Abstract
Background
Relapses of immune‐mediated hemolytic anemia (IMHA), thrombocytopenia (ITP), or polyarthropathy (IMPA) occur despite normal hematologic and cytologic parameters. Thymidine kinase 1 (TK1), canine C‐reactive protein (c‐CRP), haptoglobin (HPT), and 25‐Hydroxyvitamin‐D (25(OH)D) might be adjunct to current monitoring strategies.
Hypothesis/Objectives
Compare serum concentrations of TK1, c‐CRP, HPT, and 25(OH)D in dogs with well‐ and poorly controlled primary IMHA, ITP, or IMPA.
Animals
Thirty‐eight client‐owned dogs.
Methods
Prospective descriptive study. Dogs diagnosed with IMHA, ITP, or IMPA had serum biomarker concentrations measured commercially. Disease control was assessed by hematocrit/PCV and reticulocyte count, platelet count, and synovial fluid cytology for IMHA, ITP, and IMPA, respectively. Statistical analysis performed by Mann‐Whitney rank‐sum tests and receiver operating characteristic curves.
Results
TK1 and c‐CRP, but not HPT significantly decreased with well‐ versus poorly controlled IMHA (P = 0.047, P = 0.028, P = 0.37). C‐CRP, but not TK or HPT was significantly lower with well‐ versus poorly controlled IMPA (P = 0.05, P = 0.28, P = 0.84). Sensitivity and specificity of TK and c‐CRP (simultaneously) for detecting dogs with poorly controlled IMHA were 88 and 100%, respectively. Sensitivity and specificity of c‐CRP for detecting poorly controlled dogs with IMPA were 13 and 100%, respectively. 92% of dogs were vitamin D insufficient (<100 ng/mL) regardless of disease control.
Conclusions and Clinical Importance
Combining TK1 and c‐CRP might act markers of disease control in dogs with IMHA. Canine‐CRP cannot be recommended as an independent marker of disease control in IMPA. 25(OH)D insufficiency in immune‐mediated disorders might benefit from further study to determine if supplementation could improve therapeutic response or reduce disease risk.
Keywords: Autoimmune, Hemolysis, Insufficiency
Abbreviations
- 25(OH)D
25‐hydroxyvitamin D
- APP
acute phase proteins
- c‐CRP
canine C‐reactive protein
- dc‐CLIA
direct competitive chemiluminescence immunoassay
- ELISA
enzyme‐linked immunosorbent assay
- HPT
haptoglobin
- ic‐CLIA
indirect, competitive chemiluminescent immunoassay
- IMHA
immune‐mediated hemolytic anemia
- IMPA
immune‐mediated polyarthropathy
- ITP
immune‐mediated thrombocytopenia
- ROC
receiver operating characteristic
- TK1
thymidine kinase 1
- VDI
Veterinary Diagnostics Institute
- WBC
white blood cell
Management of immune‐mediated hemolytic anemia (IMHA), immune‐mediated thrombocytopenia (ITP), and immune‐mediated polyarthropathy (IMPA) typically requires long‐term immunosuppressive therapy. While gradual tapering might allow cessation of treatment, relapsing disease might result in substantial morbidity and mortality.1, 2, 3 Determining risk of relapse and when to taper, represents a clinical challenge as relapses can occur despite normal hematologic variables, cytological variables, or both. Additionally, frequent monitoring might represent a financial hardship or negatively impact the perception of a patient's quality of life.4 Adjunctive or alternative monitoring tools are needed to improve clinical management and to address client and patient variables limiting treatment.
Serum biomarkers can detect subclinical changes in inflammation and cellular proliferation in dogs and might reduce the need for repeated costly or invasive screening procedures.5, 6, 7 As disease‐specific biomarkers are rare, multiple biomarkers can be used to develop clinical screening algorithms.8 Acute phase proteins (APPs) are often included in screening panels as sensitive markers of inflammation. Concentrations are low in healthy animals but can rise rapidly with inflammation.9, 10 Canine C‐reactive protein (C‐CRP) and serum haptoglobin (HPT) are APPs, and sensitive markers of inflammation for a number of conditions, including immune‐mediated disease.9, 11 In dogs, changes in concentrations of APPs over time have prognostic capabilities with neoplastic, and non‐neoplastic hematologic conditions suggesting possible utility in monitoring disease control in dogs with immune‐mediated disorders.12, 13
Acute phase proteins lack specificity, and this necessitates inclusion of additional biomarkers, in combination with APPs, to improve disease specificity. Thymidine Kinase 1 (TK1) is a cell cycle‐dependent enzyme used as a biomarker for cell proliferation. While concentrations are low‐absent in nonproliferating cells (G1), serum concentrations increase 10–20× by the mitotic (M) phase.14 TK1 is commonly associated with neoplastic disease.8 However, increased TK1 activity is documented in humans with hemolysis and erythro‐megakaryocytopoiesis.15, 16 TK1 may have utility as a marker of disease control in conditions characterized by cellular proliferation, such as immune‐mediated cytopenias.
Conditions predisposing to immune‐mediated diseases are poorly understood but broadly are associated with inappropriate targeting of host antigens by the immune system. Human studies implicate vitamin D (25(OH)D) insufficiency in immune‐mediated cytopenias.17 Vitamin D promotes a shift from CD4 T‐helper (Th)1 to Th2 polarity in several autoimmune conditions promoting a state of immune “tolerance.”18 In humans, 25(OH)D supplementation improves treatment response in refractory ITP19 and reduced CRP concentrations in hemolytic anemia.18, 19, 20, 21 Whereas additional studies are needed, 1 report in healthy dogs showed c‐CRP concentrations decreased with increasing 25(OH)D concentrations suggesting an inverse relationship.22 Serum 25(OH)D concentrations have not been previously evaluated in dogs with immune‐mediated disease. It remains to be seen if identifying 25(OH)D insufficiency in these dogs might result in additional screening and therapeutic opportunities.
The primary objective of this study was to evaluate TK1, c‐CRP, HPT, and 25(OH)D concentrations in dogs with primary IMHA, ITP, and IMPA, and compare concentrations across disease type and disease control (well‐ versus poorly controlled). The secondary objective was to investigate relevant biomarkers in combination with determining sensitivity and specificity for disease control. A third objective was to report statistically significant correlations between serum biomarker concentrations and cytological/hematologic parameters where identified. Compared with poorly controlled disease, we hypothesized that serum TK1, c‐CRP, and HPT concentrations would be significantly lower in dogs with well‐controlled IMHA and ITP; that c‐CRP and HPT would be significantly lower in well‐controlled IMPA. We hypothesized that dogs with all classes of immune‐mediated disease would be 25(OH)D insufficient.
Materials and Methods
Animals
Serum was collected from 38 companion dogs presenting to the University of Missouri Veterinary Heath Center between 2013 and 2014. Samples were collected with informed owner consent and under the IACUC protocol #7728 after diagnosis of primary IMHA, ITP, or IMPA. No dog was diagnosed with immune‐mediated disease affecting more than 1 cell lineage (Evan's syndrome) or with evidence of polysystemic immune‐mediated disease, such as systemic lupus erythematosus.
Dogs were diagnosed with IMHA if they had evidence of hemolysis with a regenerative anemia and a moderate‐to‐severe spherocytosis, ±micro/macro‐agglutination, or a positive Coombs test. ITP was identified as severe thrombocytopenia with platelet count <20,000/uL identified by the use of automated cell counters plus manual slide evaluation. Dogs with IMPA had a supportive clinical history and arthrocentesis with cytology containing >10% nondegenerate neutrophils of multiple distal joints.
Cases were considered primary if no underlying cause of disease was identified during routine diagnostic evaluation. All dogs underwent physical examination, complete blood count including reticulocyte percentage and red blood cell morphology, serum biochemical profile, and urinalysis. All dogs underwent testing for vector‐borne disease by a 4DX SNAP1 test. Dogs which tested positive for rickettsial diseases were excluded from the study population. Doxycycline was prescribed at the discretion of the attending clinician if there was concern for early disease before seroconversion. Dogs with IMHA and ITP also underwent thoracic and abdominal radiography and abdominal ultrasound. Dogs with IMPA that were >7 years old underwent thoracic and abdominal imaging. Bone marrow evaluation was not routinely performed. All dogs with neoplasia or other forms of lymphoproliferative disease were excluded from the study.
After diagnosis, dogs were evaluated by physical examination and serial CBCs or PCV/TS for IMHA, CBCs for ITP, and joint cytology for IMPA reflecting typical serial evaluations in clinical practice. Dogs with IMHA were considered controlled if there was no evidence of anemia or clinical signs of disease. Where a CBC was performed dogs with normal slide differential evaluations, and reticulocyte percentages <1% were considered controlled. Dogs with anemia, persistent spherocytosis, agglutination, or evidence of regeneration were considered poorly controlled. Dogs with ITP were considered controlled based on a platelet count of >200,000/μL with no evidence of clinical disease. Dogs with persistent thrombocytopenia (<200,000/μL) or evidence of clinical disease (petchiations, ecchymosis) were considered poorly controlled. Dogs with IMPA were considered controlled based on unremarkable joint fluid cytology on multiple distal joints. Dogs where joint fluid cytology contained >10% nondegenerate neutrophils were considered poorly controlled.
Sample Collection
Whole blood was collected via venipuncture into a tube without additives by a veterinarian, veterinary technician, or veterinary student (under direct supervision). All samples were collected before blood transfusion. Samples were centrifuged, and a minimum of 0.5 mL of serum was banked and stored at −20°C within 1 hour of sample collection. Some dogs were sampled serially. In dogs that underwent serial evaluation, samples were collected no sooner than 3 week apart.
Thymidine Kinase 1 Assay
Serum TK1 was evaluated by a commercial laboratory via indirect, competitive chemiluminescent immunoassay (ic‐CLIA).8 The minimum detectable activity for this assay is 0.2 U/L. Reference values provided for this assay are normal (≤1.9 U/L), increased (2–8.9 U/L), and markedly increased (≥9 U/L).
CAnine C‐Reactive Protein Assay
Serum c‐CRP was evaluated commercially by a canine‐specific enzyme‐linked immunosorbent assay (ELISA).8 The minimum detectable concentration for this assay is 0.2 mg/mL. Reference ranges for this assay are normal (≤3.9 mg/L), mildly increased (4–9.9 mg/L), moderately increased (10–39.9 mg/L), and markedly increased (≥40 mg/L).
Haptoglobin Assay
Serum HPT concentrations were evaluated commercially by a canine‐specific sandwich ELISA.23 The minimum detectable concentration for this assay is 10 mg/dL. Reference ranges for this assay are decreased (<30 mg/dL), normal (30–250 mg/dL) and increased (>250 mg/dL).
Vitamin D assay
Serum 25(OH) D concentrations were evaluated commercially using a direct competitive chemiluminescence immunoassay (dc‐CLIA) as previously described.22 Concentrations between 30 and 100 ng/mL were considered insufficient.22 Dogs were considered deficient of 25(OH)D concentrations were <30 ng/mL. 25(OH)D concentrations in dogs that had received treatment with glucocorticoids were compared with those that had not to determine the impact of prior treatment on serum vitamin D concentrations.
Statistical Analysis
Statistical analysis was performed by SigmaPlot data analysis software2 (version 12.3). A Kruskal‐Wallis 1‐way analysis of variance (ANOVA) on ranks was used to compare serum TK1, c‐CRP, HPT, and 25(OH)D concentrations among the IMHA, ITP, and IMPA groups. A Mann‐Whitney rank‐sum test was used to compare serum TK1, c‐CRP, HPT, and 25(OH)D concentrations between well‐ and poorly controlled patients with IMHA or IMPA. TK1, c‐CRP, HPT, and 25(OH)D concentrations were evaluated between dogs that received treatment with glucocorticoids and those that had not using a 2‐way ANOVA. A Dunn's test or Holm‐Sidak test for multiple comparisons was used where appropriate. Given the very small numbers of dogs with ITP, comparisons between well‐ and poorly controlled dogs were not analyzed for statistical differences but were provided descriptively. Where a significant difference in serum biomarker concentrations between well‐ and poorly controlled dogs was identified, receiver operating curves (ROC) were performed to determine the sensitivity and specificity of each biomarker as an independent predictor of disease control. In a single disease syndrome, where more than 1 biomarker demonstrated statistical significance for disease control, the net sensitivity and specificity were calculated for simultaneous evaluation. Disease positives were defined as those that tested positive by either 1 test or both tests. Disease negatives are defined as those who test negative by both tests. Evaluation for significant correlations was performed using a Pearson product moment correlation. Descriptive statistics were performed when deemed appropriate. A P value of ≤0.05 was considered significant.
Results
Study Population
Thirty‐eight dogs were evaluated between 2013 and 2014. Group distribution and demographics are shown in Table 1. Twenty‐three breeds were represented. The most commonly identified breeds were Chihuahuas (n = 4), Labrador retrievers (n = 4), Dachshunds (n = 3), and mixed breeds (n = 3). All other breeds were represented by a single individual: Basset Hound, Alaskan Malamute, Airedale, Rat Terrier, American Eskimo, Miniature Schnauzer, Bichon Frise, Pug, Rottweiler, Poodle, Border Collie, Cocker Spaniel, Australian Shepherd, Welsh Corgi, Coonhound, Beagle, German Shepherd dog, and Doberman Pinscher. Ten dogs were sampled serially with an average ± SD of 3.4 ± 1.8 samples per dog.
Table 1.
A summary of demographics of dogs with immune‐mediated hemolytic anemia (IMHA), immune‐mediated thrombocytopenia (ITP), and immune‐mediated polyarthropathy (IMPA) having evaluation of biomarkers. Immunosuppressive therapy reflects the drug protocol for a particular patient at the time of sample collection. Dogs not receiving immunosuppressant treatment at the time of sample collection are omitted
| Disease Group | |||
|---|---|---|---|
| IMHA | ITP | IMPA | |
| Number of dogs | 17 | 6 | 15 |
| Average age at diagnosis: years, x ± SD | 7.2 ± 2.9 | 7.4 ± 3.4 | 4.7 ± 2.7 |
| Reproductive status | |||
| Spayed female | 11 | 3 | 9 |
| Castrated male | 6 | 2 | 6 |
| Intact male | 0 | 1 | 0 |
| Dogs with multiple collections | 5 | 0 | 6 |
| Treatment | |||
| Prior treatment (dogs, #samples) | 11, 22 | 3, 3 | 11, 24 |
| No prior treatment (dogs, #samples) | 6, 6 | 3, 3 | 4, 4 |
| Immunosuppressant therapy | |||
| Glucocorticoids alone | 1 | 0 | 3 |
| Glucocorticoids + cyclosporine | 8 | 2 | 10 |
| Glucocorticoids + mycophenolate mofetil | 4 | 0 | 6 |
| Glucocorticoids + azathioprine | 1 | 1 | 5 |
| Glucocorticoids + leflunomide | 0 | 0 | 1 |
| Cyclosporine alone | 0 | 0 | 2 |
| Glucocorticoids + mycophenolate + cyclosporine | 1 | 0 | 0 |
Glucocorticoids, either alone or in combination, were the most common immunosuppressant used in dogs already undergoing treatment at the time of sample collection (95.6%). Four percent of dogs received cyclosporine alone. Combinations of mycophenolate mofetil, cyclosporine, leflunomide, and azathioprine with glucocorticoids were also used (Table 1).
Thymidine Kinase 1 Activity, and c‐CRP, HPT, and 25(OH)D Concentrations Between Disease States (IMHA, ITP, IMPA)
Thymidine kinase 1 activity was significantly increased in dogs with IMHA compared to dogs with IMPA (P < 0.05). No significant differences were noted for TK1 activity between the IMHA and ITP groups. There were no significant differences among IMHA, ITP, and IMPA for c‐CRP, HPT, or 25(OH)D (Fig 1a–d). Median ± IQR biomarker concentrations for each group are provided in Table 2.
Figure 1.
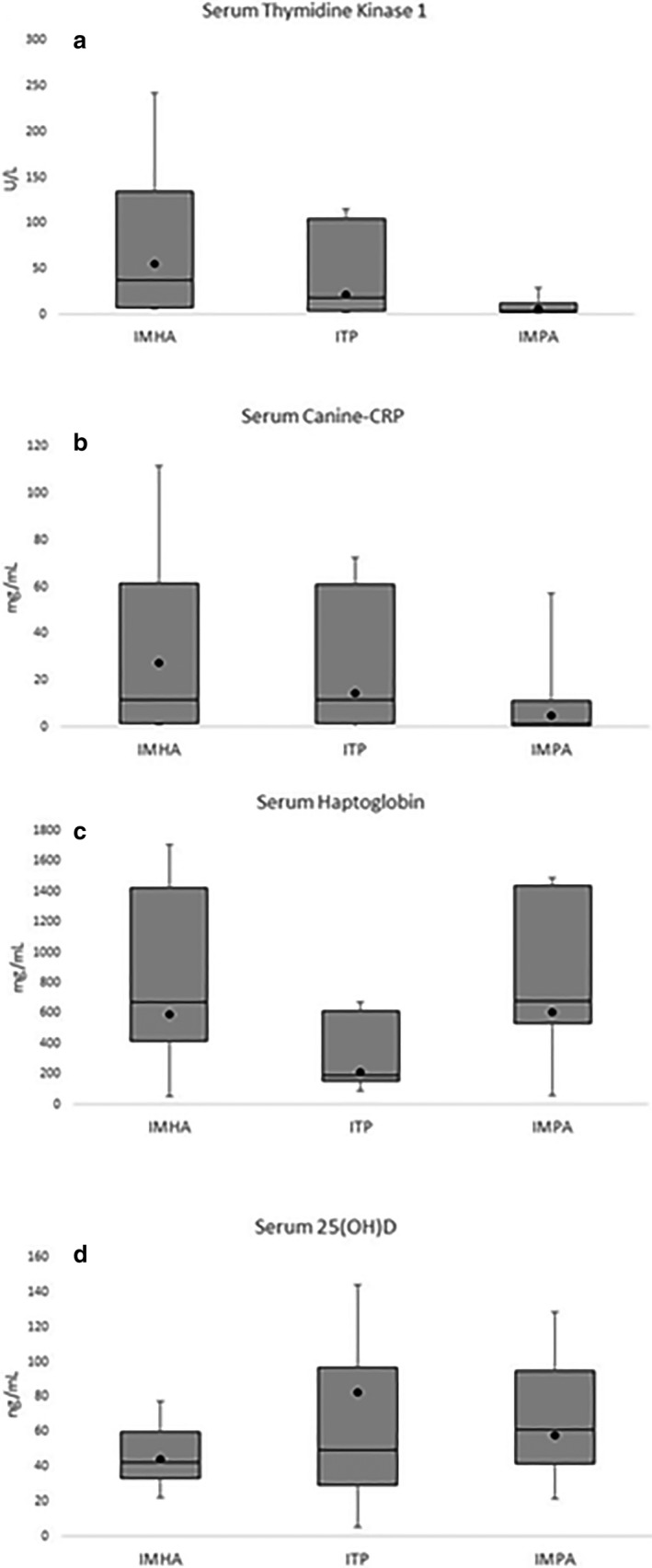
Samples from 38 client‐owned dogs diagnosed with immune‐mediated hemolytic anemia (IMHA) (n = 27), immune‐mediated thrombocytopenia (n = 6), or immune‐mediated polyarthropathy (IMPA) (n = 28) were evaluated for differences in serum thymidine kinase 1 (TK1), canine C‐reactive protein, haptoglobin, and 25(OH)D with data shown in box and whisker plots. (a) Patients with IMHA had significantly greater Serum TK1 activity compared those with IMPA (P ≤ 0.05). No other significant differences were identified for serum canine‐CRP (b), haptoglobin (c), or 25(OH)D (d) across disease states. The lower and upper boxes represent the 25th and 75th percentiles and the line represents the median. The black circle represents the mean score from each group. The upper and lower whiskers represent the minimum and maximum values.
Table 2.
Serum concentrations (median ± IQR) of TK, c‐CRP, HPT, and 25(OH)D are shown for the 3 types of immune‐mediated disease studied. Significant differences between conditions were only identified between IMHA and IMPA for TK1 (P < 0.001)
| Disease Group | |||
|---|---|---|---|
| IMHA | ITP | IMPA | |
| TK1 (U/L) | 36.75 ± 84.38 | 21.1 ± 38.75 | 4.15 ± 3.68 |
| c‐CRP (mg/mL) | 11.70 ± 48.18 | 11.55 ± 26.55 | 1.90 ± 7.00 |
| HPT (mg/mL) | 675.00 ± 294.30 | 230.85 ± 130.65 | 649.4 ± 216.40 |
| 25 (OH)D (ng/mL) | 43.50 ± 20.80 | 62.8 ± 55.60 | 63.20 ± 30.72 |
c‐CRP, canine C‐reactive protein; HPT, haptoglobin; IMHA, immune‐mediated hemolytic anemia; IMPA, immune‐mediated polyarthropathy; ITP, immune‐mediated thrombocytopenia; 25(OH)D, 25‐hydroxyvitamin D; TK1, thymidine kinase 1.
Thymidine Kinase 1 Activity, and c‐CRP, HPT, and 25(OH)D Concentrations in Dogs with IMHA, ITP, and IMPA Compared to Reference Standards
Serum samples were collected at times reflective of poorly controlled (n = 34) and well‐controlled (n = 27) immune‐mediated disease. Samples were stratified by disease syndrome and compared to the reference standard for each biomarker (Table 3).
Table 3.
Serum biomarker concentrations for well‐controlled (n = 27) and poorly controlled (n = 34) dogs were stratified according to type of immune‐mediated disease and compared to industry‐established reference ranges. One dog was not evaluated for HPT, and 2 dogs were not evaluated for 25(OH)D concentrations due to complications with the assay
| Serum Biomarker Concentrations | Disease Group | |||||
|---|---|---|---|---|---|---|
| IMHA | ITP | IMPA | ||||
| Well Controlled (%) | Poorly Controlled (%) | Well Controlled (%) | Poorly Controlled (%) | Well Controlled (%) | Poorly Controlled (%) | |
|
TK1 (≤1.9 U/L) Normal |
1/5 (20) |
1/22 (4.6) | 0/2 | 0/4 |
2/20 (10) |
1/8 (12.5) |
|
TK1 (2–8.9 U/L) Increased |
2/5 (40) |
4/22 (18.2) |
1/2 (50) |
1/4 (25) |
15/20 (75) |
5/8 (62.5) |
|
TK1 (≥9 U/L) Markedly Increased |
2/5 (40) |
17/22 (77.3) |
1/2 (50) |
3/4 (75) |
3/20 (15) |
2/8 (25) |
|
Canine‐CRP (≤3.9 mg/L) Normal |
4/5 (80) |
7/22 (31.8) |
2/2 (100) |
0/4 |
15/20 (75) |
3/8 (37.5) |
|
Canine‐CRP (4–9.9 mg/L) Mildly Increased |
1/5 (20) |
1/22 (4.6) |
0/2 |
1/4 (25) |
1/20 (5) |
2/8 (25) |
| Canine‐CRP (10–39.9 mg/L) Moderately Increased | 0/5 |
4/22 (18.2) |
0/2 |
2/4 (50) |
2/20 (10) |
2/8 (25) |
|
Canine‐CRP (≥40 U/L) Markedly Increased |
0/5 |
10/22 (45.5) |
0/2 |
1/4 (25) |
2/20 (10) |
1/8 (12.5) |
|
HPT (<30 mg/dL) Decreased |
0/5 | 0/21 | 0/2 | 0/4 | 0/20 | 0/8 |
|
HPT (30–250 mg/dL) Normal |
0/5 |
3/21 (14.3) |
0/2 |
3/4 (75) |
1/20 (5) |
2/8 (25) |
|
HPT (250.1–400 mg/dL) Moderately Increased |
0/5 |
2/21 (9.5) |
1/2 (50) |
1/4 (25) |
1/20 (5) |
2/8 (25) |
|
HPT (≥400.1 mg/dL) Markedly Increased |
5/5 (100) |
16/21 (76.2) |
1/2 (50) |
0/4 |
18/20 (90) |
4/8 (50) |
| 25(OH)D (˂30 ng/mL) Deficient |
1/5 (20) |
3/20 (15) |
1/2 (50) |
0/2 |
1/20 (5) |
0/8 |
|
25(OH)D (30–100 ng/mL) Insufficient |
4/5 (80) |
16/20 (80) |
1/2 (50) |
2/3 (66.7) |
19/20 (95) |
7/8 (87.5) |
|
25(OH)D (>100 ng/mL) Sufficient |
0/5 |
1/20 (5) |
0/2 |
1/3 (33.3) |
0/20 |
1/8 (12.5) |
c‐CRP, canine C‐reactive protein; HPT, haptoglobin; IMHA, immune‐mediated hemolytic anemia; IMPA, immune‐mediated polyarthropathy; ITP, immune‐mediated thrombocytopenia; 25(OH)D, 25‐hydroxyvitamin D; TK1, thymidine kinase 1.
Serum 25(OH)D concentrations were insufficient (<100 ng/mL) in 92% of dogs.22 The 3 dogs with vitamin D sufficiency had concentrations of 116.0, 106.0, and 150.0 ng/mL.
Thymidine Kinase 1 Activity, and c‐CRP, HPT, and 25(OH)D Concentrations in Dogs with IMHA, ITP, and IMPA in Dogs Receiving Steroids and Those Not Receiving Steroids
No statistically significant difference was noted in serum TK1 activity (P = 0.18), or c‐CRP (P = 0.10), and 25(OH)D (P = 0.64) concentrations for dogs that had received glucocorticoids and those that had not. Serum HPT was significantly higher in dogs treated with steroids compared to those that were not (P < 0.001).
Thymidine Kinase 1 Activity, and c‐CRP, HPT, and 25(OH)D Concentrations in Well‐ and Poorly Controlled Dogs (IMHA, ITP, IMPA)
Samples obtained during poor control were from dogs with IMHA (n = 22), ITP (n = 4), and IMPA (n = 8). Samples indicative of well‐controlled disease were from dogs with IMHA (n = 5), ITP (n = 2), and IMPA (n = 20; Table 3). Given the small numbers of dogs with ITP, no statistical comparisons were made between well‐ and poorly controlled disease, and descriptive data are provided in Table 4.
Table 4.
Serum biomarker concentrations (median ± IQR) between poorly and well‐controlled dogs for IMHA, ITP, and IMPA are shown. Significant differences between were found between TK1 and c‐CRP for dogs with IMHA and c‐CRP for dogs with IMPA (asterisk). There was an insufficient number of dogs perform statistical comparison between well‐ and poorly controlled dogs with ITP
| (a) IMHA | Poorly Controlled (n = 22) | Well Controlled (n = 5) | P value |
|---|---|---|---|
| TK1 (U/L) | 47.90 ± 84.95 | 7.90 ± 2.20 | 0.047* |
| c‐CRP (mg/mL) | 32.10 ± 47.75 | 1.10 ± 1.40 | 0.028* |
| HPT (mg/mL) | 677.20 ± 372.95 | 660.30 ± 144.10 | 0.37 |
| 25 (OH)D (ng/mL) | 48.30 ± 25.20 | 71.6 ± 50.10 | 0.069 |
| (b) ITP (n = 6) | Poorly Controlled (n = 4) | Well Controlled (n = 2) | P value |
|---|---|---|---|
| TK1 (U/L) | 40.40 ± 60.28 | 6.50 ± 2.50 | |
| c‐CRP (mg/mL) | 25.30 ± 35.05 | 1.35 ± 0.25 | |
| HPT (mg/mL) | 183.6 ± 54.58 | 545.5 ± 222.4 | |
| 25 (OH)D (ng/mL) | 86.80 ± 43.60 | 29.30 ± 1.90 |
| (c) IMPA (n = 15) | Poorly Controlled (n = 20) | Well Controlled (n = 8) | P value |
|---|---|---|---|
| TK1 (U/L) | 3.40 ± 3.68 | 4.70 ± 4.30 | 0.28 |
| c‐CRP (mg/mL) | 5.45 ± 10.60 | 1.30 ± 2.40 | 0.050* |
| HPT (mg/mL) | 668.20 ± 464.20 | 668.60 ± 185.80 | 0.83 |
| 25 (OH)D (ng/mL) | 54.95 ± 24.88 | 64.20 ± 30.30 | 0.88 |
c‐CRP, canine C‐reactive protein; HPT, haptoglobin; IMHA, immune‐mediated hemolytic anemia; IMPA, immune‐mediated polyarthropathy; ITP, immune‐mediated thrombocytopenia; 25(OH)D, 25‐hydroxyvitamin D; TK1, thymidine kinase 1.
In dogs with well‐controlled IMHA, serum TK1 activity and c‐CRP concentrations were both significantly decreased compared with poorly controlled IMHA (P = 0.047 and P = 0.028, respectively; Fig 2a,b). No significant differences between disease control status were detected for HPT or 25(OH)D (P = 0.37 and P = 0.069, respectively; Fig 2c,d) Median ± IQR biomarker concentrations are provided in Table 4.
Figure 2.
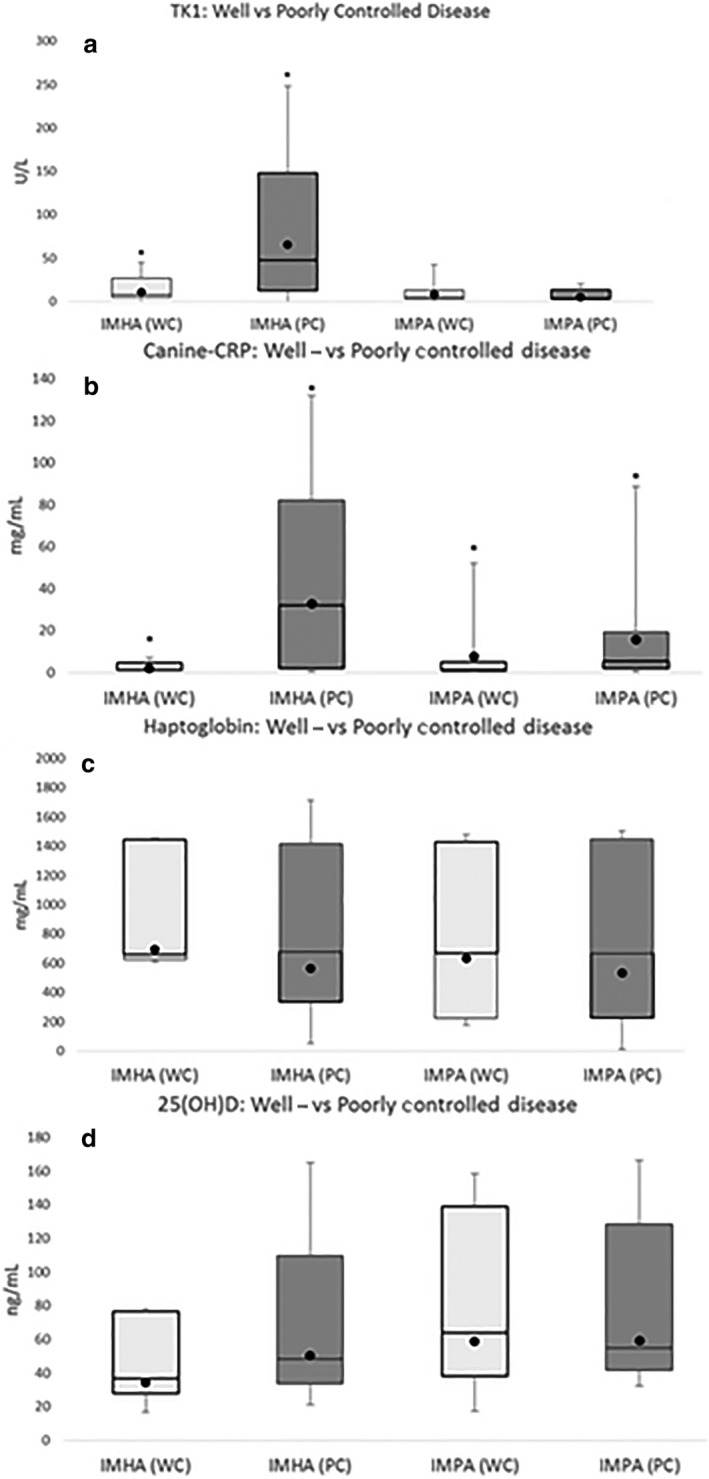
(a–d) Samples from 38 client‐owned dogs diagnosed with immune‐mediated hemolytic anemia (IMHA) (n = 27), immune‐mediated thrombocytopenia (ITP) (n = 6), or immune‐mediated polyarthropathy (IMPA) (n = 28) were evaluated. Box and whisker plots showing differences in biomarkers between well‐controlled (WC) shown in light gray and poorly controlled (PC) dogs shown in dark gray. For IMHA (PC: n = 22, WC: n = 5), for ITP (PC; n = 4, WC: n = 2), and for IMPA (PC: n = 8, WC: n = 20). The lower boxes represent the 25th and 75th percentiles. The line represents the median. The black circle represents the mean. The range of the data is represented by the whiskers. Dogs were categorized as well‐ or poorly controlled based on HCT (%) and reticulocyte percentage and multiple joint fluid cytology. Dogs with well‐controlled IMHA had significantly lower thymidine kinase 1 (TK1) activity compared with poorly controlled dogs (P = 0.047). Dogs with well‐controlled IMHA and IMPA had significantly lower canine C‐reactive protein concentrations compared to poorly controlled dogs (P = 0.028 and P = 0.05, respectively).
The sensitivity and specificity for detecting poor control of canine IMHA using serum TK1 activity were 0.66 and 1.00, respectively, using a cut off of 29.4 U/L. The sensitivity and specificity for detecting a poorly regulated dog with IMHA using c‐CRP were 0.65 and 1.00, respectively, using a cut off value of 4.85 mg/mL (Fig 3a–c). When adjusted for simultaneous testing, TK1 plus c‐CRP had a sensitivity and specificity for detecting a poorly regulated dog with IMHA of 0.88 and 1.00, respectively.
Figure 3.
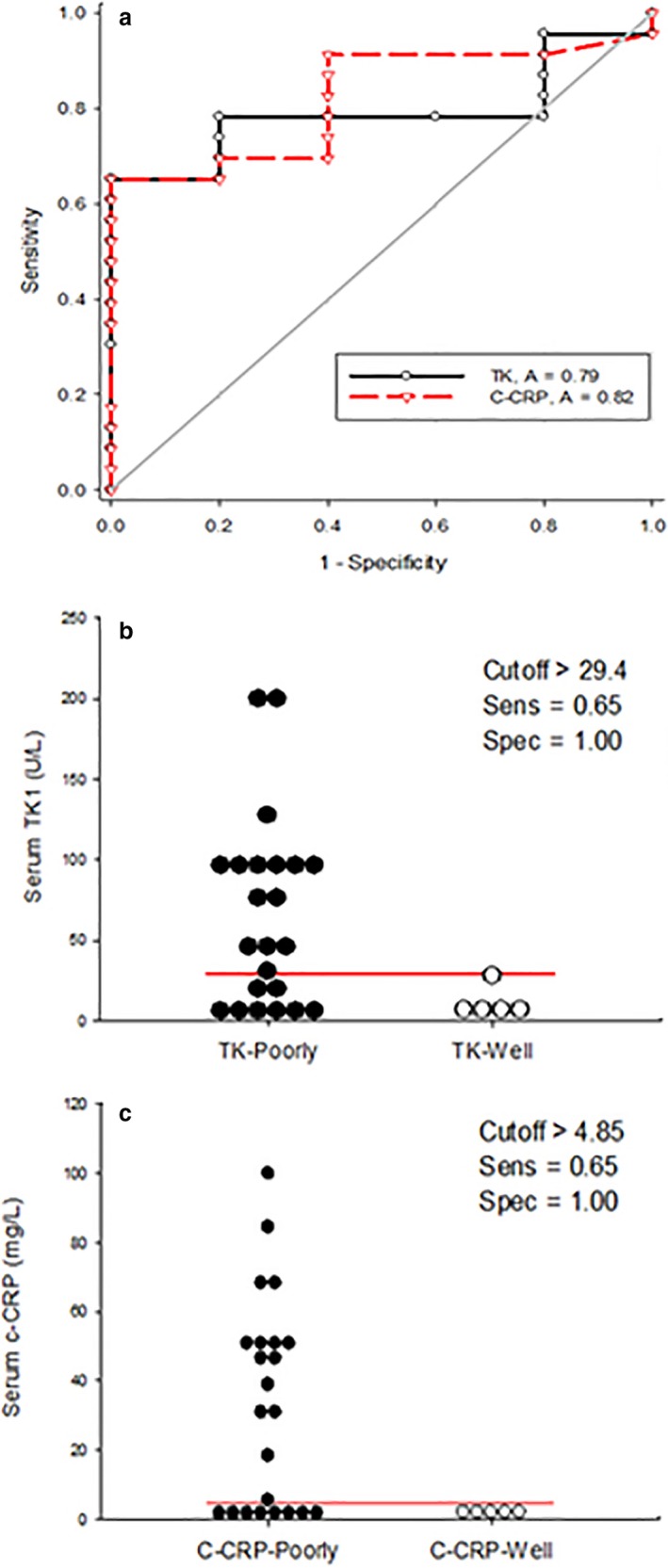
(a) depicts the combined receiver operating characteristic (ROC) curves for thymidine kinase 1 (TK1) (solid) and canine C‐reactive protein (c‐CRP) (dashed) in dogs with immune‐mediated hemolytic anemia (IMHA). The area under the curve (AUC) is 0.79 and 0.82 for TK1 and c‐CRP, respectively. (b and c) are dot plots for TK1 and c‐CRP. Each dot represents the serum biomarker concentration for an individual dog with either poorly (solid) or well‐(open) controlled IMHA. The line is a graphical depiction of the “cutoff” values to maximize sensitivity and specificity for the detection of dogs with poorly regulated disease. A sensitivity and specificity of 65 and 100% were calculated for both TK1 and c‐CRP for the detection of dogs with poorly regulated IMHA.
In dogs with IMHA, significant strong positive correlations were found between serum TK1 activity and both reticulocyte percentage and total white blood cell (WBC) count (P = 0.01, P = 0.023; r = 0.52, r = 0.60; Fig 4a,b). For c‐CRP in IMHA, significant strong negative correlation was found between c‐CRP and hematocrit (HCT) (P = <0.001; r = −0.61; Fig 5). No significant correlations were detected between c‐CRP and WBC count or 25(OH)D concentrations (P = 0.11, P = 0.83, respectively).
Figure 4.
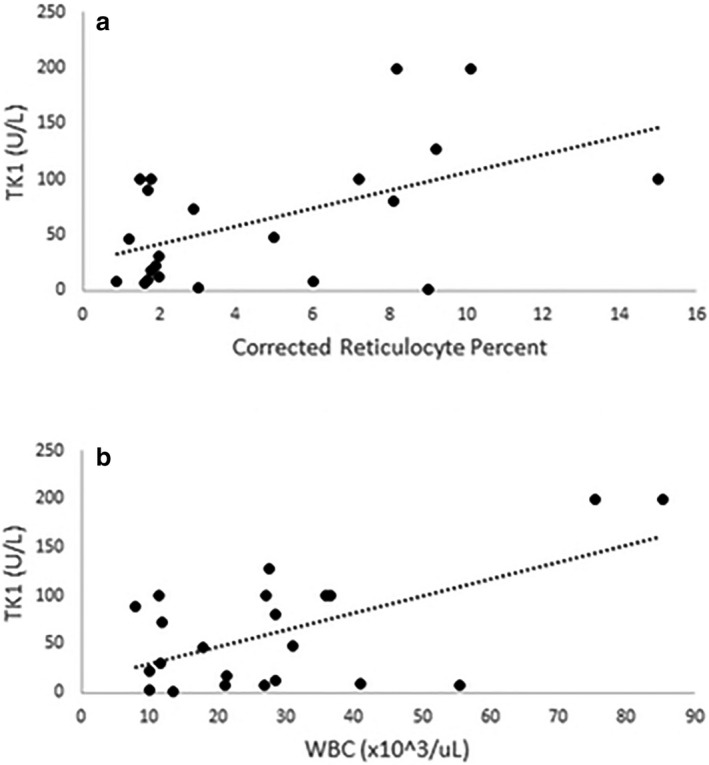
Thymidine kinase 1 (TK1) versus reticulocytes and white blood count in dogs with immune‐mediated hemolytic anemia, TK 1 showed significant, strong, positive correlations with (a) reticulocyte percentage and (b) white blood cell count (P = 0.01, P = 0.023; r = 0.52, r = 0.60, respectively).
Figure 5.
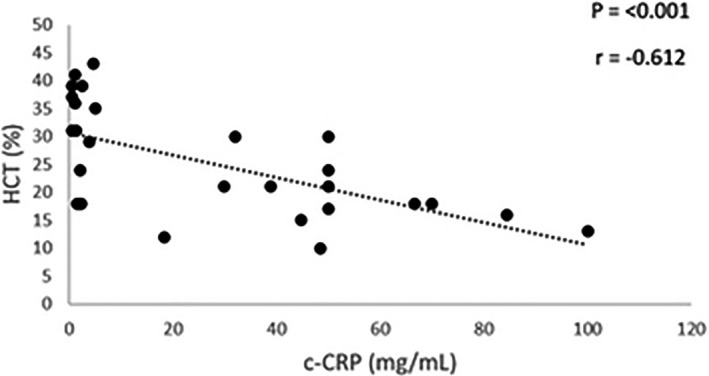
C‐reactive protein (CRP) versus hematocrit in dogs with immune‐mediated hemolytic anemia. Canine CRP showed a significant, moderate, negative correlation with hematocrit (P < 0.001, r = −0.61).
Unfortunately, not enough well‐controlled dogs were recruited to all statistical comparison between poorly and well‐controlled dogs with ITP. Median ± IQR biomarker concentrations are provided in Table 4. However, within the group as a whole, a strong negative correlation was identified between serum TK1 and c‐CRP and hematocrit (P = 0.047, P = 0.046; r = −0.82, r = −0.82; Fig 6a–b).
Figure 6.
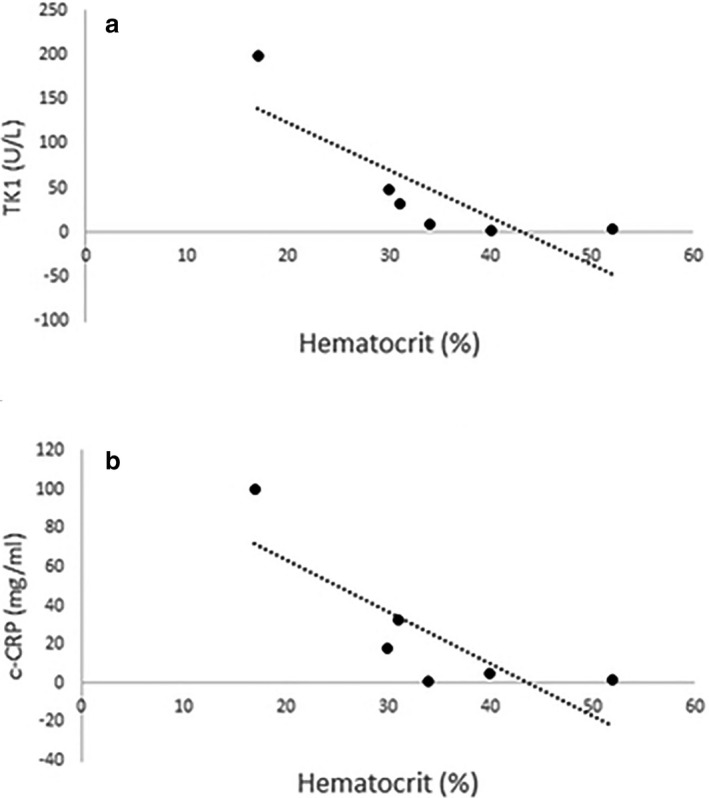
(a, b) In dogs with immune‐mediated thrombocytopenia, thymidine kinase 1 and canine C‐reactive protein showed significant, strong, positive correlations with patient hematocrit (P = 0.047, P = 0.046; r = −0.82, r = −0.82, respectively).
No significant differences were detected for serum TK, HPT, or 25(OH)D between well‐ and poorly controlled dogs with IMPA (Fig 2a,c,d). Dogs with well‐controlled IMPA had significantly lower serum c‐CRP concentrations compared to dogs with poorly controlled disease (P = 0.05; Fig 2b). Median ± IQR biomarker concentrations are provided in Table 4c. Using a cutoff value of 66.3 mg/L, the sensitivity and specificity of detecting a poorly controlled dog with IMPA using c‐CRP were 0.13 and 1.00, respectively (Fig 7a,b). Canine‐CRP was not significantly correlated with WBC count (P = 0.74) in this population.
Figure 7.
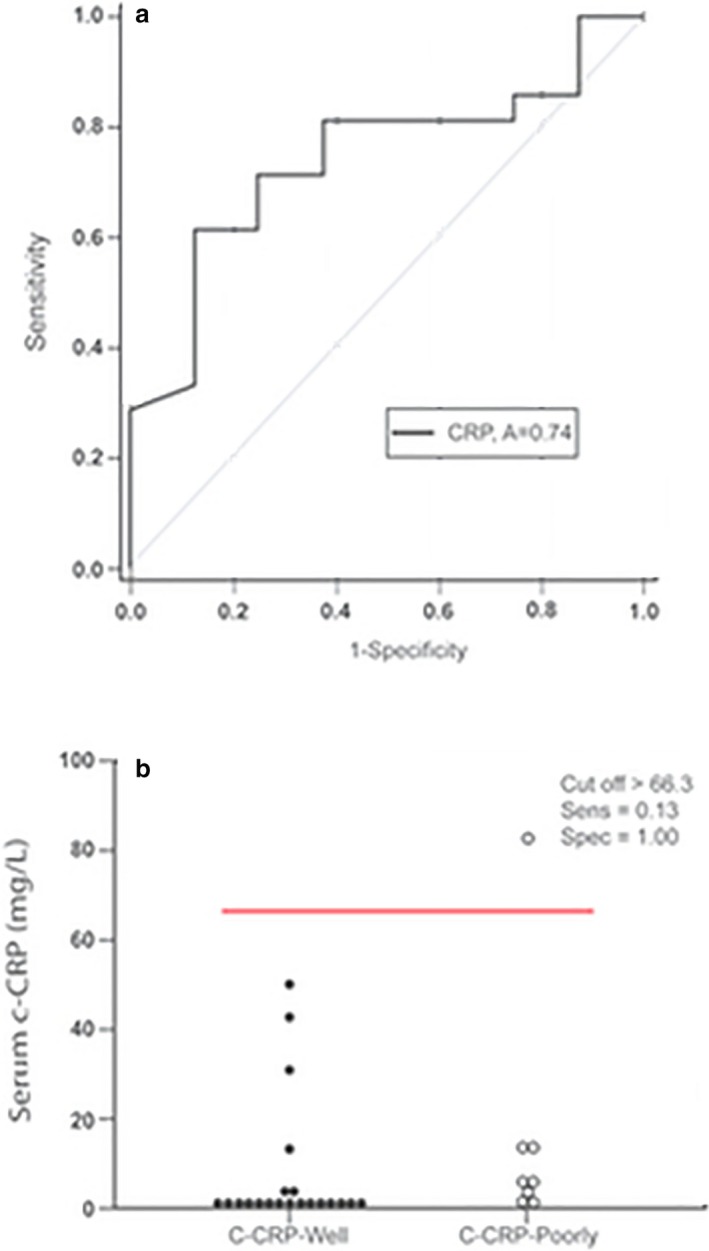
(a) Receiver operating characteristic curve for canine C‐reactive protein in dogs with immune‐mediated polyarthropathy (IMPA). The area under the curve (AUC) is 0.74. (b) Each dot represents the serum biomarker concentration for an individual dog with either poorly (solid) or well‐(open) controlled IMPA. The line is a graphical depiction of the “cutoff” values to maximize sensitivity and specificity for the detection of dogs with poorly regulated disease. Using a cutoff value of >66.3 mg/L, a sensitivity and specificity of 13 and 100% were calculated for the detection of dogs with poorly regulated IMPA.
Discussion
This study evaluates serum concentrations of a panel of biomarkers including TK1, c‐CRP, HPT, and 25(OH)D in dogs with IMHA, ITP, or IMPA. To discriminate between well‐ and poorly controlled canine immune‐mediated disease, declines in TK and c‐CRP were useful biomarkers for IMHA remission. Decreasing c‐CRP was potentially useful for IMPA remission; however, c‐CRP was only modestly elevated in most dogs with poorly controlled IMPA and though highly specific, lacked sensitivity for detecting patients with poorly controlled disease. The use of c‐CRP in place of joint fluid cytology for determining disease control in cases of IMPA cannot be recommended based on these study results. Unexpectedly, HPT was not able to discriminate status of disease control for IMHA or IMPA as concentrations generally remained high. Based on reference ranges provided by the laboratory for 25(OH)D, 92% dogs were vitamin D insufficient even when in clinical remission. As expected with inflammatory disease, dogs with IMHA, ITP, or IMPA at any time sampled had no significant differences in concentrations of the APPs c‐CRP and HPT indicating these biomarkers are not specific for the type of immune‐mediated disease. However, within the group of dogs with IMHA, in comparing well‐ versus poorly controlled disease, decreases in TK and c‐CRP had modest sensitivities of 66 and 65%, respectively and excellent specificity of 100%. When used simultaneously, decreases in TK and c‐CRP were 88% sensitive and 100% specific for detecting dogs with poorly controlled IMHA. This suggests possible clinical utility of combined biomarkers when disease control is questionable when evaluated by conventional methods.
Biomarkers can inform clinical decision making through improved therapeutic monitoring, screening for occult disease, and identifying dogs who might be at risk for disease development. Biomarkers have a number of advantages over conventional monitoring strategies, making them useful in adjunct to standard diagnostics. Acute phase proteins are a highly sensitive means of detecting inflammation and are increased in dogs unable to mount an obvious inflammatory reaction.7 This is likely to be relevant for dogs treated with immunosuppressive therapy. In this study, c‐CRP was not correlated with WBC count for dogs with either IMHA or IMPA. Biomarkers are also predictive for the development of clinical disease including relapses after remission. Measurement of APPs helped identify dogs with subclinical disease that later developed disease and died despite being asymptomatic at the time of sample collection.24 Biomarkers can be easy to analyze with serum and plasma samples that can be frozen and banked. The stability of these samples might increase their utility among clinicians that do not have the capability to perform in‐house hematologic evaluations or arthrocentesis. Finally, sedation is less likely to be required for sample collection and is comparatively less invasive in dogs with IMPA.
Despite the aforementioned advantages of using biomarkers, there are also important limitations. Biomarkers such as APPs lack specificity for use in diagnosis as many types of inflammatory processes will results in increased concentrations as was shown for all immune‐mediated diseases in this study. Some common therapeutic interventions, particularly glucocorticoids, can result in interference in some APP assays.10 In fact, glucocorticoid therapy is one explanation for the persistently elevated concentration of HPT in dogs across disease states regardless of control status. For this reason, a pair of highly sensitive APP biomarkers (c‐CRP and HPT) was combined with a cell cycle‐dependent biomarker (TK1) to attempt to increase specificity.
Thymidine kinase 1 is increased in highly proliferative cells. Although most commonly associated with neoplastic disease,8, 25 human studies have identified increased in serum TK1 in with patients with hemolytic disease.15 In a similar fashion, TK1 activity was increased in dogs with IMHA and concentrations of TK1 declined with control of disease as indicated by resolution of hemolysis. Of note, well‐controlled dogs with IMHA, ITP, and IMPA still demonstrated serum TK1 activity above the reference standard. Due to small sample size, statistical differences between well‐ and poorly controlled dogs with ITP could not be performed; however, correlations were identified between TK1 and reticulocyte count and total WBC concentrations. Increases in TK1 observed in this population may reflect regeneration of erythrocyte precursors secondary to hemorrhage and systemic inflammation. Although TK1 is involved in early megakaryocyte precursor development, no correlations were noted between TK1 and platelet count. In cases of ITP where the immune reaction is directed against the platelet precursors, there might not be detectable contributions to serum TK1. The dogs in this study did not have routine bone marrow evaluation so the presence of platelet precursors could not be determined. The importance of these correlations is unknown given small number of dogs in this population and should be considered a limitation of this study. Overall concentrations of TK1 were lower in dogs with IMPA compared with IMHA, and significant increases in TK1 were not identified in poorly vs well‐controlled dogs with IMPA. In this case, increased leukocyte production might be inadequate to produce detectable increases in serum TK1 activity in the absence of other sources of cellular proliferation. Serum activity of TK1 found in this study show marked overlap with those presented in previous studies in dogs with neoplastic disease.8 This suggests that serum TK1 activity should not be used as an independent biomarker for malignant disease, and that like APPs, as a screening test, serum TK1 lacks specificity when considered in isolation.
The significant decrease in c‐CRP concentrations with IMHA and IMPA remission agrees with previous studies documenting decreased concentrations of c‐CRP with the introduction of therapy.11, 26 Although declining serum concentrations of c‐CRP were associated with disease control, there was no relationship with WBC count (P = 0.15 and 0.94 for IMHA and IMPA, respectively), suggesting that common objective parameters such as total white blood count may not accurately gauge inflammation. Despite the reported sensitivity of c‐CRP for inflammatory disease, nearly a quarter of dogs with poorly controlled disease had normal c‐CRP concentrations. Cases of ITP and IMHA where c‐CRP concentration was within the reference range have been previously reported, although the underlying mechanism is unclear.27 The majority of dogs had serum activity of TK1 and c‐CRP concentrations above the reference range established by Veterinary Diagnostics Institute (VDI). These likely reflect poor specificity for immune‐mediated disease, however, biomarker concentrations lagging behind clinical resolution of inflammation/cellular proliferation, and type 2 statistical error cannot be entirely ruled out. Furthermore, when evaluated independently, the sensitivity of c‐CRP for detecting poorly controlled dogs with IMPA was poor, making it an inappropriate substitute for arthrocentesis. It should be noted that the well‐controlled dogs included an outlier which may have impacted the sensitivity and specificity for c‐CRP to screen for poorly controlled disease in this population.
There were no significant differences in serum HPT concentrations between dogs with IMHA, ITP, and IMPA, nor between well‐ and poorly controlled dogs for any disease. Immune‐mediated disorders are examples of inflammatory disease in which HPT increases consistent with its role as a positive acute phase protein.7 In cases of hemolysis, HPT binds free hemoglobin and is cleared via the reticuloendothelial system resulting in decreased serum HPT concentrations.28 The failure of HPT to decrease with disease control might reflect a number of factors including the degree of hemolysis, the balance between inflammation and HPT‐hemoglobin clearance, and treatment with corticosteroids. Concurrent treatment with glucocorticoids increases in serum HPT concentrations.10 These findings were supported by this study and given the pervasiveness of glucocorticoids in treating immune‐mediated disease, this might significantly limit clinical the utility of HPT in these patients.
All but 3 dogs in this population were vitamin D insufficient,22 irrespective of disease, disease control, or prior treatment with corticosteroids. The majority of dogs having decreased serum 25(OH)D concentrations might reflect a predisposition to immune dysregulation. The impact of steroid administration on 25(OH)D concentrations was considered as a possible source of low vitamin D concentrations.29 However, no difference in 25(OH)D concentrations was detected between dogs that had received prior steroid treatment and those that had not, making this unlikely.
Conclusion
The combined use of biomarkers TK1 and c‐CRP appears to have value in monitoring disease control in IMHA. Despite its high specificity, c‐CRP has a low sensitivity for detection of poorly controlled IMPA and cannot be advocated in lieu of serial synovial fluid cytology. Additional study needs to be performed with larger numbers of dogs with ITP, although both TK1 and c‐CRP appear to have some promise as the group mean concentrations were elevated in poorly controlled disease and decreased in well‐controlled disease. The low serum 25(OH)D concentrations identified in most dogs with immune‐mediated disorders regardless of disease control might reflect underlying immune dysregulation. Although further study is required, low serum 25(OH)D concentrations might help identify a population at risk for the development of immune‐mediated disease and open avenues for therapeutic intervention.
Acknowledgments
The authors thank Matt Haight for his assistance in sample collection throughout this study, and Steve Gauthier and Randy Ringold at VDI for facilitating biomarker analysis. Dr. Grobman's salary is supported by the Boehringer Ingelheim Resident‐Postdoctoral Scholar Program.
Grant support
This study was supported internally by the Cisco and Izzy fund for Immunologic Research (USA).
Conflict of Interest Declaration
The assays in this study were performed by the Veterinary Diagnostic Institute (Simi Valley, CA).
Off‐Label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
All work was conducted at the University of Missouri in cooperation with the Veterinary Diagnostic Institute (Simi, Valley, CA).
Footnotes
IDEXX Laboratories, Westbrook, ME
Systat Software, San Jose, CA
References
- 1. Whitley NT, Day MJ. Immunomodulatory drugs and their application to the management of canine immune‐mediated disease. J Small Anim Pract 2011;52:70–85. [DOI] [PubMed] [Google Scholar]
- 2. Goggs R, Dennis SG, Di Bella A, et al. Predicting outcome in dogs with primary immune‐mediated hemolytic anemia: Results of a multicenter case registry. J Vet Intern Med 2015;29:1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piek CJ, Junius G, Dekker A, et al. Idiopathic immune‐mediated hemolytic anemia: Treatment outcome and prognostic factors in 149 dogs. J Vet Intern Med 2008;22:366–373. [DOI] [PubMed] [Google Scholar]
- 4. Yeates J, Main D. Assessment of companion animal quality of life in veterinary practice and research. J Small Anim Pract 2009;50:274–281. [DOI] [PubMed] [Google Scholar]
- 5. Bathen‐Noethen A, Carlson R, Menzel D, et al. Concentrations of acute‐phase proteins in dogs with steroid responsive meningitis‐arteritis. J Vet Intern Med 2008;22:1149–1156. [DOI] [PubMed] [Google Scholar]
- 6. Lowrie M, Penderis J, Eckersall PD, et al. The role of acute phase proteins in diagnosis and management of steroid‐responsive meningitis arteritis in dogs. Vet J 2009;182:125–130. [DOI] [PubMed] [Google Scholar]
- 7. Solter PF, Hoffmann WE, Hungerford LL, et al. Haptoglobin and ceruloplasmin as determinants of inflammation in dogs. Am J Vet Res 1991;52:1738–1742. [PubMed] [Google Scholar]
- 8. Selting KA, Sharp CR, Ringold R, et al. Serum thymidine kinase 1 and C‐reactive protein as biomarkers for screening clinically healthy dogs for occult disease. Vet Comp Oncol 2015;13:373–384. [DOI] [PubMed] [Google Scholar]
- 9. Ceron JJ, Eckersall PD, Martynez‐Subiela S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet Clin Pathol 2005;34:85–99. [DOI] [PubMed] [Google Scholar]
- 10. Harvey JW, West CL. Prednisone‐induced increases in serum alpha‐2‐globulin and haptoglobin concentrations in dogs. Vet Pathol 1987;24:90–92. [DOI] [PubMed] [Google Scholar]
- 11. Griebsch C, Arndt G, Raila J, et al. C‐reactive protein concentration in dogs with primary immune‐mediated hemolytic anemia. Vet Clin Pathol 2009;38:421–425. [DOI] [PubMed] [Google Scholar]
- 12. Tecles F, Spiranelli E, Bonfanti U, et al. Preliminary studies of serum acute‐phase protein concentrations in hematologic and neoplastic diseases of the dog. J Vet Intern Med 2005;19:865–870. [DOI] [PubMed] [Google Scholar]
- 13. Gebhardt C, Hirschberger J, Rau S, et al. Use of C‐reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care 2009;19:450–458. [DOI] [PubMed] [Google Scholar]
- 14. Kauffman MG, Kelly TJ. Cell cycle regulation of thymidine kinase: Residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol Cell Biol 1991;11:2538–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagberg H, Gronowitz S, Killander A, et al. Serum thymidine kinase in vitamin B12 deficiency. Scand J Haematol 1984;32:41–45. [DOI] [PubMed] [Google Scholar]
- 16. Tronik‐Le Roux D, Roullot V, Schweitzer A, et al. Suppression of erythro‐megakaryocytopoiesis and the induction of reversible thrombocytopenia in mice transgenic for the thymidine kinase gene targeted by the platelet glycoprotein alpha IIb promoter. J Exp Med 1995;181:2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fattizzo B, Zaninoni A, Giannotta JA, et al. Reduced 25‐OH vitamin D in patients with autoimmune cytopenias, clinical correlations and literature review. Autoimmun Rev 2016;15:770–775. [DOI] [PubMed] [Google Scholar]
- 18. Baeke F, Takiishi T, Korf H, et al. Vitamin D: Modulator of the immune system. Curr Opin Pharmacol 2010;10:482–496. [DOI] [PubMed] [Google Scholar]
- 19. Bockow B, Kaplan TB. Refractory immune thrombocytopenia successfully treated with high‐dose vitamin D supplementation and hydroxychloroquine: Two case reports. J Med Case Rep 2013;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dougherty KA, Bertolaso C, Schall JI, et al. Safety and efficacy of high‐dose daily vitamin D3 supplementation in children and young adults with sickle cell disease. J Pediatr Hematol Oncol 2015;37:e308–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J Mol Med 2010;88:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selting KA, Sharp CR, Ringold R, et al. Serum 25‐hydroxyvitamin D concentrations in dogs—Correlation with health and cancer risk. Vet Comp Oncol 2014;14:295–305. [DOI] [PubMed] [Google Scholar]
- 23. Dabrowski R, Kostro K, Lisiecka U, et al. Usefulness of C‐reactive protein, serum amyloid A component, and haptoglobin determinations in bitches with pyometra for monitoring early post‐ovariohysterectomy complications. Theriogenology 2009;72:471–476. [DOI] [PubMed] [Google Scholar]
- 24. Ohwada K, Tamura K. Usefulness of alpha 1 acid glycoprotein (alpha 1‐AG) values in screening pound dogs acquired from animal shelters for experimental use. Jikken Dobutsu 1993;42:627–630. [DOI] [PubMed] [Google Scholar]
- 25. Zhou J, He E, Skog S. The proliferation marker thymidine kinase 1 in clinical use (review). Mol Clin Oncol 2013;1:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohno K, Yokoyama Y, Nakashima K, et al. C‐reactive protein concentration in canine idiopathic polyarthritis. J Vet Med Sci 2006;68:1275–1279. [DOI] [PubMed] [Google Scholar]
- 27. Nakamura M, Takahashi M, Ohno K, et al. C‐reactive protein concentration in dogs with various diseases. J Vet Med Sci 2008;70:127–131. [DOI] [PubMed] [Google Scholar]
- 28. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol 2002;69:258–271. [DOI] [PubMed] [Google Scholar]
- 29. Searing DA, Zhang Y, Murphy JR, et al. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 2010;125:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]


