Abstract
Intravenous fluid therapy can alter plasma acid‐base balance. The Stewart approach to acid‐base balance is uniquely suited to identify and quantify the effects of the cationic and anionic constituents of crystalloid solutions on plasma pH. The plasma strong ion difference (SID) and weak acid concentrations are similar to those of the administered fluid, more so at higher administration rates and with larger volumes. A crystalloid's in vivo effects on plasma pH are described by 3 general rules: SID > [] increases plasma pH (alkalosis); SID < [] decreases plasma pH (alkalosis); and SID = [] yields no change in plasma pH. The in vitro pH of commercially prepared crystalloid solutions has little to no effect on plasma pH because of their low titratable acidity. Appreciation of IV fluid composition and an understanding of basic physicochemical principles provide therapeutically valuable insights about how and why fluid therapy can produce and correct alterations of plasma acid‐base equilibrium. The ideal balanced crystalloid should (1) contain species‐specific concentrations of key electrolytes (Na+, Cl−, K+, Ca++, Mg++), particularly Na+ and Cl−; (2) maintain or normalize acid‐base balance (provide an appropriate SID); and (3) be isosmotic and isotonic (not induce inappropriate fluid shifts) with normal plasma.
Keywords: Acid‐base balance, Base replacement, Fluid therapy, Metabolic acidosis, Physiology
Abbreviations
- AG
anion gap
- Atot
nonvolatile weak acids
- NHE1
Na+/H+ exchanger
- PCO2
partial pressure of carbon dioxide
- pKa
pH at which the acid and conjugate base are equal
- SIDa
apparent SID
- SIDe
effective SID
- SIDif
crystalloid in vivo SID
- SID
strong ion difference
- SIG
strong ion gap
- THAM
trishydroxymethyl aminomethane
- UA
unmeasured anion
Intravenous salt solutions (“crystalloids”) are routinely administered to animals and are considered an established standard of care in many veterinary practices. They are administered to maintain or restore vascular volume, electrolyte concentrations, and acid‐base balance and are utilized as diluents for large (>30 kD) molecular weight insoluble molecules to produce a colloidal suspension (“colloids”) that helps preserve plasma colloid osmotic pressure.1 Fluids are drugs, and although often considered to produce beneficial effects, they should only be administered after thorough consideration of the indication for which they are prescribed.2, 3 Reasons for administering IV fluids include the prevention or treatment of dehydration, replacement of ongoing fluid losses, correction of electrolyte imbalances, restoration of tissue perfusion, treatment of hypotension, and correction of acid‐base abnormalities.1, 4 Although the beneficial volume effects of IV fluids have been appreciated for more than 100 years, their impact on the extracellular and intracellular concentrations of electrolytes, acid‐base balance, and survival is only beginning to be appreciated.5, 6, 7, 8, 9, 10
Improvements in hydration status and hemodynamic parameters (macrocirculatory features such as arterial blood pressure, blood flow) are frequently the primary goals of IV fluid therapy. Macrocirculatory improvements, however, do not always insure an improvement in capillary perfusion (microcirculatory effect), cellular homeostasis, or survival.11, 12, 13 The maintenance of normal blood hydrogen ion (H+) activity is a key factor linked to survival, and its regulation is one of the most tightly controlled homeostatic processes in the body.14, 15, 16, 17, 18 Use of the term hydrogen ion concentration ([H+]), although commonplace in the acid‐base literature, is misleading and should be discouraged because it is hydrogen ion activity (pH) that is measured by pH electrodes. Hydrogen ions are considerably smaller than other chemicals in aqueous solutions but have the highest charge density of any electrolyte in plasma.19 Hydrogen ions (protons) are chemically active because of the electromotive force (activity) they produce. Furthermore, hydrogen ion concentration cannot be accurately determined in vivo because it is calculated assuming an activity coefficient of 1, but its activity coefficient in plasma is uncertain. Comparatively small changes in H+ activity (pH) can produce substantial and potentially life‐threatening alterations in cellular metabolism (Fig 1).20, 21 Acidemia (decreased blood pH) and alkalemia (increased blood pH) directly impact morbidity and mortality and are decidedly influenced by the administration of IV fluids.21, 22, 23, 24 This review will summarize the various methods used to identify and describe acid‐base abnormalities, provide a modern definition for what is considered to be a balanced crystalloid solution, and explain how IV fluid therapy can alter acid‐base balance. The use of different IV fluids for the treatment of metabolic acidosis will be reviewed, and the influence of commercially prepared IV fluid solutions on acid‐base balance will be discussed.
Figure 1.
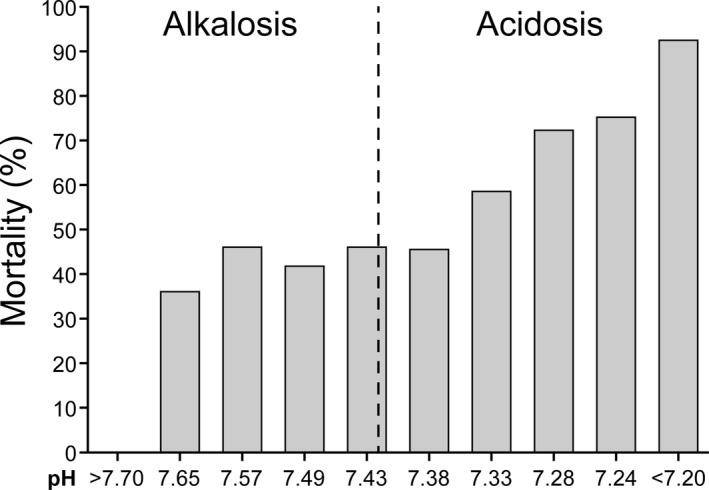
Relationship between approximate pH values and mortality in 754 critically ill human patients.21
Diagnosing and Describing Acid‐Base Disorders
Regrettably, conflicting opinions, ambiguous terminology, computational complexity, and, until recently, the absence of simplified and versatile monitoring equipment have hindered the assessment and diagnosis of acid‐base abnormalities in veterinary clinical practice.25 The various approaches employed for the diagnosis and description of acid‐base abnormalities are based upon changes in blood pH (negative base 10 logarithm of activity) or the principal analytes responsible for its alteration (Fig 2).19, 25 They include (1) the Henderson‐Hasselbalch approach; (2) the anion gap (AG) approach; (3) the Astrup and Siggaard‐Andersen (base excess [BE]) approach; and (4) the physiochemical or Stewart approach.25, 26, 27, 28, 29, 30, 31 The Henderson‐Hasselbalch approach is based on the relationship among pH, PCO2, and (pH = pK + log []/.0308 × PCO2, where PCO2 is measured in mmHg at 37°C and is the basis of [] determination by blood gas analyzers. The anion gap (AG) approach attempts to identify changes in acid‐base balance by determining the difference between the principal cations and anions (AG = ([Na+] + [K+]) − ([Cl−] + [])).19, 26 An increase in the AG is considered indicative of an increase in fixed (nonvolatile) acid and metabolic (nonrespiratory) acidosis. Increased AG acidosis (e.g, lactic or ketoacidosis) is characterized by decreased plasma bicarbonate concentration without hyperchloremia.27 Increased plasma chloride concentration (i.e, hyperchloremia) is accompanied by a decrease in plasma [] with no increase in the AG, a condition referred to as “normal AG acidosis” or “hyperchloremic acidosis”.27, 28, 29 Discontent with the Henderson‐Hasselbalch approach prompted Singer and Hastings to introduce the BE concept and propose that plasma pH be determined by 2 independent factors, PCO2 and net strong (highly dissociable) ion charge, equivalent to the SID.32 Base excess is comparable to the difference between an animal's SID and the normal SID for that species, assuming a fixed and normal plasma protein concentration.33 The Astrup‐Siggaard‐Andersen approach utilizes the PCO2 and BE.30 The BE is defined as the concentration of titratable acid or alkali required to return the in vitro pH of whole blood to 7.4 at 37°C when the PCO2 is equal to 40 mmHg. Base excess incorporates the buffering capacity of hemoglobin and was the first measure of metabolic acid‐base status independent of PCO2.29 Base excess is calculated by most acid‐base analyzers or can be derived from the Siggaard‐Andersen nomogram.30 The physiochemical or Stewart approach posits that [H+] and [] are dependent variables and categorizes acid‐base disturbances based upon changes in the 3 independent factors: PCO2, SID, and Atot (Fig 3).15, 31, 34 Bicarbonate is a dependent variable and does not determine hydrogen ion activity; both [] and hydrogen ion activity are determined by PCO2, SID, and Atot. The Stewart approach does not account for the buffering capacity of hemoglobin and is considered “quantitatively cumbersome” and to have “no advantage for diagnostic or prognostic purposes” by some.35 These opinions aside, the Stewart approach is utilized hereafter because (1) hydrogen ion activity and [] are dependent not independent variables; (2) it incorporates the influence of strong anions and cations on acid‐base balance (anion gap approach); and (3) it provides a more clinically intuitive, descriptive, and quantitative understanding of how variations in plasma constituents and the administration of crystalloid solutions can alter acid‐base balance (Table 1).15, 31, 33, 34, 35, 36, 37, 38, 39
Figure 2.
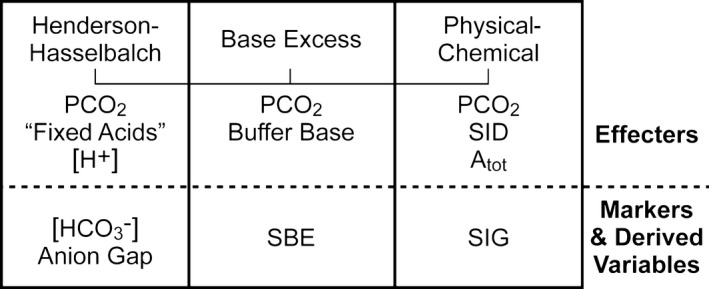
The different approaches used to diagnose and describe acid‐base disorders can be categorized as descriptive, semiquantitative, and quantitative. The physicochemical (Stewart) approach can be used in all 3 capacities. Atot = total weak acids; PCO 2 = partial pressure of carbon dioxide; SBE = standard base excess; SID = strong ion difference; SIG = strong ion gap.26
Figure 3.
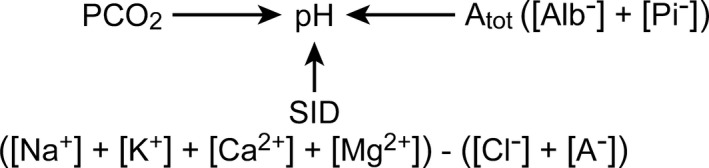
Principal independent factors that determine pH.
Table 1.
Stewart approach to acid‐base balance
| Independent Variable | Change | Acid‐base Effect | pH |
|---|---|---|---|
| PCO2 mmHg | ↑ | Respiratory acidosis | ↓ |
| ↓ | Respiratory alkalosis | ↑ | |
| SID mEq/L | ↑ | Metabolic alkalosis | ↑ |
| ↓ | Metabolic acidosis | ↓ | |
| Atot mmol/L | ↑ | Metabolic acidosis | ↓ |
| ↓ | Metabolic alkalosis | ↑ |
↑ = increase; ↓ = decrease.
Physicochemical or Stewart Approach to Acid‐base Abnormalities
Strong ions behave as though nearly completely dissociated at physiologic pH, and the SID characterizes the net charge that must be balanced by all of the nonvolatile weak acids (Atot, primarily albumin, and phosphate) in order to maintain electrical neutrality.31 The plasma SID is typically calculated as the difference between all measurable strong cations ([Na+] + [K+] + [Ca++] + [Mg++]) and anions ([Cl−] + [other strong anions, e.g, lactate]) and is frequently referred to as the apparent SID (SIDa) because this value is representative of the majority of ions found in plasma. The SIDa is normally positive (+40–45 mEq/L), and this net charge is balanced by the negative charge of the fixed weak anion components of phosphate and proteins (Atot), and bicarbonate.40, 41, 42 As stated earlier, the sum of the nonvolatile weak acids, Atot, is an independent variable that impacts hydrogen ion activity and therefore pH (Fig 3). An increase in the concentration of lactate and unmeasured anions (other organic acids) from any cause (e.g, hemorrhage, trauma, hypoxia) will increase hydrogen ion activity creating metabolic acidosis.43, 44, 45 Importantly, fluid selection and infusion strategies are key factors that can influence the production of unmeasured anions (UA).46 The [UA] can be derived by subtracting the effective SID (SIDe, the sum of the negatively charged substances) from SIDa, thereby defining the strong ion gap (SIG, SIG = SIDa–SIDe = UA; Fig 4).47 Delayed metabolism or elimination of any UA− decreases SIDe, thereby increasing the SIG and contributing to metabolic acidosis.47 Notably, the SIDa minus SIDe (SIG) is equal to or near zero when plasma pH is 7.4 and PCO2 is 40 mmHg. The SIDa and SIG have been used clinically in both humans and animals to identify acid‐base imbalances and predicting mortality, respectively.47, 48, 49, 50, 51, 52, 53, 54, 55 Current evidence, however, suggests that directly measured serial arterial lactate concentrations may provide as good or better prognostic ability than SIG for discriminating between survivors and nonsurvivors.53, 54 Simplified versions of the Stewart approach have substantially improved the recognition and contribution of electrolyte abnormalities (alterations in SID) in maintaining acid‐base balance and highlight the importance of fluid selection as a potential therapy.34, 37, 38, 39, 52, 53, 54, 55, 56
Figure 4.
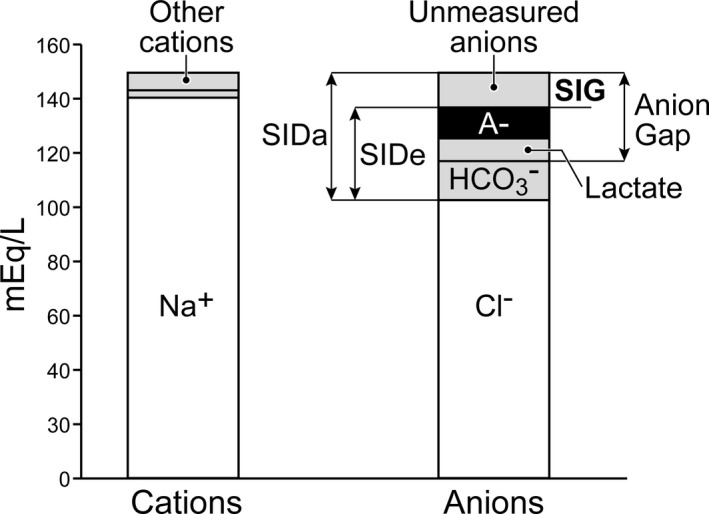
Strong ion gap (SIG) is the difference SID a and SID e. The SIG is an accurate measure of the unmeasured anions present in plasma.81
Crystalloids and Balanced Crystalloids
Crystalloid solutions are prepared by diluting relatively small amounts of the salts of physiologically relevant elements (Na+, K+, Ca++, Mg++, Cl−) in water. These elements are electrically balanced (law of electrical neutrality) but fully dissociate (e.g, strong cations and anions) in water because their pKa (pH at which the acid and conjugate base are equal) value is considerably different (e.g, the pKa of lactic acid = 3.86, and therefore it is fully dissociated) from that of normal plasma pH (7.40). Compounded crystalloids may contain added NaHCO3 in order to treat (buffer) or resist pH changes from nonrespiratory causes of acidosis. Most commercial manufacturers of crystalloid solutions have abandoned the addition of Na+ to replace chloride ion when stored in plastic bags that allow equilibration with atmospheric CO2 because of the potential to form divalent carbonate () and precipitates with calcium and magnesium.57, 58 Most manufacturers have replaced with an organic anion (lactate, acetate, citrate) with the expectation that it will act as a “precursor” or stable surrogate for bicarbonate (Table 2).59, 60 In addition, replacing some of the Cl− with an organic anion maintains electrical neutrality and lowers the solution's [Cl−].59 Organic anions are strong anions (pK < 4) combined with Na+. Their role is not to generate , as often taught, but to be rapidly metabolized and disappear from solution, thus increasing the in vivo SIDa.60, 61, 62, 63 This requirement is met in most animals but is likely to be species dependent, compromised in sick animals, and dependent upon the rate and amount of organic ion administered.64, 65, 66, 67, 68, 69 The in vivo fate of organic anions as generators in animals with naturally occurring diseases (hypovolemic shock; sepsis) requires further investigation.
Table 2.
Characteristics of crystalloid and colloid solutions
| Fluid | pH | Na+ (mEq/L) | Cl− (mEq/L) | K+ (mEq/L) | Ca++ (mEq/L) | Mg++ (mEq/L) | Buffer (mEq/L) | Osmolarity (mOsm/L) | COP (mmHg) | SID (mEq/L) | Viscosity (cP) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.9% NaCl | 5.5 | 154 | 154 | 0 | 0 | 0 | 0 | 308 | 0 | 0 | ≈1 |
| 7.5% Saline | 5.5 | 1283 | 1283 | 0 | 0 | 0 | 0 | 2,566 | 0 | 0 | ≈1 |
| 1.4% NaHCO3 | 167 | HCO3 | 300 | 167 | |||||||
| 8.4% NaHCO3 | 8.0 | 1,000 | 1,000 | 0 | 0 | 0 | HCO3 | 2,000 | 0 | 1,000 | ≈1 |
| 3% Na Lactatec | 7.0 | 504 | 7 | 4 | 2.7 | 0 | Lactate 504 | 1,020 | 0 | 500 | ≈1 |
| LRS | 6.5 | 130 | 109 | 4 | 3 | 0 | Lactate 28 | 273 | 0 | 27 | |
| Normosol‐Ra | 7.4 | 140 | 98 | 5 | 0 | 3 | Acetate 27 Gluconated23 | 295 | 0 | 27–50 | ≈1 |
| Plasma‐Lyte Ab | 7.4 | 140 | 98 | 5 | 0 | 3 | Acetate 27 Gluconated23 | 294 | 0 | 27–50 | ≈1 |
| Plasma‐Lyte 148b | 6.0 | 140 | 98 | 5 | 0 | 3 | Acetate 27 Gluconated23 | 294 | 0 | 27–50 | ≈1 |
| 5% Albumin | 5.5 | 154 | 154 | 0 | 0 | 0 | 0 | 308 | 19 | 0 | 1.2–1.5 |
| 6% Het/Saline | 5.5 | 154 | 154 | 0 | 0 | 0 | 0 | 308 | 3 | 0 | 4.3 |
| 6% Het/LRS | 6.5 | 143 | 124 | 3 | 5 | 0.9 | 28 | 303 | 32 | 28 | 4.3 |
| 6% Tetra/Saline | 5.5 | 154 | 154 | 0 | 0 | 0 | 0 | 308 | 42 | 0 | ≈4 |
| Blood | 7.4 | ≈150 | ≈105 | ≈4 | ≈5 | ≈2 | 40 | 300–305 | 20–25 | 40 | 3.5 |
Common properties of crystalloid and colloid solutions used for fluid therapy. LRS, Lactated Ringer's solution.
Hospira, Inc., Lake Forest, IL 60045.
Baxter Healthcare Corporation Deerfield, IL 60015.
l‐lactate; Het, hetastarch; Tetra, tetrastarch.
Gluconate is a mixed nonmetabolizable strong ion.
The term “balanced” was originally devised to describe mixtures of various salts in water that produced electrolyte concentrations similar to normal plasma.5, 6, 7, 56, 57, 59 This term, however, has become both confusing and misleading because it is not always apparent what is balanced (e.g, [Na+] or [Cl−], effective osmolality, pH relative to plasma) and because the fate of organic anions is not always predictable.70, 71 Normal or physiologic saline (0.9% NaCl), Ringer's solution, lactated Ringer's solution (LRS), and compound sodium lactate (Hartmann's) are historically popular resuscitation fluids in both human and veterinary medicine.5, 6, 7, 72, 73, 74 Saline (0.6–0.9% NaCl) evolved from in vitro experiments designed to prevent hemolysis of human red blood cells whereas Ringer's solution was formulated in order to improve the contraction of beating frog hearts in vitro.72, 73 Lactated Ringer's and Hartmann's solutions soon followed by adding lactate to Ringer's solution in order to buffer dehydration‐associated acidosis in humans.74 Although the concentrations of 1 or more electrolytes in each of these 3 solutions are comparable to those found in plasma, none are truly “normal,” “physiologic,” “plasma adapted,” or balanced, especially when different species of animals are considered (Table 2).56, 57, 69, 71 If “balanced” is meant to imply a solution that has an electrolyte composition close to plasma, a normal [Cl−], and 1 that maintains normal (effective) osmolality, tonicity, and pH values, then the composition of most commercially prepared “balanced” crystalloids can be challenged.56, 75, 76, 77 I propose that balanced crystalloid solutions should (1) contain species‐specific concentrations of key electrolytes (Na+, Cl−, K+, Ca++, Mg++), particularly [Na+] and [Cl−]; (2) maintain or normalize acid‐base balance (provide an appropriate SID); and (3) be isosmotic and isotonic (not induce inappropriate fluid shifts) with normal plasma.
pH of Commercial Crystalloid Solutions
The pH of most commercially prepared crystalloids varies between 4.0 and 6.5 unless specified otherwise (e.g, Normosol‐R1 [pH 7.4], Plasma‐Lyte A2 [pH 7.4], Plasma‐Lyte 1482 [pH 7.4]). A solution's in vitro pH is a measure of the degree of acidity or alkalinity of the solution and not the total reservoir (or lack thereof) of hydrogen ions available.78 Three factors determine the in vitro pH of commercially prepared solutions: the container (glass or polyvinyl chloride [PVC]), the temperature‐dependent solubility of CO2 in water, and the concentration of electrolytes (strong ions) added to the solution. Glass containers are considered to be inert, but autoclaving of PVC packaged solutions generates small quantities of acetic and formic acid, lowering the solution's pH.58, 78, 79 This source of acidity, however, is inconsequential based upon the miniscule amounts of H+ produced.73 Carbon dioxide absorbed from air is the largest contributor to hydrogen ion activity and a decrease in pH in commercial solutions. Finally, the addition of physiologically relevant concentrations of salts to water is believed to influence the formation of hydronium ions (H3O+) favoring an increase in hydrogen ion activity and a decrease in pH.79 The concentration of the electrolytes in 0.9% NaCl, for example, is responsible for lowering the pH approximately 0.01 pH unit.79 Sterile distilled water has a pH of approximately 5.6 at sea level and at 25°C due to the absorption of CO2 from the atmosphere;79 the same process is the largest contributor to hydrogen ion activity in commercial solutions stored in plastic containers.70 Titratable acidity, as measured by the titration of any commercial crystalloid solution with NaOH to pH = 7.4, is clinically negligible in all commercial crystalloids and ranges from 0.126–0.152 mEq/L for 0.9% NaCl.70 The low titratable acidity of 0.9% NaCl implies that it is not the solution's in vitro pH that is responsible for its potential to produce an in vivo metabolic acidosis but rather the solution's effect (0.9% NaCl = 0) on the in vivo SID.56, 57, 59, 71, 79
The Acid‐Base Effects of Intravenous Fluid Therapy
All crystalloids have the potential to significantly alter acid‐base balance because of differences in their physicochemical composition relative to plasma.56, 71
The SIDa of normal plasma is approximately 40–44 mEq/L (mM/L), suggesting that administration of a crystalloid with an in vivo SID (SIDif) of 40–44 mEq/L should maintain plasma SID within the normal reference range. Infusion of a solution with an SIDif of 40–44 mEq/L, however, results in the development of metabolic alkalosis because of progressive dilution of Atot.56 Acid‐base balance is achieved when the SIDif of the infused fluid is similar to the normal plasma [] of the species (omnivore versus herbivore) being treated.
The in vitro SID of all crystalloid and colloid solutions is zero (law of electrical neutrality) but ranges from 0 to 50 mEq/L in vivo because of the addition of metabolizable organic anions (e.g, lactate, acetate, citrate, gluconate; Table 2). Because commercially prepared crystalloids do not contain , final plasma pH is determined by the net charge difference produced by the crystalloid's SIDif after metabolism of the crystalloid's organic anion(s) in proportion to the rate and volume of fluid administered.70 The rapid infusion of large volumes of any crystalloid, for example, will cause the extracellular fluid (plasma and interstitial fluid) to drift toward the SIDif of the infused crystalloid, changing hydrogen ion activity and pH accordingly. The SIDif of any crystalloid can be calculated by subtracting the major strong anions from strong cations after removal of the metabolizable organic anion (Table 2). Plasma pH can be predictably maintained, increased, or decreased based upon the difference between the crystalloid's SIDif and the animal's baseline [].80 This usually corresponds to SIDif values ranging from 22 to 32 mEq/L for normal healthy animals (depending upon the species being treated) and can be formulated into general rules: if the crystalloid's SIDif > plasma [], pH increases; if SIDif < plasma [], pH decreases; and if SIDif equals plasma [], pH does not change.81, 82, 83, 84, 85, 86 These general rules also hold true for all commercially prepared colloids except those that contain charged ionic macromolecules (e.g, albumin, gelatins) as apposed to nonionic colloids (e.g, starches, dextrans). Nonionic colloids do not alter acid‐base balance. Ionic colloids (e.g, albumin), particularly those diluted in 0.9% NaCl, have the potential to acidify plasma as a consequence of the increase in Atot and decrease in SID.85 The clinical importance of the acid‐base effects of current commercially prepared ionic colloidal solutions, however, is minimal compared to that produced by their diluent.
As described above, a crystalloid's SIDif, the rate and total volume of fluid administered, and metabolism of organic anions present in the fluid are the principal determinants of the solution's effect on plasma pH. A lower infusion rate (10 mL/kg/h) of 0.9% NaCl, Hartmann's solution, or a polyionic glucose‐free maintenance solution for 2 hours (total volume = 20 mL/kg) to 60 normal dogs, for example, produced no significant differences in plasma electrolytes, total protein, plasma volume, SIG, or pH.87 The infusion of 30 mL/kg/h LRS (273 mOsm/L) for 1 hour consistently decreased packed cell volume (PCV), total protein (TP), albumin, colloid osmotic pressure (COP), and extracellular tonicity in the plasma of healthy isoflurane‐anesthetized dogs but did not change plasma pH or lactate concentrations.88 Lactate concentrations, however, were increased (>2 mmol/L) for up to 60 minutes when LRS was rapidly administered (180 mL/kg/h) for 1 hour to conscious healthy dogs, suggesting that the capacity for lactate metabolism had been exceeded.67, 69 In addition, IV administration of LRS (4.1 mL/kg/h for 6 hours) increased venous blood lactate concentrations in dogs with stage IIIa and IVa lymphoma.64 Fluids containing gluconate (Normosol‐R1; Plasma‐Lyte A2; Plasma‐Lyte 1482) may be particularly inefficient for correcting metabolic acidosis in dogs and calves because of gluconate's poor metabolism.61, 62, 69 Other issues associated with the infusion of organic anions, in addition to their impaired or delayed metabolism in various disease states, include but are not limited to poor metabolism (d‐lactate), proinflammatory effects (d‐lactate, acetate), neurologic and cardiac toxicity (d‐lactate), and hypotension (acetate).66, 89, 90
Acute Metabolic Acidosis: Causes, Consequences, and Treatment
Acute metabolic (nonrespiratory) acidosis is caused by diseases that increase the production or decrease the elimination of nonvolatile fixed acids or decrease the body's buffering capabilities. Metabolic acidosis can be characterized by a decrease in SIDa or increase in Atot with resultant decreases in [] and secondary (compensatory) decreases in PCO2 (approximately 0.7 mmHg for each 1 mEq/L decrease in []). Metabolic acidosis also may coexist with respiratory acidosis in animals that have impaired pulmonary function.91, 92 Acute metabolic acidosis can be further classified as normal (nonion gap acidosis, hyperchloremic metabolic acidosis) or high anion gap acidosis.28, 93 Lactic acidosis (hyperlactatemia > 2 mmol/L), a high anion gap acidosis, is considered evidence for tissue hypoxia, tissue hypoperfusion, and anaerobic metabolism and is used as a prognostic indicator for increased morbidity and mortality in humans and animals.94, 95, 96, 97, 98, 99, 100, 101, 102, 103 Lactic acidosis in sepsis is multifactorial and may be caused by regional tissue hypoxia and increased aerobic glycolysis secondary to cytokine‐stimulated cellular glucose uptake and catecholamine stimulation of Na‐K ATPase.94, 104, 105
Acidemia decreases cardiac contractility, decreases blood flow (cardiac output), increases susceptibility to cardiac arrhythmias, impairs responsiveness to catecholamines, alters the immune response, and promotes a systemic inflammatory state.94, 106, 107, 108 Severe metabolic acidosis (pH < 7.15) may decrease systemic and increase pulmonary vascular resistance, worsening hypotension and tissue perfusion.109, 110, 111 Intracellular acidification (decreased pHi) also activates Na‐dependent acid/base transporters.112, 113, 114 The Na+/H+ exchanger (NHE1) is a ubiquitous and integral membrane transporter involved in regulating cell volume and pHi.115, 116 Activation of NHE1 increases intracellular [Na+] and [Ca++] resulting in alterations in cellular metabolism and cell membrane potential and is likely the principal cause for poor cardiac function, arrhythmias, and increased concentrations of proinflammatory cytokines.114, 115, 116
Treating acute acidemia (pH < 7.2) is not simple and should be based upon identification and control of the pathophysiologic process(s) responsible for its production.117 Ideally, species‐specific crystalloids containing appropriate quantities of electrolytes and metabolizable organic anions are preferred for maintaining tissue perfusion and correcting mild acid‐base abnormalities in otherwise normal healthy animals.36, 59, 70, 77, 118 Alkaline therapies including NaHCO3 or carbon dioxide‐consuming bases (trishydroxymethyl aminomethane: THAM3) and Carbicarb4 (combination of NaHCO3 and Na2CO3) are more effective treatments for acute severe metabolic acidosis than balanced crystalloids because of their high SIDif (Table 2).5 , 119 Carbicarb4 (SIDif = 210; 300 mOsm/L) generates less CO2 than NaHCO3 and therefore is less likely to produce intracellular acidosis whereas THAM3 (SIDif = 201; 300 mOsm/L) increases the buffering capacity of blood without generating CO2 and does not produce hypernatremia or hypokalemia.120, 121, 122, 123 Neither THAM3 nor Carbicarb4, however, has therapeutic advantages compared to NaHCO3 in clinical practice.124, 125 All 3 therapies remain controversial because of selective or uncertain long‐term benefit and the potential for complications.126, 127 More specifically, the Surviving Sepsis Campaign guidelines of 2016 recommend “against the use of sodium bicarbonate therapy to improve hemodynamics or to reduce vasopressor requirements in patients with hypoperfusion‐induced lactic acidemia.”128 In addition, sodium bicarbonate has the potential to produce hypersosmolality, hypernatremia, hypokalemia, decreased ionized calcium concentration, and increased hemoglobin affinity for oxygen.129, 130, 131, 132 Sodium bicarbonate also is believed to produce paradoxical intracellular and CNS acidosis (decreased pHi as plasma pH increases) an effect that is not observed in ventilated animals.133, 134 Regardless, the IV administration or addition of various commercially available or compounded hypertonic or isotonic NaHCO3 solutions (8.4%: SID = 1,000 mEq/L, 2,000 mOsm/L; 5.0%: SID = 595 mEq/L, 1190 mOsm/L; 1.3%: SID = 154 mEq/L, 310 mOsm/L) have been shown to be more effective than balanced crystalloid solutions for treating diarrheic calves.135, 136 More recently, the co‐administration of NHE exchange inhibitors (e.g, sabiporide) and sodium bicarbonate or hypertonic sodium l‐lactate (3%) has been shown to restore acid‐base balance, improve cardiac performance and hemodynamic parameters, promote urine formation, improve endothelial function, and decrease extracellular fluid accumulation in acidotic, hemorrhaged, hypotensive, endotoxic, and traumatized animals, in addition to preventing intracranial hypertension after severe traumatic brain injury.137, 138, 139, 140, 141, 142, 143 Hypoosmolar sodium L‐lactate also may serve as a potential energy substrate (3.61 kcal/g).144 Collectively, these studies suggest that restoration of vascular volume and tissue perfusion and removal of tissue oxygen debt in conjunction with normalization of plasma pH are key factors in the success or failure of alkalinizing and cell protective therapies.136, 137, 141, 144, 145, 146, 147, 148
Concluding Comments
Misconceptions regarding the interpretation and influence of crystalloid therapy on acid‐base balance can confound fluid selection (Table 3). Balanced crystalloids should (1) contain species‐specific concentrations of key electrolytes (Na+, Cl−, K+, Ca++, Mg++), particularly Na+ and Cl−; (2) maintain or normalize acid‐base balance (provide an appropriate SID); (3) be isosmotic and isotonic (not induce inappropriate fluid shifts) with normal plasma; and (4) consider the temperature dependence of H2CO3. New insights regarding the mechanisms responsible for acid‐base balance, the role and efficacy of organic anions as buffers, and the importance of a crystalloid's SIDif have helped clarify and determine fluid selection. The administration of large volumes of solutions containing high concentrations of chloride (e.g, 0.9% NaCl) can no longer be supported because of their potential to produce hyperchloremic metabolic acidosis, impair renal function, and increase mortality.75, 147, 148, 149, 150 A small volume of hypertonic sodium bicarbonate solution is an effective treatment for metabolic acidosis and hyperkalemia in dehydrated, hypovolemic, septic animals as long as larger volumes of balanced solutions also are administered to restore tissue perfusion.135, 136 More species‐specific research is required to identify appropriate fluid choices within the context for which they are prescribed.3 Ideally, fluid administration should be continuously monitored, frequently reassessed, and focused upon the restoration cardiovascular function, vascular volume, electrolyte concentrations, effective osmolality, tonicity, plasma SID, and pH.
Table 3.
Misconceptions of acid‐base balance and fluid therapy
| Misconception | Fact |
|---|---|
| The pH of plasma is determined by the partial pressure of carbon dioxide (PCO2) and the bicarbonate ion [H+CO3 −] | Partially true: the plasma pH is determined by 3 primary independent variables: PCO2, Atot, and SID. Changes in [H+CO3 −] are dependent on these same 3 factors |
| Most commercially available fluids produce no effect on plasma acid‐base balance | All commercially available fluids produce changes in plasma acid‐base balance dependent upon their ability to change in vivo strong ion difference: Their effects on plasma SID become more pronounced when larger fluid volumes are administered rapidly |
| Fluid administration produces acidosis by dilution of plasma [H+CO3 −] | Fluid administration does dilute [H+CO3 −] producing metabolic acidosis but also dilutes Atot producing metabolic alkalosis. Crystalloid‐induced changes in plasma pH are primarily caused by a change in SID, not dilution |
| The in vitro pH of commercial crystalloid solutions can acidify the plasma | The titratable acidity of all commercially available IV crystalloid solutions has no clinically relevant effect on plasma pH |
| Physiologic saline solution (0.9% Na+Cl−) has no effect on plasma pH | 0.9% Na+Cl− in vivo SID (SIDif) = 0 and produces hyperchloremic metabolic acidosis; effect is related to dose and administration rate |
| Physiologic saline solution is harmful to animals | 0.9% Na+Cl− effect on [H+] is usually negligible in normal healthy animals unless large volumes (>30 mL/kg) are administered over a short period (<1 h) or administered to animals that already have metabolic acidosis |
| Lactated Ringer's solution (LRS) is a “balanced” crystalloid | LRS is hypotonic (273 mOsm/L): Tonicity is the effective osmolality of a solution |
| The lactate in LRS is a bicarbonate precursor or bicarbonate substitute | The role of lactate (like all organic anions) in LRS is to be rapidly metabolized (disappear), thereby increasing SID |
| The lactate in LRS is an ideal organic anion to substitute for bicarbonate | LRS contains a racemic mixture of d and l‐lactate as an inorganic ion. d‐lactate is proinflammatory and can cause CNS depression |
| Normosol‐R and Plasma‐Lyte maintain normal plasma pH | Normosol‐R and Plasma‐Lyte have an SIDif = 50 increasing plasma pH |
| Sodium bicarbonate solution administration produces CNS and intracellular acidosis (paradoxical acidosis) | Possibly true but the effect is dose and rate of administration related, usually transient and clinically irrelevant in animals that are adequately ventilated |
[], concentration; pH, negative log of [H+]; PCO2, partial pressure of carbon dioxide; Atot, weak nonvolatile acids, inorganic phosphate, serum proteins, and albumin; SID, difference between strong cations and strong anions.
“We are still confused – but on a much higher level” W. Churchill.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Footnotes
Hospira, Inc., Lake Forest, IL 60045
Baxter Healthcare Corporation Deerfield, IL 60015
Abbott Laboratories, North Chicago, Ill 60064
International Medication Systems, South El Monte, CA 91733
Berchtold, J., H. Hartmann, and W. Hofmann. “The comparative effectiveness of Carbicarb‐R, Tribonate‐R and bicarbonate in the treatment of acidosis in neonatal calves.” In: Proceedings of the 30th Annual Conference of the American Association of Bovine Practitioners, Montreal, CAN. 1997.p.135
References
- 1. Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013;369:1243–1251. [DOI] [PubMed] [Google Scholar]
- 2. Raghunathan K, Shaw AD, Bagshaw SM. Fluids are drugs: Type, dose and toxicity. Curr Opin Crit Care 2013;19:280–298. [DOI] [PubMed] [Google Scholar]
- 3. James MFM. Context‐sensitive fluid administration: What, when and how much. South Afr J Anaesth Analg 2015;21:38–39. [Google Scholar]
- 4. Davis H, Jensen T, Johnson A, et al. American Association of Feline Practictioners; American Animal Hospital Association. 2013 AAHA/AAFP Fluid therapy guidelines for dogs and cats. J Am Animal Hosp Assoc 2013;49:149–159. [DOI] [PubMed] [Google Scholar]
- 5. Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr 2008;27:170–188. [DOI] [PubMed] [Google Scholar]
- 6. Srinivasa S, Hill AG. Perioperative fluid administration: Historical highlights and implications for practice. Ann Surg 2012;256:1113–1118. [DOI] [PubMed] [Google Scholar]
- 7. Kampmeier T, Rehberg S, Ertmer C. Evolution of fluid therapy. Best Pract Res Clin Anaesthesiol 2014;28:207–216. [DOI] [PubMed] [Google Scholar]
- 8. Raghunathan K, Bonavia A, Hathanson BH, et al. Association between initial fluid choice and subsequent in‐hospital mortality curing the resuscitation of adults with septic shock. Anesthesiology 2015;123:1385–1393. [DOI] [PubMed] [Google Scholar]
- 9. Todd SR, Malinoski D, Muller PJ, et al. Lactated Ringer's is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma 2007;62:636–639. [DOI] [PubMed] [Google Scholar]
- 10. Magder S. Balanced versus unbalanced salt solutions: What difference does it make? Best Pract Res Clin Anaesthesiol 2014;28:235–247. [DOI] [PubMed] [Google Scholar]
- 11. Dubin A, Pozo MO, Rerrara G, et al. Systemic and microcirculatory responses to progressive hemorrhage. Intensive Care Med 2009;35:556–564. [DOI] [PubMed] [Google Scholar]
- 12. Dyson A, Cone S, Singer M, et al. Microvascular and macrovascular flow are uncoupled in early polymicrobial sepsis. Br J Anaesth 2012;108:973–978. [DOI] [PubMed] [Google Scholar]
- 13. Tachon G, Harrois A, Tanaka S, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med 2014;42:1433–1441. [DOI] [PubMed] [Google Scholar]
- 14. Adrogué HE, Adrogué HJ. Acid‐base physiology. Respir Care 2001;46:328–341. [PubMed] [Google Scholar]
- 15. Corey HE. Bench‐to‐bedside review: Fundamental principles of acid‐base physiology. Crit Care 2005;9:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clancy J, McVicar A. Short‐term regulation of acid‐base homeostasis of body fluids. Br J Nurs 2007;16:1016–1021. [DOI] [PubMed] [Google Scholar]
- 17. Clancy J, McVicar A. Intermediate and long‐term regulation of acid‐base homeostasis. Br J Nurs 2007;16:1076–1079. [DOI] [PubMed] [Google Scholar]
- 18. Van Hall G. Lactate as a fuel for mitochondrial respiration. Acta Physiol Scand 2000;168:643–656. [DOI] [PubMed] [Google Scholar]
- 19. Constable PD. Clinical assessment of acid‐base status: Comparison of the Henderson‐Hasselbalch and strong ion approaches. Vet Clin Path 2000;29:115–128. [DOI] [PubMed] [Google Scholar]
- 20. Collins KD, Neilson GW, Enderby JE. Ions in water: Characterizing the forces that control chemical processes and biological structure. Biophys Chem 2007;128:95–104. [DOI] [PubMed] [Google Scholar]
- 21. Gattinoni L, Carlesso E. Supporting hemodynamics: What should we target? What treatments should we use? Crit Care 2013;17(Suppl 10):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stillion JR, Fletcher DJ. Admission base excess as a predictor of transfusion requirement and mortality in dogs with blunt trauma: 52 cases (2007–2009). J Vet Emerg Crit Care 2012;22:588–594. [DOI] [PubMed] [Google Scholar]
- 23. Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl‐Stewart method predict mortality better than base excess, anion gap, and lactate in patients in the pediatric intensive care unit. Crit Care Med 1999;27:1577–1581. [DOI] [PubMed] [Google Scholar]
- 24. Ho KM, Lan NS, Williams TA, et al. A comparison of prognostic significance of strong ion gap (SIG) with other acid‐base markers in the critically ill: A cohort study. J Intensive Care 2016;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berend K. Acid‐base pathophysiology after 130 years: Confusing, irrational and controversial. J Nephrol 2013;26:254–265. [DOI] [PubMed] [Google Scholar]
- 26. Kellum JA. Clinical review: Reunification of acid‐base physiology. Crit Care 2005;9:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Constable PD, Hinchcliff KW, Muir WW 3rd. Comparison of anion gap and strong ion gap as predictors of unmeasured strong ion concentration in plasma and serum from horses. Am J Vet Res 1998;59:881–887. [PubMed] [Google Scholar]
- 28. Kraut JA, Kurtz I. Treatment of acute non‐anion gap metabolic acidosis. Clin Kidney J 2015;8:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fidkowski C, Helstrom J. Diagnosing metabolic acidosis in the critically ill: Bridging the anion gap, Stewart, and base excess methods. Can J Anaesth 2009;56:247–256. [DOI] [PubMed] [Google Scholar]
- 30. Siggaard‐Andersen O, Engel K, Jorgensen K, et al. A Micro method for determination of pH, carbon dioxide tension, base excess and standard bicarbonate in capillary blood. Scand J Clin Lab Invest 1960;12:172–176. [DOI] [PubMed] [Google Scholar]
- 31. Stewart PA. Modern quantitative acid‐base chemistry. Can J Physiol Pharmacol 1983;61:1444–1461. [DOI] [PubMed] [Google Scholar]
- 32. Singer RB, Hastings AB. An improved clinical method for the estimation of disturbances of the acid‐base balance of human blood. Medicine 1948;27:22–242. [DOI] [PubMed] [Google Scholar]
- 33. Constable PD. A simplified strong ion model for acid‐base equilibria: Application to horse plasma. J Appl Physiol 1997;83:297–311. [DOI] [PubMed] [Google Scholar]
- 34. Magder S, Emani A. Practical approach to physical‐chemical acid‐base management. Stewart at the bedside. Ann Am Thorac Soc 2015;12:111–117. [DOI] [PubMed] [Google Scholar]
- 35. Masevicius FO, Dubin A. Has the Stewart approach improved our ability to diagnose acid‐base disorders in critically ill patients? World J Crit Care Med 2015;4:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morgan TJ, Venkatesh B, Hall J. Crystalloid strong ion difference determines metabolic acid‐base change during acute normovolaemic haemodilution. Intensive Care Med 2004;30:1432–1437. [DOI] [PubMed] [Google Scholar]
- 37. Morgan TJ. Clinical review: The meaning of acid‐base abnormalities in the intensive care unit‐ effects of fluid administration. Crit Care 2005;9:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seifter JL. Integration of acid‐base and electrolyte disorders. N Engl J Med 2014;371:1821–1831. [DOI] [PubMed] [Google Scholar]
- 39. Story DA. Stewart acid‐base: A simplified bedside approach. Anesth Analg 2016;123:511–515. [DOI] [PubMed] [Google Scholar]
- 40. McCullough SM, Constable PD. Calculation of the total plasma concentration of nonvolatile weak acids and the effective dissociation constant of nonvolatile buffers in plasma for use in the strong ion approach to acid‐base balance in cats. Am J Vet Res 2003;64:1047–1051. [DOI] [PubMed] [Google Scholar]
- 41. Constable PD, Stampfli HR. Experimental determination of net protein charge and A(tot) and K(a) of nonvolatile buffers in canine plasma. J Vet Intern Med 2005;19:507–514. [PubMed] [Google Scholar]
- 42. Constable PD, Stampfli HR, Navetat H, et al. Use of a quantitative strong ion approach to determine the mechanism for acid‐base abnormalities in sick calves with or without diarrhea. J Vet Intern Med 2005;19:581–589. [DOI] [PubMed] [Google Scholar]
- 43. Forni LG, McKinnon W, Hilton P. Unmeasured anions in metabolic acidosis: Unraveling the mystery. Crit Care 2006;10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruegger D, Kemming GI, Jacob M, et al. Causes of metabolic acidosis in canine hemorrhagic shock: Role of unmeasured ions. Crit Care 2007;11:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaplan LJ, Kellum JA. Comparison of acid‐base models for prediction of hospital mortality after trauma. Shock 2008;29:662–666. [DOI] [PubMed] [Google Scholar]
- 46. Kaplan LJ, Philbin N, Arnaud F, et al. Resuscitation from hemorrhagic shock: Fluid selection and infusion strategy drives unmeasured ion genesis. J Trauma 2006;61:9–98. [DOI] [PubMed] [Google Scholar]
- 47. Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med 2004;32:1120–1128. [DOI] [PubMed] [Google Scholar]
- 48. Chappell D, Hofmann‐Kiefer K, Jacob M, et al. Metabolic alkalosis despite hyperlactatemia and hypercapnia. Interpretation and therapy with help of the Stewart concept. Anaesthesist 2008;57:139–142. [DOI] [PubMed] [Google Scholar]
- 49. Stampfli HR, Schoster A, Constable PD. Clinical utility of serum biochemical variables for predicting acid–base balance in critically ill horses. Vet Clin Pathol 2014;43:547–556. [DOI] [PubMed] [Google Scholar]
- 50. Siegling‐Vlitakis C, Kohn B, Kellermeier C, et al. Qualification of the Stewart variables for the assessment of the acid‐base status in healthy dogs and dogs with different diseases. Berl Munch Tierarztl Wochenschr 2007;120:148–155. [PubMed] [Google Scholar]
- 51. Reinhold P, Hartmann H, Constable PD. Characterization of acid‐base abnormalities in pigs experimentally infected with Chlamydia suis. Vet J 2010;184:212–218. [DOI] [PubMed] [Google Scholar]
- 52. Constable PD. Acid‐base assessment: When and how to apply the Henderson‐Hasselbalch equation and strong ion difference theory. Vet Clin North Am Food Anim Pract 2014;30:295–316. [DOI] [PubMed] [Google Scholar]
- 53. Kwok MH, Lan NSH, Williams A, et al. A comparison of prognostic significance of strong ion gap (SIG) with other acid‐base markers in the critically ill. A cohort study. J Intensive Care 2016;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kotake Y. Unmeasured anions and mortality in critically ill patients in 2016. J Intensive Care 2016;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kaplan LJ, Cheung NH, Maerz L, et al. A physicochemical approach to acid‐base balance in critically ill trauma patients minimizes errors and reduces inappropriate plasma volume expansion. J Trauma 2009;66:1045–1051. [DOI] [PubMed] [Google Scholar]
- 56. Langer T, Santini A, Scotti E, et al. Intravenous balanced solutions: From physiology to clinical evidence. Anaesthesiol Intensive Ther 2015;47:s78–s88. [DOI] [PubMed] [Google Scholar]
- 57. Reddy S, Weinberg L, Young P. Crystalloid fluid therapy. Crit Care 2016;20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wear J, McPherson TB, Kolling WM. Stability of sodium bicarbonate solutions in polyolefin bags. Am J Healt‐Syst Pharm 2010;67:1026–1029. [DOI] [PubMed] [Google Scholar]
- 59. Morgan TJ, Venkatesh B. Designing ‘balanced’ crystalloids. Crit Care Resusc 2003;5:284–291. [PubMed] [Google Scholar]
- 60. Hartsfield SM. Sodium bicarbonate and bicarbonate precursors for treatment of metabolic acidosis. J Am Vet Med Assoc 1981;179:914–916. [PubMed] [Google Scholar]
- 61. Naylor JM, Fosryth GW. The alkalinizing effects of metabolizable bases in the healthy calf. Can J Vet Res 1986;50:509–516. [PMC free article] [PubMed] [Google Scholar]
- 62. Kirkendol PL, Starrs J, Tonzalez FM. The effects of acetate, lactate, succinate and gluconate on plasma pH and electrolytes in dogs. Trans Am Soc Artif Intern Organs 1980;26:323–327. [PubMed] [Google Scholar]
- 63. Ke L, Calzavacca P, Bailey M, et al. Acid‐base changes after fluid bolus: Sodium chloride vs. sodium octanoate. Intensive Care Med Exp 2013;1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vail DM, Ogilvie GK, Fettman MJ, et al. Exacerbation of hyperlactatemia by infusion of lactated Ringer's solution in dogs with lymphoma. J Vet Intern Med 1990;4:228–232. [DOI] [PubMed] [Google Scholar]
- 65. Didwania A, Miller J, Kassel D, et al. Effect of intravenous lactated Ringer's solution infusion on the circulating lactate concentration: Part 3: Results of a prospective, randomized, double‐blind, placebo‐controlled trial. Crit Care Med 1997;25:1851–1854. [DOI] [PubMed] [Google Scholar]
- 66. Lorenz I, Gentile A, Klee W. Investigations of D‐lactate metabolism and the clinical signs of D‐lactataemia in calves. Vet Rec 2005;156:412–415. [DOI] [PubMed] [Google Scholar]
- 67. Boysen SR, Dorval P. Effects of rapid intravenous 100% L‐isomer lactated Ringer's administration on plasma lactate in healthy dogs. Vet Emerg Crit Care 2014;24:571–577. [DOI] [PubMed] [Google Scholar]
- 68. Morris C. Exacerbation of hyperlactatemia by infusion of lactated Ringer's solution in dogs with lymphoma. Anaesthesia 2008;64:703–705. [DOI] [PubMed] [Google Scholar]
- 69. Ergin B, Kapuca A, Guerci P, Ince C. The role of bicarbonate precursors in balanced fluid during haemorrhagic shock with and without compromised liver function. Br J Anaesth 2016;117:521–528. [DOI] [PubMed] [Google Scholar]
- 70. Morgan TJ. The ideal crystalloid ‐ what is ‘balanced’? Curr Opin Crit Care 2013;19:299–307. [DOI] [PubMed] [Google Scholar]
- 71. Guidet B, Soni N, Della Rocca G, et al. A balanced view of balanced solutions. Crit Care 2010;14:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hamburger HJ. A discourse on permeability in physiology and pathology. Lancet 1921;198:1039–1045. [Google Scholar]
- 73. Ringer S. Concerning the influence exerted by each of the constituents of the blood on the contraction of the ventricle. J Physiol 1882;3:380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hartmann AF. Theory and practice of parenteral fluid administration. J Am Med Assoc 1934;4:1349–1354. [Google Scholar]
- 75. Li H, Sun S‐R, Hap JQ, et al. 0.9% saline is neither normal nor physiological. Biomed Biotechol 2016;17:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vincent J‐L, DeBacker D. Saline versus balanced solutions: Are clinical trials comparing two crystalloid solutions really needed? Crit Care 2016;20:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Raghunathan K, Nalier P, Knoske R. What is the ideal crystalloid? Curr Opin Crit Care 2015;21:309–314. [DOI] [PubMed] [Google Scholar]
- 78. Lebowitz MH, Masuda JY, Beckerman JH. The pH and acidity of intravenous infusion solutions. J Am Med Assoc 1971;215:1937–1940. [PubMed] [Google Scholar]
- 79. Reddi BAJ. Why is saline so acidic (and does it really matter?). Int J Med Sci 2013;10:747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kaplan LJ, Frangos S. Clinical review: Acid‐base abnormalities in the intensive care unit‐part II. Crit Care 2005;9:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Constable PD. Hyperchloremic acidosis: The classic example of strong ion acidosis. Anesth Analg 2003;96:919–922. [DOI] [PubMed] [Google Scholar]
- 82. Constable PD. In response: Letters to the editor (response). Anesth Analg 2004;98:271–272. [DOI] [PubMed] [Google Scholar]
- 83. Constable PD. Iatrogenic hyperchloremic acidosis due to large volume fluid administration: Mechanism, diagnosis, and management. Int J Intens Care 2005;12:111–122. [Google Scholar]
- 84. Carlesso E, Maiocchi G, Tallarini F, et al. The rule regulating pH changes during crystalloid infusion. Intensive Care Med 2011;37:461–468. [DOI] [PubMed] [Google Scholar]
- 85. Langer T, Ferrari M, Zazzeron L, et al. Effects of intravenous solutions on acid‐base equilibrium: From crystalloids to colloids and blood components. Anaesth Int Ther 2014;46:350–360. [DOI] [PubMed] [Google Scholar]
- 86. Langer T, Carlesso E, Praotti A, et al. In vivo conditioning of acid‐base equilibrium by crystalloid solutions: An experimental study on pigs. Intensive Care Med 2012;38:686–693. [DOI] [PubMed] [Google Scholar]
- 87. West E, Pettitt R, Jones RS, et al. Acid‐base and electrolyte balance following administration of three crystalloid solutions in dogs undergoing elective orthopaedic surgery. Vet Anaesth Analg 2013;40:482–493. [DOI] [PubMed] [Google Scholar]
- 88. Muir WW, Kijtawornrat A, Ueyama Y, et al. Effects of intravenous administration of lactated Ringer's solution on hematologic, serum biochemical, rheological, hemodynamic and renal measurements in healthy isoflurane anesthetized dogs. J Am Vet Med Assoc 2011;239:630–637. [DOI] [PubMed] [Google Scholar]
- 89. Chan L, Slater J, Hasbargen J, et al. Neurocardiac toxicity of racemic D. L‐lactate fluids. Integr Physiol Behav Sci 1994;29:383–394. [DOI] [PubMed] [Google Scholar]
- 90. Veech RL, Litomer WL. The medical and metabolic consequences of administration of sodium acetate. Adv Enyme Regul 1988;27:313–343. [DOI] [PubMed] [Google Scholar]
- 91. Kraut JA, Madias NE. Metabolic acidosis: Pathophysiology, diagnosis and management. Nat Rev Nephrol 2010;6:274–285. [DOI] [PubMed] [Google Scholar]
- 92. Hopper K, Epstein SE. Incidence, nature, and etiology of metabolic acidosis in dogs and cats. J Vet Intern Med 2012;26:1107–1114. [DOI] [PubMed] [Google Scholar]
- 93. Kraut JA, Medias NE. Differential diagnosis of non‐gap metabolic acidosis: Value of a systematic approach. Clin J Am Sc Nephrol 2012;7:671–679 95. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014;371:2309–2319. [DOI] [PubMed] [Google Scholar]
- 95. Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non‐lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit Care 2006;10:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Regnier MA, Raux M, Le Manach Y, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology 2012;117:1276–1288. [DOI] [PubMed] [Google Scholar]
- 97. Johnston K, Holcombe SJ, Hauptman JG. Plasma lactate as a predictor of colonic viability and survival after 360 degrees volvulus of the ascending colon in horses. Vet Surg 2007;36:563–567. [DOI] [PubMed] [Google Scholar]
- 98. Figueiredo MD, Nydam DV, Perkins GA, et al. Prognostic value of plasma L‐lactate concentration measured cow‐side with a portable clinical analyzer in Holstein dairy cattle with abomasal disorders. J Vet Intern Med 2006;20:1463–1470. [DOI] [PubMed] [Google Scholar]
- 99. Pang DE, Boysen S. Lactate in veterinary critical care: Pathophysiology and management. J Am Animal Hosp Assoc 2007;43:270–279. [DOI] [PubMed] [Google Scholar]
- 100. Tennent‐Brown B. Blood lactate measurement and interpretation in critically ill equine adults and neonates. Vet Clin North Am Eq Pract 2014;30:399–413. [DOI] [PubMed] [Google Scholar]
- 101. Hopper K, Borchers A, Epstein SE. Acid base, electrolyte, glucose, and lactate values during cardiopulmonary resuscitation in dogs and cats. J Vet Emerg Crit Care 2014;24:208–214. [DOI] [PubMed] [Google Scholar]
- 102. Ateca LB, Dombrowski SC, Silverstein DC. Survival analysis of critically ill dogs with hypotension with or without hyperlactatemia: 67 cases (2006‐2011). J Am Vet Med Assoc 2015;246:100–104. [DOI] [PubMed] [Google Scholar]
- 103. Reineke E, Rees C, Drobatz KJ. Association of blood lactate concentration with physical perfusion variables, blood pressure, and outcome for cats treated at an emergency service. J Am Vet Med Assoc 2015;247:79–84. [DOI] [PubMed] [Google Scholar]
- 104. Wutrich Y, Barraud D, Conrad M, et al. Early increase in arterial lactate concentration under epinephrine infusion is associated with a better prognosis during shock. Shock 2010;34:4–9. [DOI] [PubMed] [Google Scholar]
- 105. Marik PE, Bellomo R. Stress hyperglycemia: An essential survival response. Crit Care 2013;41:e93–e94. [DOI] [PubMed] [Google Scholar]
- 106. Orchard CH, Cingolani HE. Acidosis and arrhythmias in cardiac muscle. Cardiovasc Res 1994;28:1312–1319. [DOI] [PubMed] [Google Scholar]
- 107. Kellum JA, Song M, Li J. Science review: Extracellular acidosis and the immune response: Clinical and physiological implications. Crit Care 2004;8:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kmonishy DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol 2010;184:4062–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mitchell JH, Wildenthal K, Johnson RL Jr. The effects of acid‐base disturbances on cardiovascular and pulmonary function. Kidney Int 1972;1:375–389. [DOI] [PubMed] [Google Scholar]
- 110. Brimioulle S, Lejeune P, Vachiery JL, et al. Effects of acidosis and alkalosis on hypoxic pulmonary vasoconstriction in dogs. Am J Physiol 1990;258:H347–H353. [DOI] [PubMed] [Google Scholar]
- 111. Adrogue HF, Madias NE. Management of life‐threatening acid‐base disorders. First of two parts. N Engl J Med 1998;338:26–34. [DOI] [PubMed] [Google Scholar]
- 112. Demaurex N. Grinstein. Na+/H+ Antiport: Modulation by ATP and role in cell volume regulation. J Exp Biol 1994;196:389–404. [DOI] [PubMed] [Google Scholar]
- 113. Wu D, Kraut JA. Potential role of NHE1 (sodium‐hydrogen exchanger 1) in the cellular dysfunction of lactic acidosis: Implications for treatment. Am J Kidney Dis 2011;57:781–787. [DOI] [PubMed] [Google Scholar]
- 114. Wu D, Kraut JA. Role of NHE1 in the cellular dysfunction of acute metabolic acidosis. Am J Nephrol 2014;40:36–42. [DOI] [PubMed] [Google Scholar]
- 115. Fliegel L. Functional and cellular regulation of the myocardial Na+/H+ exchanger. J Thromb Thrombolysis 1999;8:9–14. [DOI] [PubMed] [Google Scholar]
- 116. Vaughan‐Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol 2009;46:318–331. [DOI] [PubMed] [Google Scholar]
- 117. Kraut JA, Madias NE. Lactic acidosis: Current treatments and future directions. Am J Kidney Dis 2016;68:473–482. [DOI] [PubMed] [Google Scholar]
- 118. Severs D, Hoorn EJ, Bookmaaker MB. A critical appraisal of intravenous fluids: From physiological basis to clinical evidence. Nephrol Dial Transplant 2015;30:178–187. [DOI] [PubMed] [Google Scholar]
- 119. Constable P. Fluid and electrolyte therapy in ruminants. Vet Clin North Am Food Anim Pract 2003;19:557–597. [DOI] [PubMed] [Google Scholar]
- 120. Shapiro JI, Whalen M, Kucera R, et al. Brain pH responses to sodium bicarbonate and Carbicarb during systemic acidosis. Am J Physiol 1989;256:H1316–H1321. [DOI] [PubMed] [Google Scholar]
- 121. Sonett J, Baker LS, His C, et al. Sodium bicarbonate versus Carbicarb in canine myocardial hypercarbic acidosis. J Crit Care 1993;8:1–11. [DOI] [PubMed] [Google Scholar]
- 122. Moon PF, Gabor L, Gleed RD, et al. Acid‐base, metabolic, and hemodynamic effects of sodium bicarbonate or tromethamine administration in anesthetized dogs with experimentally induced metabolic acidosis. Am J Vet Res 1997;58:771–776. [PubMed] [Google Scholar]
- 123. Holmdahl MH, Wiklund L, Wetterberg T, et al. The place of THAM in the management of acidemia in clinical practice. Acta Anaesthesiol Scand 2000;44:524–527. [DOI] [PubMed] [Google Scholar]
- 124. Leung JM, Landow L, Franks M, et al. Safety and efficacy of intravenous Carbicarb in patients undergoing surgery: Comparison with sodium bicarbonate in the treatment of mild metabolic acidosis. SPI Research Group. Study of Perioperative Ischemia. Crit Care Med 1994;22:1540–1549. [PubMed] [Google Scholar]
- 125. Hoste EA, Colpaert K, Vanholder RC, et al. Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis. J Nephrol 2005;18:303–307. [PubMed] [Google Scholar]
- 126. Jung B, Rimmele T, Le Goff C, et al. Severe metabolic or mixed acidemia on intensive care unit admission: Incidence, prognosis and administration of buffer therapy. A prospective, multiple‐center study. Crit Care 2011;15:R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Valissaris D, Karamouzos V, Ktenopoulos N, et al. The use of sodium bicarbonate in the treatment of acidosis in sepsis: A literature update on a long term debate. Crit Care Res Pract 2015;2015:605830 https://doi.org/10.1155/2015/605830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International guidelines for the management of sepsis and septic shock: 2016. Intensive Care Med 2017;45:1–67. [DOI] [PubMed] [Google Scholar]
- 129. Arieff AI, Leach W, Park R, et al. Systemic effects of NaHCO3 in experimental lactic acidosis in dogs. Am J Physiol 1982;242:F586–F591. [DOI] [PubMed] [Google Scholar]
- 130. Boyd JH, Walley KR. Is there a role for sodium bicarbonate in treating lactic acidosis form shock? Curr Opin Crit Care 2008;14:379–383. [DOI] [PubMed] [Google Scholar]
- 131. Aschner JL, Poland RL. Sodium bicarbonate: Basically useless therapy. Pediatrics 2008;122:831–835. [DOI] [PubMed] [Google Scholar]
- 132. Sabatini S, Kurtzman NA. Bicarbonate therapy in severe metabolic acidosis. J Am Soc Nephrol 2009;20:692–695. [DOI] [PubMed] [Google Scholar]
- 133. Berchtold JF, Constable PD, Smith GW, et al. Effects of intravenous hyperosmotic sodium bicarbonate on arterial and cerebrospinal fluid acid‐base status and cardiovascular function in calves with experimentally induced respiratory and strong ion acidosis. J Vet Intern Med 2005;19:240–251. [DOI] [PubMed] [Google Scholar]
- 134. Abeysekars S, Zello GA, Lohmann KL, et al. Infusion of sodium bicarbonate in experimentally induced metabolic acidosis does not provoke cerebrospinal fluid (CSF) acidosis in calves. Can J Vet Res 2012;76:16–22. [PMC free article] [PubMed] [Google Scholar]
- 135. Muller KR, Gentile A, Klee W, et al. Importance of effective strong ion difference of an intravenous solution in the treatment of diarrheic calves with naturally acquired acidemia and strong ion (metabolic) acidosis. J Vet Intern Med 2012;26:674–683. [DOI] [PubMed] [Google Scholar]
- 136. Coskun A, Sen I, Guzelbektes H, et al. Comparison of the effects of intravenous administration of isotonic and hypertonic sodium bicarbonate solutions on venous acid‐base status in dehydrated calves with strong ion acidosis. J Am Vet Med Assoc 2010;236:1098–1103. [DOI] [PubMed] [Google Scholar]
- 137. Wu D, Kraut JA, Abraham WM. Sabiporide improves cardiovascular function, decreases the inflammatory response and reduces mortality in acute metabolic acidosis in pigs. PLoS ONE 2013;8:e53932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lin X, Kraut JA, Wu D. Coadministration of a Na+H+ exchange inhibitor and sodium bicarbonate for the treatment of asphyxia‐induced cardiac arrest in piglets. Pediatr Res 2014;76:118–126. [DOI] [PubMed] [Google Scholar]
- 139. Lin X, More AS, Kraut JA, et al. Interaction of sodium bicarbonate and Na+/H+ exchanger inhibition in the treatment of acute metabolic acidosis in pigs. Crit Care Med 2015;43:e160–e169. [DOI] [PubMed] [Google Scholar]
- 140. Somasetia DH, Setiati TE, Sjahrodji AM, et al. Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium‐lactate: A randomized single‐blind clinical trial of efficacy and safety. Crit Care 2014;5:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Duburcq T, Favory R, Mathieu D, et al. Hypertonic sodium lactate improves fluid balance and hemodynamics in porcine endotoxic shock. Crit Care 2014;18:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nalos M, Leverve X, Huang S, et al. Half‐molar sodium lactate infusion improves cardiac performance in acute heart failure: A pilot randomised controlled clinical trial. Crit Care 2014;18:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nalos M, Tang BM, Nanan R. Is lactate the new panacea for endothelial dysfunction? Crit Care 2014;18:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fontaine E, Orban JC, Ichai C. Hyperosmolar sodium‐lactate in the ICU: Vascular filling and cellular feeding. Crit Care 2014;18:599 https://doi.org/10.1186/s13054-014-0599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rixen D, Raum M, Hozgraete B, et al. A pig hemorrhagic shock model: Oxygen debt and metabolic acidemia as indicators of severity. Shock 2001;16:239–244. [DOI] [PubMed] [Google Scholar]
- 146. Rixen D, Siegel JH. Bench‐to‐besdside review: Oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post traumatic shock. Crit Care 2005;9:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sen A, Keener CM, Sileanu FE, et al. Chloride content of fluids used for large‐volume resuscitation is associated with reduced survival. Crit Care Med 2017;45:e146–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shaw AD, Raghunathan K, Peyeri FW, et al. Association between intravenous chloride load during resuscitation and in‐hospital mortality among patients with SIRS. Intensive Care Med 2014;40:1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Raghunathan K, Shaw A, Nathanson B, et al. Association between choice of IV crystalloid and in‐hospital mortality among critically ill adults with sepsis. Crit Care Med 2014;42:1585–1591. [DOI] [PubMed] [Google Scholar]
- 150. Soussi S, Ferry A, Chaussard M, Legrand M. Chloride tonicity in critically ill patients: What's the evidence? Anaesth Crit Care Pain Med 2017;36:125–130. [DOI] [PubMed] [Google Scholar]


