Abstract
Background
When dogs are transfused, blood compatibility testing varies widely but may include dog erythrocyte antigen (DEA) 1 typing and rarely cross‐matching.
Objectives
Prospective study to examine naturally occurring alloantibodies against red blood cells (RBCs) and alloimmunization by transfusion using 2 antiglobulin‐enhanced cross‐match tests.
Animals
Eighty client‐owned anemic, 72 donor, and 7 control dogs.
Methods
All dogs were typed for DEA 1 and some also for DEA 4 and DEA 7. Major cross‐match tests with canine antiglobulin‐enhanced immunochromatographic strip and gel columns were performed 26–129 days post‐transfusion (median, 39 days); some dogs had an additional early evaluation 11–22 days post‐transfusion (median, 16 days). Plasma from alloimmunized recipients was cross‐matched against RBCs from 34 donor and control dogs.
Results
The 2 cross‐match methods gave entirely concordant results. All 126 pretransfusion cross‐match results for the 80 anemic recipients were compatible, but 54 dogs died or were lost to follow up. Among the 26 recipients with follow‐up, 1 dog accidently received DEA 1‐mismatched blood and became cross‐match‐incompatible post‐transfusion. Eleven of the 25 DEA 1‐matched recipients (44%) became incompatible against other RBC antigens. No naturally occurring anti‐DEA 7 alloantibodies were detected in DEA 7− dogs.
Conclusions and clinical importance
The antiglobulin‐enhanced immunochromatographic strip cross‐match and laboratory gel column techniques identified no naturally occurring alloantibodies against RBC antigens, but a high degree of post‐transfusion alloimmunization in dogs. Cross‐matching is warranted in any dog that has been previously transfused independent of initial DEA 1 typing and cross‐matching results before the first transfusion event.
Keywords: Alloantibodies, Blood compatibility, Canine, Dog erythrocyte antigen, Hemolytic transfusion reaction
Abbreviations
- ACD
acid citrate dextrose
- DAT
direct antiglobulin test
- DEA
dog erythrocyte antigen
- FACS
fluorescence‐activated cell sorter
- Ig
immunoglobulin
- MFI
mean fluorescence intensity
- PBS
phosphate‐buffered saline
- RBC
red blood cell
- WB
whole blood
Acute hemolytic transfusion reactions due to blood group incompatibilities between recipient and donor are serious complications, but could be mostly avoided when transfusing dog erythrocyte antigen (DEA) 1‐matched and cross‐matched blood in previously transfused dogs.1, 2, 3 The DEA 1 is considered the most important blood group in dogs due to its strong antigenicity and nearly equal distribution of DEA 1+ and DEA 1− dogs among many breeds worldwide. In‐clinic kits with monoclonal anti‐DEA 1 antibodies are available for DEA 1 typing.4, 5, 6, 7, 8 In contrast, only polyclonal typing reagents are available on a limited basis for DEA 3, 4, and 7.1 Furthermore, polyclonal or monoclonal typing reagents for Dal and Kai 1 & 2, respectively, recently have been introduced by specific laboratories.3, 9, 10, 11, 12
Based upon clinical transfusion practices and limited surveys, there appear to be no or rare naturally occurring alloantibodies in dogs and their clinical relevance has not been shown.2, 3 Early literature,2,13 and recent reports14, 15 suggest the occurrence of weak anti‐DEA 7 alloantibodies leading possibly to the so‐called but not yet documented delayed transfusion reactions. Currently, canine donors and recipients that have not been previously transfused are considered to have no clinically important alloantibodies and thus are expected to be compatible in a minor and major cross‐match test.1 However, after transfusion, canine recipients may become sensitized, even when DEA 1 matched, which may lead to blood type incompatibilities recognized by incompatible major cross‐match results, acute hemolytic transfusion reactions, or both (even when using the same donor again, which is wrongly believed to be safer).3, 16, 17 Acute hemolytic transfusion reactions and incompatible cross‐matches have been reported clinically in previously transfused dogs receiving a transfusion ≥4 days after the first transfusion.3, 5, 17 However, documentation of post‐transfusion alloimmunization by a major cross‐match test is sparse, and the RBC antigen specificity is rarely if ever identified in any transfused dog.3, 5, 17
Major and minor cross‐match testing is offered by clinical pathology laboratories which use the standard tube, microtiter plate, or neutral saline gel column method without canine antiglobulin at either room temperature or 37°C.3, 9 Because of the need for washing RBCs and the involvement of several steps, cross‐matching of dogs is rarely done in veterinary practice. A gel tube‐based cross‐match kit has been available for in‐clinic use. It recently was assessed in a limited study, but transfused patients either were not studied or no alloantibodies were detected.18, 19 Moreover, an antiglobulin‐enhanced immunochromatographic strip kit, similar to the direct antiglobulin test (DAT),20 recently has been introduced for cross‐matching dogs, but has not been assessed in clinical settings.
The objective of our prospective clinical study was to investigate pre‐ and post‐transfusion alloimmunization after administration of DEA 1‐matched blood products with a novel immunochromatographic strip cross‐match test kit and compare the results to a standard antiglobulin‐enhanced gel column laboratory method.20, 21
Materials and Methods
Animals and Blood Sample Collection
Anemic dogs at the intensive care unit of the VetAgro Sup Campus Vétérinaire de Lyon, France (VetAgro Sup) receiving fresh or stored whole blood (WB) or stored packed RBC transfusions between September 2012 and December 2014 were eligible for inclusion in this prospective study. Dogs were excluded from the study if they weighed ≤3 kg, previously received any blood products, were dialyzed, had incomplete medical records, or some combinations of these. Blood samples were collected immediately before transfusion from all enrolled dogs, at a follow‐up period (26–129 days), and when possible at an early follow‐up period (11–22 days). The DAT was performed before transfusion and at each follow‐up testing.
In addition, blood samples were collected from blood donors and control dogs owned by veterinary students or hospital staff during the study period for typing, cross‐matching, and panel RBC alloantibody testing. These dogs were considered healthy based on history, physical examination, and CBC results. The study was approved by the Ethical Committee of VetAgro Sup (#1267), and owner consent was obtained before enrollment in the study.
Small (2–6 mL) blood samples were collected from dogs in tubes containing acid citrate dextrose solution (ACD)3 or obtained from blood bag segments4 and stored at 4°C for typing and cross‐matching within 3 days. The ACD tubes were centrifuged at 1,300 × g for 10 minutes, and the plasma was used for major cross‐matching with the donor RBCs before transfusion. The remaining plasma was frozen at −20°C for later testing against panel RBCs.
At the follow‐up time periods, 2–6 mL ACD blood samples were obtained from the recipients, and the plasma was processed and frozen as described above. Fresh ACD blood samples also were obtained from donor and control dogs for follow‐up cross‐matching and RBC panel testing for alloantibodies. Plasma from donor and control dogs, which was typed as DEA 7− also was frozen for later identification of anti‐DEA 7 alloantibodies against DEA 7+ RBCs from 1 control dog. Plasma samples were stored frozen at −20°C up to 6 months until testing.
Laboratory Methods
DEA 1 Typing
Two DEA 1 typing methods utilizing the same monoclonal murine anti‐DEA 1 antibody5 were used. For the immunochromatographic strip kit, 10 μL ACD blood was used, and the results were graded either DEA 1− (no band) or subjectively graded weakly, moderately or strongly DEA 1 positive according to the band intensity, following manufacturer's instructions,6 and as previously described.4, 8
For flow cytometric DEA 1 typing,4 10 μL of packed RBCs was washed 3 times with phosphate‐buffered saline (PBS), and the last pellet was mixed with 90 μL of PBS. Then, 10 μL of the 10% washed RBC suspension was mixed with 100 μL of a diluted monoclonal murine anti‐DEA 1 antibody5 and incubated at 37°C for 30 minutes. Thereafter, the RBC suspension was washed with PBS, and 20 μL of a fluorescein isothiocyanate (FITC)‐conjugated polyclonal goat anti‐mouse antibody7 solution, diluted 20‐fold in PBS, was added to the RBC pellet. The suspension was mixed and incubated at 22°C for 15 minutes. The suspension was washed again in PBS, and the pellet was resuspended in 500 μL of PBS before flow cytometric analysis with a FACSCalibur.8 Data were collected for 10,000 events through a gated region, analyzed with the CellQuest Pro software,8 and mean fluorescence intensity (MFI) was obtained. The DEA 1 antigen expression was designated as negative (MFI < 10), weakly positive (10 ≤ MFI < 100), moderately positive (100 ≤ MFI < 300), or strongly positive (MFI ≥ 300).
DEA 4 Typing
A DEA 4 agglutination test was performed with a special paper card.9 In the marked field, 10 μL of the 10% RBC suspension, described above, was mixed with 10 μL of canine polyclonal antisera against DEA 4 10 on the card. Then, the card was slightly rotated and tilted (to‐and‐from motions) by hand for 30–60 seconds. The occurrence of agglutination was assessed by visual examination within 3 minutes; a sample was considered DEA 4+ when strong (>2+) agglutination was observed.
DEA 7 Typing
For DEA 7 typing, an approximately 4% RBC suspension was prepared with 20 μL washed RBCs in 500 μL of modified low ionic strength solution (LISS)11 and neutral saline gel columns.12 In a 3‐mL polystyrene test tube, 25 μL of the 4% RBC suspension was mixed with 25 μL of canine polyclonal anti‐DEA 7 antisera13 and incubated at 4°C for 1 hour. Then, the RBC suspension was centrifuged at 1,000 × g for 2 minutes, and the pellet was washed 2 times with PBS, and the PBS was removed. The pellet was mixed with 10 μL of polyclonal anti‐canine globulin14 and incubated at 22°C for 15 minutes. Then, the suspension was pipetted on top of the gel column, and the gel columns were centrifuged in a special gel column card centrifuge15 at 80 × g for 10 minutes. The results of the gel column cards were analyzed: When agglutination was observed at the top of (4+) or within the gel (2+; 3+), the blood sample was typed as DEA 7+, whereas if all RBCs passed through the gel and resided at the bottom, the sample was considered DEA 7−. For all samples, the same amount of RBCs and anti‐canine globulin14 was used in the negative control column, but instead of anti‐DEA 7 antisera,13 PBS was added.
Direct Antiglobulin Test (DAT)
A DAT, also known as direct Coombs' test, was performed with washed RBCs by an immunochromatographic strip test according to manufacturer's instructions16 and as previously described.20
Major Cross‐match Test Between Recipient and Donor(s)
A novel immunochromatographic strip kit and laboratory gel column cross‐match technique utilizing the same anti‐canine antiglobulin14 were performed between recipient and its donor(s) (major cross‐match), before transfusion, and at each follow‐up period when samples were obtained. Furthermore, plasma from DEA 7− donor and control dogs was assessed for the presence of alloantibodies by a cross‐match with RBCs of 1 DEA 7+ control dog by the same immunochromatographic strip technique. Autocontrols with RBCs and plasma from the same animal always were included.
Immunochromatographic Cross‐match Strip Ki17 (antiglobulin‐enhanced cross‐match)
Three drops of suspension buffer (buffer 117), 10 μL of packed RBCs from the donor, and 120 μL of plasma from the recipient were placed into a 3‐mL polystyrene tube. The suspension was gently mixed, and, after incubation at 22°C for 10 minutes, the RBCs were washed 2 times with PBS and centrifuged at 1,000 × g for 2 minutes. Then, 2 drops of migration buffer (buffer 217) were added to the pellet, and the suspension was gently mixed. The tip of the chromatographic strip was placed into the RBC suspension for 4 minutes to allow the RBC suspension to diffuse to the top of the strip. After incubation, the strip was removed, and the banding pattern was read immediately.
The strip had been impregnated with different antibodies at 2 levels 1 cm apart to form the following bands: a control anti‐glycophorin antibody5 (labeled “C”) that bound to all canine RBCs (thus, a strong red band at “C” indicated that the test was valid) and the anti‐canine antiglobulin at the testing site (labeled “XM”) binding only RBCs coated with immunoglobulin (Ig) G, IgM, complement C3, or some combination of these. Any band intensity at “XM” was considered positive (graded 1+ to 4+) and indicated the presence of antibodies against IgG, IgM, C3 or some combination of these on the RBC surface and thus incompatibility between RBCs and plasma tested.
Gel Column Cross‐match (Antiglobulin‐enhanced Cross‐match)
In a 3‐mL polystyrene test tube, 50 μL of 2% packed donor RBCs in a low ionic strength solution (LISS) was added to 50 μL of recipient plasma, briefly mixed, and incubated at 37°C for 15 minutes. After the incubation, the RBC suspension was washed 2 times in PBS at 1,000 × g for 2 minutes to remove free antibodies. The pelleted RBCs were aspirated and added in the incubation chamber of the gel column card with 10 μL of polyclonal anti‐canine antiglobulin.14 The gel column cards were centrifuged in a special centrifuge15 at 80 × g for 10 minutes, and the location of the migrated RBCs was recorded. In the absence of agglutination, the RBC passed through the gel to the bottom, which was scored as “compatible,” whereas agglutination on the top of or within the gel was considered “incompatible.” Autocontrols (with RBCs and plasma from the same dog) and positive controls (with the canine polyclonal DEA 4 antisera10 [as all dogs were DEA 4+]) were included for all cross‐match tests performed.
Major Cross‐match Test Between Recipients and Panel RBCs
Alloimmunized recipient plasma, which was collected and frozen at the last follow‐up period, was further tested in a major cross‐match test against a panel of RBCs from donors (including their actual donor[s]) and control dogs. Panel RBCs subsequently were assigned to 4 subpanels covering different DEA 1 and DEA 7 type constellations. Plasma from 1 nonalloimmunized dog was used as a negative control. Each alloimmunized recipient plasma sample was separately tested with RBCs from each dog of the 4 panels, and percentages of incompatibility were obtained for each panel.
Identification of naturally occurring anti‐DEA 7 alloantibodies
Plasma from DEA 7− donor and control dogs was cross‐matched against RBCs from 1 DEA 7+ control dog by cross‐match strip method as described above.
Statistical Analysis
Descriptive data are presented as percentages, medians, and ranges. Post‐transfusion immunochromatographic and gel column cross‐match test results were compared by linear regression by Excel software.18 Post‐transfusion cross‐match test results between recipient and donor parings, which were (mis)matched for DEA 7, were compared by Fisher's exact test. Cross‐matches between samples from recipient and donor dogs with different DEA 7 types and potentially sensitized by prior transfusion were compared by the chi‐square test. Cross‐match test results from once or twice transfused recipients also were compared by Fisher's exact test. The statistical analyses were performed by a commercially available statistical program,19 and P ≤ 0.05 was considered significant.
Results
During a 28‐month study period from September 2012 to December 2014, 80 client‐owned anemic dogs were DEA 1‐typed and cross‐matched before receiving 126 DEA 1‐matched RBC transfusions from 72 blood donors at VetAgro Sup. Because 22 dogs died within 11–72 days, and owners of additional 32 dogs did not follow up at VetAgro Sup, and only 26 transfusion recipients (Table S1, Table S2) were evaluated with extended DEA 7 typing and cross‐matching between 26 and 129 days post‐transfusion (median, 39 days); 10 dogs had an additional early evaluation between 11 and 22 days post‐transfusion (median, 16 days).
None of the recipients appeared to experience any acute hemolytic transfusion reactions as assessed by a lack of acute signs of icterus, hypotension, and hemoglobinuria during and after transfusion. Furthermore, the hematocrit increased after transfusion and did not decrease rapidly thereafter. Among those recipients, 1 DEA 1− dog was found to have accidently received a unit of DEA 1+ RBCs (and thus a DEA 1 mismatched RBC transfusion), and 11 other recipients became sensitized based upon incompatible major cross‐match test results. Samples of plasma were further screened for alloimmunization against DEA 1‐, DEA 4‐, and DEA 7‐typed RBCs from 34 blood bank dogs (27 donors and 7 control dogs).
Blood Type
Among the 80 recipients and 79 healthy dogs (72 donors and 7 control dogs), 21.3% and 43.0% were DEA 1−, respectively, and the others were to variable degrees DEA 1+ based upon flow cytometry (Table 1, Fig 1). No categorical discrepancies were observed in the DEA 1 typing results between the flow cytometry and immunochromatographic strip typing technique.
Table 1.
Dog erythrocyte antigen (DEA) 1 blood typing results of anemic patients and healthy dogs assessed by flow cytometry
| Blood typea | Dogs | DEA 1 MFI |
|---|---|---|
| n (%) | Median (range) | |
| Anemic patients | 80 | |
| DEA 1− | 17 (21.3)b | 4 (2–9) |
| DEA 1+ | 63 (78.7)b | 248 (22–686) |
| Weakly DEA 1+ | 18 (28.6)c | 46 (22–74) |
| Moderately DEA 1+ | 22 (34.9)c | 183 (109–287) |
| Strongly DEA 1+ | 23 (36.5)c | 467 (308–686) |
| Healthy dogs | 79 | |
| DEA 1− | 34 (43.0)b | 5 (3–8) |
| DEA 1+ | 45 (57.0)b | 272 (14–954) |
| Weakly DEA 1+ | 9 (20.0)c | 46 (14–70) |
| Moderately DEA 1+ | 19 (42.2)c | 200 (114–283) |
| Strongly DEA 1+ | 17 (37.8)c | 473 (316–954) |
n (%), number (percentage) of dogs, MFI, Mean Fluorescence Intensity.
DEA, Dog erythrocyte antigen. DEA 1 expression was classified as negative (MFI < 10), weakly positive (10 ≤ MFI < 100), moderately positive (100 ≤ MFI < 300), and strongly positive (MFI ≥ 300).
Categorical data of DEA 1+ and DEA 1− were completely concordant with immunochromatographic strip technique.
Percentage of total dogs.
Percentage of DEA 1+ dogs to weak moderate and strong.
Figure 1.
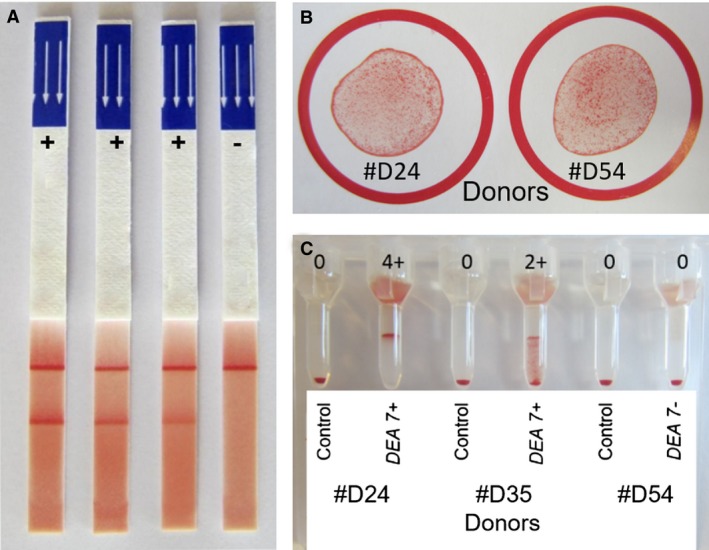
Dog erythrocyte antigen (DEA) 1,DEA 4 and DEA 7 typing results. (A) Dog erythrocyte antigen (DEA) 1 immunochromatographic typing strip results were graded from positive (strong, moderate, and weak) to negative (no band). (B) Typing results of 2 DEA 4+ donors showing many small agglutinates by the paper card method. No DEA 4− dogs were found. (C) DEA 7 typing results of 3 donors by the gel column method. Blood samples agglutinating at the top of (4+) and/or within the gel (2+; 3+) were typed as DEA 7+, whereas when all RBCs resided at the bottom, the sample was considered DEA 7−. For all samples, a negative control column with phosphate‐buffered saline (PBS) was included.
All 72 donor and 7 control dogs as well as the 26 recipients with follow‐up evaluation were typed as DEA 4+ with moderate agglutination reactions on the paper card (Fig 1). Among them, 56 healthy dogs (49 donors and 7 control dogs) and 15 recipients with follow‐up evaluation were typed for DEA 7, and 41.1% and 40.0% were DEA 7+, respectively, showing moderate (2+) to strong (3+, 4+) agglutination reactions by gel column technique (Fig 1). Other blood types such as Dal and Kai were not evaluated because of a lack of available reagents at the time of the study.
Direct Antiglobulin (DAT) Results
All 79 healthy dogs (72 donors, 7 control dogs) were found to be DAT−, but 8 of 80 recipients were DAT+ and considered clinically to have immune‐mediated hemolytic anemia (IMHA). Their DAT test results were moderately to strongly positive. In addition, a 9‐year‐old female Bernese Mountain dog (R22) with malignant histiocytosis was weakly DAT+ by strip but negative by the standard microtiter method at the routine laboratory.
Only 2 recipients, which were DAT+, could be followed post‐transfusion. One (R9, DEA 1 mismatch transfused dog) was strongly DAT+ and remained DAT+ at the second follow‐up (day 67). The second dog (R15) was moderately DAT+ and became DAT− at the time of second follow‐up (day 26). Identical DAT results were observed with the standard microtiter method at the routine laboratory.
Pretransfusion Major Cross‐match Results
None of the 80 recipients and 79 healthy dogs had previously received a transfusion. All major cross‐match test results with their respective donor(s) were compatible (negative) as assessed by both antiglobulin‐enhanced immunochromatographic strip kit and laboratory gel column cross‐match technique.
The 8 DAT+ recipients also had negative cross‐match results, except the Bernese Mountain dog (R22) with malignant histiocytosis, which showed a weakly positive major cross‐match result against its donor, which was considered unspecific because its autocontrol was also weakly positive.
Post‐transfusion Major Cross‐match Results
The 26 recipients, which could be followed once (n = 16) or twice (n = 10), received 1–2 RBC units from 29 donors totaling 32 transfusion events, and 12 dogs (46%) became alloimmunized (Table 2 & Table S2). T0 was the first transfusion time point. The delay between transfusions in the 6 dogs that were transfused twice was 1–4 days (median, 2.5 days). At the post‐transfusion follow‐up period between 11 and 22 days, only 10 recipients were cross‐matched, and 2 of them (R13, R15) became incompatible to their donor(s) between 15 and 19 days. At the follow‐up period between 16 and 129 days, 12 of 26 cross‐matched recipients (46%) showed an incompatible major cross‐match result to their respective donor(s). This included the 2 dogs, which were already incompatible at first follow‐up (Fig 2), but also 3 dogs (R10, R14, R22), which were compatible at first follow‐up, between 11 and 13 days and became cross‐match‐incompatible by 31–39 days. Furthermore, 7 dogs (R6, R7, R8, R9, R16, R17, R23), which were not cross‐matched at the first follow‐up, became incompatible to their respective donor(s) by the second follow‐up period (Table 2 & Table S2). Among the 6 dogs, which received 2 transfusions, 3 recipients (R3, R18, R26) remained compatible with both of their donors, and 1 (R17) and 2 dogs (R13, R22) became incompatible to 1 or both of their donors, respectively (Table S2).
Table 2.
Major cross‐match test results for 26 anemic dogs pre‐ and post‐transfusion
| Days after transfusion | Recipients n | Major cross‐match, n (%) | ||
|---|---|---|---|---|
| Median (range) | Compatible | Incompatible | ||
| Pre‐transfusion | 0 | 26 | 26 (100) | 0 (0) |
| Post‐transfusion | ||||
| 1st follow‐up period | 16 (11–22) | 10 | 8 (80) | 2 (20) |
| 2nd follow‐up period | 39 (26–129) | 26 | 14 (54) | 12 (46) |
n (%), number (percentage) of recipients.
Figure 2.
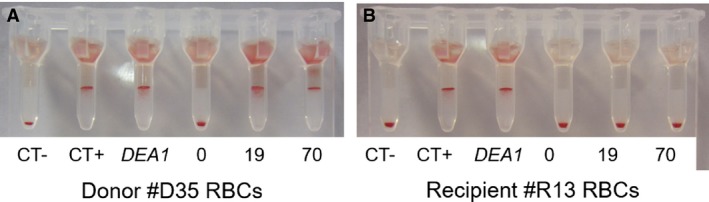
Example of Dog erythrocyte antigen (DEA) 1 and Cross‐match test results for recipient R13 and its donor D35 pre‐ and post‐transfusion by the gel column method. For each series of cross‐match tests, a negative control (CT‐; plasma from a nonalloimmunized dog), a positive control (CT+; DEA 4 antisera), and a DEA 1 blood typing (DEA 1) were performed on the RBCs from recipient R13 and its donor D35. Both recipient R13 and its donor D35 are DEA 1+. (A) Donor D35 RBCs: major cross‐match with recipient R13 plasma pre‐ (0) and post‐transfusion at days 19 and 70 (19 and 70). (B) Recipient R13 RBCs: autocontrol pre‐ (0) and post‐transfusion at days 19 and 70 (19 and 70).
The immunochromatographic strip kit method for an antiglobulin‐enhanced cross‐match was simple to perform and produced band strengths of 1 + (n = 1), 2 + (9), 3 + (6), and 4 + (1) at “XM” position when incompatible with a consistently 4+ control (“C”) band (Fig 3). The laboratory gel column cross‐match assay also gave moderate 2 + (n = 1) and strong 3+ and 4+ (16) agglutination reactions (Fig 3). The cross‐match results were concordant between the immunochromatographic strip and gel column methods (r 2 = 0.96) (Fig 4). Furthermore, results for both techniques could be readily captured by photography or scanning (Fig 3).
Figure 3.
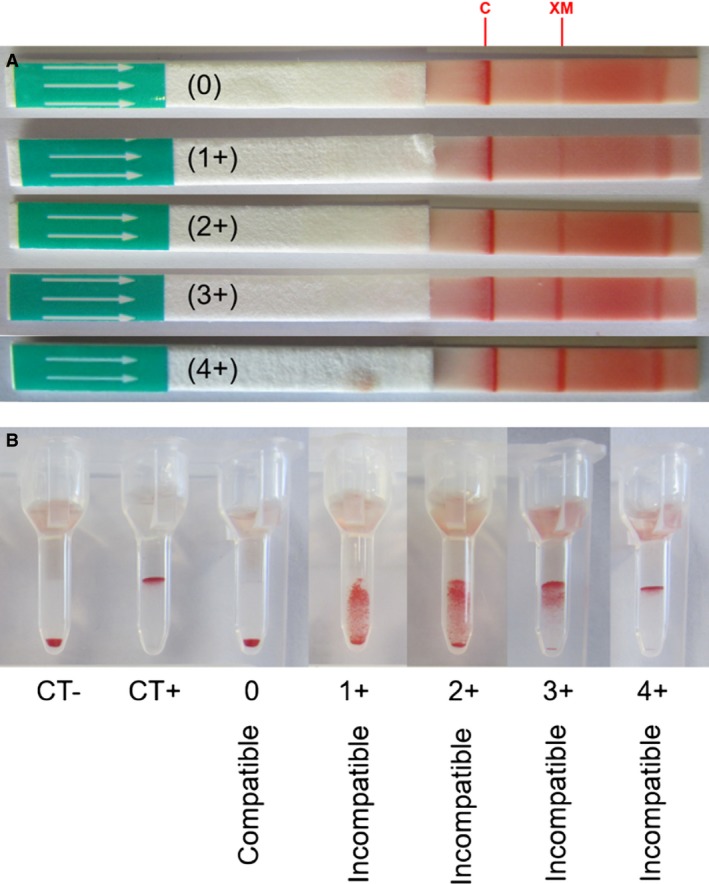
Example of different cross‐match test results with the direct antiglobulin‐enhanced immunochromatographic strip and gel column methods. (A) Cross‐match strip method: Any band intensity at “XM” is considered incompatible (graded 1+ to 4+). (B) Cross‐match gel column method: For each series of cross‐matches, a negative control (CT‐) and a positive control (CT+) were performed with the donor RBCs. In the absence of agglutination, all RBCs resided at the bottom of the gel, which was scored as “compatible” (0), whereas agglutination on the top of or within the gel was considered “incompatible” (graded 1+ to 4+).
Figure 4.
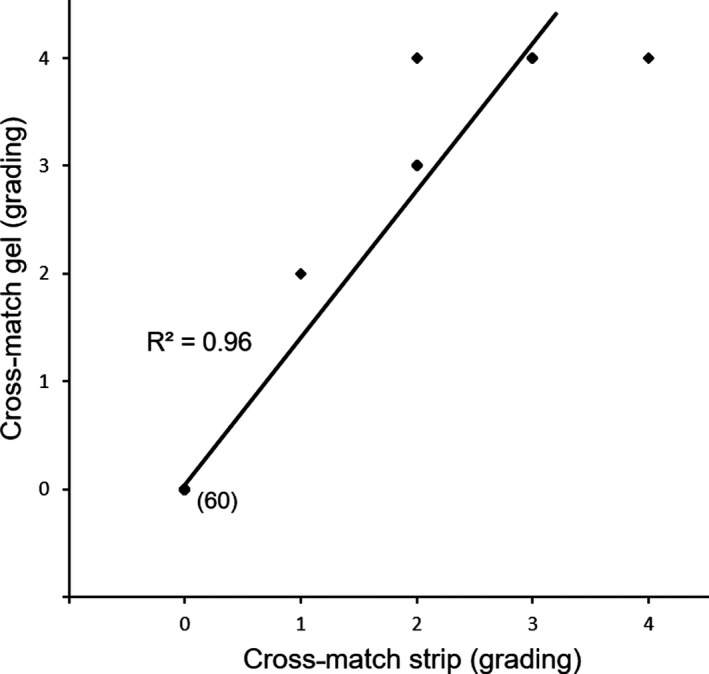
Comparison of pre‐ and post‐transfusion immunochromatographic and gel column cross‐match test results for 26 recipient dogs. Cross‐match strip and gel grading is shown linearly. Each bullet (◆) represents results from both methods compared for each sample with a linear regression (—). The bullets were overlapping for several dogs. The number of dogs allocated to each bullet is written next to each bullet.
Two DAT+ recipients could be followed post‐transfusion. One (R9) DEA 1 mismatched dog (see below) was incompatible to its donor at the second follow‐up period (day 67). The second 1 (R15) was incompatible to its donor at first (day 15) and second (day 26) follow‐up period.
Alloimmunization Due to DEA 1 Mismatch
At VetAgro Sup, DEA 1− recipients exclusively receive DEA 1− blood, whereas DEA 1+ recipients are transfused with either DEA 1+ (preferred to preserve DEA 1− donor blood for DEA 1− recipients) or DEA 1− blood depending on availability1. Thus, the dogs in our study received DEA 1‐matched blood and no anti‐DEA 1 alloimmunization was expected. However, 1 DEA 1− recipient (R9) accidently received moderately DEA 1+ blood, which was missed upon initial typing before transfusing the dog (Table S2 & S3). The hematocrit increased as predicted based upon volume administered from 15 to 21% and 24%, 2 hours and 4 days after the transfusion, respectively. The patient was discharged, and no further data were available except at the follow‐up date (67 days) after which the dog died from gastric dilatation and volvulus. At that time, the hematocrit was 49%, and the major cross‐match was strongly incompatible to the original donor as well as to 3 other weakly to strongly DEA 1+ dogs, whereas the recipient plasma did not react against RBCs from 2 DEA 1− dogs or its own RBCs by either cross‐match method.
Screening for Alloantibodies Against 4 RBC Panels from 34 Dogs
The post‐transfusion plasma from 7 of the sensitized recipients (excluding the DEA 1 mismatch transfused dog described above) was screened against 4 panels of RBCs representing the different DEA 1 and DEA 7 type constellations from 34 healthy dogs (27 donors and 7 control dogs) including their respective donors. Plasma from 1 transfused dog, which was cross‐match‐compatible with its donor, also was found to be compatible with the 4 RBC panels, and was used as negative control (Table 3).
Table 3.
Post‐transfusion major cross‐match test results (DEA 1‐matched blood; 2nd follow‐up period) between plasma samples from 8 recipients and RBCs from 34 dogs (representing 4 different RBC panels based on DEA 1 and DEA 7 typing)
| Panel blood type | n | Number of incompatible cross‐match results with each recipient | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R6 | R7 | R10 | R13 | R14 | R17 | R22 | ||
|
Panel 1 DEA 1−, 7− |
11 | 0 | 10 | 1 | 0 | 11 | 6 | 0 | 11 |
|
Panel 2 DEA 1−, 7+ |
4 | 0 | 4 | 1 | 0 | 4 | 2 | 0 | 4 |
|
Panel 3 DEA 1+, 7− |
11 | 0 | 9 | 0 | 9 | 11 | 9 | 1 | 9 |
|
Panel 4 DEA 1+, 7+ |
8 | 0 | 6 | 2 | 5 | 8 | 7 | 1 | 8 |
| All panels | 34 | 0 | 29 | 4 | 14 | 34 | 24 | 2 | 32 |
| Overall incompatibility | % | 0 | 85 | 12 | 41 | 100 | 71 | 6 | 94 |
DEA, Dog erythrocyte antigen, R, dog recipient number; n, number of dogs in each panel.
R1: negative control, DEA 1−, 7+; R6: DEA 1−, DEA 7 blood type was not determined; R7: DEA 1−, 7−; R10 & R13: DEA 1+, 7−; R14 & R17: DEA 1+, 7+; R22: DEA 1+, DEA 7 blood type was not determined.
Of the 34 healthy dogs (27 donors, 7 control dogs), 11 dogs were DEA 1− and DEA 7− (Panel 1), 4 were DEA 1− and DEA 7+ (Panel 2), 11 were DEA 1+ and DEA 7− (Panel 3) and 8 were DEA 1+, and DEA 7+ (Panel 4).
All 7 screened recipients with incompatible major cross‐match test results with their respective donor(s) were found to be incompatible to an additional 1–33 dogs (Table 3). The sensitization rates of 7 dogs receiving 1 or 2 blood units was not significantly different (P = 1.00). The sensitization rate of the 5 DEA 7− recipients transfused with DEA 7+ RBCs (Table S3) was not significantly different from DEA 7+ dogs receiving DEA 7− blood (P = 0.17). Furthermore, the sensitization rates were not significantly different for the recipients that received DEA 7‐matched transfusion(s) compared with the dogs that received DEA 7−incompatible transfusion(s) (P = 0.94).
Screening for Naturally Occurring Anti‐DEA 7 Alloantibodies
No anti‐DEA 7 alloantibodies were detected in the pretransfusion plasma of 5 DEA 7− recipients tested against RBCs of their respective DEA 7+ donors as well as in the plasma samples of 33 healthy DEA 7− dogs (28 donor and 5 control dogs) that were tested against RBCs from 1 DEA 7+ control dog.
Discussion
In veterinary transfusion medicine, cross‐match tests are rarely performed. If they are done, antiglobulin is not used, whereas in human medicine cross‐match testing utilizing antiglobulins is routinely done before the first and any subsequent transfusion events.1, 3, 21, 22, 23 The difference is related to the presence or absence of naturally occurring alloantibodies, distribution of blood groups, antigenicity of RBC membrane antigens, and availability, sensitivity, and specificity of cross‐match tests.15, 18, 19 In our prospective clinical study of transfused dogs and canine blood donors, we documented the lack of any pretransfusion alloantibodies and the frequent alloimmunization post‐transfusion in dogs receiving DEA 1‐matched transfusions based upon antiglobulin‐enhanced cross‐match tests. Furthermore, an accidental DEA 1 mismatched transfusion leading to the expected development of anti‐DEA 1 alloantibodies in the DEA 1− recipient also was detected, but other alloimmunizations in DEA 1‐matched recipients could not be related to DEA 4 and DEA 7.5, 16, 17 The benefit of extending blood typing to other RBC antigens, such as Dal and Kai 1 and Kai 2, was not investigated because those reagents were not available at the time of our study.9, 10 Our study further supports the need for initial DEA 1 typing to select DEA 1‐matched donors, and additional DEA 1 matching and cross‐matching for subsequent transfusions with practical in‐clinic or laboratory major cross‐match techniques to assure safe and effective transfusion treatment in dogs.
In our study, all plasma samples from the 80 recipients showed major cross‐match compatibility before receiving any blood products. Thus, none of the 80 cross‐matched dogs had any naturally occurring agglutinating alloantibodies against RBCs based upon the antiglobulin‐enhanced cross‐match. This finding is consistent with current transfusion practices and experience1, 11 and limited past studies done with regular agglutination‐enhanced cross‐match tests3, 13, 18, 19, but contrasts with some reports in the past1 , 2 , 24, 25 as well as with recent studies.14, 15 The latter studies report the presence of anti‐DEA 7 antibodies in 38.1% of DEA 7− dogs not previously transfused. The DEA 7 antigen is unique as it is acquired onto the RBC surface and thus varies in expression, making DEA 7 typing difficult. Although different techniques may explain some of the differences, clinical experience does not suggest any anti‐DEA 7 alloantibodies. In fact, no DEA 7‐related acute hemolytic transfusion reactions have ever been reported in any clinical situation. Others15 have proposed that anti‐DEA 7 alloantibodies were related to “delayed transfusion reactions,” but those refer to the development of new antibodies after sensitization by transfusion. However, even in previously transfused dogs, no DEA 7‐related delayed hemolytic transfusion reactions have been clinically documented. Neither did the 9 DEA 7− patients receiving DEA 7+ blood develop any specific anti‐DEA 7 alloantibody responses in our study.
The rate of alloimmunization by transfusion has not been systematically evaluated because it is clinically difficult to obtain post‐transfusion samples.2, 3 Indeed, of the 80 transfusion recipients in our study, 67% of recipients died or were lost to follow up. However, 2/10 (20%) dogs and 12/26 (46%) dogs at the first and second follow‐up periods, respectively, were sensitized by the transfused RBCs as shown by incompatible major cross‐match results. Similarly, 9 dogs transfused with DEA 1‐matched packed RBC units for RBC sensitization by cross‐matching were followed 14–28 days post‐transfusion, and 78% developed alloantibodies against at least 1 of their donors, but none could be associated with DEA 7 or other tested blood types.3
The rate of alloimmunization assessed by screening recipient plasma against RBCs from 34 healthy dogs was found to be similar to the rate of the recipients' alloimmunization against their respective donor(s). Thus, cross‐matching any previously transfused dogs is highly recommended to avoid any acute hemolytic transfusion reactions because of prior alloimmunization. This recommendation applies to the same donor previously used and to any other donor. However, we did not screen the plasma from recipients that remained cross‐match‐compatible to their original donors with the RBC panel (except for R1) and thus cannot assure that a compatible cross‐match against its original donor could assure compatibility to all donors.
The canine‐specific antiglobulin‐enhanced cross‐match tests for the laboratory26 utilizing a gel column technique and in‐clinic kit with an immunochromatographic strip as for the DAT20 and DEA 1 typing gave weakly (1+) to strongly (4+) positive results that were readily differentiated from those that were compatible (negative). Furthermore, the results were categorically concordant between the 2 test methods with the same antibody. And although ours was not a formal validation study (which had been done by the company5), both tests appeared to detect cross‐match incompatibilities equally well and thereby alloimmunization when expected. Both antiglobulin‐enhanced cross‐match techniques seem to be easy to perform according to the described instructions and are readily usable in laboratory or clinical settings. Both methods also permit easy interpretation and recording of actual test results, might be more sensitive than cross‐match without antiglobulin, and represent the required standard cross‐match technique in blood banking for humans. However, a direct comparison to the regular non‐antiglobulin‐enhanced cross‐match test was not performed in our study.
As generally recommended in canine transfusion medicine,1, 2, 11, 24, 25 all recipients and blood donors in our study were typed for DEA 1, and all DEA 1− patients received DEA 1− blood, whereas DEA 1+ patients received DEA 1+ or DEA 1− blood. Thus, each recipient and donor pair in our study was matched for DEA 1 to avoid any alloimmunization against DEA 1 (except for 1 accidental DEA 1‐mismatched transfusion referred to below). Excellent typing kits are available for DEA 1 typing in clinical settings.4, 8 In our study, the chromatographic strip method used in clinics and routine laboratories gave completely concordant results to the research flow cytometric DEA 1 technique as also shown previously.4, 7, 10
One DEA 1− recipient accidently received moderately DEA 1+ blood because of a clerical error (mislabelling) in the intensive care unit rather than a technical failure. Although every effort is made to match transfusions based upon blood types, clerical errors are the main reasons for mismatched transfusions and acute hemolytic transfusion reactions in humans.27 Although no acute hemolytic transfusion reaction was observed in this patient receiving its first unit of blood, this dog developed anti‐DEA 1 alloantibodies based upon cross‐matching with its donor and 3 other DEA 1+ dogs performed 67 days after the DEA 1‐mismatched transfusion. This case also illustrates that even moderately DEA 1+ RBCs can induce anti‐DEA 1 alloantibodies in a DEA 1− dog. As recommended in recent studies on DEA 1,20 , 4 a weak band at the DEA 1 position of the strip should be considered as DEA 1+ when referring to a donor to avoid any sensitization. In contrast, a weakly DEA 1+ patient preferably should receive DEA 1− blood.28
The frequency of DEA 4+ has been documented to be >99% across all breeds studied, and thus, DEA 4 should be considered a high‐frequency RBC antigen rather than a blood group.1, 24, 25 Indeed, all typed recipient and donor dogs in our study were DEA 4+ and thereby no anti‐DEA 4 sensitization could be evaluated. On the other hand, the anti‐DEA 4 reagent worked well as a positive control for each test. When our study was performed, anti‐DEA 3, anti‐DEA 5, and anti‐Dal antisera were not commercially available and neither were the anti‐Kai 1 and anti‐Kai 2 monoclonal antibodies recently described.9, 10, 24 Thus, we cannot speculate further on the target of the alloimmunization in the 12 sensitized recipients beyond the 1 caused by a DEA 1 mismatch.
Although the DAT for IMHA and DAT‐enhanced major cross‐match are similar, a positive DAT did not lead to a positive cross‐match result. In fact, all 8 DAT+ dogs showed a compatible cross‐match result (the weakly DAT+ dog with malignant histiocytosis was considered to be a nonspecific reaction). Although this is a small group of DAT+ dogs, it is encouraging to see that these dogs with IMHA can be cross‐matched, which is consistent with the clinical experience in canine transfusion medicine except for those dogs in which autoagglutination persists after washing 3 times.
Immunosuppressive treatment might blunt alloimmunization in humans.29 However, the 2 dogs with IMHA (DAT+) that had received immunosuppressive doses of glucocorticosteroids in our study (including the DEA 1 mismatch transfused dog) became sensitized against their respective donors. Thus, like any other patient requiring multiple transfusions, DEA 1‐matched blood and cross‐matching after the first transfusion are recommended even when the patient is immunocompromised.
Number of transfusions increases the risk of alloimmunization against RBC in humans.30, 31 However, we did not find any significant difference in alloimmunization between dogs receiving 1 versus 2 units of RBCs in our study. Again, this is a small number of patients and it may take >2 donors or units to show the increased risk of alloimmunization.
Acute hemolytic transfusion reactions only have been reported clinically in previously transfused dogs.5, 16, 17 Our study could not evaluate whether or not the cross‐match incompatibilities correspond to decreased in vivo RBC survival and cause acute hemolytic reactions in patients requiring additional transfusions. At the time of follow‐up, these patients did not require any blood transfusions, and it would have been unethical to administer cross‐match‐incompatible blood.3 Our study did not evaluate for delayed transfusion reactions, which occur when new alloantibodies develop and result in a more rapid decrease in PCV after several days to week (after sensitization).32
In conclusion, the antiglobulin‐enhanced immunochromatographic strip kit as well as the antiglobulin‐enhanced gel column technique are practical tests for cross‐matching in the clinic and laboratory, respectively, and identified alloantibodies on RBCs of some transfused dogs. In our study, no naturally occurring alloantibodies against RBC antigens were found in any recipients before transfusion, confirming clinical experience and the practice of not cross‐matching at the time of a first transfusion. Because the post‐transfusion alloimmunization rate was high, major cross‐matching is warranted in any dog that previously had received DEA 1‐matched RBCs before the next transfusion (at least 4 days apart).
Supporting information
Table S1. Signalment and cause of anemia of 26 recipient dogs.
Table S2. Dog Erythrocyte Antigen (DEA) 1 and DEA 7 typing and major crossmatch results of 26 recipient dogs pre‐ and post‐transfusion with 29 donors totaling 32 transfusion events.
Table S3. Dog Erythrocyte Antigen (DEA) 1 and DEA 7 mismatched transfused recipient dogs pre‐ and post‐transfusion.
Acknowledgment
The immunochromatographic DEA 1 typing and canine cross‐match strips were kindly provided by Alvedia, Limonest, France. The authors thank the nursing staff in the intensive care unit for their assistance with blood sampling and typing and Mathieu Magnin for statistical analysis, all at VetAgro Sup.
Conflict of Interest Declaration
B. Canard, M. Guidetti and B. Chaprier were employed, and I. Goy‐Thollot and U. Giger have been scientific advisors to Dianov. Reagents were received for these studies from Alvedia. Alvedia is commercially offering typing and cross‐match kits. However, the design and execution of the study, data analysis, and writing of the manuscript have been done independently. Urs Giger is the director of PennGen at the University of Pennsylvania, which is a non‐for‐profit laboratory offering blood typing and compatibility testing.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Presented, in part, at the Forum of the International Veterinary Emergency and Critical Care Society, Grapevine, Tx, September 2016.
This work was performed in the intensive care unit of VetAgro Sup campus vétérinaire de Lyon, Marcy l'Etoile, France and Dianov Laboratories, Limonest, France.
Source of Funding: This study was in part supported by Dianov Laboratories and the National Institutes of Health OD 010939
Footnotes
Hale AS, Werfelman J, Lemmons M, et al. An evaluation of 9,570 dogs by breed and dog erythrocyte antigen typing (abstr). J Vet Intern Med 2008;22:740
Hale AS, Werfelmann J. Incidence of canine serum antibody to known dog erythrocyte antigens in potential donor population (abstr). J Vet Intern Med 2006;20:768‐769
ACD Solution B, Becton Dickinson, Plymouth, UK
ACD Blood Pack Units, Macopharma, Tourcoing, France
Alvedia, Limonest, France
Canine LabTest DEA 1 Blood Typing, Alvedia, Limonest, France
Polyclonal Goat Anti‐Mouse Immunoglobulins/FITC Goat F(ab')2, Dako Denmark A/S, Glostrup, Denmark
Becton Dickinson & Co, Franklin Lakes, NJ
Plaques d'examens SF200, BSA Concept Lab, Mont Dol, France
Canine DEA 4 anti‐sera, Animal Blood Resources International, Stockbridge, MI
Bio‐Rad ID‐Diluent 2, modified LISS solution, DiaMed GmbH, Cressier, Switzerland
Bio‐Rad ID‐Cards, NaCl, Enzyme Test and Cold Agglutinins, DiaMed GmbH, Cressier, Switzerland
Canine DEA 7 anti‐sera, Animal Blood Resources International, Stockbridge, MI
Canine Coombs Reagent, MP Biomedicals, Solon, OH
ID ‐Centrifuge 24 S, Diamed‐ID, Microtyping System, DiaMed GmbH, Cressier, Switzerland
Canine LabTest Direct Antiglobulin (DAT), Alvedia, Limonest, France
Canine LabTest Crossmatch (XM), Alvedia, Limonest, France
Excel 2013, Microsoft Ltd, Reading, UK
Prism 6, GraphPad Software, La Jolla, CA
Canard B, Barthelémy A, Felix N, et al. Stability of DEA1+ antigen expression and production of alloantibodies after transfusion in dogs. A preliminary study (abstr). J Vet Emerg Crit Care 2013; doi: 10.1111/vec.12088-S30
References
- 1. Giger U. Blood typing and crossmatching to ensure compatible transfusions In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy, 15th ed St. Louis, MO: Elsevier Saunders; 2014:e143. [Google Scholar]
- 2. Holowaychuk MK, Leader JL, Monteith G. Risk factors for transfusion‐associated complications and nonsurvival in dogs receiving packed red blood cell transfusions: 211 cases (2008‐2011). J Am Vet Med Assoc 2014;244:431–437. [DOI] [PubMed] [Google Scholar]
- 3. Kessler RJ, Reese J, Chang D, et al. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7, and Dal blood typing and cross‐matching by gel column technique. Vet Clin Pathol 2010;39:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Acierno MM, Raj K, Giger U. DEA 1 expression on dog erythrocytes analyzed by immunochromatographic and flow cytometric techniques. J Vet Intern Med 2014;28:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giger U, Gelens CJ, Callan MB, et al. An acute hemolytic transfusion reaction caused by dog erythrocyte antigen 1.1 incompatibility in a previously sensitized dog. J Am Vet Med Assoc 1995;206:1358–1362. [PubMed] [Google Scholar]
- 6. Giger U, Stieger K, Palos H. Comparison of various canine blood‐typing methods. Am J Vet Res 2005;66:1386–1392. [DOI] [PubMed] [Google Scholar]
- 7. Polak K, Acierno MM, Raj K, et al. Dog erythrocyte antigen 1: Mode of inheritance and initial characterization. Vet Clin Pathol 2015;44:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seth M, Jackson KV, Winzelberg S, et al. Comparison of gel column, card, and cartridge techniques for dog erythrocyte antigen 1.1 blood typing. Am J Vet Res 2012;73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blais MC, Berman L, Oakley DA, et al. Canine Dal blood type: A red cell antigen lacking in some Dalmatians. J Vet Intern Med 2007;21:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Euler CC, Lee JH, Kim HY, et al. Survey of two new (Kai 1 and Kai 2) and other blood groups in dogs of North America. J Vet Intern Med 2016;30:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hohenhaus AE. Importance of blood groups and blood group antibodies in companion animals. Transfus Med Rev 2004;18:117–126. [DOI] [PubMed] [Google Scholar]
- 12. Goulet S, Giger U, Arsenault J, et al. Prevalence and mode of inheritance of the Dal blood group in dogs in North America. J Vet Intern Med 2017;31:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blais MC, Rozanski EA, Hale AS, et al. Lack of evidence of pregnancy‐induced alloantibodies in dogs. J Vet Intern Med 2009;23:462–465. [DOI] [PubMed] [Google Scholar]
- 14. Spada E, Proverbio D, Baggiani L, et al. Activity, specificity, and titer of naturally occurring canine anti‐DEA 7 antibodies. J Vet Diagn Invest 2016;28:705–708. [DOI] [PubMed] [Google Scholar]
- 15. Spada E, Proverbio D, Vinals Florez LM, et al. Prevalence of naturally occurring antibodies against dog erythrocyte antigen 7 in a population of dog erythrocyte antigen 7‐negative dogs from Spain and Italy. Am J Vet Res 2016;77:877–881. [DOI] [PubMed] [Google Scholar]
- 16. Callan MB, Jones LT, Giger U. Hemolytic transfusion reactions in a dog with an alloantibody to a common antigen. J Vet Intern Med 1995;9:277–279. [DOI] [PubMed] [Google Scholar]
- 17. Melzer KJ, Wardrop KJ, Hale AS, et al. A hemolytic transfusion reaction due to DEA 4 alloantibodies in a dog. J Vet Intern Med 2003;17:931–933. [PubMed] [Google Scholar]
- 18. Guzman LR, Streeter E, Malandra A. Comparison of a commercial blood cross‐matching kit to the standard laboratory method for establishing blood transfusion compatibility in dogs. J Vet Emerg Crit Care (San Antonio) 2016;26:262–268. [DOI] [PubMed] [Google Scholar]
- 19. Villarnovo D, Burton SA, Horney BS, et al. Preliminary evaluation of a gel tube agglutination major cross‐match method in dogs. Vet Clin Pathol 2016;45:411–416. [DOI] [PubMed] [Google Scholar]
- 20. Caviezel LL, Raj K, Giger U. Comparison of 4 direct Coombs' test methods with polyclonal antiglobulins in anemic and nonanemic dogs for in‐clinic or laboratory use. J Vet Intern Med 2014;28:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lapierre Y, Rigal D, Adam J, et al. The gel test: A new way to detect red cell antigen‐antibody reactions. Transfusion 1990;30:109–113. [DOI] [PubMed] [Google Scholar]
- 22. Lamba DS, Mittal K, Sood T, et al. Antibody screening in multitransfused patients: A prerequisite before each transfusion. Transfus Apher Sci 2014;51:132–133. [DOI] [PubMed] [Google Scholar]
- 23. Novaretti MC, Jens E, Pagliarini T, et al. Comparison of conventional tube test technique and gel microcolumn assay for direct antiglobulin test: A large study. J Clin Lab Anal 2004;18:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hale AS. Canine blood groups and blood typing In: Day MJ, Kohn B, eds. BSAVA Manual of Canine and Feline Haematology and Transfusion Medicine, 2nd ed Quedgeley, Gloucester, England: British Small Animal Veterinary Association; 2012:280–283. [Google Scholar]
- 25. Hale AS. Canine blood groups and their importance in veterinary transfusion medicine. Vet Clin North Am Small Anim Pract 1995;25:1323–1332. [DOI] [PubMed] [Google Scholar]
- 26. Sriwichai C, Jiraudommongkol M, Wutti‐In Y, et al. Production of an anticanine globulin (polyspecific) reagent for laboratory investigation. J Small Anim Pract 2011;52:476–483. [DOI] [PubMed] [Google Scholar]
- 27. Bolton‐Maggs PH. SHOT conference report 2016: Serious hazards of transfusion – human factors continue to cause most transfusion‐related incidents. Transfus Med 2016;26:401–405. [DOI] [PubMed] [Google Scholar]
- 28. Flegel WA. How I manage donors and patients with a weak D phenotype. Curr Opin Hematol 2006;13:476–483. [DOI] [PubMed] [Google Scholar]
- 29. Asfour M, Narvios A, Lichtiger B. Transfusion of RhD‐incompatible blood components in RhD‐negative blood marrow transplant recipients. MedGenMed 2004;6:22. [PMC free article] [PubMed] [Google Scholar]
- 30. Zalpuri S, Zwaginga JJ, le Cessie S, et al. Red‐blood‐cell alloimmunization and number of red‐blood‐cell transfusions. Vox Sang 2012;102:144–149. [DOI] [PubMed] [Google Scholar]
- 31. Evers D, Middelburg RA, de Haas M, et al. Red‐blood‐cell alloimmunisation in relation to antigens' exposure and their immunogenicity: A cohort study. Lancet Haematol 2016;3:e284–e292. [DOI] [PubMed] [Google Scholar]
- 32. Swisher SN, Izzo MJ, Young LE. Post‐transfusion survival of fresh and stored dog erythrocytes measured by differential agglutination. J Lab Clin Med 1953;41:946–952. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Signalment and cause of anemia of 26 recipient dogs.
Table S2. Dog Erythrocyte Antigen (DEA) 1 and DEA 7 typing and major crossmatch results of 26 recipient dogs pre‐ and post‐transfusion with 29 donors totaling 32 transfusion events.
Table S3. Dog Erythrocyte Antigen (DEA) 1 and DEA 7 mismatched transfused recipient dogs pre‐ and post‐transfusion.


