Supplemental Digital Content is available in the text.
Keywords: atherosclerosis; malapposition; stents; thrombosis; tomography, optical coherence; uncovered struts
Abstract
Background:
Stent thrombosis (ST) is a serious complication following coronary stenting. Intravascular optical coherence tomography (OCT) may provide insights into mechanistic processes leading to ST. We performed a prospective, multicenter study to evaluate OCT findings in patients with ST.
Methods:
Consecutive patients presenting with ST were prospectively enrolled in a registry by using a centralized telephone registration system. After angiographic confirmation of ST, OCT imaging of the culprit vessel was performed with frequency domain OCT. Clinical data were collected according to a standardized protocol. OCT acquisitions were analyzed at a core laboratory. Dominant and contributing findings were adjudicated by an imaging adjudication committee.
Results:
Two hundred thirty-one patients presenting with ST underwent OCT imaging; 14 (6.1%) had image quality precluding further analysis. Of the remaining patients, 62 (28.6%) and 155 (71.4%) presented with early and late/very late ST, respectively. The underlying stent type was a new-generation drug-eluting stent in 50.3%. Mean reference vessel diameter was 2.9±0.6 mm and mean reference vessel area was 6.8±2.6 mm2. Stent underexpansion (stent expansion index <0.8) was observed in 44.4% of patients. The predicted average probability (95% confidence interval) that any frame had uncovered (or thrombus-covered) struts was 99.3% (96.1–99.9), 96.6% (92.4–98.5), 34.3% (15.0–60.7), and 9.6% (6.2–14.5) and malapposed struts was 21.8% (8.4–45.6), 8.5% (4.6–15.3), 6.7% (2.5–16.3), and 2.0% (1.2–3.3) for acute, subacute, late, and very late ST, respectively. The most common dominant finding adjudicated for acute ST was uncovered struts (66.7% of cases); for subacute ST, the most common dominant finding was uncovered struts (61.7%) and underexpansion (25.5%); for late ST, the most common dominant finding was uncovered struts (33.3%) and severe restenosis (19.1%); and for very late ST, the most common dominant finding was neoatherosclerosis (31.3%) and uncovered struts (20.2%). In patients presenting very late ST, uncovered stent struts were a common dominant finding in drug-eluting stents, and neoatherosclerosis was a common dominant finding in bare metal stents.
Conclusions:
In patients with ST, uncovered and malapposed struts were frequently observed with the incidence of both decreasing with longer time intervals between stent implantation and presentation. The most frequent dominant observation varied according to time intervals from index stenting: uncovered struts and underexpansion in acute/subacute ST and neoatherosclerosis and uncovered struts in late/very late ST.
Clinical Perspective.
What Is New?
This report represents the largest available series of patients with optical coherence tomography imaging during stent thrombosis presentation and includes findings from patients treated predominantly with current-generation drug-eluting stents.
The dominant findings at optical coherence tomography varies according to the time interval between index stenting and presentation with stent thrombosis.
Uncovered struts and stent underexpansion were the most common observations in acute/subacute stent thrombosis and neoatherosclerosis and uncovered struts were the most common findings in late/very late stent thrombosis.
What Are the Clinical Implications?
Improved acute deployment techniques with post–stent dilatation where appropriate may significantly impact stent thrombosis by reducing underexpansion and malapposition.
The impact of dedicated clinical strategies for the prevention and treatment of neoatherosclerosis should be investigated in future clinical studies.
Percutaneous coronary intervention with stent implantation is a successful treatment for patients with obstructive coronary artery disease and has been shown to improve symptoms, and to reduce mortality in certain settings, as well.1,2 The most serious complication of coronary stenting is abrupt thrombotic occlusion, which often results in Q-wave myocardial infarction and significantly impacts life expectancy.3–5 Although the absolute risk of stent thrombosis is low after treatment with current-generation drug-eluting stents (DES),6 the large number of patients implanted with coronary stents worldwide makes this condition a significant health issue.
Numerous risk factors for stent thrombosis have been identified from case-control studies.7 These factors are related to specific patient characteristics and the types of disease patterns treated. In addition, risk characteristics attributed to the type of implanted device have been well defined.7 Specifically, higher risk was observed with first-generation DES in comparison with bare metal stents, although new-generation DES seem to be associated with a comparable or lower risk of stent thrombosis in comparison with uncoated stents.8–10
Detailed understanding of the underlying conditions in the stented segment at the time of the stent thrombosis event remains an important clinical need. In particular, although imaging with intravascular ultrasound can help define stent-vessel interactions, this modality is limited by modest axial resolution.11 Recently, intravascular optical coherence tomography (OCT) has become widely available in clinical practice. This technology permits rapid evaluation of stent coverage and apposition, and detailed characterization of neointimal tissue and vessel wall pathology.12 In the setting of the PRESTIGE registry (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European) effort, we collected information on patients presenting with stent thrombosis undergoing intracoronary imaging with OCT at a network of centers across Europe. In the present report, we present the key findings from our analysis.
Methods
Study Population and Patient Treatment
Consecutive patients presenting with definite stent thrombosis, undergoing percutaneous coronary intervention at 29 participating centers with OCT imaging capability, were prospectively enrolled in the multicenter PRESTIGE registry using a centralized telephone registration system. A list of participating centers is provided in the Appendix in the online-only Data Supplement.13 Definite stent thrombosis was defined according to Academic Research Consortium criteria.14 Clinical, procedural, and imaging data were collected according to a standardized protocol and entered by site investigators in a central electronic database (Open Clinica, Leuven Coordinating Center, Leuven, Belgium) checked by centralized monitoring queries. Platelet reactivity was measured according to the available assays per center using the VerifyNow P2Y12 (Accriva Diagnostics) or Multiplate ADP assay (Roche Diagnostics International Ltd) according to manufacturer’s instructions. High platelet reactivity was defined as HPR-ADP >208 P2Y12 Reaction Units by the VerifyNow P2Y12, and as >46 U (U: 1 U=10 AU×min) by the Multiplate ADP assay. Type of underlying stent was classified as a bare metal stent, early-generation DES (durable polymer sirolimus-eluting stents [Cypher, Cordis], durable polymer paclitaxel-eluting stents [Taxus, Boston Scientific], or durable polymer zotarolimus-eluting stents [Endeavor, Medtronic Inc]), newer-generation DES (all other metallic-backbone DES), or bioresorbable DES.9 The study complied with the Declaration of Helsinki. The ethical review committee at each participating institution approved the study, and all patients provided written informed consent. The study was funded by the European Union under the Seventh Framework Program FP7/2007 to 2013, grant agreement HEALTH-F2-2010 to 260309 (PRESTIGE).
Study Procedures
Patients enrolled in the PRESTIGE registry underwent percutaneous coronary intervention according to local practices. After angiographic confirmation of stent thrombosis, a guidewire was advanced distally in the culprit vessel across the site of occlusion. The use of OCT before and after percutaneous coronary intervention procedures was recommended in all patients. Use of thrombectomy with manual aspiration was encouraged to restore effective flow and to reduce residual thrombus before OCT image acquisition.15 In selected cases, small balloon dilation (≤2.0 mm in diameter) at low pressure was permitted if image quality remained insufficient after thrombectomy. OCT image acquisition was discouraged in patients presenting in a medical condition precluding safe OCT acquisition (eg, unstable electric or hemodynamic conditions or reported chronic renal insufficiency).15 During the procedure, patients were treated with intravenous heparin or bivalirudin. Use of glycoprotein inhibitors was at the discretion of the treating physician.
OCT Data Acquisition
Following administration of intracoronary nitrates, OCT was performed with a nonocclusive imaging technique using commercially available frequency domain OCT imaging systems (C7XR, Ilumien or Ilumien Optis, St. Jude Medical). In brief, after restoration of flow, a rapid exchange imaging catheter (Dragonfly or Dragonfly Duo, St. Jude Medical) was advanced beyond the stented segment. An OCT pullback of the entire stented segment, including distal and proximal reference sites, was performed with contrast injection through the guiding catheter at 3 to 5 mL/s. If the stented segment was too long to be imaged in a single pullback, an additional pullback was acquired using angiographic landmarks for appropriate imaging catheter position and view.
OCT Quantitative Analysis
Raw data of OCT image acquisitions were collected and sent to a centralized core laboratory (ISAResearch Center) for off-line analyses. Each OCT sequence was assessed and measured by independent readers experienced in OCT imaging analysis, blinded to patient characteristics and timing of stent thrombosis (see the Appendix and Figure I in the online-only Data Supplement). Initially, a quality screening of the entire sequence was performed to confirm sufficient quality of imaging to permit the analysis. Reasons for exclusion were insufficient image quality because of the poor clearance of blood, missed region of interest with incomplete stent visualization, excessive remaining thrombus obscuring the stent assessment, or the presence of imaging artifacts precluding the analysis. Nonanalyzable frames were defined as frames with <45° of visible lumen border (eg, attributable to the presence of thrombus or side branch). Stent struts located across the ostium of side branches were excluded from the analysis of coverage and apposition. Quantitative and morphometric analyses were performed every 1 mm along the entire target segment. Dedicated software (St. Jude Medical) was used for quantification. Further details and definitions are provided in the Appendix in the online-only Data Supplement.
Imaging Adjudication Committee OCT Analysis
An imaging adjudication committee adjudicated the findings at the time of stent thrombosis based on systematic review of all acquired OCT pullbacks in dedicated sessions at the central core laboratory. The committee reviewed each pullback in detail, scoring for the presence or absence of findings according to a prespecified protocol. OCT images were analyzed without the knowledge of patient/stent characteristics or timing of stent thrombosis. The composition of the committee is listed in the Appendix in the online-only Data Supplement. Each OCT pullback was assessed in both longitudinal and cross-sectional views for the presence of a single dominant finding at stent thrombosis (Figure I in the online-only Data Supplement). If no single dominant finding was assessed, this was recorded. Additional findings assessed to be of lesser relevance were adjudicated as contributory. In case of disagreement, a decision was made by consensus. The following categories were considered for visual adjudication: stent underexpansion (defined as minimum stent area <80% of the mean of proximal and distal vessel reference area), edge dissection (proximal or distal to the stented segment, involving the intima and media with a circumferential extent at least of one-third of the vessel contour and a longitudinal extent >3 mm), overlapping stents, stent fracture, uncovered stent struts, malapposed stent struts, presence of interstrut cavities, in-stent restenosis, and neoatherosclerosis (in the presence of plaque rupture or in association with thin cap fibroatheroma adjacent to the site of maximum thrombus burden). Restenosis was defined as the presence of >50% diameter stenosis in the stented segment. In the presence of both in-stent restenosis and neoatherosclerosis, neoatherosclerosis was considered as the dominant finding if either of the 2 associated factors listed above were present. If none of these characteristics was present, the label no contributing factor identifiable was recorded.
Statistical Analysis
Continuous data are presented as mean (SD) or median (25th–75th percentiles). Categorical data are presented as observed frequencies and proportions (%). Patients were analyzed according to the time interval between index stenting and stent thrombosis presentation, classified as acute (<24 hours), subacute (24 hours to 30 days), late (>30 days to 1 year), and very late (>1 year).14 Differences between groups were assessed for statistical significance using a Wilcoxon rank sum test or a Kruskal-Wallis test for continuous data and χ2 test (or Fisher exact test where the expected cell value was <5) for categorical variables. Repeatability data were analyzed by calculating the within-patient SD and repeatability coefficient,16 and the intraclass correlation coefficient, described by Shrout and Fleiss17 for the case when all patients are rated by the same raters who are assumed to be a random subset of all possible raters (data reported in the Results section of the online-only Data Supplement). Predicted average probability for a frame to have uncovered struts, thrombus-covered struts, malapposed struts, struts with neoatherosclerosis, and the presence of interstrut cavities was assessed by using generalized linear mixed regression models to account for clustering effects. The generalized model used a logit-link with a binary distribution for the residuals (ie, logistic regression model). Interdependencies within patients were accounted for by including a random intercept per patient. Time between index stenting and stent thrombosis (ST) was used as a fixed categorical effect. All tests were 2-sided and assessed at a significance level of 5%. Because of the exploratory nature of the analysis, no adjustment was made for multiple testing. The statistical analysis was performed using SAS/STAT software, Version 9.4 (TS2M3, SAS Institute).
Results
A total of 675 patients with ST were enrolled in the PRESTIGE ST registry at participating centers. Of these patients, 231 underwent OCT imaging at the time of presentation (Figure II in the online-only Data Supplement). Fourteen patients had image quality precluding further analysis (poor quality attributable to blood contamination [n=6], or excessive remaining thrombus [n=8]). The remaining 217 patients comprised the primary study cohort for the current analysis.
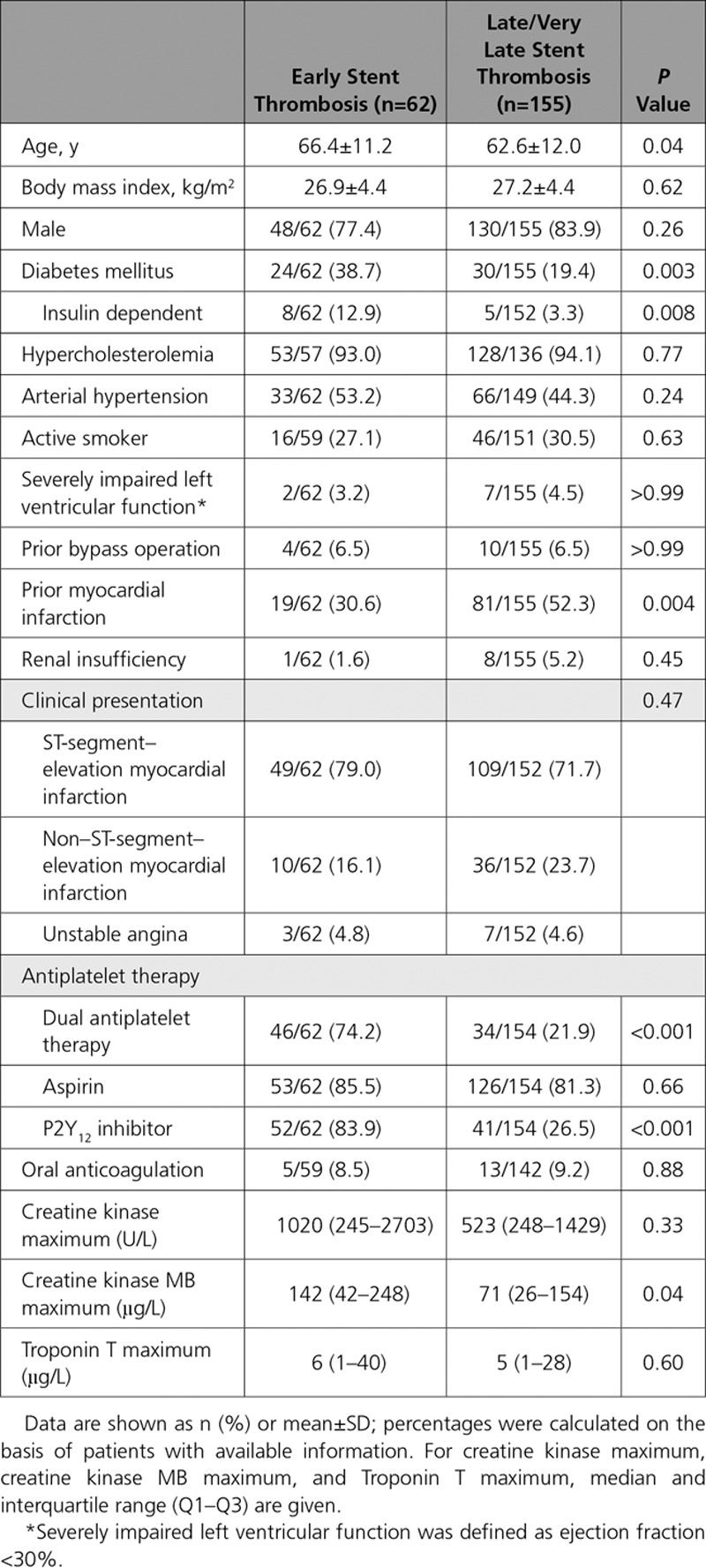
Baseline clinical characteristics of patients according to presentation with acute/subacute (≤30 days) or late/very late (>30 days) ST are shown in Table 1. Overall, 62 (28.6%) patients presented with acute/subacute and 155 (71.4%) presented with late/very late ST. Median time from index stenting to presentation with ST was 4 (2–8) days and 1804 (692–2953) days, respectively. Patients with acute/subacute ST in comparison with late/very late were older, more likely to have diabetes mellitus, more likely to be on dual antiplatelet therapy, and less likely to have had a prior myocardial infarction. Regarding clinical presentation, 158 patients (73.8%) presented with ST-segment–elevation myocardial infarction, 46 (21.5%) with non–ST- segment–elevation myocardial infarction, and 10 (4.7%) with unstable angina.
Table 1.
Patient Clinical Characteristics at Time of Presentation With Stent Thrombosis
Blood sampling for platelet function testing was available in 81 of 217 (37.3%) of the patients. Data from Verify Now P2Y12 assay was available in 49 patients and Multiplate ADP assay in 51 patients. The results are shown in Table I in the online-only Data Supplement. Overall, 37 of 49 (75.5%) and 34 of 51 (66.7%) patients had high platelet reactivity. There was no difference in the proportion of patients with high platelet reactivity between early and late ST groups with either assay (P=0.18 and 0.31, respectively).
In terms of the index stenting procedure, there were no significant differences between the groups regarding presentation (P=0.064): overall 85 of 209 patients (40.6%) presented with ST-segment–elevation myocardial infarction, 79 of 209 (37.8%) patients presented with acute coronary syndrome, and 45 of 209 (21.5%) patients presented with stable coronary disease. Ten of 204 (4.9%) patients underwent intravascular imaging guidance at the time of index intervention.
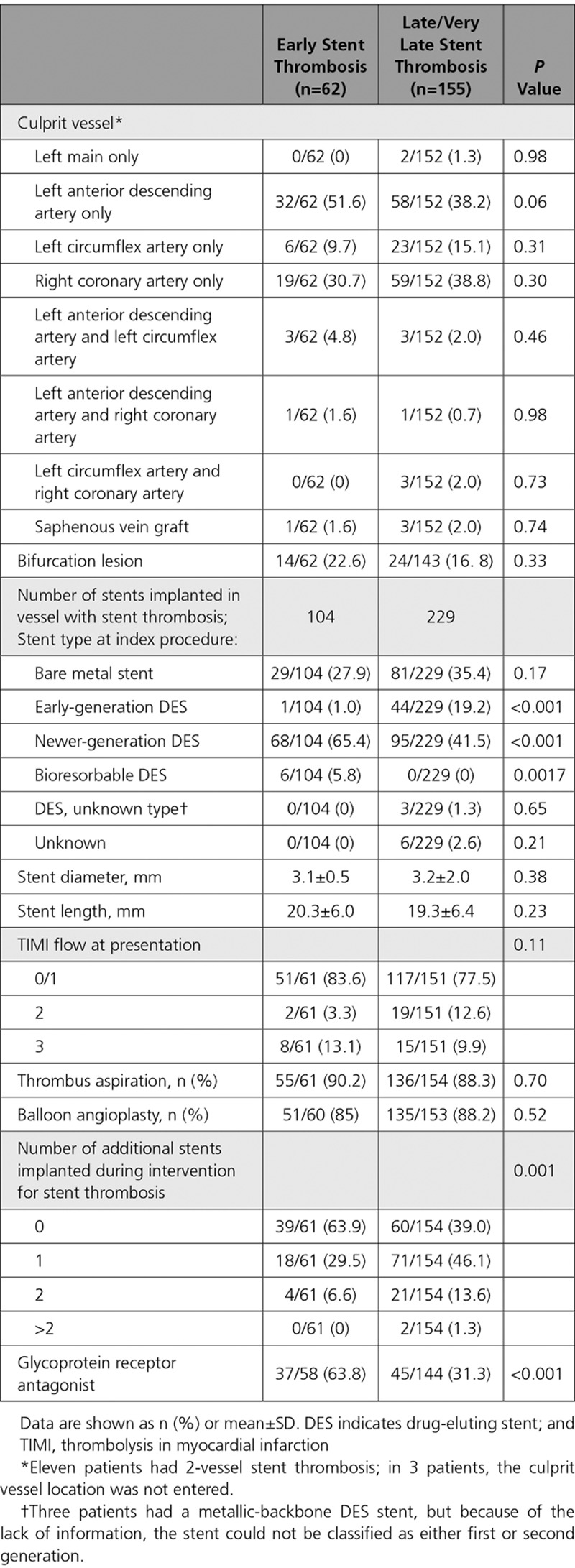
Lesion and procedural characteristics of patients according to presentation are reported in Table 2. The left anterior descending artery was the most commonly involved culprit vessel. A total of 333 stents were implanted in target vessels presenting ST: 110 (33.0%) bare metal stents, 45 (13.9%) first-generation DES, 163 (50.3%) newer-generation DES, 3 DES of unknown type, and 6 (1.8%) bioresorbable stents; in 6 patients (1.8%), the stent type could not be determined.
Table 2.
Angiographic and Procedural Characteristics of Patients With Stent Thrombosis
There were no significant differences between patients with ST with analyzable OCT imaging who were included in the present analysis (n=217) versus those without (n=458) in terms of proportion of patients with acute/subacute and late/very late ST (see the Appendix in the online-only Data Supplement). In comparison with patients with OCT imaging, there was a higher proportion of patients with prior myocardial infarction, ST in a saphenous vein graft, and unknown stent type at baseline in patients without OCT imaging. Otherwise there were no significant differences between the groups (Table II in the online-only Data Supplement).
OCT Core Laboratory Analysis
OCT morphometric data in patients with acute, subacute, late, and very late ST are reported in Table 3. Overall mean reference vessel diameter was 2.9±0.6 mm and mean reference vessel area was 6.8±2.6 mm2. Mean minimum stent diameter was 2.6±0.6 mm and mean minimum stent area was 5.8±2.5 mm2. A stent expansion index <0.8 was observed in 44.4% of all patients (Figure 1). In the subgroup of patients with subacute ST, mean expansion index was 0.7±0.2, and a stent expansion index <0.8 was observed in 65.8% of these patients.
Table 3.
Optical Coherence Tomography Morphometric Analysis in Patients Presenting With Stent Thrombosis Classified According to Time
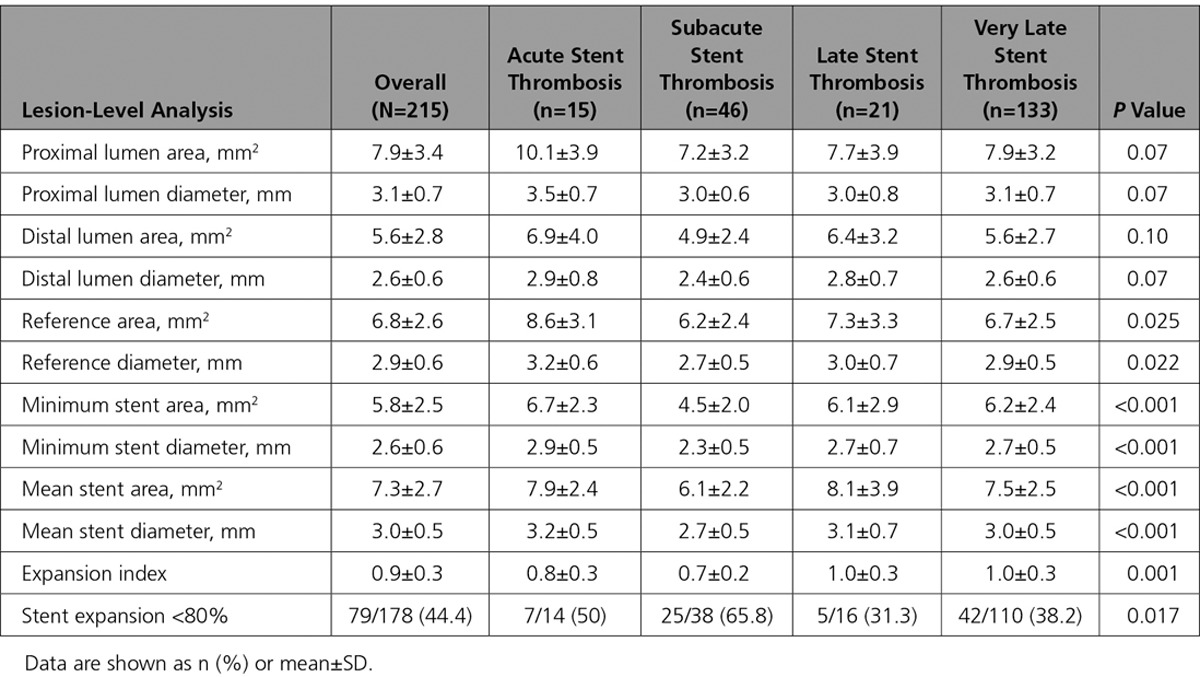
Figure 1.
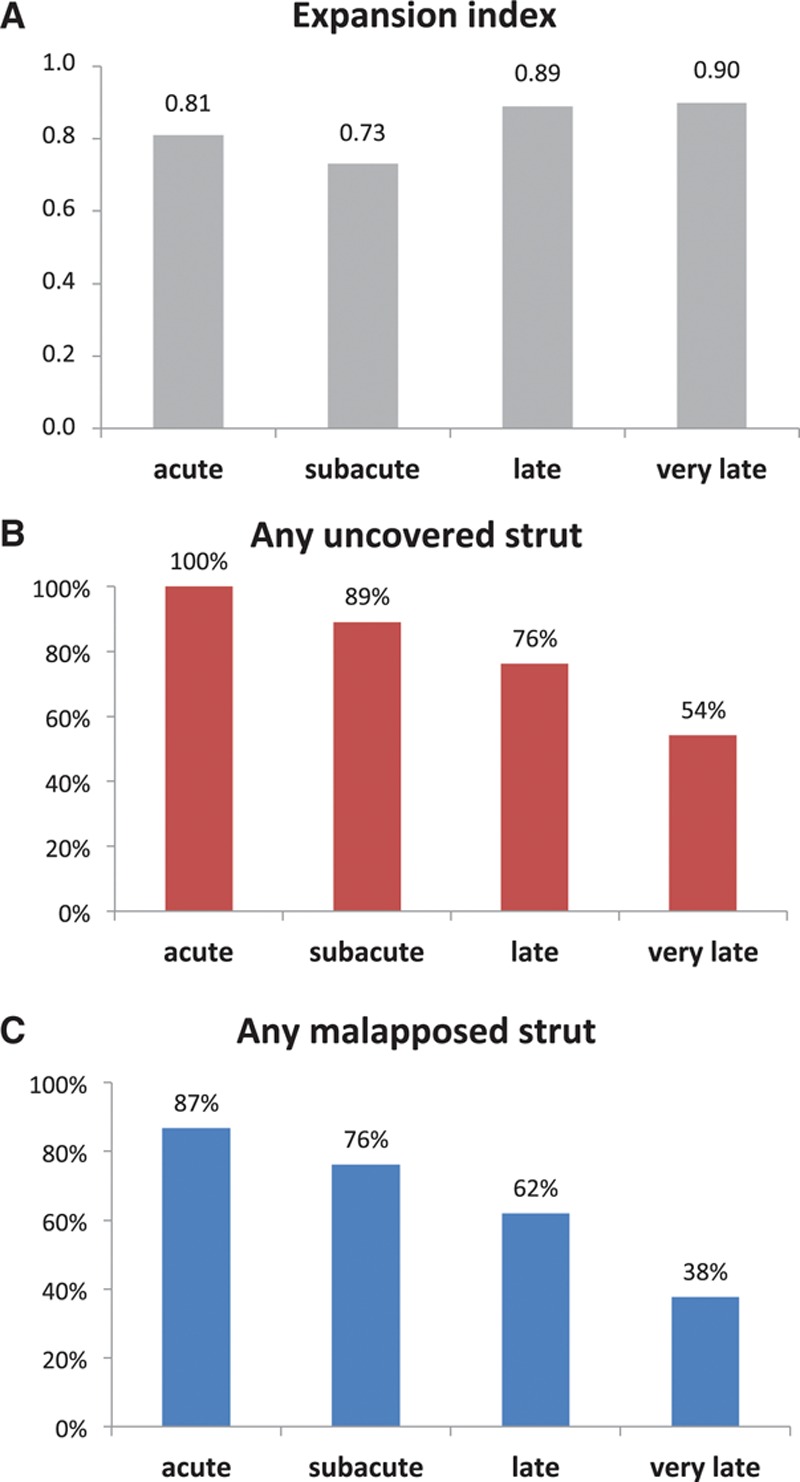
Optical coherence tomography findings in patients presenting with stent thrombosis classified according to time. A, Mean stent expansion index. B, Proportion of patients with at least 1 frame with uncovered struts. C, Proportion of patients with at least 1 frame with malapposed struts.
OCT analysis of stent-vessel interaction for each lesion according to the time of presentation is reported in Table 4. A total of 5704 frames were quantitatively analyzed with a mean number of 26.5±13.2 frames per patient; 96.7% of target regions showed at least 1 frame with any remaining thrombus.
Table 4.
Optical Coherence Tomography Analysis of Stent-Vessel Interaction in Patients Presenting With Stent Thrombosis Classified According to Time
The number of ST lesions with any frame of the pullback showing uncovered struts significantly decreased according to the time of presentation: 100%, 89.1%, 76.2%, and 54.1% for acute, subacute, late, and very late ST, respectively (P<0.001) (Figure 1). In patients presenting with acute or subacute ST, a median of 100% of frames were uncovered or covered with thrombus, in comparison with 50.0% and 11.4% in patients with late or very late ST (P<0.001). The maximum longitudinal extent of thrombus and uncovered struts was greatest in patients with acute and subacute ST and decreased over time.
The number of lesions with any frame showing malapposed struts significantly decreased according to the time of presentation: 86.7%, 76.1%, 61.9%, and 37.6% for acute, subacute, late, and very late ST, respectively (P<0.001). A median of 25% of frames showed malapposition in patients with acute ST in comparison with 10.6%, 4.3%, and 0.0% in patients with subacute, late, and very late ST, respectively (P<0.001) (Figure 1).
The predicted average probability (95% confidence interval) for any frame to have uncovered (or thrombus-covered) struts was 99.3% (96.1–99.9), 96.6% (92.4–98.5), 34.3% (15.0–60.7), and 9.6% (6.2–14.5), and malapposed struts was 21.8% (8.4–45.6), 8.5% (4.6–15.3), 6.7% (2.5–16.3), and 2.0% (1.2–3.3) for acute, subacute, late, and very late ST, respectively (Table III in the online-only Data Supplement).
The number of lesions showing any frames with core-laboratory–adjudicated neoatherosclerosis or interstrut cavity significantly increased over time. In patients with acute, subacute, or late ST, neoatherosclerosis was not observed. Of lesions in patients presenting with very late ST, 43.6% had at least 1 frame with neoatherosclerosis. In these patients, the mean number of frames with neoatherosclerosis was 3.8.
The predicted average probability (95% confidence interval) for any frame to have struts covered with neoatherosclerosis or interstrut cavities was 0.0% (0.0–0.0), 0.0% (0.0–0.0), 0.0% (0.0–0.0), and 2.8% (1.3–5.9) and 0.0% (0.0–0.0), 0.1% (0.0–0.3), 1.2% (0.3–4.5), and 0.7% (0.3–1.4) for acute, subacute, late, and very late ST, respectively (Table III in the online-only Data Supplement).
Imaging Adjudication Committee Analysis of Findings
Representative images of dominant findings for acute and subacute ST and late and very late ST are shown in Figure 2 and Figure 3, respectively. The results of the imaging adjudication committee analysis for dominant findings for acute and subacute ST and late and very late ST are shown in Figure 4; the results for contributory findings are shown in Table IV in the online-only Data Supplement. The most commonly adjudicated dominant finding according to presentation was: acute, persistence of uncovered struts (66.7%); subacute, persistence of uncovered struts (61.7%) and underexpansion (25.5%); late, uncovered struts (33.3%) and severe restenosis (19.1%); and very late, neoatherosclerosis (31.3%) and uncovered struts (20.2%).
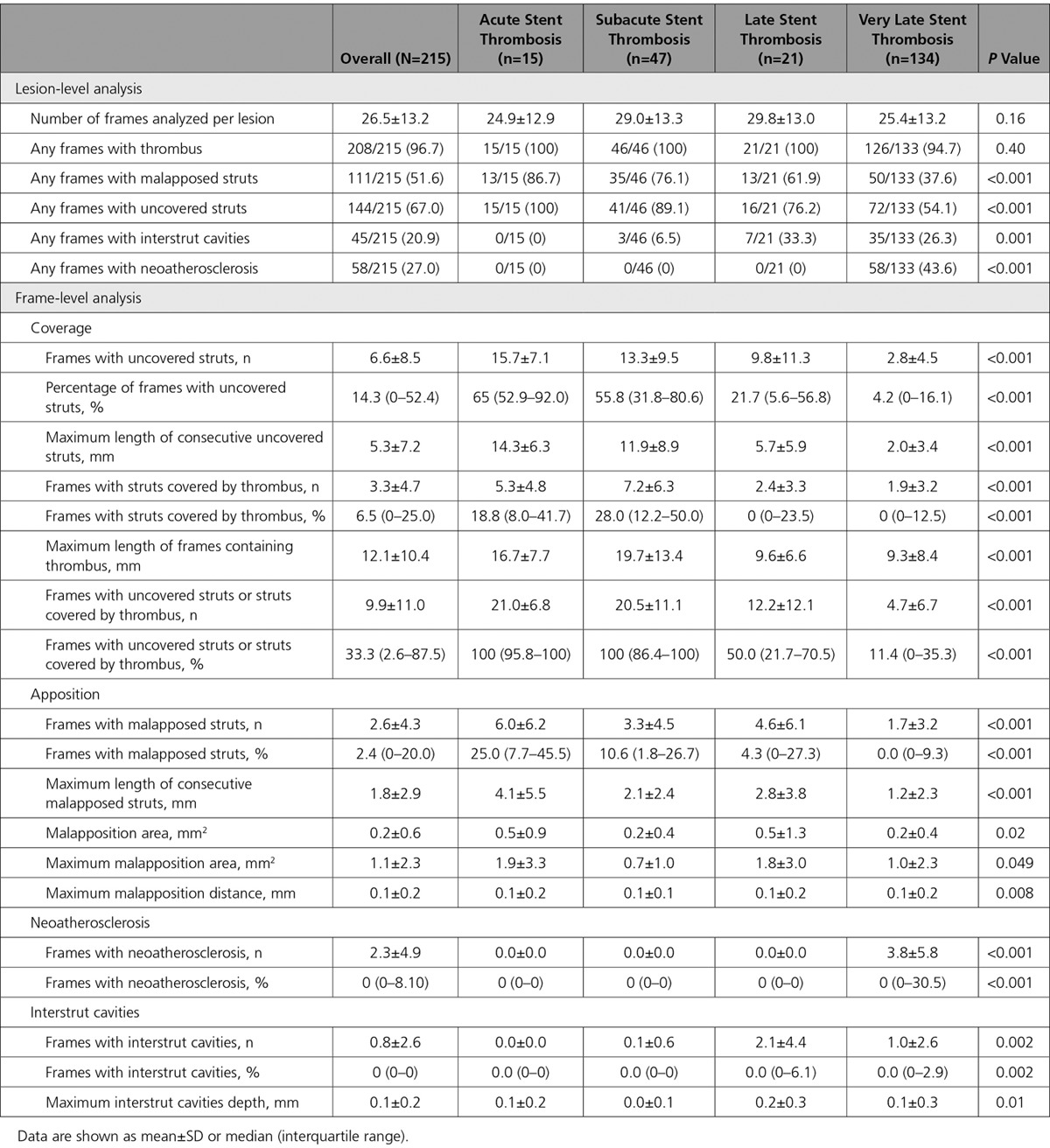
Figure 2.
Representative images of optical coherence tomography findings in patients presenting with acute/subacute stent thrombosis. A, Edge dissection, with a dissection flap separating the true lumen (TL) from the false lumen (FL). B, Acute stent thrombosis with thrombus accumulation on uncovered stent struts. C, Multiple layers of overlapping struts in a segment with marked stent underexpansion and proximal area of thrombus (see also Figure 2E). D, Malapposed struts with thrombus accumulation. E, Corresponding longitudinal view of patient shown in Figure 2C with stent thrombosis in a very long stented segment with overlapping struts and marked stent underexpansion (C indicates the location of the cross section in Figure 2C). *Shadow artifact caused by guidewire.
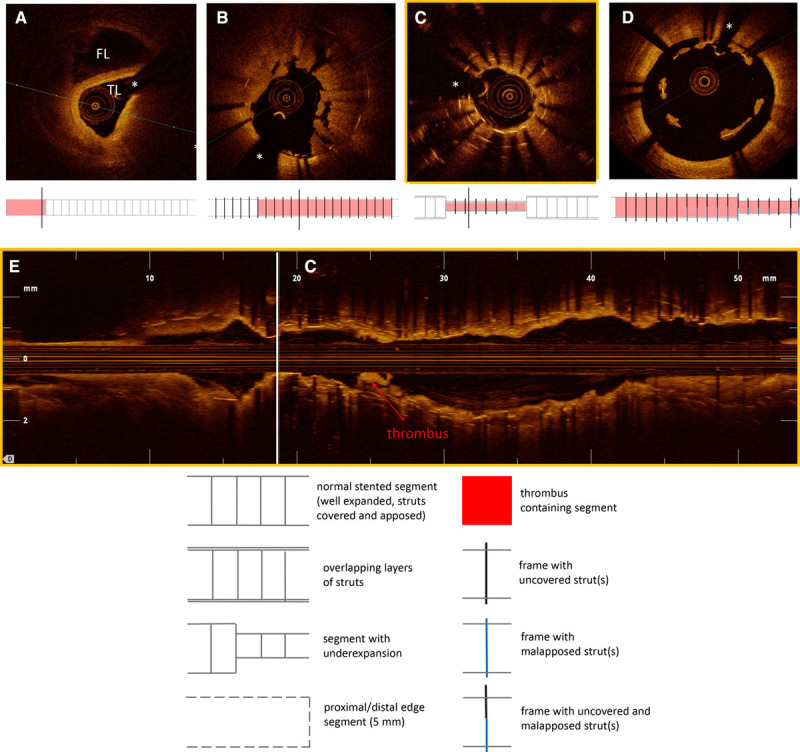
Figure 3.
Representative images of optical coherence tomography findings in patients presenting with late/very late stent thrombosis. A, Uncovered struts, with local accumulations of white thrombus (thr) (see also Figure 3E). B, Interstrut cavities (IC) with small thrombus deposition (thr). C, Severe restenosis with superimposed thrombus (thr). D, Neoatherosclerosis with lipid-rich plaque (L) and plaque rupture (indicated with red arrow). E, Corresponding longitudinal view of the patient with stent thrombosis and uncovered struts shown in Figure 3A. The length of the stented segment is indicated in blue. Thrombus is adherent to uncovered struts along the stented segment, visible as cauliflower-like structures protruding into the lumen (A indicates the location of the cross section in Figure 3A). SB indicates side branch. *Shadow artifact caused by guidewire.
Figure 4.
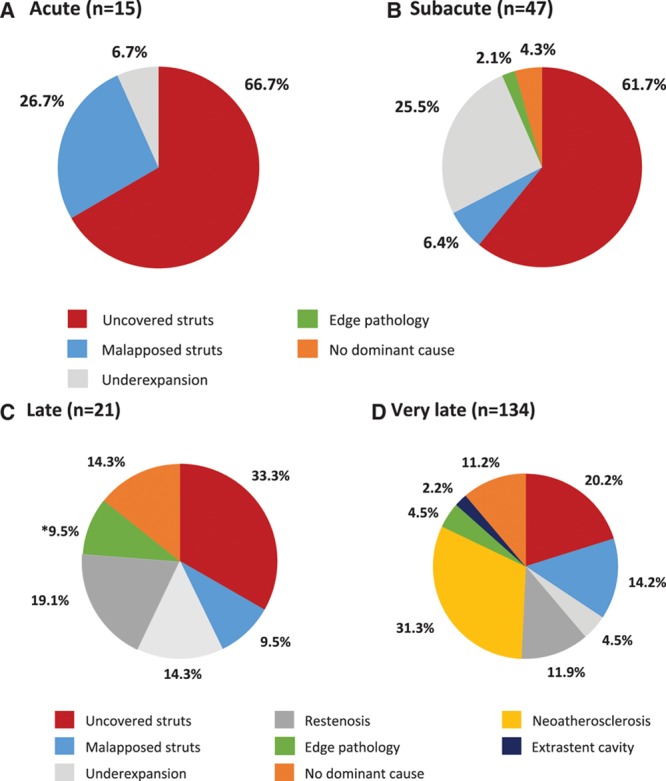
Dominant findings identified by optical coherence tomography imaging according to time interval from index stenting to presentation.A, Acute stent thrombosis (<24 hours). B, Subacute stent thrombosis (24 hours to 30 days). C, Late stent thrombosis (>30 days to 1 year). D, Very late stent thrombosis (>1 year).
In patients presenting with very late ST, an analysis of the imaging adjudication findings according to stent type is shown in Figure 5. A variety of causes was seen at each time interval analyzed. Uncovered stent struts comprised a higher proportion of dominant findings in patients treated with DES. In patients with time interval >5 years from stenting, most had been treated with bare metal stents, and the most frequent dominant finding in these patients was neoatherosclerosis (30/45 [66.7%]). Neoatherosclerosis was a frequent finding in patients presenting with very late ST (>1 year, 59/134 patients). Although a higher proportion of patients with neoatherosclerosis had severe stenosis (23/59 [39.0%]) in comparison with those without neoatherosclerosis (19/75 [25.3%]), this was not statistically significant (relative risk, 1.54; 95% confidence interval, 0.93–2.54; P=0.09).
Figure 5.
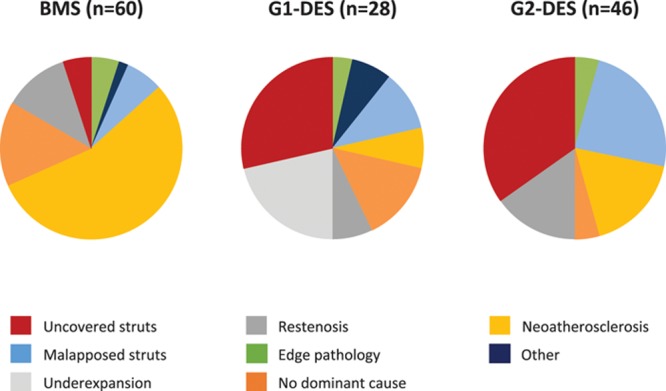
Dominant findings identified by optical coherence tomography imaging in very late stent thrombosis according to type of stent.BMS indicates bare metal stent; G1-DES, first-generation drug-eluting stent; and G2-DES, second-generation drug-eluting stent.
Discussion
Treatment with current generation stents is generally safe and highly efficacious in preventing restenosis. However, abrupt stent failure attributable to thrombotic stent occlusion continues to occur at a low rate and this typically results in myocardial infarction, not inconsiderable mortality, and a higher rate of subsequent adverse events in comparison with patients presenting with de novo myocardial infarction.18 A better understanding of the underlying pathophysiological process leading to ST is an important clinical need. In this respect, the increasing availability of high-resolution intravascular imaging with OCT affords new opportunities for clinical evaluation at the time of presentation.
The aim of the present study was to evaluate and summarize OCT imaging findings in patients presenting with ST at a network of centers across Europe. In a population presenting with both early and late/very late ST containing a high proportion of current generation DES and undergoing imaging in the acute setting using current-generation OCT systems the key findings were: (1) detailed analysis of OCT images acquired in the setting of ST was feasible in the selected patients included in this registry; (2) the rate of stent underexpansion was high across all groups and highest in patients with subacute ST; (3) both uncovered struts and malapposed struts were frequently found in patients with ST; both decreased over time, although more than half of lesions with very late ST had frames with uncovered struts and more than a third had malapposed struts; and (4) neoatherosclerosis was a relatively frequent finding in patients with very late ST.
When categorizing patients according to the timing of ST, some clear messages emerge. In patients presenting with acute/subacute ST, uncovered and malapposed stent struts along with underexpansion of the stented coronary segment were identified as key morphological features of ST by OCT. Although it is not unexpected that uncovered stent struts were frequently observed early after stent implantation, this emphasizes the inherent thrombogenicity of stents in this phase, when neointimal healing and reendothelialization are incomplete. Similarly, although the relevance of stent malapposition in isolation is somewhat unclear,19 the finding of high rates of malapposition in our report is in line with other reports.20,21 Indeed, the relevance of flow disturbance, especially occurrence of nonstreamlined flow along malapposed stent struts has recently been shown to be of relevance with regard to acute thrombogenicity of stents.22 Nevertheless, it may well be that during this time mechanisms other than those detectable by OCT predominate. These include inadequate response to antiplatelet therapy, genetic predisposition to hypercoagulability, and patient comorbidities such as diabetes mellitus, reduced left ventricular function, and renal failure. It is interesting to note that residual dissection proximal or distal to the stent edge was relatively infrequently observed in our study in comparison with some other reports,23 despite systematic analysis of stent edge segments in our analysis protocol.
The high prevalence of stent underexpansion is a noteworthy observation and is in keeping with the known association between acute procedural results and ST.24 Moreover, residual stenosis within the stented segment or small minimal stent area is a well-recognized independent predictor of ST.9,25 A stent expansion index <0.8 was observed in >40% of patients, and approximately two-thirds of patients with subacute ST had stent expansion indices <0.8. Indeed, the observed rate in our study is in line with that observed in a recent case-control study, which found a >2-fold higher rate of stent underexpansion in patients with ST in comparison with patients undergoing routine surveillance without clinical events (ST group 42.8% versus control group 16.7%, P=0.05).23 Moreover, stent underexpansion was adjudicated as the dominant cause of ST in 25% of these patients. It is noteworthy that the rate of intravascular imaging guidance at the time of the original intervention was low (4.9%). These findings suggest that improved recognition and correction of suboptimal stent deployment (eg, stent underexpansion, marked malapposition), perhaps with more liberal use of intravascular imaging-guided stenting, is likely to impact significantly rates of ST.
In patients presenting with late/very late ST, a more heterogeneous profile was observed, with uncovered/malapposed stent struts, underexpansion, and severe restenosis predominant features within the first year and in-stent neoatherosclerosis beyond 1 year. Indeed, although both uncovered and malapposed struts decreased over time, more than half of lesions with very late ST had frames with uncovered struts and more than one-third had malapposed struts. This is in keeping with a prior report by Guagliumi et al26 showing higher rates of both uncovered and malapposed struts in patients with ST in comparison with control patients with prior stent implantation. In that study at a median of 615 days after implant, patients with ST had a higher percentage of uncovered (median [interquartile range]) (12.27 [5.50–23.33] versus 4.14 [3.00–6.22], P<0.001) and malapposed (4.60 [1.85–7.19] versus 1.81 [0.00–2.99], P<0.001) struts. This is also concordant with pathological observations of systematic delayed arterial healing after DES implantation.27 In addition, severe restenosis in the treated segment was relatively frequently observed. This underlines the association between ST and in-stent restenosis as part of the spectrum of stent failure.7,28 The link can be explained by deceleration of flow within the restenotic stented segment, which causes a shift toward a procoagulant state.29 In a minority of late ST cases, additional factors such as residual edge dissection and progression of atherosclerosis with plaque rupture within the proximal or distal edge segment were considered to be the dominant pathology.
In our study, we found that in-stent neoatherosclerosis, ie, development of de novo atherosclerosis inside the implanted stent, was an important association with ST beyond 1-year cases. Although the diagnosis of neoatherosclerosis can be challenging in the presence of residual overlying thrombus, and its definition remains a matter of some debate, these observations are consistent with findings in late stent failure from autopsy studies, which have documented the presence of neoatherosclerosis in ≈30% of selected autopsy cases and premature and accelerated formation in DES in comparison with uncoated stents.30 One explanation for the more accelerated course in DES is the greater delay in endothelial healing and lack of endothelial integrity within the stented segments of DES in comparison with bare metal stents. It is interesting to note that, in the current study, neoatherosclerosis was frequent in bare metal stents, which is likely explained by the longer duration of follow-up in these patients. Indeed, the majority of patients with a time interval >5 years from stenting had been treated with bare metal stents, and neoatherosclerosis was observed as the dominant finding in the overwhelming majority of cases. In line with this, previous registries showed that the duration of follow-up is the most important risk factor for the occurrence of neoatherosclerosis.30 Effective measures to overcome neoatherosclerosis represent an important unmet need for future clinical investigation. The link between the development of neoatherosclerosis and the progression of native atherosclerotic disease suggests an important role for therapies targeted at secondary prevention of atherosclerosis.31 These observations also highlight the importance of further investigation of alternative approaches to conventional metallic stents in patients who are likely to survive long-term after stent implantation.
The results of our study should be interpreted against the background of recent reports from other investigators. Souteyrand and colleagues20 reported on imaging findings in ST from a cohort of 120 patients who presented with acute coronary syndrome and had OCT performed predominantly in a second sitting according to a deferred intervention model. Similar to our report, they also found that malapposition (34%), stent underexpansion (11%), and neoatherosclerosis (23%) were well-represented underlying mechanisms. In addition, uncovered struts and neoatherosclerosis were frequently observed in patients presenting very late ST. In contrast to our report, malapposition was adjudicated to be a key finding in both early and late ST. Differences in the definition of malapposition as a dominant observation for ST may explain some of the discrepancy with our data set.
Taniwaki and colleagues21 also reported on OCT findings in 64 patients with ST, focusing only on those with late or very late thrombosis. In line with our findings, they observed neoatherosclerosis as a major underlying risk factor in a significant proportion of patients (27.6% of cases). The most common finding however, was malapposition, which was the main risk factor in 34.5% of cases beyond 1 year. Uncovered stent struts (12.1%) and stent underexpansion (6.9%) were also identified as additional major causes, which is in agreement with the findings of our study.
Our report has a number of important strengths. First, the analysis represents the largest number of patients presenting with ST and undergoing OCT imaging in the literature to date. In addition, in contradistinction to earlier reports, a high proportion of the thrombosed stents were current-generation DES (≈50%), which are the dominant devices in clinical use at present, and imaging was performed using exclusively contemporary frequency domain OCT. Second, imaging was performed in the acute setting rather than at a deferred time point. Third, data from all participating centers were collected in a prospective manner and recorded in a centralized electronic database. Fourth, analysis of all pullbacks was done in a central core laboratory according to a predefined protocol. In addition, all images were also reviewed by an imaging adjudication panel in dedicated sessions in the core laboratory, with the aim of defining the presence or absence of predetermined characteristics and adjudicating on the dominant cause of the ST based on imaging findings.
Several limitations should be considered when interpreting the results of our report. First, the impact of patient selection must be considered. Only patients presenting to OCT-capable centers were included. Moreover, in patients presenting at OCT-capable centers that did not undergo OCT imaging, the reasons for this were not available for analysis. However, the exclusion of patients presenting with hemodynamic and electric instability was recommended; patients with vessel characteristics unfavorable for image acquisition are also likely unrepresented. Second, the presence of residual thrombus burden in >95% of cases impacts the assessment of the underlying stent and vessel wall findings. In particular, the proportion and extent of stent strut coverage and malapposition may be underestimated. Moreover, offline grayscale signal intensity analysis, a promising approach to characterize neointimal tissue within stents,32 cannot be reliably undertaken. Third, we did not include a control group of patients with prior stent implantation but without ST. This limits our ability to determine the association of observed factors with the clinical presentation of ST, although our findings are concordant with prior case-control studies.26 Fourth, manual thrombectomy was often required to reestablish target vessel patency before imaging. This means that stent/vessel interactions may be altered before image acquisition. Fifth, OCT was not available at the time of index stent implantation. Accordingly, in patients with malapposition at the time of ST, insight into whether this was present at implantation or acquired during follow-up is not available. Finally, strut fracture was infrequently observed. This may be because of the challenges of OCT imaging when residual thrombus is present, and its impact on 3-dimensional reconstruction analysis, as well.
Conclusions
In patients presenting with ST, a variety of factors are observed on OCT that may contribute to the pathophysiology of this condition. Uncovered and malapposed struts were frequently observed and the prevalence of both decreased with increasing time interval from the index stenting. Moreover, core laboratory–assessed stent underexpansion was present in >40% of cases. The dominant observation, however, varied according to presentation: uncovered struts and underexpansion were most common in acute/subacute ST, and uncovered struts and neoatherosclerosis were most common in late/very late ST. Improved acute deployment techniques with poststent dilatation where appropriate may impact significantly ST by reducing underexpansion and malapposition. In addition, improved management strategies for the prevention and treatment of neoatherosclerosis should be investigated in dedicated studies.
Sources of Funding
The research leading to these results has received funding from the European Union Seventh Framework Program FP7/2007 to 2013 under grant agreement HEALTH-F2-2010 to 260309 (PRESTIGE). Funding for open access publication is provided by the European Commission.
Disclosures
Dr Adriaenssens is a consultant for St Jude Medical. Dr Joner is a consultant for Biotronik, Orbus Neich, AUM Medical and reports speakers fees from Biotronik, Orbus Neich, Boston Scientific, Abbott Vascular, and Medtronic, and has received research grants from St Jude Medical. Dr Feldman has received research grants from Sanofi and Bristol-Myers Squibb, and is a consultant for St Jude Medical. Dr Goodall reports lecture fees/advisory board fees/travel bursary from Abbott Vascular, AstraZeneca, Boston Scientific, Medicines Company, and Medtronic. Dr Guagliumi is a consultant for Boston Scientific and St Jude Medical and reports research grants from Abbott Vascular, Boston Scientific, and St Jude Medical. Dr Kastrati reports submission of patent applications in relation to drug-eluting stent technology. Dr Byrne reports lectures fees from B. Braun Melsungen, Biotronik, and Boston Scientific and research grants from Boston Scientific and Heartflow.
Supplementary Material
Footnotes
Drs Adriaenssens and Joner contributed equally.
Drs Guagliumi and Byrne contributed equally (see page 1018).
Sources of Funding, see page 1019
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.026788/-/DC1.
Circulation is available at http://circ.ahajournals.org.
References
- 1.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A Authors/Task Force Members. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 2.Windecker S, Stortecky S, Stefanini GG, da Costa BR, daCosta BR, Rutjes AW, Di Nisio M, Silletta MG, Siletta MG, Maione A, Alfonso F, Clemmensen PM, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head S, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter D, Schauerte P, Sousa Uva M, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Kolh P, Jüni P, Juni P. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ. 2014;348:g3859. doi: 10.1136/bmj.g3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz S, Schuster T, Mehilli J, Byrne RA, Ellert J, Massberg S, Goedel J, Bruskina O, Ulm K, Schömig A, Kastrati A. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J. 2009;30:2714–2721. doi: 10.1093/eurheartj/ehp275. doi: 10.1093/eurheartj/ehp275. [DOI] [PubMed] [Google Scholar]
- 4.Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T RESTART Investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation. 2010;122:52–61. doi: 10.1161/CIRCULATIONAHA.109.903955. doi: 10.1161/CIRCULATIONAHA.109.903955. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong EJ, Feldman DN, Wang TY, Kaltenbach LA, Yeo KK, Wong SC, Spertus J, Shaw RE, Minutello RM, Moussa I, Ho KK, Rogers JH, Shunk KA. Clinical presentation, management, and outcomes of angiographically documented early, late, and very late stent thrombosis. JACC Cardiovasc Interv. 2012;5:131–140. doi: 10.1016/j.jcin.2011.10.013. doi: 10.1016/j.jcin.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, Joner M, Oktay S, Jüni P, Kastrati A, Sianos G, Stefanini GG, Wijns W, Windecker S. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J. 2015;36:2608–2620. doi: 10.1093/eurheartj/ehv203. doi: 10.1093/eurheartj/ehv203. [DOI] [PubMed] [Google Scholar]
- 7.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015;36:3320–3331. doi: 10.1093/eurheartj/ehv511. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Räber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, Daemen J, Meier B, Jüni P, Serruys PW, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–1121. doi: 10.1161/CIRCULATIONAHA.111.058560. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 9.Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013;6:1267–1274. doi: 10.1016/j.jcin.2013.06.015. doi: 10.1016/j.jcin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, Nilsen DW, Kløw NE, Uchto M, Trovik T, Bendz B, Stavnes S, Bjørnerheim R, Larsen AI, Slette M, Steigen T, Jakobsen OJ, Bleie Ø, Fossum E, Hanssen TA, Dahl-Eriksen Ø, Njølstad I, Rasmussen K, Wilsgaard T, Nordrehaug JE NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–1252. doi: 10.1056/NEJMoa1607991. doi: 10.1056/NEJMoa1607991. [DOI] [PubMed] [Google Scholar]
- 11.Alfonso F, Dutary J, Paulo M, Gonzalo N, Pérez-Vizcayno MJ, Jiménez-Quevedo P, Escaned J, Bañuelos C, Hernández R, Macaya C. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart. 2012;98:1213–1220. doi: 10.1136/heartjnl-2012-302183. doi: 10.1136/heartjnl-2012-302183. [DOI] [PubMed] [Google Scholar]
- 12.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW Expert’s OCT Review Document. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 13.Adriaenssens T, Byrne R. PREvention of late Stent Thrombosis by an Interdisciplinary Global European effort: PRESTIGE. Eur Heart J. 2014;35:2128–2129. [PubMed] [Google Scholar]
- 14.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 15.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Dudeck D, Falk E, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Räber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Troels T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT) Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 17.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 18.Belle L, Mahmoudi M, Delhaye C, Ben-Dor I, Maluenda G, Gaglia MA, Jr, Torguson R, Satler LF, Pichard AD, Waksman R. Do patients with drug-eluting stent thrombosis have a similar prognosis to patients presenting with ST-elevation myocardial infarction of de novo lesions? J Interv Cardiol. 2011;24:320–325. doi: 10.1111/j.1540-8183.2011.00643.x. doi: 10.1111/j.1540-8183.2011.00643.x. [DOI] [PubMed] [Google Scholar]
- 19.Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, Versaci F, Marco V, Di Vito L, Imola F, Paoletti G, Trani C, Tamburino C, Tavazzi L, Mintz GS. Clinical impact of OCT findings during PCI: The CLI-OPCI II Study. JACC Cardiovasc Imaging. 2015;8:1297–1305. doi: 10.1016/j.jcmg.2015.08.013. doi: 10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Souteyrand G, Amabile N, Mangin L, Chabin X, Meneveau N, Cayla G, Vanzetto G, Barnay P, Trouillet C, Rioufol G, Rangé G, Teiger E, Delaunay R, Dubreuil O, Lhermusier T, Mulliez A, Levesque S, Belle L, Caussin C, Motreff P PESTO Investigators. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37:1208–1216. doi: 10.1093/eurheartj/ehv711. doi: 10.1093/eurheartj/ehv711. [DOI] [PubMed] [Google Scholar]
- 21.Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, Jørgensen E, Kelbæk H, Pilgrim T, Caussin C, Zanchin T, Veugeois A, Abildgaard U, Jüni P, Cook S, Koskinas KC, Windecker S, Räber L. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–660. doi: 10.1161/CIRCULATIONAHA.115.019071. doi: 10.1161/CIRCULATIONAHA.115.019071. [DOI] [PubMed] [Google Scholar]
- 22.Koppara T, Cheng Q, Yahagi K, Mori H, Sanchez OD, Feygin J, Wittchow E, Kolodgie FD, Virmani R, Joner M. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ Cardiovasc Interv. 2015;8:e002427. doi: 10.1161/CIRCINTERVENTIONS.115.002427. doi: 10.1161/CIRCINTERVENTIONS.115.002427. [DOI] [PubMed] [Google Scholar]
- 23.Prati F, Kodama T, Romagnoli E, Gatto L, Di Vito L, Ramazzotti V, Chisari A, Marco V, Cremonesi A, Parodi G, Albertucci M, Alfonso F. Suboptimal stent deployment is associated with subacute stent thrombosis: optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: the CLI-THRO study. Am Heart J. 2015;169:249–256. doi: 10.1016/j.ahj.2014.11.012. doi: 10.1016/j.ahj.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 24.van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399–1409. doi: 10.1016/j.jacc.2008.12.055. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, Kim SJ, Vergallo R, Minami Y, Ong DS, Gao L, Lee H, Zhang S, Yu B, Saito Y, Jang IK. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation. 2015;132:1020–1029. doi: 10.1161/CIRCULATIONAHA.114.014704. doi: 10.1161/CIRCULATIONAHA.114.014704. [DOI] [PubMed] [Google Scholar]
- 26.Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, Ladich E, Lortkipanidze N, Matiashvili A, Valsecchi O, Virmani R, Stone GW. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012;5:12–20. doi: 10.1016/j.jcin.2011.09.018. doi: 10.1016/j.jcin.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 28.Alfonso F, Fernandez-Viña F, Medina M, Hernandez R. Neoatherosclerosis: the missing link between very late stent thrombosis and very late in-stent restenosis. J Am Coll Cardiol. 2013;61:e155. doi: 10.1016/j.jacc.2012.09.071. doi: 10.1016/j.jacc.2012.09.071. [DOI] [PubMed] [Google Scholar]
- 29.Koskinas KC, Chatzizisis YS, Antoniadis AP, Giannoglou GD. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. J Am Coll Cardiol. 2012;59:1337–1349. doi: 10.1016/j.jacc.2011.10.903. doi: 10.1016/j.jacc.2011.10.903. [DOI] [PubMed] [Google Scholar]
- 30.Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36:2147–2159. doi: 10.1093/eurheartj/ehv205. doi: 10.1093/eurheartj/ehv205. [DOI] [PubMed] [Google Scholar]
- 31.Taniwaki M, Windecker S, Zaugg S, Stefanini GG, Baumgartner S, Zanchin T, Wenaweser P, Meier B, Jüni P, Räber L. The association between in-stent neoatherosclerosis and native coronary artery disease progression: a long-term angiographic and optical coherence tomography cohort study. Eur Heart J. 2015;36:2167–2176. doi: 10.1093/eurheartj/ehv227. doi: 10.1093/eurheartj/ehv227. [DOI] [PubMed] [Google Scholar]
- 32.Malle C, Tada T, Steigerwald K, Ughi GJ, Schuster T, Nakano M, Massberg S, Jehle J, Guagliumi G, Kastrati A, Virmani R, Byrne RA, Joner M. Tissue characterization after drug-eluting stent implantation using optical coherence tomography. Arterioscler Thromb Vasc Biol. 2013;33:1376–1383. doi: 10.1161/ATVBAHA.113.301227. doi: 10.1161/ATVBAHA.113.301227. [DOI] [PubMed] [Google Scholar]


