Abstract
A 6‐year old male neutered Scottish Terrier was referred with a 1 week history of progressive lethargy and anorexia. Neurological examination localized a lesion to the forebrain and hormonal testing showed panhypopituitarism. Magnetic resonance imaging (MRI) of the brain revealed a rounded, well‐defined, suprasellar central mass. The mass was slightly hyperintense to the cortical grey matter on T2‐weighted (T2W), hypointense on T1‐weighted (T1W) images and without T2* signal void. There was a central fusiform enhancement of the mass after contrast administration which raised the suspicion of a pituitary neoplasm. Rapid deterioration of the dog prevented further clinical investigations. Histopathologic examination revealed a lymphocytic panhypophysitis of unknown origin suspected autoimmune involving the hypothalamus (hypothalamitis). This is a unique case report of a dog presenting with inflammatory hypophysitis and hypothalamitis of suspected autoimmune origin with detailed clinical, MRI, histology and immunohistochemistry findings.
Keywords: Autoimmune, Diabetes insipidus, Hypoadrenocorticism, MRI, Neoplasia, Pituitary
Abbreviations
- GME
granulomatous meningoencephalitis
- LH
lymphocytic hypophysitis
- MRI
magnetic resonance imaging
- RI
reference interval
- TSH
thyroid‐stimulating hormone
Panhypopituitarism refers to decreased secretion of pituitary hormones, which can result from different diseases (nonfunctional pituitary neoplasia, the local effects of another expanding mass, or secondary to other systemic or regional condition among others) affecting the hypothalamus or the pituitary gland.1, 2 Primary inflammatory lesions of the pituitary gland are rare in dogs, and in humans, they occur much less commonly than pituitary adenomas.3 Lymphocytic hypophysitis (LH) is the most common primary hypophyseal inflammatory disorder in humans, and it is speculated to have an autoimmune basis with a focal or diffuse inflammatory infiltration of the pituitary gland and varying degrees of gland destruction.4 Endocrine clinical signs might include partial or total hypopituitarism, with ACTH deficiency being the earliest and most frequent alteration.4 The hypophyseal form of diabetes insipidus develops as a result of damage of the pars nervosa, infundibular stalk, or supraoptic nucleus in the hypothalamus.2
Few cases of a lymphocytic adenohypophysitis are reported in dogs so far,5, 6, 7, 8, 9 and none have reported extension of the inflammation to the neurohypophysis or hypothalamus along with detailed clinical findings and MRI. In people, inflammatory hypophysitis is a rare but well‐described condition known to mimic pituitary neoplasia on computed tomography scans and MRI, and its treatment options are promising.10, 11 Therefore, hypophysitis should be included in the list of differential diagnoses of a presumptive mass located in the hypophyseal or suprasellar area as timely anti‐inflammatory management provides a potential treatment option.9 This case report presents the clinical, radiologic, and pathologic characteristics of this rare condition in a dog.
Case History and Clinical Findings
A 6‐year‐old male neutered Scottish Terrier was referred to the Pride Veterinary Centre (United Kingdom) for progressive lethargy and anorexia of a week duration. On clinical examination, he was lethargic with no other clinical abnormalities detected. The only hematologic abnormality detected was a borderline erythrocytosis. Serum biochemical abnormalities included mildly increased creatinine (129 mmol/L; reference interval (RI) 27–124 mmol/L) and mild hyponatremia (135 mmol/L; RI 138–160 g/L). Thoracic radiographs did not reveal abnormalities. Abdominal ultrasonography revealed a subjectively small liver with 1–2 mm hyperechoic areas with occasional distal shadowing, and liver cytology revealed moderate vacuolar hepatopathy. The dog was hospitalized, and isotonic crystalloid fluids were administered IV, and supplemental feeding through a nasoesophageal feeding tube was provided, but remained obtunded. On the next day, the dog showed a low carriage of the head and hindlimb ataxia. Neurologic examination revealed a decreased nasal sensation bilaterally, but the rest of cranial nerve examination, postural reactions, spinal reflexes, and spinal pain was normal.
MRI of the brain was performed with a 1.5T permanent open magnet (General Electric MR; UK). T2‐weighted (T2W), T1‐weighted (T1W) pre‐ and postintravenous contrast administration (0.1 mmol/kg body weight of gadopentetate dimeglumine1) in transverse and sagittal planes. Fluid‐attenuated inversion recovery (FLAIR) and T2* gradient echo sequences in transverse plane and T1W pre‐ and postcontrast in dorsal plane were also acquired. Dorsal and slightly rostral to the pituitary fossa, there was a rounded well‐defined central mass measuring about 12 mm in diameter. The mass was slightly hyperintense to the cortical gray matter on T2W, hypointense on T1W, without T2* signal void (Fig 1A–C). There was a central fusiform enhancement of the mass after administration of contrast medium (Fig 2). There was a regional meningeal enhancement ventrally, mostly rostral to the described mass. Both medial retropharyngeal LN were enlarged with heterogeneous enhancement. The suprasellar mass was initially suspected consistent with a neoplasm such as a macroadenoma or carcinoma, or less likely lymphoma.
Figure 1.
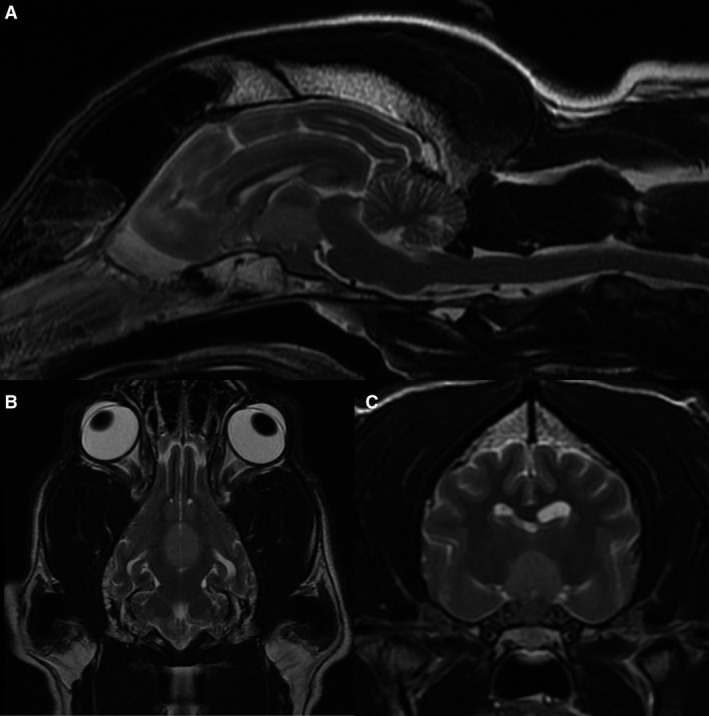
Midsagittal (A), dorsal (B), and transverse (C) T2‐weighted images of the brain. There is a well‐defined, slightly hyperintense to the normal cortical gray matter central mass in the hypophysis/hypothalamic region.
Figure 2.
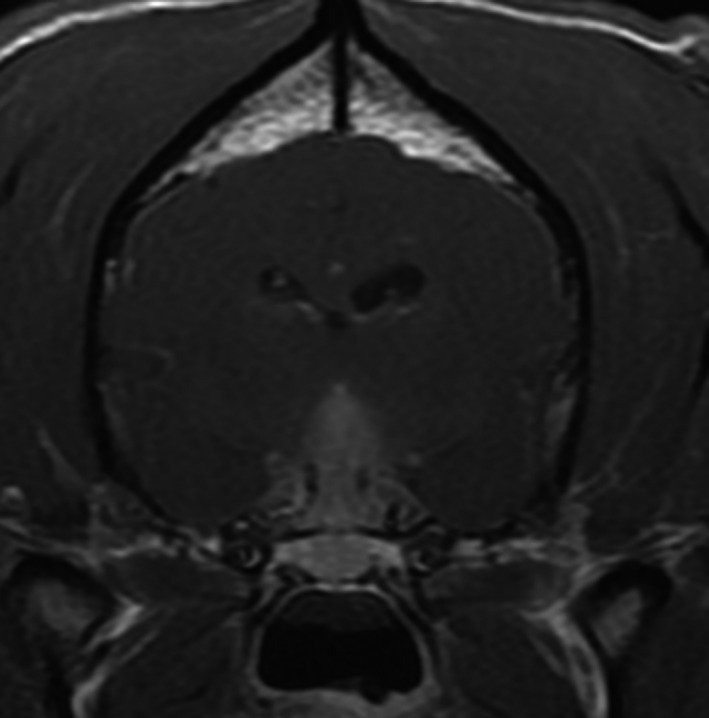
Transverse T1‐weighted image after administration of contrast showing central fusiform enhancement of the mass.
Total thyroxine (T4) and thyroid‐stimulating hormone (TSH) were both low at <5.15 nmol/L (RI): 13.5–50) and <0.03 ng/mL (RI: 0–0.6 ng/mL), respectively. Secondary hypothyroidism was strongly suspected due to the relatively low TSH and documented pituitary lesion, and supplementation with levothyroxine sodium2 was initiated at 0.02 mg/kg PO q24h. Due to marked deterioration, prednisolone at 0.25 mg/kg PO q24h was added to treatment, while endogenous ACTH and basal cortisol results were pending.
Despite the hormonal supplementation, the dog had a marked deterioration and became stuporous. An increase in body temperature was also noticed (40°C). Blood tests revealed a mild neutrophilic leukocytosis, presence of toxic neutrophils, and increased serum sodium concentration (200 mmol/L; RI: 139–150 mmol/L). Urine analysis identified a mild bacterial urinary tract infection. The acute hypernatremia was suspected to be due to a decompensated diabetes insipidus. Supplementation with desmopressin acetate3 into the conjunctival sac was initiated at 5 μg/dog q12hours was started, and fluid therapy was calculated to gradually decrease the serum sodium concentrations. Cefuroxime axetil4 at 15 mg/Kg PO q8hours was introduced to treat the lower urinary tract infection. Although serum sodium concentration decreased gradually, the dog deteriorated very quickly, went into respiratory failure, and died. The endogenous ACTH and basal cortisol results were received after the dog died. Basal cortisol was low <20 nmol/L (RI: 28‐250), but endogenous ACTH was not increased at 41 pg/mL (RI: 10–110 pg/mL). The absence of an elevated ACTH in combination with low cortisol was suggestive of secondary hypoadrenocorticism in the absence of presample glucocorticoid therapy.
Postmortem Examination and Histopathology
The body was submitted to the Veterinary Pathology Service of the University of Nottingham for postmortem examination. Upon removing the brain, the sella turcica appeared empty, and no pituitary gland was clearly identifiable. There was no visible mass or mass effect macroscopically. Both adrenal glands showed moderate thinning (atrophy) of the adrenal cortices with a corticomedullary ratio of 1 : 1 (reference range is 2 : 1). Examination of thyroid glands revealed bilateral mild‐to‐moderate atrophy. The parathyroid glands were of normal size.
Microscopic examination of remnant pituitary tissue showed a severe lymphocytic panhypophysitis with a marked loss of glandular parenchyma (Fig 3). A similar leukocytic infiltrate with a higher proportion of plasma cells and few histiocytes infiltrated diffusely the hypothalamus, affecting paraventricular and supraoptic nuclei and hypothalamic periventricular areas with a focal mild infiltration into the ventral third ventricle (Fig 4). A few periventricular vessels showed an eosinophilic, diffuse expansion of the vascular wall which was negative on Congo red and positive on Periodic Schiff acid stain (plasma leakage, fibrinoid degeneration). Other periventricular vessels showed a variable but mainly lymphoplasmacytic infiltration into the Virchow‐Robin space with a multifocal, mild‐to‐moderate lymphocytic vasculitis. The presence of a small number of intralesional histiocytes rendered a focal granulomatous meningoencephalitis (GME) a possible differential diagnosis, even though the lack of angiocentricity and the pituitary involvement would be unusual. Samples of the pituitary gland and hypothalamus were also stained by Giemsa and Gram stains which were negative for bacterial, fungal, and protozoal agents.
Figure 3.
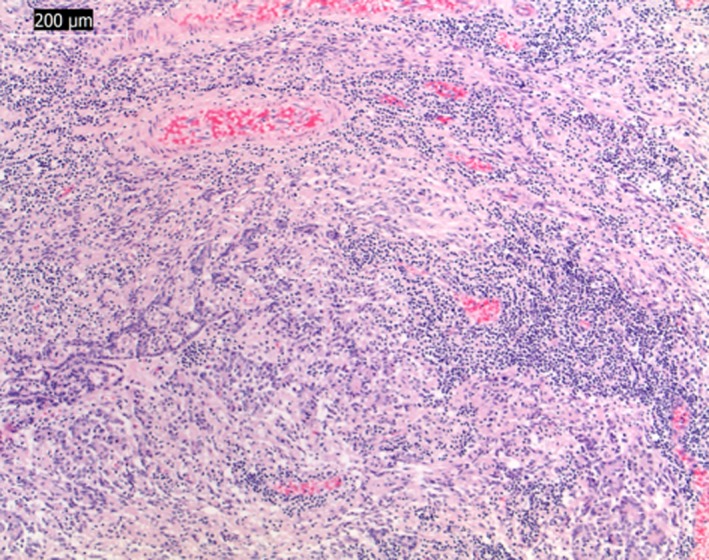
Light micrographs of paraffin‐embedded section stained with hematoxylin and eosin. Diffuse lymphocytic inflammation of the hypophysis. Bar indicates magnification (×10).
Figure 4.
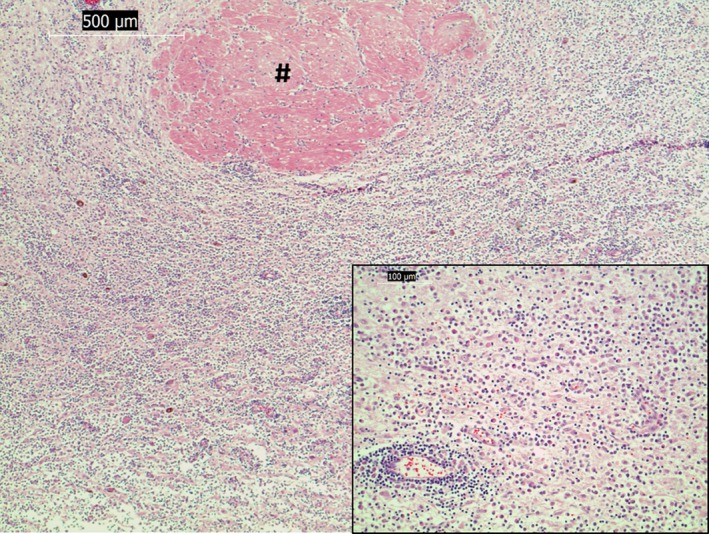
Light micrographs of paraffin sections stained with hematoxylin and eosin. Diffuse lymphoplasmacytic inflammation of the hypothalamus with perivascular cuffing (# shows the nonaffected left rostral commissure) (×5 magnification). Inset: higher magnification (×20) of infiltrating leukocytic cell population. Bar indicates magnification.
Immunohistochemistry (IHC) was conducted on tissue sections using antibodies against canine CD3, CD20, and Neospora caninum with appropriate positive and negative controls. IHC of the pituitary gland showed a predominantly T‐lymphocytic‐rich infiltrate with lower numbers of B cells (Fig 5A and B). The inflammation of the hypothalamus showed a higher component of plasma cells, and the IHC test for Neospora caninum antigen was negative.
Figure 5.
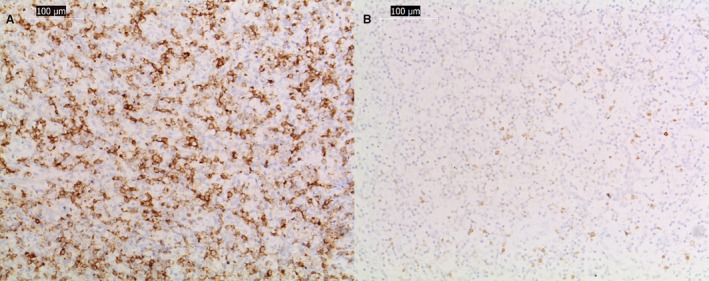
CD3+ (A) and CD20+ (B) immunohistochemical‐stained serial sections of the inflammatory infiltrate of the hypophysis. Images (A) show high numbers of lymphocyte T (CD3+ positive) with less but also abundant B cells (CD20+ positive). Bar indicates magnification (×20).
The fascicular and the reticular layers of the adrenal cortex from both glands were moderately reduced in thickness (cortical adrenal atrophy) with no signs of inflammation.
Discussion
Histopathologic examination of this dog revealed a global infiltration of the pituitary gland extending to the hypothalamus with lymphocytic panhypophysitis and hypothalamitis. In people, lymphocytic hypophysitis (LH) is a neuroendocrine disorder characterized by an autoimmune inflammation of the pituitary gland with various degrees of pituitary dysfunction, which most commonly affects females in late pregnancy or early postpartum period.12 There are human cases with a hypothalamic involvement due to antibodies targeting neurohypophyseal hormone‐secreting neurons.11
After imaging, the case was initially interpreted as a suprasellar mass, and the correct diagnosis was made at postmortem. It is known that MRI findings in people with lymphocytic hypophysitis are challenging as neither clinical presentation nor MRI findings allow a clear differentiation of hypophysitis from pituitary masses. In pituitary macroadenomas in dogs, MRI findings usually report a relatively well‐circumscribed expansile T1W iso‐ to hypointense and T2W/FLAIR hyperintense mass with strong contrast enhancement. However, pituitary hemorrhage might occur in any pituitary macrotumor, resulting in susceptibility artefacts on T2* images and alteration of signal intensity of the mass before and after contrast images. In the present case, the pattern of the mass on MRI was unusual for an adenoma, with inhomogeneous central fusiform enhancement, while marked and relatively diffuse enhancement would be expected in a non‐necrotic and nonhemorrhagic standard macroadenoma. In people, MRI features more suggestive of autoimmune inflammatory hypophysitis, as compared to pituitary adenoma, include a symmetric enlargement of the pituitary gland, a thickened, nontapering pituitary stalk and an intact sellar floor and may help to distinguish from pituitary adenoma.10, 12 To date, no single radiologic sign has proven to have sufficient accuracy in distinguishing with certainty LH from pituitary adenomas.
In human patients, the type, phase, and degree of lymphocytic hypophyseal infiltration determine the clinical signs. Most frequently, diabetes insipidus, hypopituitarism, headache, and visual defects are reported.12, 13 The sudden deterioration of this dog could have prompted a clinical diagnosis of “pituitary apoplexy,” a syndrome resulting from sudden infarction, hemorrhage, or both, in a normal or an adenomatous pituitary gland.14 There are few descriptions of pituitary apoplexy in dogs in the veterinary literature,15 but in people, there is usually signal void on T2* (hemorrhagic lesion) and no central contrast enhancement (ischemic infarction)16, 17 which did not fit with our case.
Pituitary insufficiency might affect all pituitary hormones, but normal and elevated plasma concentrations occur, probably representing different stages of the disease.18 In the present case, there was reduced TSH and T4, but normal plasma ACTH concentration. Unfortunately, the dog died before the cortisol, and ACTH results were obtained. For this reason, an ACTH stimulation test was not performed, but the basal cortisol and ACTH were suggestive of a secondary hypoadrenocorticism. Hypothalamic involvement in autoimmune hypophysitis has rarely been described and usually manifests as central diabetes insipidus as seen in our case.19, 20 Although previously considered to be due to possible expansion of an inflammatory process from cranial to caudal lobe, with consequent inhibition of the axonal transport of antidiuretic hormone, hypothalamic involvement has now been shown to be due to the antihypothalamic antibodies targeting arginine vasopressin‐secreting neurons, strongly suggesting the presence of hypothalamic autoimmunity in autoimmune hypophysitis.11
In the present case, the etiopathogenesis of the lymphocytic hypophyseal and lymphoplasmacytic hypothalamic inflammation remains uncertain: there was no evidence of an infectious agent. Known idiopathic inflammatory diseases such as canine meningoencephalitides of unknown origin were considered unlikely, with the possible exception of a focal GME which is usually more angiocentric and histiocytic, less diffuse within the neuroparenchyma and when close to the leptomeninges often incites a stronger meningeal inflammation than that observed in this case. To our knowledge, a pituitary involvement has not been described in GMEs in dogs or in other forms of meningoencephalitis of unknown origin.
The hypophysitis was predominantly lymphocytic with most lymphocytes being CD3‐positive T cells, and the adenohypophysis as well as the pars intermedia and neurohypophysis were affected; therefore, we suggest an autoimmune, subacute, lymphocytic, panhypophysitis as a likely diagnosis. The increased number of large plasma cells and B cells in the hypothalamus might represent, as described in the medical literature, an antibody‐mediated immune reaction against neurosecretory products like vasopressin. In people, the initial pituitary enlargement in LH, secondary to inflammatory infiltration and edema, can evolve to progressive diffuse pituitary destruction,12 and this mechanism can explain why the sellar region appeared “empty” on postmortem examination, so‐called empty sella syndrome. In humans, panhypophysitis affecting the entire pituitary gland is rare but there are some very similar reports to the present case: Two human patients with reported necrotizing infundibulo‐hypophysitis presented a combination of diabetes insipidus and hypopituitarism, and both were suspected to have pituitary tumors with suprasellar extension. Each one was found to have a suprasellar mass lesion with an abnormally thickened enlarged pituitary stalk that intensively enhanced on contrast MRI. However, tissue obtained at transsphenoidal surgery revealed necrosis, fibrosis, and chronic inflammation; there was no evidence of infiltrative, infective, or neoplastic disease processes.21 These disorders are generally considered idiopathic or autoimmune in origin.22 The treatment in these cases consists of replacement of deficient hormones and anti‐inflammatory therapy.23, 24 One case of presumptive LH in a dog resolved after corticosteroid treatment, so both hormonal supplementation and corticosteroids may be considered a promising option when a case is suspected.9
After diagnostic investigation, the dog developed high temperature. Given the intensive inflammatory and destructive changes within the hypothalamus, the fever was thought to be of central origin affecting the temperature regulating center within the hypothalamus25, 26 but the mild bacterial lower urinary tract infection might have played a contributory role.
In conclusion, the clinical diagnosis of lymphocytic hypophysitis and hypothalamitis in dogs can be challenging as the clinical presentation does not differ from that of more common pituitary masses and MRI imaging findings, like in humans, might not allow differentiation of LH from other pituitary masses. An inhomogeneous enhancement of a pituitary mass‐like lesion can raise the suspicion of an inflammatory process. In these cases, imaging findings should be combined with other diagnostic techniques to reach a final diagnosis. Considering that infectious diseases play an important role in development of hypophysitis, laboratory tests to diagnose infectious diseases, might be necessary. In humans, transsphenoidal biopsy is performed for conclusive histopathologic diagnoses; however, the biopsy procedure can be traumatic and complicated. We conclude that lymphocytic hypophysitis, although very rare, is a differential diagnosis that should be considered when evaluating pituitary changes detected on MRI in dogs, because it warrants very different management and treatment and may have a better prognosis.
Acknowledgments
We are grateful to Annette Wessmann, Marc Dhumeaux, Ranieri Verin, Carlo Cantile, Simone de Brot and Llorenc Grau Roma for insightful discussions and recommendations. We also thank Alan Lasslett, Joanne Sanders, and Pauline Brind for their excellent technical work.
Conflict of Interest Declaration
The authors declared no conflict of interests with respect to the research, authorship, and/or publication of this article.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The work was performed at the University of Nottingham and Pride Veterinary Centre (clinical associate veterinary practice of the University of Nottingham).
The authors received no financial support for the research, authorship, or publication of this article.
Parts of this case were presented as a poster at the 29th Annual Symposium of the European Society of Veterinary Neurology and European College of Veterinary Neurology (2016) on September 15 to 17th, Edinburgh, United Kingdom.
Footnotes
Dotarem, Guerbet, Solihull, UK
Thyforon, Dechra, Northwich, UK
DDAVP, Ferring Pharmaceuticals, West Drayton, UK
Cefuroxime, Teva UK limited, East Sussex, UK
References
- 1. Maitra A. The Endocrine System. Robbins & Cotran Pathologic Basis of Disease, 9th ed. Philidelphia, PA: Elsevier Health Sciences; 2015; 1074–1137. [Google Scholar]
- 2. Rosol T, Grone A. Endocrine Glands. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. Vol. 3, 6th ed St. Louis, Missouri: Elsevier; 2016; 269–343. [Google Scholar]
- 3. Lury KM. Inflammatory and infectious processes involving the pituitary gland. Top Magn Reson Imaging 2005;16:301–306. [DOI] [PubMed] [Google Scholar]
- 4. Carpinteri R, Patelli I, Casanueva FF, Giustina A. Inflammatory and granulomatous expansive lesions of the pituitary. Best Pract Res Clin Endocrinol Metab 2009;23:639–650. [DOI] [PubMed] [Google Scholar]
- 5. Wolfesberger B, Fuchs‐Baumgartinger A, Schwendenwein I, et al. Sudden death in a dog with lymphoplasmacytic hypophysitis. J Comp Pathol 2011;145:231–234. [DOI] [PubMed] [Google Scholar]
- 6. Meij BP, Voorhout G, Gerritsen RJ, et al. Lymphocytic hypophysitis in a dog with diabetes insipidus. J Comp Pathol 2012;147:503–507. [DOI] [PubMed] [Google Scholar]
- 7. Adissu H, Hamel‐Jolette A, Foster R. Lymphocytic adenohypophysitis and adrenalitis in a dog with adrenal and thyroid atrophy. Vet Pathol 2010;47:1082–1085. [DOI] [PubMed] [Google Scholar]
- 8. McAllister MM. Adenohypophysitis associated with sebaceous gland atrophy in a dog. Vet Pathol 1991;28:340–341. [DOI] [PubMed] [Google Scholar]
- 9. Rzechorzek NM, Liuti T, Stalin C, Marioni‐Henry K. Restored vision in a young dog following corticosteroid treatment of presumptive hypophysitis. BMC Vet Res 2016;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gutenberg A, Larsen J, Lupi I, et al. A radiologic score to distinguish autoimmune hypophysitis from nonsecreting pituitary adenoma preoperatively. Am J Neuroradiol 2009;30:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bianchi A, Mormando M, Doglietto F, et al. Hypothalamitis: A diagnostic and therapeutic challenge. Pituitary 2014;17:197–202. [DOI] [PubMed] [Google Scholar]
- 12. Falorni A, Minarelli V, Bartoloni E, et al. Diagnosis and classification of autoimmune hypophysitis. Autoimmun Rev 2014;13:412–416. [DOI] [PubMed] [Google Scholar]
- 13. Caturegli P, Lupi I, Landek‐Salgado M, et al. Pituitary autoimmunity: 30 years later. Autoimmun Rev 2008;7:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long SN, Michieletto A, Anderson TJ, et al. Suspected pituitary apoplexy in a German shorthaired pointer. J Small Anim Pract 2003;44:497–502. [DOI] [PubMed] [Google Scholar]
- 15. Bertolini G, Rossetti E, Caldin M. Pituitary apoplexy‐like disease in 4 dogs. J Vet Intern Med 2007;21:1251–1257. [DOI] [PubMed] [Google Scholar]
- 16. Tosaka M, Sato N, Hirato J, et al. Assessment of hemorrhage in pituitary macroadenoma by T2*‐weighted gradient‐echo MR imaging. Am J Neuroradiol 2007;28:2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ostrov S, Quencer R, Hoffman J, et al. Hemorrhage within pituitary adenomas: How often associated with pituitary apoplexy syndrome? Am J Roentgenol 1989;153:153–160. [DOI] [PubMed] [Google Scholar]
- 18. Shimono T, Hatabu H, Kasagi K, et al. Rapid progression of pituitary hyperplasia in humans with primary hypothyroidism: Demonstration with MR imaging. Radiology 1999;213:383–388. [DOI] [PubMed] [Google Scholar]
- 19. Garg MK, Gundgurthi A, Dutta MK, et al. Hypopituitarism due to isolated lymphocytic hypothalamitis in a young girl. World J Endocr Surg 2011;3:33–37. [Google Scholar]
- 20. Stelmachowska M, Bolko P, Wasko R, et al. Lymphocytic hypophysitis and hypothalamitis‐case report. Endokrynol Pol 2006;57:648–653. [PubMed] [Google Scholar]
- 21. Ahmed SR, Aiello DP, Page R, et al. Necrotizing infundibulo‐hypophysitis: A unique syndrome of diabetes insipidus and hypopituitarism. J Clin Endocrinol Metab 1993;76:1499–1504. [DOI] [PubMed] [Google Scholar]
- 22. Guo S, Wang C, Zhang J, et al. Diagnosis and management of tumor‐like hypophysitis: A retrospective case series. Oncol Lett 2015;11:1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lupi I, Manetti L, Raffaelli V, et al. Diagnosis and treatment of autoimmune hypophysitis: A short review. J Endocrinol Invest 2011;34:245–252. [DOI] [PubMed] [Google Scholar]
- 24. Hypophysitis Faje A. Evaluation and management. Clin Diabetes Endocrinol 2016;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 2011;16:74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honig A, Michael S, Eliahou R, Leker RR. Central fever in patients with spontaneous intracerebral hemorrhage: Predicting factors and impact on outcome. BMC Neurol 2015;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]


