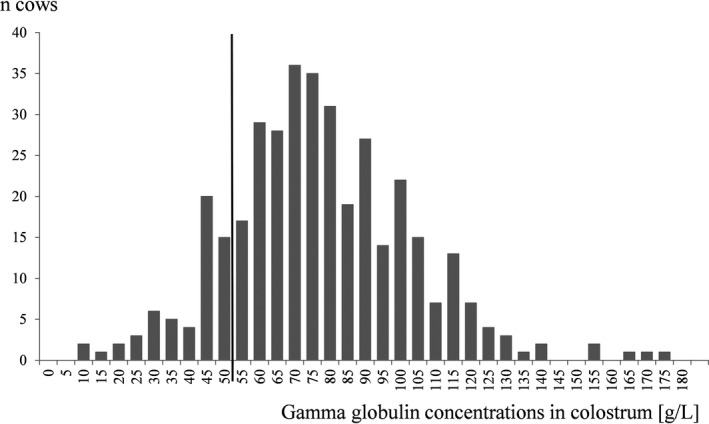
Distribution of gamma globulin concentrations in colostrum samples in g/L.
Of the 373 analyzed colostrum samples, 58 (15.5%) had a Gg content <50 g/L (95% CI, 12.0–19.6%). These 58 deficient samples originated from 46 of the 141 participating farms (32.6%).
Farmers indicated that 201 cows (53.9%) were milked for the first time <2 hours after parturition (post‐partum, PP), 141 cows (37.8%) between 2 and 6 hours PP, and 31 cows (8.3%) >6 hours PP. The second milking took place <2 hours PP for 59 cows (15.9%), between 2 and 6 hours PP for 215 cows (57.8%), and >6 hours PP for 98 cows (26.3%; Table 1).
Of the 370 calves with complete questionnaire data, 189 (51.1%) received their first colostrum within 2 hours of birth, 149 calves (40.3%) between 2 and 6 hours, and 32 calves (8.6%) more than 6 hours after birth. Most calves (339, 90.9%) ingested the colostrum supplied by bottle, and 34 (9.1%) were fed by an esophageal tube feeder. The calves consumed a mean of 1.9 L (SD, 0.7) colostrum at first feeding and 2.2 L (SD, 0.7) at second feeding. At first feeding, 149 calves (40.4%) ingested <2 L colostrum, of these 79 also had <2 L with the second feeding. A total of 96 calves (26.3%) ingested <2 L colostrum at their second feeding. Colostrum from the first milking was warmed to be fed to the calves as their second meal in 49 cases (13.1%), and the other calves received colostrum from the second milking for their second meal (Table 2).
Serum Gamma Globulin Concentrations in Calves
The mean Gg concentration of the calf serum samples was 12.3 g/L (SD, 6.7 g/L; minimum, 1.6 g/L; maximum, 37.0 g/L; Fig 2). The serum Gg concentration was <10 g/L in 162 of 373 calves (43.4%; 95% CI, 38.4–48.8%) distributed in 105 of 141 participating farms (74.5%).
Figure 2.

Distribution of gamma globulin concentrations in calf serum samples in g/L.
Three serum samples were collected before the first colostrum feeding, and their Gg concentrations were 0.8, 1.1, and 1.1 g/L, respectively. These samples were excluded from further analyses because they were not taken in the time frame defined in the study.
A moderate correlation was observed between the dams’ colostrum and the corresponding calves’ serum Gg concentrations (Spearman's rank correlation coefficient, 0.48).
Risk Factor Analysis
The analysis of the data regarding breed, duration of gestation, herd size, stall system, feeding management, and selenium supplementation of the dams during the dry period had no significant association with deficient Gg concentration in colostrum.
Likewise, increased incidence of reproductive disorders, puerperal diseases, or mastitis at the herd level, as reported by the farmers, was not significantly associated with deficient Gg concentration in colostrum samples (Table 1). No significant association was found between hypogammaglobulinemia in calves and increased incidence of calf diseases (diarrhea, pneumonia, umbilical infections, otitis) at the herd level (Table 2).
Also, no significant association was found between hypogammaglobulinemia of the calves and the way by which the colostrum was ingested (from a bucket with nipple or from a bottle vs. via drenching) or the calves’ age at the time of blood sampling (data not shown).
Explanatory Variables for Colostrum Quality
The results of the univariable logistic regression for the binary outcome of sufficient and deficient colostral Gg concentrations are shown in Table 1.
Leakage of colostrum from the udder before parturition, during parturition, or both was observed in 169 cows (46.0%; in 31 cows with intensive colostrum flow, and in 138 with weak flow). Univariable analysis identified a highly significant association (P < 0.001) between intensive leakage and poor quality of colostrum. Prolonged time lag until first milking PP (>6 hours) also was significantly associated with low colostrum quality.
A significant association was observed between the occurrence of puerperal diseases at the individual level (hypocalcemia or retention of fetal membranes in 38 cows [10.2%]) and a decreased risk (OR < 1) of insufficient colostrum quality.
No significant association was found between colostral Gg concentration and individual lactation number or in categorical analysis after the cows were distributed to 2 groups (primiparous and multiparous). In contrast, if the cows were grouped in 4 categories (first, second, third lactation, and fourth lactation and higher), a higher proportion of cows with low colostrum quality in cows in second lactation and a lower proportion in cows in third as well as in fourth and higher lactation were observed in comparison with primiparous cows. These differences, however, were not significant. The duration of the dry period (<6 weeks 5.7%; >6 weeks 94.3%) was not significantly associated with colostrum quality.
In the multivariable analysis, the variables colostrum leakage and time to first milking remained significantly associated with poor‐quality colostrum, whereas the occurrence of puerperal diseases at the individual level remained significantly associated with decreased risk of poor colostrum quality. The variable categorized as numbers of lactations was significantly associated with colostrum quality in the multivariable analysis (Table 3).
Table 3.
Multivariable logistic regression for factors associated with insufficient Gg concentrations (<50 g/L) in colostrum
| OR (95% CI) | LR Χ2 a | P (LR Χ2)b | |
|---|---|---|---|
| Duration of gestation | |||
| ≥280 days | 1 | ||
| <280 days | 2.2 (0.8–5.8) | 2.3 | 0.13 |
| Lactation number | |||
| 1 | 1 | ||
| 2 | 2.7 (1.2–6.3) | ||
| 3 | 1.2 (0.4–3.1) | ||
| ≥4 | 0.9 (0.4–2.4) | 8.6 | 0.035 |
| Leakage of colostrum AP | |||
| None | 1 | ||
| Weak | 2.3 (1.1–4.6) | ||
| Intensive | 5.9 (2.2–16.3) | 12.9 | 0.002 |
| Time to first milking | |||
| <2 hours | 1 | ||
| 2–≤6 hours | 1.6 (0.8–3.1) | ||
| >6 hours | 3.8 (1.4–10.0) | 7.3 | 0.027 |
| Normal parturition | |||
| Yes | 1 | ||
| No (assistance by farmer or veterinarian) | 1.7 (0.8–3.6) | 2.2 | 0.14 |
| Puerperal diseases (individual) | |||
| No | 1 | ||
| Yes | 0.1 (0.01–0.9) | 7.8 | 0.005 |
Chi‐square value of the likelihood ratio statistic.
P‐value of the LR Χ2.
See abbreviations and footnotes in Table 1.
Explanatory Variables for the Gg Concentration in the Calves’ Serum
The results of the univariable logistic regression for hypogammaglobulinemia of the calves are given in Table 2.
The quality of the colostrum fed, the volume of colostrum fed, and the timing of the first colostrum meal (after birth) were significantly associated with the calves’ serum Gg concentrations.
A significant association of serum Gg concentrations with farm type (78.4% exclusively dairy, 21.6% with additional sources of income) was not observed although the percentage of calves with FPT was higher in farms with alternative sources of income than in exclusively dairy farms.
In the multivariable analysis, a colostrum Gg concentration <50 g/L for the first feeding was associated with the highest OR value for FPT. The timing of the first feeding and the volume of colostrum fed with the first and second meals also were significantly associated with risk of FPT (Table 4).
Table 4.
Multivariable logistic regression for factors associated with insufficient serum Gg concentrations (<10 g/L) in newborn dairy calves
| OR (95% CI) | LR Χ2a | P (LR Χ2)b | |
|---|---|---|---|
| Gg colostrum | |||
| Gg ≥50 g/L | 1 | ||
| Gg <50 g/L | 10.7 (4.7–24.2) | 44.7 | <0.001 |
| Time to first colostrum feeding | |||
| <2 hours | 1 | ||
| 2–≤6 hours | 1.8 (1.0–3.1) | ||
| >6 hours | 3.1 (1.1–8.6) | 6.7 | 0.035 |
| Time to second colostrum feeding | |||
| <6 hours | 1 | ||
| 6–≤ 12 hours | 1.2 (0.5–2.7) | ||
| >12 hours | 1.3 (0.5–3.5) | 0.3 | 0.9 |
| Colostrum of first milking fed at second feeding | |||
| No | 1 | ||
| Yes | 1.3 (0.6–2.5) | 0.4 | 0.5 |
| Volume at first feeding | |||
| ≥2 L | 1 | ||
| <2 L | 2.0 (1.2–3.5) | 7.0 | 0.008 |
| Volume at second feeding | |||
| ≥2 L | 1 | ||
| <2 L | 2.0 (1.2–3.7) | 5.7 | 0.017 |
Discussion
The mean colostrum Gg content determined in the Swiss dairy herds participating in our study was 74.8 (SD, 25.8) Gg/L. Similar concentrations were found in colostrum samples of dairy cows in studies performed in other countries.5, 11, 12, 13, 14, 15, 16, 17, 18 More pronounced differences, however, are evident when comparing the percentage of colostrum samples of poor quality in various reports. A cut‐off of 50 g Gg/L generally is chosen to differentiate high‐ and low‐quality colostrum.4, 5, 9, 10, 11, 13, 18 Using this cut‐off, a proportion of colostrum samples of poor quality of 15.5% was found in our study. Proportions between 4 and 57.8% have been observed in other studies.6, 11, 13, 18, 19 These differences may be due to the high variability in colostrum Gg concentrations4, 13, 18, 19 (ie, to a different distribution of individual Gg concentrations despite comparable mean concentrations). Colostral Gg concentrations between 5.4 and 173.8 g/L were measured in our study. This wide range confirms the importance of objective measurements of colostral Gg content, especially in herds with a high incidence of neonatal calf diseases.
Colostrum samples with Gg concentrations <50 g/L were found in 46 of 141 farms. The fact that no poor‐quality colostrum samples were identified in 67.4% of the participating farms suggests an influence of management factors in colostrum quality. An influence on the Gg content of colostrum has been described for the following factors: time to first milking PP, volume milked at first milking, season, herd‐specific factors, and parity of the dam.4, 6, 11, 13, 18 Time to first milking PP also was identified as an important factor in our study. Delayed first milking should be addressed in recommendations for farmers for improved colostrum management. First colostrum milking within 8 hours PP has been described as optimal.6 A cut‐off of 6 hours, as recommended by others,4, 10 was used in our study, because a maximal delay of 6 hours until first milking also appears advisable with regard to optimal colostrum intake and transfer of passive immunity in newborn calves.
The most significant risk factor associated with poor‐quality colostrum identified in our study was leaking of colostrum before or during parturition (ie, loss of the Gg‐rich secretion stored in the udder at the end of the dry period).4 This factor has not been investigated in other studies. Colostrum leaking during parturition is not very amenable to control measures by farmers and can take place in well‐managed farms. Milking the mare as soon as colostrum is seen to be dripping from the udder just before or during parturition is common practice in horses20 and also could be performed in cattle to prevent the colostral fraction richest in Gg from being lost on the stall floor in the course of parturition. Colostrum leakage during parturition is easy to notice, and thus could be used by farmers as an indicator of increased risk of poor‐quality colostrum (if the dam is only milked PP). Using only colostrum from dams with no milk loss ante‐partum (AP) or milking the colostrum as soon as leakage is observed therefore should be recommended in combination with milking the dam as soon as possible PP. These measures are easy to implement for farmers and will ensure optimal colostrum quality and thus improved colostral immunity in newborn calves.
The relationship between parity and colostrum quality has been investigated by several authors, all observed either significantly higher Ig concentrations in the colostrum of older cows or at least a trend toward higher Ig concentrations in the colostrum of cows of higher parity.6, 13, 17, 18, 21 In our study, a significant association between parity and colostrum quality also was found in the multivariable analysis after categorization of the dams in first, second, third, and fourth or higher lactation. As already reported by others,6, 18 the highest proportion of dams with colostrum Gg concentrations <50 g/L was observed in cows starting their second lactation. Reasons for this finding are not well understood.6, 18 A possible explanation may be that a faster onset of lactation after second parturition (larger volume of milk produced), in comparison with primiparous cows, has a stronger effect than the moderate increase of Gg content observed with increasing age. Our results also confirm that the once‐popular recommendation not to use the colostrum of primiparous cows to feed newborn calves can no longer be considered valid.4, 17, 18, 19 These observations suggest that the effects of parity on colostrum quality must be interpreted with caution because other factors may have a stronger influence on colostrum quality in many cases. Based on our results, for reserves of frozen colostrum, if the Ig content is not assessed, the risk of freezing low‐quality colostrum can be minimized by selecting colostrum of cows in fourth or higher lactation harvested within 6 hours PP from dams that did not leak colostrum in the course of parturition.
An interesting finding of our study was the association observed between the occurrence of puerperal diseases (ie, hypocalcemia and retained fetal membranes, when assessed at the individual level) and colostrum quality, whereby puerperal diseases were significantly associated with decreased risk of poor colostrum quality. No significant association was found between colostrum leaking (as the factor with the strongest influence on colostrum quality) and occurrence of puerperal disorders, but a highly significant association was present between colostrum leaking and parity (less colostrum leaking in cows of higher parity, results not shown). Cows in their fourth or higher lactation were more prone to puerperal disease and their colostrum tended to be of poor quality less often than that of cows in first to third lactation. The fact that colostrum is produced and harvested before the occurrence of puerperal diseases suggests that these 2 variables are linked only indirectly (ie, by age of the animals as a confounding factor). In accordance with the observations of others,18 the occurrence of puerperal disease was not significantly associated with colostrum quality when assessed at the herd level, likely because of limited occurrence of puerperal disease as a herd problem.
The prevalence of FPT was 43.5% in our study. A similar prevalence (42.9%) has been observed in healthy calves,10 whereas a distinctly higher prevalence of FPT (97.2%) was found in calves with neonatal diarrhea9 in previous studies performed in the same geographic region. Others have observed lower (16–28%)3, 6, 22 or similar (41%)23 prevalence for FPT. Colostrum of poor quality was the main risk factor associated with FPT in our study. Consequently, 15.5% of the calves in our study were in an unfavorable situation from the beginning because they received the colostrum of their dams with <50 g Gg/L at first feeding. This adverse effect can be compensated by other factors in the management of the calves (eg, good hygiene) or be aggravated by additional management deficits. In our study, 5% of the calves with serum Gg concentrations ≥10 g/L had been fed poor‐quality colostrum, but 70% of the calves with FPT had received colostrum of good quality. This observation emphasizes the important role played by other factors besides colostrum Gg concentration on the success (or lack thereof) of passive transfer of immunity in neonatal dairy calves.
In a recent study,6 the most important factor associated with the rate of FPT in calves was the individual farm, and the herd‐level prevalence of FPT in the 7 farms included in that study ranged from 5 to 51%. In our study, the herd influence was decreased by inclusion of 3 cow‐calf pairs per farm on average, in order to obtain a reliable assessment of individual management factors amenable to improvement to ensure optimal transfer of passive immunity in newborn dairy calves. In fact, clustering of poor‐quality colostrum samples or of calves with FPT in specific farms was not observed. The majority of the 141 participating farms (77%) had at least 1 colostrum or serum sample with insufficient Gg concentrations. This finding suggests that uniform practices were used for colostrum management in the study farms, which is not unexpected because all farms were located in the same region within a maximal radius of 40 km from each other.
Another factor significantly associated with FPT in calves in a previous study6 was the course of parturition (ie, lower plasma protein concentrations if assistance was needed for calving). In our study, a significant association between colostrum quality and course of parturition only was observed in the univariable analysis (increased risk of Gg concentration <50 g/L in cases of dystocia requiring veterinary assistance). However, a significant association also was observed between colostrum leakage and assistance during parturition: leaking of colostrum AP was recorded in 13.6% of cows calving without assistance, whereas this proportion increased to 24.3% in cows with dystocia requiring assistance (from the farmer or from a veterinarian). Thus, the association between dystocia and FPT may be indirect and at least in part caused by increased colostrum leakage during prolonged parturition. A direct effect of hypoxia or respiratory acidosis as a consequence of dystocia on the absorptive capacity of the calf's gut for Ig has been postulated but has yet to be demonstrated convincingly.24, 25, 26, 27
In our study, the factors significantly associated with the calves’ serum Gg concentrations beside colostrum quality included the volume of colostrum ingested at first feeding, the time lag between birth and first feeding, and the volume of colostrum ingested at second feeding.
The high proportion of calves (40.3%) that received <2 L colostrum at first feeding combined with the large proportion of calves (49.1%) that were fed for the first time >2 hours after birth may explain why FPT was diagnosed in 43.5% of the calves whereas only 15.5% of the colostrum samples were classified as being of insufficient quality. Other authors have reported similarly suboptimal colostrum management practices in Switzerland and elsewhere.28, 29 These observations confirm that no improvement in colostrum management has occurred in the last few years although farmers and veterinarians are aware of the importance of colostral immunity for calf health.
A significant association between the volume of colostrum fed at second feeding (>6 hours after birth) and the serum Ig concentration of the calves has been described in a previous study7 and was observed in our study as well. In contrast, no significant difference was found in the serum Gg concentrations of the calves whether colostrum from the first (with an expected higher Gg concentration) or from the second milking was fed at second feeding. This observation may be explained by the fact that optimal Ig absorption in the gastrointestinal tract of newborn calves is limited to the first 4 hours of life27 so that the Gg concentration at second feeding may not have a strong effect on the calves’ final serum Gg concentrations. Based on this consideration, a significant effect of the volume of colostrum given at second feeding on the serum Gg concentration also would not be expected, but was observed in our study. The reason for this discrepancy remains unclear.
Additional studies also will be necessary to investigate the discrepancy between the highly significant (negative) association between the occurrence of puerperal diseases (ie, indirectly the dam's age), and prevalence of FPT and the lack of significant association between parity and colostrum Gg concentration. Differences in time to first feeding or volume fed to the calves of primiparous vs. multiparous cows were not observed in our study.
No significant association between the prevalence of FPT and increased occurrence of calf diseases was evident, likely because the occurrence of calf diseases was assessed retrospectively by questionnaire at the herd level and not prospectively for every individual calf.
The main limitation of our study is that most data used to assess colostrum management practices were based on the farmers’ responses. However, the farmers’ participation was voluntary, all data were collected prospectively, and the samples along with the information regarding each individual calving and the consequent colostrum feedings were collected within 2–5 days of the calving. Furthermore, the samples and data were collected by the regular farm veterinarians who had previous knowledge of the general management of the farms and a trusted relationship with the farmers. Thus, we believe that the information received from the farmers was accurate and of high quality.
Conclusions
Our study allowed us to assess risk factors for FPT in calves with paired colostral and serum Gg measurement in a large population and based on detailed data on management factors at the individual and herd level. Our results confirm a large potential for improvement in colostrum management practices in Swiss dairy farms. Although the importance of classical risk factors associated with FPT, such as colostrum quality, and volume and timing of first feeding, was confirmed, additional factors were newly identified as relevant (eg, colostrum leakage during parturition, volume of colostrum given at second feeding), which can readily be given more attention by farmers to improve the supply of colostral Gg to newborn calves.
Funding
This research work was funded by the Clinic for Ruminants, Vetsuisse Faculty, University of Bern, Switzerland.
Acknowledgments
The authors thank the farmers who participated in the study, the veterinary practice Max Schiffmann in Steffisburg for support with sample collection, and Dr Judith Howard, Clinical Laboratory of the Vetsuisse Faculty, University of Bern, for support with sample analyses.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This work was performed in private veterinary practices in the region of Bern, at the Clinic for Ruminants of the Vetsuisse Faculty, University of Bern, and at the Swiss Tropical and Public Health Institute, University of Basel, Switzerland.
Footnotes
Paragon Serum Protein Electrophoresis‐Kit, Beckmann Coulter, Brea CA
MicrosoftCorp, Redmont WA
StataCorp LP, College Station TX
References
- 1. Waldner CL, Rosengren LB. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can Vet J 2009;50:275–281. [PMC free article] [PubMed] [Google Scholar]
- 2. Lorenz I, Mee JF, Earley B, More SJ. Calf health from birth to weaning. I. General aspects of disease prevention. Ir Vet J 2011;64:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filteau V, Bouchard E, Fecteau G, et al. Health status and risk factors associated with failure of passive transfer of immunity in newborn beef calves in Québec. Can Vet J 2003;44:907–913. [PMC free article] [PubMed] [Google Scholar]
- 4. Godden S. Colostrum management for dairy calves. Vet Clin North Am Food Anim Pract 2008;24:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kananub S, Rukkwamsuk T, Arunvipas P. Influence of colostral quality on serum proteins in dairy calves raised in smallholder farms in Thailand. Trop Anim Health Prod 2013;45:1687–1690. [DOI] [PubMed] [Google Scholar]
- 6. MacFarlane JA, Grove‐White DH, Royal MD, Smith RF. Identification and quantification of factors affecting neonatal immunological transfer in dairy calves in the UK. Vet Rec 2015;176:625. [DOI] [PubMed] [Google Scholar]
- 7. Morin DE, McCoy GC, Hurley WL. Effects of quality, quantity, and timing of colostrum feeding and addition of a dried colostrum supplement on immunoglobulin G1 absorption in Holstein bull calves. J Dairy Sci 1997;80:747–753. [DOI] [PubMed] [Google Scholar]
- 8. Osaka I, Matsui Y, Terada F. Effect of the mass of immunoglobulin (Ig) G intake and age at first colostrum feeding on serum IgG concentration in Holstein calves. J Dairy Sci 2014;97:6608–6612. [DOI] [PubMed] [Google Scholar]
- 9. Lanz‐Uhde F, Kaufmann T, Sager H, et al. Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland. Vet Rec 2008;163:362–366. [DOI] [PubMed] [Google Scholar]
- 10. Lejeune B, Schelling E, Meylan M. Gammaglobulin and selenium status in healthy neonatal dairy calves in Switzerland. Schweiz Arch Tierheilkd 2012;154:389–396. [DOI] [PubMed] [Google Scholar]
- 11. Chigerwe M, Tyler JW, Summers MK, et al. Evaluation of factors affecting serum IgG concentrations in bottle‐fed calves. J Am Vet Med Assoc 2009;234:785–789. [DOI] [PubMed] [Google Scholar]
- 12. Furman‐Fratczak K, Rzasa A, Stefaniak T. The influence of colostral immunoglobulin concentration in heifer calves’ serum on their health and growth. J Dairy Sci 2011;94:5536–5543. [DOI] [PubMed] [Google Scholar]
- 13. Conneely M, Berry DP, Sayers R, et al. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Animal 2013;7:1824–1832. [DOI] [PubMed] [Google Scholar]
- 14. Chigerwe M, Hagey JV. Refractometer assessment of colostral and serum IgG and milk total solids concentrations in dairy cattle. BMC Vet Res 2014;10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore M, Tyler JW, Chigerwe M, et al. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J Am Vet Med Assoc 2005;226:1375–1377. [DOI] [PubMed] [Google Scholar]
- 16. Rivero MJ, Valderrama X, Haines D, Alomar D. Prediction of immunoglobulin G content in bovine colostrum by near‐infrared spectroscopy. J Dairy Sci 2012;95:1410–1418. [DOI] [PubMed] [Google Scholar]
- 17. Tyler JW, Steevens BJ, Hostetler DE, et al. Colostral immunoglobulin concentrations in Holstein and Guernsey cows. Am J Vet Res 1999;60:1136–1139. [PubMed] [Google Scholar]
- 18. Gulliksen SM, Lie KI, Sølverød L, Østerås O. Risk factors associated with colostrum quality in Norwegian dairy cows. J Dairy Sci 2008;91:704–712. [DOI] [PubMed] [Google Scholar]
- 19. McGuirk SM, Collins M. Managing the production, storage, and delivery of colostrum. Vet Clin North Am Food Anim Pract 2004;20:593–603. [DOI] [PubMed] [Google Scholar]
- 20. Chavatte‐Palmer P. Lactation in the mare. Equine Vet Educ 2002;14:88–93. [Google Scholar]
- 21. Morrill KM, Conrad E, Lago A, et al. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J Dairy Sci 2012;95:3997–4005. [DOI] [PubMed] [Google Scholar]
- 22. Moraes MP, Weiblen R, Rebelatto MC, da Silva AM. Relationship between passive immunity and morbidity and weight gain in dairy cattle. Ciência Rural 2000;30:299–304. [Google Scholar]
- 23. National Animal Health Monitoring System . National dairy health evaluation project. Dairy heifer morbidity, mortality, and health management focusing on preweaned heifers: Transfer of maternal immunity to calves. USDA‐APHIS Veterinary Services 1993. [cited 2017 Feb 9]. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/ndhep/NDHEP_Immunity.pdf
- 24. Besser TE, Szenci O, Gay CC. Decreased colostral immunoglobulin absorption in calves with postnatal respiratory acidosis. J Am Vet Med Assoc 1990;196:1239–1243. [PubMed] [Google Scholar]
- 25. Tyler H, Ramsey H. Hypoxia in neonatal calves: Effect on intestinal transport of immunoglobulins. J Dairy Sci 1991;74:1953–1956. [DOI] [PubMed] [Google Scholar]
- 26. Drewry JJ, Quigley JD, Geiser DR, Welborn MG. Effect of high arterial carbon dioxide tension on efficiency of immunoglobulin G absorption in calves. Am J Vet Res 1999;60:609–614. [PubMed] [Google Scholar]
- 27. Weaver DM, Tyler JW, VanMetre DC, et al. Passive transfer of colostral immunoglobulins in calves. J Vet Intern Med 2000;14:569–577. [DOI] [PubMed] [Google Scholar]
- 28. Pipoz F, Meylan M. Calf health and antimicrobial use in Swiss dairy herds: Management, prevalence and treatment of calf diseases. Schweiz Arch Tierheilkd 2016;158:389–396. [DOI] [PubMed] [Google Scholar]
- 29. National Animal Health Monitoring Syst . National dairy health evaluation project. Dairy heifer morbidity, mortality, and health management focusing on preweaned heifers. USDA‐APHIS Veterinary Services. 1993. [cited 2017 Feb 9]. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/ndhep/NDHEP_dr_Report.pdf


