Abstract
Background
A hyperbolic relationship between β‐cell response and insulin sensitivity (IS) has been described in several species including rodents, dogs, and humans. This relationship has not been elucidated in the horse.
Hypothesis/Objectives
To determine whether the hyperbolic relationship between β‐cell response and IS exists in horses by using indices of β‐cell response from the oral sugar test (OST) and IS measurements from the euglycemic hyperinsulinemic clamp (EHC). A second aim was to compare how well IS estimates from the OST and EHC correlate.
Animals
Forty‐nine horses with different degrees of insulin regulation (normal‐to‐severe insulin dysregulation).
Methods
Cross‐sectional study. Horses were examined with an OST and an EHC.
Results
Decreased IS was associated with increased β‐cell response in the horses. Nine of 12 comparisons between indices of β‐cell response and IS measures fulfilled the criteria for a hyperbolic relationship. Indices of IS calculated from the OST correlated highly with the insulin‐dependent glucose disposal rate (M) and the insulin‐dependent glucose disposal rate per unit of insulin (M/I) determined from the EHC (r = 0.81–0.87).
Conclusions and Clinical Importance
A hyperbolic relationship between β‐cell response and IS exists in horses, which suggest that horses with insulin dysregulation respond not only with postprandial hyperinsulinemia but are also insulin resistant. The OST is primarily a test for β‐cell response rather than a test for IS, but calculated indices of IS from the OST may be useful to estimate IS in horses, especially when the horse is insulin resistant.
Keywords: Equine metabolic syndrome, Hyperbolic relationship, Insulin dysregulation, Insulin resistance
Abbreviations
- AUCINS/GLU‐30
incremental area under the curve for insulin/glucose at 30 minutes of OST
- AUCINS/GLU‐120
incremental area under the curve for insulin/glucose at 120 minutes of OST
- AUCINS/GLU‐180
incremental area under the curve for insulin/glucose at 180 minutes of OST
- AUCINS‐180
incremental area under the curve for insulin at 180 minutes of OST
- BCS
body condition score
- CNS
cresty neck score
- CV
coefficient of variation
- DI
disposition index
- EHC
euglycemic hyperinsulinemic clamp
- EMS
equine metabolic syndrome
- ID
insulin dysregulation
- INSINDEX
insulinogenic index
- IR
insulin resistance
- IS
insulin sensitivity
- ISI60
60‐minutes insulin sensitivity index
- ISI90
90‐minutes insulin sensitivity index
- ISICOMP
composite whole‐body insulin sensitivity index
- ISIPEAK
peak insulin sensitivity index
- M
mean insulin‐dependent glucose disposal rate
- M/I
mean insulin‐dependent glucose disposal rate per unit of insulin
- NIMGU
non‐insulin‐mediated glucose uptake
- NIR
normal insulin regulation
- OST
oral sugar test
- PeakINS
peak insulin concentration during OST
- SD
standard deviation
Insulin dysregulation (ID), a key feature of the equine metabolic syndrome (EMS), is a nonspecific term used to describe horses with abnormalities in their insulin metabolism encompassing either fasting hyperinsulinemia, excessive insulin response to sugars, or insulin resistance (IR).1 The euglycemic hyperinsulinemic clamp (EHC) is considered to be the gold standard for measurements of tissue IR, but the test is costly, complex, and time‐consuming.2, 3 The recently developed oral sugar test (OST),4 a modified variant of the oral glucose tolerance test (OGTT),5 is easier to perform and generates information about both glucose disposal and insulin secretion from the β‐cells in the pancreas.3, 6 Theoretically, the oral tolerance tests (OST and OGTT) are therefore suitable for estimating both insulin sensitivity (IS) and β‐cell response.6 The oral tolerance tests generally are considered to be less specific for measuring β‐cell response compared to the hyperglycemic clamp, because the insulin secretion pattern after oral glucose administration reflects the combined response to several physiological factors such as gastric emptying, degree of hepatic glucose trapping, and incretin effects. Still, administration of sugars PO more closely mimics a physiological stimulation of insulin secretion compared to the IV tests.3, 6, 7 The oral tolerance tests also have been used to calculate indices of IS (eg, composite whole‐body insulin sensitivity index [ISICOMP]) in both humans and horses.8, 9 These indices are not specific quantifications of IS but have been shown to correlate well with IS measured by the EHC in humans.8, 10
The β‐cell response and IS are dependent on each other, whereby changes in 1 feature are mirrored by a reciprocal adaptation in the other.11 A nonlinear inverse relationship between β‐cell response and IS was first described in humans in 1981,12 and this relationship was further characterized and described in 1993 as rectangular hyperbolic.13 The hyperbolic relationship between β‐cell response and IS has, during the last few decades, been described in several species including rodents, dogs, and humans13, 14, 15 but has, to the authors’ knowledge, not been elucidated in horses. In a rectangular hyperbolic relationship, the product of β‐cell response and IS is constant for a given degree of glucose tolerance. This product is called disposition index (DI) and represents an index of β‐cell function related to the patient's IS. A decrease in DI is thus an indication of altered β‐cell function, resulting in inability to compensate for decreased IS (ie, impaired glucose tolerance and type 2 diabetes mellitus).16
The aim of our study was to test whether a rectangular hyperbolic relationship exists between β‐cell response and IS, by comparing OST‐derived indices of β‐cell response with IS measured by the EHC, in horses with variable degrees of insulin regulation (normal‐to‐severe ID). One further aim was to compare how well indices of IS derived from the OST correlate with quantitative measurements of IS determined by the gold standard EHC.
Materials and Methods
Horses
The study was approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden. Forty‐nine horses with different degrees of insulin regulation (normal‐to‐severe ID) were used in the study. The horses were owned by the Swedish University of Agricultural Sciences, the National Council Horse Facility, or privately owned. Criteria for inclusion were as follows: normal findings on clinical examination except for general obesity, regional obesity, or both, age ≥4 years, negative test results for pituitary pars intermedia dysfunction (normal plasma ACTH concentration) at the start of the study as well as no ongoing episode of laminitis. The privately owned horses were recruited from a group of horses previously diagnosed with ID and referred to the University Animal Hospital, Swedish University of Agricultural Sciences for an extended examination. The horses owned by the Swedish University of Agricultural Sciences or by the National Council Horse Facility were examined in their home environment. All horses in the study were fed a hay or haylage diet supplemented with minerals. Horses were housed in individual box stalls and allowed daily turnout in a dirt or sand paddock. None of the horses had been on grass pasture for at least 2 months before testing.
Experimental Design
All horses were acclimatized for at least 48 hours to the environment where sampling was to take place. After acclimatization, horses were screened with an OST followed by an EHC the next day. Both the OST and the EHC commenced after 12 hours of feed withdrawal overnight, but with free access to water. Based on results from the OST performed as part of the study, horses were allocated into 2 different groups: normal insulin regulation (NIR; peak insulin concentration <45 μIU/mL) and ID (peak insulin dysregulation ID>45 μIU/mL).1
OST—Oral Sugar Test
The day before testing an IV catheter2 was inserted aseptically into 1 of the jugular veins under local anesthesia.3 The OST procedure was conducted as described previously.17 Briefly, Dan Sukker Glykossirap4 was syringed PO at a dosage of 0.2 mL/kg body weight. Blood samples were collected from the jugular catheter into evacuated tubes5 containing lithium heparin, immediately before (−5 minutes), and at 30, 60, 90, 120, 150, and 180 minutes after the PO administration of syrup. Tubes were placed on ice immediately after sampling and centrifuged within 5 minutes for 10 minutes at 2,700 × g. Plasma was subsequently harvested, frozen rapidly, and stored at −80°C until later analysis of plasma glucose and plasma insulin concentrations (see below).
Plasma glucose and plasma insulin concentrations from the OST were used to calculate 6 indices of β‐cell response: peak insulin concentration (PeakINS), insulinogenic index (INSINDEX),18, 19 incremental area under the insulin curve for the time interval 0–180 minutes (AUCINS‐180),20, 21 and the ratio of the incremental area under the insulin curve to the incremental area under the glucose curve for the time intervals 0–30, 0–120, and 0–180 minutes (AUCINS/GLU‐30, AUCINS/GLU‐120 and AUCINS/GLU‐180).22 The glucose and insulin data from the OST also were used for calculation of an IS index: ISICOMP.8 In addition, new simplified methods to calculate ISICOMP using fewer time points were introduced: peak insulin sensitivity index (ISIPEAK), 60‐minutes insulin sensitivity index (ISI60), and 90‐minutes insulin sensitivity index (ISI90).
The INSINDEX was calculated as (I 30−I 0)/(G 30−G 0), where I 30 = insulin concentration at 30 minutes of OST, I 0 = baseline insulin concentration, G 30 = glucose concentration at 30 minutes of OST, and G 0 = baseline glucose concentration.
The AUCINS‐180, AUCINS/GLU‐30, AUCINS/GLU‐120, and AUCINS/GLU‐180 were calculated by use of the trapezoid method.6
The ISICOMP was calculated using the following formula: 1,000/, where G = baseline glucose concentration, I = baseline insulin concentration, MG = mean glucose concentration during the OST, and MI = mean insulin concentration during the OST.
The ISIPEAK, ISI60, and ISI90 were calculated as: 100/, 100/, and 100/ respectively, where G peak = peak glucose concentration, I peak = peak insulin concentration, G60 = glucose concentration at 60 minutes, I60 = insulin concentration at 60 minutes, G90 = glucose concentration at 90 minutes, I90 = insulin concentration at 90 minutes during the OST.
EHC—Euglycemic Hyperinsulinemic Clamp
After the OST examination, a second IV catheter5 was inserted into the contralateral jugular vein. On the morning of the following day, blood samples for determination of baseline concentrations of plasma glucose and plasma insulin were drawn from 1 of the IV catheters immediately before (−10, −5, and −1 minute) the start of the EHC. The EHC procedure has previously been described for use in horses.23, 24 Briefly, a continuous rate infusion of glucose7 and recombinant human insulin8 was initiated through 1 of the jugular catheters, with a multichannel volumetric infusion pump.9 The infusion rate for insulin was held constant at 3 mIU/kg/min, and a variable rate of glucose was infused to maintain blood glucose concentration at euglycemia (5 mmol/L) during the 3‐hour infusion. The glucose infusion rate was adjusted if the concentration deviated by more than 0.2 mmol/L from euglycemia. Blood samples were obtained every 5 minutes for immediate analysis of blood glucose concentration with a handheld glucometer10 and every 10 minutes throughout the EHC for subsequent determination of plasma glucose (10‐minutes intervals) and plasma insulin (20‐minutes intervals) concentrations. These blood samples were collected into evacuated tubes5 containing lithium heparin and placed on ice for 5 minutes before centrifugation (10 minutes, 2,700 × g). Plasma was separated, frozen rapidly, and stored at −80°C until later analysis of plasma glucose and plasma insulin concentrations (see below).
The first 120 minutes of the EHC was considered an equilibration period. Plasma glucose and plasma insulin concentrations from the final 60 minutes (steady state) were used for calculation of the total mean glucose disposal rate (M 60) and the mean insulin concentration (I).23 The non‐insulin‐mediated glucose uptake (NIMGU) at euglycemia (5 mmol/L) was estimated to be 0.57 mg/kg/min. This value was obtained by extrapolating data from NIMGU during hyperglycemic clamps in horses25 using the strong linear relationship that has been demonstrated between plasma glucose concentration and NIMGU during stepwise hyperglycemic clamps.26 The intercept was set to 0. The mean insulin‐dependent glucose disposal rate (M) then was calculated as M 60 – NIMGU and expressed per unit of insulin (M/I).
Analysis of Blood Samples
Plasma glucose concentrations were measured enzymatically with an automated clinical chemistry analyzer.11 Endogenous equine plasma insulin concentrations from the OST were determined using a commercial equine‐optimized ELISA12 evaluated for use in horses,27 and insulin concentrations were controlled for with a commercial kit.13 Plasma insulin concentrations from the continuous rate infusion of recombinant human insulin during the EHC procedures were determined using a commercial ELISA14 designed for use in humans, and a commercial kit15 was used as a control. All analyses of plasma glucose and plasma insulin concentrations were performed in duplicate. The mean intra‐assay coefficients of variation (CVs) calculated from samples run in duplicate for glucose, equine insulin (equine‐optimized ELISA), and human insulin (human ELISA) were 0.6, 2.2, and 2.7%, respectively. The equine‐optimized ELISA12 previously has been evaluated in our laboratory with reported intra‐assay CVs between 2.0 and 6.5% and an interassay CV of 10.7%.27
Statistical Analysis
All data were analyzed using JMP Pro 11.2.0.16
Differences in physical characteristics (age, body condition score [BCS], cresty neck score [CNS], and body weight), EHC data (M and M/I), and time to PeakINS during OST were compared between the NIR and ID groups by use of an independent t‐test or a nonparametric test (Wilcoxon rank‐sum test) as appropriate.
The rectangular hyperbolic function (y = constant × 1/x), can be re‐expressed as a linear model by log‐transformation of data producing the following equation: Ln (y) = constant + β × Ln (x), where β is the regression coefficient. This linear function was used to determine the β for the different combinations of OST‐derived indices of β‐cell response (dependent variable) and EHC‐derived sensitivity measures (independent variable). The 95% confidence interval (CI) for β was calculated by using the bootstrap method with 1,000 replications randomly selected from the original data. A rectangular hyperbolic relationship between β‐cell response and IS was established if β was approximately equal to −1 (a criterion that was fulfilled if the 95% CI for β included −1), and if the 95% CI for β excluded zero. The linear regressions were run by the fit orthogonal command in the bivariate platform. This model adjusts for measurement errors in both the y (dependent) and x (independent) variables. The error estimates for OST‐derived indices of β‐cell response were based on repeated testing performed by our research group with 17 healthy horses, where mean CVs of 19.4, 26.1, 23.3, 25.9, 24.4, and 28.0% were found for PeakINS, INSINDEX, AUCINS‐180, AUCINS/GLU‐30, AUCINS/GLU‐120, and AUCINS/GLU‐180, respectively. The error estimates for EHC‐derived sensitivity measures were based on previously published data reporting mean CVs of 12.2 and 14.1% for M and M/I, respectively.28
To examine how well IS indices derived from the OST (ISICOMP, ISIPEAK, ISI60, and ISI90) correlated with EHC‐derived sensitivity measures (M and M/I), linear regression analyses were performed. Data were logarithmically transformed before analysis, to correct for skewed distribution of residuals.
Values of P < 0.05 were considered as significant for all analyses. All data are presented as mean ± standard deviation (SD) except for non‐normally distributed data, which are presented as median and interquartile range.
Results
Horses
All horses fulfilled the inclusion criteria for the study. Based on results from the OST, 23 and 26 horses, respectively, were allocated into the NIR and the ID groups. None of the horses included in the NIR group, but 62% (16/26) of horses, included in ID group, had a previous history of laminitis. Physical characteristics for horses included in the study are reported in Table 1. Horses in the ID group had higher BCS (P < 0.0001) and CNS (P < 0.0001) than horses in the NIR group.
Table 1.
Physical characteristics for horses (n = 49) included in the study divided into normal insulin regulation (NIR) and insulin dysregulation (ID) groups
| NIR n = 23 | ID n = 26 | |
|---|---|---|
| Sex (g/m) | 12/11 | 5/21 |
| Age (y) | 9.5 ± 3.9a | 14.1 ± 4.3b |
| Body weight | 424 ± 101a | 423 ± 116a |
| BCS (1–9) | 5.3 ± 0.6a | 6.7 ± 1.1b |
| CNS (0–5) | 2.2 ± 0.5a | 3.5 ± 0.7b |
SD, standard deviation; g, gelding; m, mare; BCS, body condition score; CNS, cresty neck score.
Data are mean ± SD. a,bMeans with different superscript letters differ significantly within row (P < 0.05).
The breeds represented in the NIR group were Standardbred (n = 10), Icelandic horse (n = 6), Swedish warmblood (n = 1), and Gotland pony (n = 6). The ID group included the following breeds: Fjord horse (n = 2), Icelandic horse (n = 10), Shetland pony (n = 2), Welsh cob (n = 1), Highland pony (n = 1), Swedish warmblood (n = 2), Standardbred (n = 4), Swedish riding pony (n = 1), crossbreed horse or pony (n = 2), and Hanoverian (n = 1).
Oral Sugar Test and Euglycemic Hyperinsulinemic Clamp
The median PeakINS for horses in the NIR and ID groups were 28 (20–36) μIU/mL and 149 (89–223) μIU/mL, respectively. There was no difference in median time to PeakINS (P = 0.01) between the NIR (90 (30–90) minutes) and ID (90 (60–120) minutes) groups. For 3 of the horses, the early glucose response, insulin response, or both were delayed. Indices for early β‐cell response (INSINDEX and AUCINS/GLU‐30) could therefore not be obtained for these horses.
The plasma glucose concentration during the final 60 minutes (steady state) of the EHC had a mean CV of 3.8 ± 1.6. Horses in the NIR group had higher M (P < 0.0001) and M/I (P < 0.0001) compared to horses in the ID group. The median values for M and M/I were 2.8 (2.2–3.6) mg/kg/min and 5.2 (4.1–6.8) (mg/kg/min × 103)/(μIU/mL) for the NIR group and 0.8 (0.5–1.1) mg/kg/min and 0.9 (0.6–2.0) (mg/kg/min × 103)/(μIU/mL) for the ID group.
Hyperbolic Relationship Between β‐cell Response and Insulin Sensitivity
The estimated regression coefficient (β), correlation coefficient (r), and the 95% CI for β obtained from regression analysis comparing OST‐derived indices of β‐cell response and EHC‐derived sensitivity measures are shown in Table 2. Scatter plots with data for 6 indices of β‐cell response (PeakINS, AUCINS‐180, INSINDEX, AUCINS/GLU‐30, AUCINS/GLU‐120, or AUCINS/GLU‐180) versus 2 measurements of IS (M or M/I) illustrate a nonlinear inverse relationship (Figs 1 and 2). A rectangular hyperbolic relationship was evident in 9 of the 12 aforementioned comparisons using the following model: Ln (y) = constant + β × Ln (x). As IS decreased, the β‐cell response increased.
Table 2.
Correlation coefficient (r), regression coefficient (β), and 95% confidence interval for β for combinations of oral sugar test derived indices of β‐cell response and euglycemic hyperinsulinemic clamp derived sensitivity measures used in the regression model: Ln (y) = constant + β × Ln (x). Three of the 49 horses had a delayed early glucose and/or insulin responses during OST, resulting in missing data for INSINDEX and AUCINS/GLU‐30
| β‐cell response | Sensitivity measures | r | β (95% CI) |
|---|---|---|---|
| PeakINS | M | −0.84 | −1.30 (−1.50 to −1.10) |
| PeakINS | M/I | −0.84 | −1.03a (−1.22 to −0.90) |
| INSINDEX | M | −0.77 | −1.03a (−1.32 to −0.92) |
| INSINDEX | M/I | −0.87 | −1.10a (−1.30 to −0.99) |
| AUCINS‐180 | M | −0.87 | −1.40 (−1.62 to −1.19) |
| AUCINS‐180 | M/I | −0.86 | −1.12a (−1.35 to −0.96) |
| AUCINS/GLU‐30 | M | −0.77 | −1.04a (−1.33 to −0.80) |
| AUCINS/GLU‐30 | M/I | −0.73 | −0.80a (−1.01 to −0.59) |
| AUCINS/GLU‐120 | M | −0.81 | −1.17a (−1.44 to −1.00) |
| AUCINS/GLU‐120 | M/I | −0.80 | −0.92a (−1.19 to −0.83) |
| AUCINS/GLU‐180 | M | −0.81 | −1.16 (−1.43 to −1.02) |
| AUCINS/GLU‐180 | M/I | −0.80 | −0.91a (−1.17 to −0.82) |
PeakINS, peak insulin concentration during OST; INSINDEX, insulinogenic index; AUCINS‐180, incremental area under the curve for insulin at 180 min of OST; AUCINS/GLU‐30, incremental area under the curve for insulin/glucose at 30 min of OST; AUCINS/GLU‐120, incremental area under the curve for insulin/glucose at 120 min of OST; AUCINS/GLU‐180, incremental area under the curve for insulin/glucose at 180 min of OST; M, mean insulin‐dependent glucose disposal rate; M/I, mean insulin‐dependent glucose disposal rate per unit of insulin.
Indicate rectangular hyperbolic relationship within row (P < 0.05).
Figure 1.
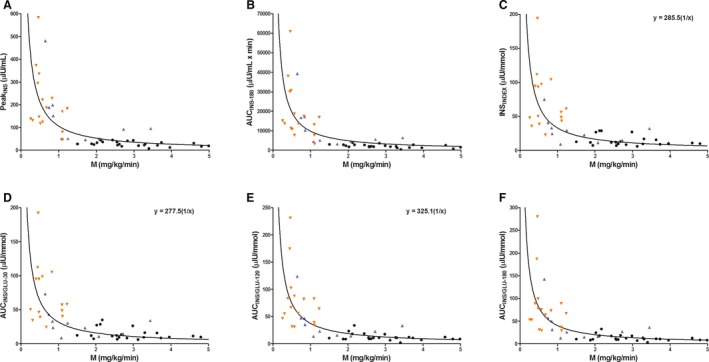
Scatter plots of individual horse data (n = 49) showing the relationship between oral sugar test derived indices of β‐cell response: PeakINS (A), AUCINS ‐180 (B), INSINDEX (C), AUCINS / GLU ‐30 (D), AUCINS / GLU ‐120 (E), and AUCINS / GLU ‐180 (F) versus the mean insulin‐dependent glucose disposal rate (M), in horses with normal insulin regulation (NIR, black circle) and insulin dysregulation (ID with previous history of laminitis, orange downward pointing triangle; ID without previous history of laminitis, blue upward pointing triangle). A solid line (line of best fit) represents a rectangular hyperbolic curve fitted to the data. The curve is based on the function (y = constant × 1/x), where the constant = disposition index. Data that fulfill the statistical criteria of a rectangular hyperbolic curve have the equation in the figure (C, D and E). Three of the horses had a delayed early glucose and/or insulin responses during OST, resulting in missing data for INSINDEX and AUCINS / GLU ‐30.
Figure 2.
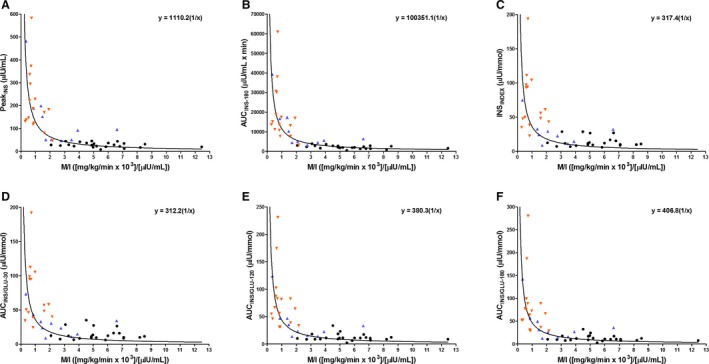
Scatter plots of individual horse data (n = 49) showing the relationship between oral sugar test derived indices of β‐cell response: PeakINS (A), AUCINS ‐180 (B), INSINDEX (C), AUCINS / GLU ‐30 (D), AUCINS / GLU ‐120 (E), and AUCINS / GLU ‐180 (F) versus the mean insulin‐dependent glucose disposal rate per unit of insulin (M/I), in horses with normal insulin regulation (NIR, black circle) and insulin dysregulation (ID with previous history of laminitis, orange downward pointing triangle; ID without previous history of laminitis, blue upward pointing triangle). A solid line (line of best fit) represents a rectangular hyperbolic curve fitted to the data. The curve is based on the function (y = constant × 1/x), where the constant = disposition index. Data that fulfill the statistical criteria of a rectangular hyperbolic curve have the equation in the figure (A ‐ F). Three of the horses had a delayed early glucose and/or insulin responses during OST, resulting in missing data for INSINDEX and AUCINS / GLU ‐30.
Correlation Between OST‐Derived IS Indices and Sensitivity Measures from the EHC
Selected scatter plots showing the correlation between OST‐derived IS indices (ISIPEAK and ISICOMP) and the M/I are presented on the original scale and after log‐transformation (Fig 3). Data on the original scale showed heteroscedasticity. All IS indices derived from the OST were highly correlated with M and M/I derived from the EHC (r = 0.81–0.87; P < 0.0001; Table 3).
Figure 3.
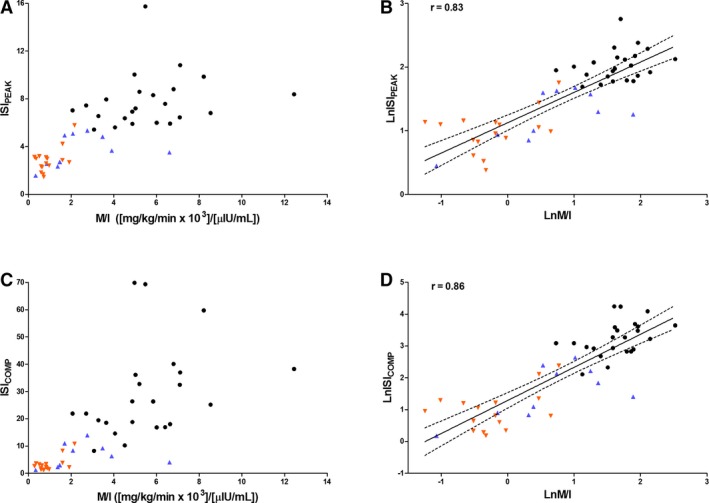
Scatter plots showing the relationship between the oral sugar test derived insulin sensitivity indices: ISIPEAK (A, B) and ISICOMP (C, D) and the mean insulin‐dependent glucose disposal rate per unit of insulin (M/I) calculated from the euglycemic hyperinsulinemic clamp, on the original scale (A, C) and after log‐transformation (B, D), in horses with normal insulin regulation (NIR, black circle) and insulin dysregulation (ID with previous history of laminitis, orange downward pointing triangle; ID without previous history of laminitis, blue upward pointing triangle). The solid line is the regression line (line of best fit), and the dashed lines are 95% CI of the best fit (CI for the mean response).
Table 3.
Linear regression analysis for oral sugar test derived insulin sensitivity indices (ISICOMP, ISIPEAK, ISI60, and ISI90) and euglycemic hyperinsulinemic clamp derived sensitivity measures (M and M/I)
| IS indices | Ln M | Ln M/I | ||
|---|---|---|---|---|
| r | P‐value | r | P‐value | |
| LnISICOMP | 0.85 | < 0.0001 | 0.86 | < 0.0001 |
| LnISIPEAK | 0.83 | < 0.0001 | 0.83 | < 0.0001 |
| LnISI60 | 0.83 | < 0.0001 | 0.82 | < 0.0001 |
| LnISI90 | 0.81 | < 0.0001 | 0.82 | < 0.0001 |
Ln, the natural logarithm; r, correlation coefficient; ISICOMP, composite whole‐body insulin sensitivity index; ISIPEAK, peak insulin sensitivity index; ISI60, 60‐min insulin sensitivity index and ISI90, 90‐min insulin sensitivity index; M, mean insulin‐dependent glucose disposal rate; M/I, mean insulin‐dependent glucose disposal rate per unit of insulin.
Discussion
We found a nonlinear inverse relationship for all 12 comparisons between indices of β‐cell response obtained from the OST and the quantitative IS measurements obtained by the EHC. A rectangular hyperbolic relationship was evident in 9 of these 12 comparisons, which justifies the use of DI as a measure of pancreas functionality under these 9 specific conditions. The results of the study confirm that a reciprocal relationship between β‐cell response and IS exists in horses and that the OST primarily is a test for β‐cell response rather than a test for IS. Despite these findings, calculated indices of IS from the OST may be useful to estimate IS in horses, especially when the horse is in a state of IR.
It is well known that IR is associated with increased insulin secretion from the β‐cells.11, 12, 13, 14 Insulin sensitivity and β‐cell response have been shown to be inversely related in order to maintain glucose tolerance in humans and dogs.13, 14, 16 Our results support that a similar relationship exists in horses. Because of the nature of this nonlinear inverse relationship between the β‐cell response and the IS, the magnitude in the β‐cell response that follows a change in IS will depend on the horse's initial degree of IS. This means that horses with high IS will have small increases in their β‐cell response following large decreases in IS, whereas horses with markedly decreased IS will have large changes in their β‐cell response associated with small changes in IS. As a result, diagnostic tests that are designed to measure the β‐cell response (eg, OST) are dependent on the patient's degree of IS. In addition, the results of the OST also are affected by several physiological factors such as gastric emptying and hepatic glucose trapping,3, 7 contributing to the relatively high variability among the indices of β‐cell response found in previous studies.4, 17 Thus, calculated indices for β‐cell response derived from the OST appear to have a lower ability to estimate changes in IS as long as the patient is within the normal sensitivity range. These inherent characteristics of the OST suggest that the test may not be the most ideal diagnostic method for identification of horses with very mild IR or horses close to developing IR, despite being a test more closely aligned with physiologic situations in which horses have been shown to have had an increased risk for developing laminitis (eg, grazing situations). However, when the degree of IS decrease is more pronounced (ie, IR), the function of the β‐cells becomes upregulated, which makes the test very useful to diagnose IR and to monitor improvements or deteriorations in IS. Several studies have linked laminitis to excessive hyperinsulinemia29, 30 or to markedly increased serum insulin concentrations in IR horses on pasture.31 Although we do not have conclusive evidence in our results, it is interesting that 14 of the 18 horses that had PeakINS >100 μIU/mL or M‐value <1.0 mg/kg/min, suggesting more advanced IR, had a previous history of laminitis. Thus, it appears that laminitis is more strongly associated with advanced IR or pronounced postprandial hyperinsulinemia rather than very mild IR, which suggests that the OST is a suitable and sensitive test for identifying horses at risk for developing laminitis secondary to ID. In a recent study, the OST was found to be highly specific but had a poor sensitivity for diagnosing IR.32 One problem with the majority of the diagnostic tests for ID is the lack of comparison to a gold standard and the use of arbitrary cutoff values. A recent study33 comparing results of OST to a gold standard showed that a much lower cutoff concentration for insulin (30.2 mIU/mL) is indicative of IR compared to the previously suggested arbitrary cutoff concentration (60 mIU/mL). The cutoff concentration for the OST used in our study is also lower (45 mIU/mL)1. This cutoff concentration for the diagnosis of IR is based on specific analyses comparing OST results to quantitative measurements of IS using a receiver operating characteristic curve.
In a rectangular hyperbolic relationship, the product of β‐cell response and IS is constant for a given degree of glucose tolerance. This product, DI,12, 13, 16 can be considered a measure of pancreas functionality (ie, a measure of the β‐cells’ ability to compensate for decreased IS). During development of IR, the β‐cells’ release of insulin is increased and compensation will be adequate as long as DI remains constant (ie, normal glucose tolerance).13, 16 When the β‐cells’ release of insulin becomes inadequate in relation to IR, glucose intolerance and type 2 diabetes mellitus develop, which is associated with a decrease in DI. The decrease in DI is represented by deviation of the hyperbolic curve closer toward the origin (Fig 4).34, 35 In horses, type 2 diabetes mellitus is rare, and the β‐cells appear to fully compensate for the IR over a long time period. Postprandial hyperinsulinemia after feeding or grazing in horses with IR is a risk factor for developing laminitis.36, 37, 38, 39 The most accepted theory is that abnormal hyperinsulinemia post‐feeding is related to the degree of decreased IS, as part of the β‐cells’ compensatory response.40, 41, 42 An alternative explanation is that hyperinsulinemia is the primary cause and IR the compensatory or adaptive response.43, 44, 45 It has recently been suggested that abnormal hyperinsulinemia during the postprandial phase may occur in horses without the presence of decreased IS.1 In contrast, the data from our study showed that postprandial hyperinsulinemia in response to oral glucose is highly related to the patient's degree of IS. Pronounced hyperinsulinemia after an oral glucose load in horses with normal IS without a compensatory decrease in IS will cause hypoglycemia (Fig 4). Instead, abnormal hyperinsulinemia as a driving force for developing ID will likely have an adaptive response in IR to prevent its occurrence.43, 45 In a recent study, horses with ID were found to have increased glucose bioavailability along with increased insulin response.46 Horses with a more exaggerated increase in glucose bioavailability and secondary hyperinsulinemia could have a delayed adaptive response in IR, which could cause an increased DI and deviation of the hyperbolic curve farer from origin (Fig 4). The deviation of DI is thus in the opposite direction compared to patients with type 2 diabetes mellitus. Our study is one of very few studies in which the degree of IS has been quantified in horses with ID and it emphasizes the importance of determining the β‐cell response in conjunction with more accurate determinations of the degree of IS (ie, the EHC or the frequently sampled IV glucose tolerance test with MinMod analysis) to better understand the pathophysiology of ID. Whether or not a distinct reciprocal association between the β‐cell response and the degree of IS was found, the results do not allow differentiation of whether IR is the cause of ID or the adaptive response to postprandial hyperinsulinemia.
Figure 4.
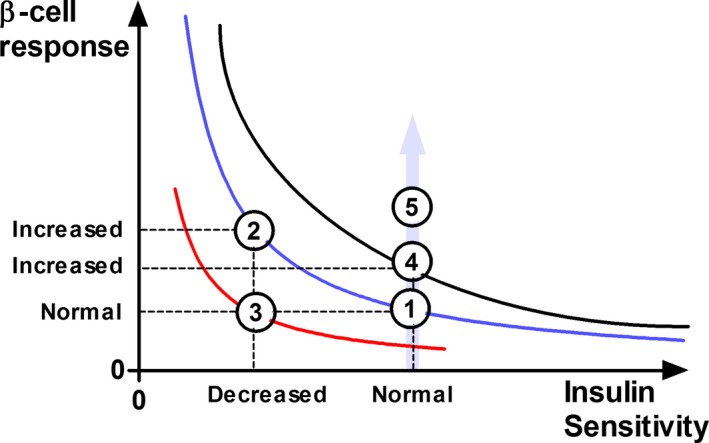
Schematic diagram illustrating the characteristics of the hyperbolic relationship between β‐cell response and insulin sensitivity (IS) and the product of the 2 parameters, the disposition index (DI). State 1: A horse with normal IS and normal β‐cell response. State 2: The horse compensates for decreased IS by increasing β‐cell responsivity, and the DI is unchanged compared to state 1 (ie, remains on the same hyperbolic curve). State 3: The horse fails to compensate for the decreased IS, the β‐cell response remains normal while the DI is decreased (a deviation of the hyperbolic curve toward origin). State 4: The horse develops an increased β‐cell response with a delayed adaptive decrease in IS (a deviation of the hyperbolic curve farer from origin; increased DI). State 5: The horse develops hyperinsulinemia without an adaptive decrease in IS. The IS is unchanged but the hyperinsulinemia is increased and follows the direction of the gray arrow. The horse will develop hypoglycemia when the increased β‐cell response reaches a certain level because there is no adaptive decrease in IS. In state 2, the glucose tolerance remains normal due to the adequate compensatory increase in β‐cell response. State 3 illustrates a typical response for a horse with impaired glucose tolerance and type 2 diabetes mellitus.
Several studies in humans have emphasized the importance of assessing both IS and β‐cell response in the same subject to reach appropriate conclusions about β‐cell function.13, 35, 47 However, measurements of IS and β‐cell response must be as independent as possible, which is not the case for all tests. For example, results for indices of β‐cell response obtained from the OST are intrinsically related to calculated indices of IS from the same test, because the same glucose and insulin data are used for both calculations. To avoid this error, we used 2 independent methods to assess the β‐cell response and the IS, the OST, and the EHC.
In humans, the DI has been calculated from IS measured by the frequently sampled IV glucose tolerance test, the EHC and the OGT in conjunction with measurements of the β‐cell response obtained from the frequently sampled IV glucose tolerance test, hyperglycemic clamp, or the OGT.13, 22, 47, 48, 49 These calculated DI are not identical, and a specific DI is only valid for comparison as long as the same test protocols and analytical methods are used for all patients. Even if there is a nonlinear inverse relationship between β‐cell response and IS to maintain glucose tolerance at a stable level, the relationship may not always fulfill the mathematical criteria for a rectangular hyperbolic relationship.50 This is not surprising because the relationship is dependent on the nature of the variables (ie, the way in which β‐cell response and IS are expressed).6 In our study, indices ranging from early insulin response (INSINDEX and AUCINS/GLU‐30) to whole insulin response (AUCINS‐180 and AUCINS/GLU‐180) demonstrated parameters for the β‐cell response that fulfilled the criteria for a rectangular hyperbolic relationship. The indices INSINDEX 7, 18, 19 and AUCINS/GLU‐30 7, 22 are considered to represent the first phase insulin response in humans,51 but a biphasic insulin release from the β‐cells has not been proven to occur in horses. The M‐values calculated from the EHC in our study were corrected for NIMGU to avoid a confounding factor in the measurement of IS. The contribution of NIMGU to total M is relatively small in individuals with normal IS and thus can be ignored.52 In IR individuals however the relative importance of NIMGU increases considerably. In our study, the estimated error of NIMGU to total M ranged between 10 and 69% and thus represented a heterogeneous error. A weakness with this correction is that it is based on calculations with the assumption of a linear relationship for NIMGU with blood glucose concentrations driven by a mass action effect of glucose similar for all horses. This glucose dose–response relationship has been proven for several species,26, 53 and it is constant as long as there is no increase in non‐insulin‐dependent glucose uptake. This situation might occur in human patients with type II diabetes54 or in critically ill patients,55 but it is not applicable to the horses in our study.
Several different IS indices calculated from the OST have been correlated to IS measured by the EHC in humans.8, 10, 56, 57, 58 We studied 1 of these indices: ISICOMP,8 previously used in horses.9, 17 In addition, we formulated 3 new simpler OST indices (ISIPEAK, ISI60, and ISI90) based on ISICOMP, but calculated them from only a single blood sample during the dynamic OST. There was a high correlation between all IS indices calculated from the OST and IS measures from the EHC, where the more simple indices (ISIPEAK, ISI60, and ISI90) showed as good correlation as the more complex index (ie, ISICOMP). The use of ISI60 or ISI90 enables IS to be estimated from the OST with only a single blood sample.
The OST indices are mathematical variants of data for the β‐cell response, which by inversion have been transformed to correlate linearly instead of hyperbolically to IS measured by the EHC. Because the OST is a quite nonspecific test for β‐cell response,50 this bias will be propagated to the estimates for IS, because it is calculated from the same data. Due to the nonlinear inverse relationship between the β‐cell response and IS, large changes in IS for horses with high IS will only result in very small changes in insulin secretion. These small variations in β‐cell response will not be accurately determined by the OST. The opposite situation with more pronounced insulin secretions occurs for IR horses, and OST‐derived indices for IS will therefore more accurately estimate IS under these conditions. This is apparent in our data as shown in Figure 3, where the relationship between IS indices and M/I showed heteroscedasticity with higher variability for the data in the range of high IS. The same pattern is evident when OST‐derived indices of IS are compared to IS quantified in humans.8
In conclusion, we have demonstrated that an inverse relationship between β‐cell response and IS exists in horses. The hyperbolic paradigm between β‐cell response and IS also holds true for the horse. Therefore, assessment of hyperinsulinemia as part of the β‐cell response should be interpreted in relation to prevailing IS. The inverse relationship between β‐cell response and IS suggests that horses with ID respond not only with postprandial hyperinsulinemia after intake of nonstructural carbohydrates but are also IR.
Acknowledgments
The authors thank Dr. Hanna Edberg for technical assistance.
Conflict of Interest Declaration
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the article.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was performed at the Swedish University of Agricultural Sciences, Uppsala, Sweden, and at the National Council Horse Facility, Wången, Sweden.
The study was funded by the Future Animal Health and Welfare Program, Swedish University of Agricultural Sciences, Sweden, and by the Swedish‐Norwegian Foundation for Equine Research.
Footnotes
Clinical Pathology Laboratory, University Animal Hospital, Uppsala, Sweden
Intranule, 2.0 × 105 mm. Vygon, Ecouen, France
EMLA, AstraZenica AB, Södertälje, Sweden
Nordic Sugar A/S, Copenhagen, Denmark
Vacuette 9 mL, Greiner Bio‐One GmbH, Kremsmünster, Austria
GraphPad Prism, version 6.0 for windows; GraphPad Software Inc, San Diego, CA
Glucose Fresenius Kabi 500 mg/mL, Fresenius Kabi AB, Uppsala, Sweden
Humulin Regular, Eli Lilly Sweden AB, Solna, Sweden
Colleague, Volumetric infusion pump, Baxter Healthcare SA, Zurich, Switzerland
Accu‐Check Aviva, Roche Diagnostics Scandinavia AB, Bromma, Sweden
YSI 2300 Stat Plus Analyzer, YSI Incorporated, Yellow Spring, Ohio
Mercodia equine insulin ELISA, Mercodia AB, Uppsala, Sweden
Mercodia animal insulin control (low, medium, high), Mercodia AB, Uppsala, Sweden
Mercodia insulin ELISA, Mercodia AB, Uppsala, Sweden
Mercodia diabetes antigen control (low, high)/human, Mercodia AB, Uppsala, Sweden
SAS Institute Inc., Cary, NC
References
- 1. Frank N, Tadros E. Insulin dysregulation. Equine Vet J 2014;46:103–112. [DOI] [PubMed] [Google Scholar]
- 2. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 1979;237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 3. Kronfeld DS, Treiber KH, Geor RJ. Comparison of nonspecific indications and quantitative methods for the assessment of insulin resistance in horses and ponies. J Am Vet Med Assoc 2005;226:712–719. [DOI] [PubMed] [Google Scholar]
- 4. Schuver A, Frank N, Chameroy KA, et al. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Equine Vet Sci 2014;34:465–470. [Google Scholar]
- 5. Smith S, Harris P, Menzies‐Gow N. Comparison of the in‐feed glucose test and the oral sugar test. Equine Vet J 2016;48:224–227. [DOI] [PubMed] [Google Scholar]
- 6. Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and β‐cell function. Best Pract Res Clin Endocrinol Metab 2003;17:305–322. [DOI] [PubMed] [Google Scholar]
- 7. Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: A critical appraisal. Diabetologia 2004;47:943–956. [DOI] [PubMed] [Google Scholar]
- 8. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 9. Pratt‐Phillips SE, Geor RJ, McCutcheon LJ. Comparison among the euglycemic‐hyperinsulinemic clamp, insulin‐modified frequently sampled intravenous glucose tolerance test, and oral glucose tolerance test for assessment of insulin sensitivity in healthy Standardbreds. Am J Vet Res 2015;76:84–91. [DOI] [PubMed] [Google Scholar]
- 10. Soonthornpun S, Setasuban W, Thamprasit A, et al. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab 2003;88:1019–1023. [DOI] [PubMed] [Google Scholar]
- 11. Kahn S. The relative contributions of insulin resistance and beta‐cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19. [DOI] [PubMed] [Google Scholar]
- 12. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta‐cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β‐cell function in human subjects: Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 14. Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat‐induced insulin resistance includes reduced insulin clearance and enhanced beta‐cell response. Diabetes 2000;49:2116–2125. [DOI] [PubMed] [Google Scholar]
- 15. Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin‐independent vs. insulin‐dependent mechanisms in mice. Am J Physiol Endocrinol Metab 2001;281:E693–E703. [DOI] [PubMed] [Google Scholar]
- 16. Bergman RN, Ader M, Huecking K, et al. Accurate assessment of β‐cell function the hyperbolic correction. Diabetes 2002;51:S212–S220. [DOI] [PubMed] [Google Scholar]
- 17. Lindåse S, Nostell K, Bröjer J. A modified oral sugar test for evaluation of insulin and glucose dynamics in horses. Acta Vet Scand 2016;58(suppl 1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemioiogic studies. Am J Epidemiol 2000;151:190–198. [DOI] [PubMed] [Google Scholar]
- 19. Phillips D, Clark P, Hales C, et al. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292. [DOI] [PubMed] [Google Scholar]
- 20. Lindeberg S, Jönsson T, Granfeldt Y, et al. A Palaeolithic diet improves glucose tolerance more than a Mediterranean‐like diet in individuals with ischaemic heart disease. Diabetologia 2007;50:1795–1807. [DOI] [PubMed] [Google Scholar]
- 21. Tura A, Ludvik B, Nolan JJ, et al. Insulin and C‐peptide secretion and kinetics in humans: Direct and model‐based measurements during OGTT. Am J Physiol Endocrinol Metab 2001;281:E966–E974. [DOI] [PubMed] [Google Scholar]
- 22. Retnakaran R, Shen S, Hanley AJ, et al. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008;16:1901–1907. [DOI] [PubMed] [Google Scholar]
- 23. Lindåse SS, Nostell KE, Müller CE, et al. Effects of diet‐induced weight gain and turnout to pasture on insulin sensitivity in moderately insulin resistant horses. Am J Vet Res 2016;77:300–309. [DOI] [PubMed] [Google Scholar]
- 24. Firshman AM, Valberg SJ, Baird JD, et al. Insulin sensitivity in Belgian horses with polysaccharide storage myopathy. Am J Vet Res 2008;69:818–823. [DOI] [PubMed] [Google Scholar]
- 25. Geor R, Stewart‐Hunt L, McCutcheon L. Effects of prior exercise on insulin‐mediated and noninsulin‐mediated glucose uptake in horses during a hyperglycaemic clamp. Equine Vet J 2010;42:129–134. [DOI] [PubMed] [Google Scholar]
- 26. Ader M, Ni T‐C, Bergman RN. Glucose effectiveness assessed under dynamic and steady state conditions. Comparability of uptake versus production components. J Clin Invest 1997;99:1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Öberg J, Bröjer J, Wattle O, et al. Evaluation of an equine‐optimized enzyme‐linked immunosorbent assay for serum insulin measurement and stability study of equine serum insulin. Comp Clin Path 2012;21:1291–1300. [Google Scholar]
- 28. Pratt SE, Geor RJ, McCutcheon LJ. Repeatability of 2 methods for assessment of insulin sensitivity and glucose dynamics in horses. J Vet Intern Med 2005;19:883–888. [DOI] [PubMed] [Google Scholar]
- 29. Asplin KE, Sillence MN, Pollitt CC, et al. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J 2007;174:530–535. [DOI] [PubMed] [Google Scholar]
- 30. De Laat M, McGowan C, Sillence M, et al. Equine laminitis: Induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J 2010;42:129–135. [DOI] [PubMed] [Google Scholar]
- 31. Treiber KH, Carter RA, Harris PA, et al. Seasonal change in energy metabolism of ponies coincides with changes in pasture carbohydrates: Implications for laminitis. J Vet Intern Med 2008;22:735. [Google Scholar]
- 32. Dunbar L, Mielnicki K, Dembek K, et al. Evaluation of four diagnostic tests for insulin dysregulation in adult light‐breed horses. J Vet Intern Med 2016;30:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manfredi J, Geor R, Weber PS, et al. Evaluation of an oral sugar test for dynamic assessment of five equine breeds’ insulin response/sensitivity. J Vet Intern Med 2016;30:1510–1511. [Google Scholar]
- 34. Pacini G. The hyperbolic equilibrium between insulin sensitivity and secretion. Nutr Metab Cardiovasc Dis 2006;16:S22–S27. [DOI] [PubMed] [Google Scholar]
- 35. Ahrén B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol 2004;150:97–104. [DOI] [PubMed] [Google Scholar]
- 36. Bailey SR, Habershon‐Butcher JL, Ransom KJ, et al. Hypertension and insulin resistance in a mixed‐breed population of ponies predisposed to laminitis. Am J Vet Res 2008;69:122–129. [DOI] [PubMed] [Google Scholar]
- 37. Geor RJ. Metabolic predispositions to laminitis in horses and ponies: Obesity, insulin resistance and metabolic syndromes. J Equine Vet Sci 2008;28:753–759. [Google Scholar]
- 38. Carter RA, Treiber K, Geor R, et al. Prediction of incipient pasture‐associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalised and localised obesity in a cohort of ponies. Equine Vet J 2009;41:171–178. [DOI] [PubMed] [Google Scholar]
- 39. Treiber KH, Kronfeld DS, Hess TM, et al. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture‐associated laminitis in ponies. J Am Vet Med Assoc 2006;228:1538–1545. [DOI] [PubMed] [Google Scholar]
- 40. Hoffman R, Boston R, Stefanovski D, et al. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J Anim Sci 2003;81:2333–2342. [DOI] [PubMed] [Google Scholar]
- 41. Toth F, Frank N, Martin‐Jimenez T, et al. Measurement of C‐peptide concentrations and responses to somatostatin, glucose infusion, and insulin resistance in horses. Equine Vet J 2010;42:149–155. [DOI] [PubMed] [Google Scholar]
- 42. Carter RA, McCutcheon LJ, George LA, et al. Effects of diet‐induced weight gain on insulin sensitivity and plasma hormone and lipid concentrations in horses. Am J Vet Res 2009;70:1250–1258. [DOI] [PubMed] [Google Scholar]
- 43. Corkey BE. Diabetes: Have we got it all wrong? Diabetes Care 2012;35:2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shanik MH, Xu Y, Škrha J, et al. Insulin Resistance and Hyperinsulinemia Is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31:S262–S268. [DOI] [PubMed] [Google Scholar]
- 45. Corkey BE. Banting lecture 2011 hyperinsulinemia: Cause or consequence? Diabetes 2012;61:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: Investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab 2016;310:E61–E72. [DOI] [PubMed] [Google Scholar]
- 47. Utzschneider KM, Prigeon RL, Carr DB, et al. Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 2006;29:356–362. [DOI] [PubMed] [Google Scholar]
- 48. Ahrén B, Larsson H. Quantification of insulin secretion in relation to insulin sensitivity in nondiabetic postmenopausal women. Diabetes 2002;51:S202–S211. [DOI] [PubMed] [Google Scholar]
- 49. Sjaarda L, Lee S, Tfayli H, et al. Measuring β‐cell function relative to insulin sensitivity in youth does the hyperglycemic clamp suffice? Diabetes Care 2013;36:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferrannini E, Mari A. β‐Cell function in type 2 diabetes. Metabolism 2014;63:1217–1227. [DOI] [PubMed] [Google Scholar]
- 51. Cersosimo E, Solis‐Herrera C, Trautmann ME, et al. Assessment of pancreatic β‐cell function: Review of methods and clinical applications. Curr Diabetes Rev 2014;10:2–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo*. Endocr Rev 1985;6:45–86. [DOI] [PubMed] [Google Scholar]
- 53. Edelman SV, Laakso M, Wallace P, et al. Kinetics of insulin‐mediated and non‐insulin‐mediated glucose uptake in humans. Diabetes 1990;39:955–964. [DOI] [PubMed] [Google Scholar]
- 54. Capaldo B, Santoro D, Riccardi G, et al. Direct evidence for a stimulatory effect of hyperglycemia per se on peripheral glucose disposal in type II diabetes. J Clin Invest 1986;77:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest 2004;114:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI 0,120): Comparison with other measures. Diabetes Res Clin Pract 2000;47:177–184. [DOI] [PubMed] [Google Scholar]
- 57. Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- 58. Cederholm J, Wibell L. Insulin release and peripheral sensitivity at the oral glucose tolerance test. Diabetes Res Clin Pract 1990;10:167–175. [DOI] [PubMed] [Google Scholar]


