Supplemental Digital Content is available in the text.
Keywords: basal forebrain, cholinergic anti-inflammatory pathway, inflammatory cytokines, optogenetics, sepsis
Abstract
Objectives:
Basal forebrain cholinergic neurons are proposed as a major neuromodulatory system in inflammatory modulation. However, the function of basal forebrain cholinergic neurons in sepsis is unknown, and the neural pathways underlying cholinergic anti-inflammation remain unexplored.
Design:
Animal research.
Setting:
University research laboratory.
Subjects:
Male wild-type C57BL/6 mice and ChAT-ChR2-EYFP (ChAT) transgenic mice.
Interventions:
The cholinergic neuronal activity of the basal forebrain was manipulated optogenetically. Cecal ligation and puncture was produced to induce sepsis. Left cervical vagotomy and 6-hydroxydopamine injection to the spleen were used.
Measurements and Main Results:
Photostimulation of basal forebrain cholinergic neurons induced a significant decrease in the levels of tumor necrosis factor-α and interleukin-6 in the serum and spleen. When cecal ligation and puncture was combined with left cervical vagotomy in photostimulated ChAT mice, these reductions in tumor necrosis factor-α and interleukin-6 were partly reversed. Furthermore, photostimulating basal forebrain cholinergic neurons induced a large increase in c-Fos expression in the basal forebrain, the dorsal motor nucleus of the vagus, and the ventral part of the solitary nucleus. Among them, 35.2% were tyrosine hydroxylase positive neurons. Furthermore, chemical denervation showed that dopaminergic neurotransmission to the spleen is indispensable for the anti-inflammation.
Conclusions:
These results are the first to demonstrate that selectively activating basal forebrain cholinergic neurons is sufficient to attenuate systemic inflammation in sepsis. Specifically, photostimulation of basal forebrain cholinergic neurons activated dopaminergic neurons in dorsal motor nucleus of the vagus/ventral part of the solitary nucleus, and this dopaminergic efferent signal was further transmitted by the vagus nerve to the spleen. This cholinergic-to-dopaminergic neural circuitry, connecting central cholinergic neurons to the peripheral organ, might have mediated the anti-inflammatory effect in sepsis.
Sepsis is a life-threatening organ dysfunction that is caused by a dysregulated host response to infection (1–3). The innate immune response provides critical protection against lethal infection and injury, but an uncontrolled hyperinflammatory response may cause tissue damage, multiple organ failure, and poor outcomes in severe sepsis (4). Recent studies have demonstrated that neuroimmune interactions function in both the CNS and peripheral inflammation in a variety of clinical conditions (5). Vagus nerve–mediated cholinergic signaling controls immune functions and the inflammatory response, and this has provided a basis for new therapeutic intervention (6). Promptly and precisely regulating inflammation via this cholinergic anti-inflammatory pathway is pivotal to strategies aimed at combatting sepsis (7).
The efferent vagus nerve, through releasing acetylcholine (8), dominates systemic inflammation and inhibits the release of pro-inflammatory cytokines, depending on the α7-nicotinic acetylcholine receptor. This network is therefore called the “cholinergic anti-inflammatory pathway” (9, 10). Excessive immune responses are controlled by this neural pathway and maintain homeostasis (11). Stimulating the efferent vagus nerve significantly decreased serum levels of tumor necrosis factor (TNF)-α in a rodent model of sepsis (10), whereas surgical vagotomy increased susceptibility to infection, suggesting that the vagus nerve plays an indispensable role in modulating peripheral inflammation (12). Cholinergic anti-inflammatory modulation is therefore an essential underlying mechanism in inflammatory diseases (13).
In the CNS, acetylcholine transmission mainly originates in groups of cholinergic neurons within the basal forebrain (BF) that densely innervate cortical and subcortical regions (14, 15). The BF contains a diverse population of neurons, including cortically projecting cholinergic and noncholinergic neurons as well as various interneurons (16). Cholinergic projection neurons in the BF originate the major cholinergic output in the CNS and have been widely studied in the sleep-wake transition and the control of cortical activation, plasticity, and cognition (17, 18). However, it remains unknown whether BF cholinergic (ch-BF) neurons exert an anti-inflammatory effect on sepsis and if so, through what central and peripheral neuronal circuits the cholinergic anti-inflammatory pathway work.
We therefore asked whether activating ch-BF neurons has an anti-inflammatory effect on sepsis using ChAT-Channelrhodopsin-2 (Chr2)-EYFP optogenetic and wild-type (WT) mice. To explore how photoactivating ch-BF neurons affect inflammation resolution, we used c-Fos expression to screen photoactivation-induced neuronal activity in the whole brain. We further clarified the central and peripheral neuronal circuits mediated the anti-inflammation.
MATERIALS AND METHODS
Animals
Adult male 6–8-week-old wild-type C57BL/6 mice and ChAT-ChR2-EYFP transgenic mice (subsequently referred to as “ChAT mice”) were used in our study. All animal experiments were performed according to procedures approved by the Animal Care and Use Committee of Zhejiang University. See the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/C782), which presents the necessary methods used in this study.
Photostimulation of ch-BF Neurons in ChAT Mice
Photostimulation was induced unilaterally on the right BF. An optical fiber was inserted into an implanted cannula 1 day before the experiments. We confirmed that ChR2 expression was selectively induced with high precision by applying blue light in ch-BF neurons of ChAT mice (18, 19). The optical fiber was coupled to a 473 nm laser under driver control. Light pulse trains (30 ms pulses at 20 Hz for 15 s once per minute for 30 min every 1.5 hr) were controlled using digital commands and a Master-8 pulse stimulator (A.M.P.I., Jerusalem, Israel).
Surgical Implantation
A guide cannula (RWD Life Science, Shenzhen, China) was unilaterally surgically implanted in all. The animals were anesthetized using chloral hydrate (400 mg/kg, i.p.) and mounted on a small animal stereotaxic frame (Stoelting Corp., RWD Life Science). A cannula was placed above the right BF (antero-posterior, 0.7 mm; mediolateral, 1.6 mm; dorsoventral, 4.0 mm) according to The Mouse Brain in Stereotaxic Coordinates, Second Edition (20). Four skull screw-holes were drilled, and tightly fitting screws were driven through the skull to the surface of the dura. After surgery, the animals were allowed to recover in individual chambers for at least 7 days.
Polymicrobial Sepsis Model
A cecal ligation and puncture (CLP)–induced sepsis model was generated as previously described (21). See the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/C782), which presents the necessary methods used in this study.
Left Cervical Vagotomy
A ventral cervical midline incision was used to expose the left cervical vagus trunk, which was ligated using 4-0 silk sutures and excised at least 1 cm. The skin was then closed using 3-0 sutures. In sham-operated mice, the left vagus nerve was exposed and isolated from the surrounding tissue but not transected. All animals were vagotomized 3 days before CLP.
Cytokine Measurements
Serum and tissue supernatants were used to analyze the protein levels of TNF-α, interleukin (IL)-6, and IL-10 using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s recommendations. See the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/C782), which presents the necessary methods used in this study.
Brain Slice Preparation
See the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/C782), which presents the necessary methods used in this study.
Immunohistochemistry
See the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/C782), which presents the necessary methods used in this study.
Administration of 6-Hydroxydopamine (6-OHDA)
Chemical splenic denervation was performed by injecting 6-OHDA (60–120 μg; Sigma-Aldrich, St. Louis, MO) or saline into exteriorized spleens according to the method described by Gigliotti et al (22). The mice were then allowed to recover for 1 week before photostimulation.
Statistical Analysis
All statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, IL) and GraphPad Prism 5.00 for Windows (GraphPad Software, La Jolla, CA). All data shown in the figures and text are presented as the mean ± sem. One-way analysis of variance followed by a Bonferroni test were performed to compare mean values between multiple groups. Survival rates were analyzed using the Mantel-Cox test. The threshold for significance was set at a p value of less than 0.05.
RESULTS
Photoactivation of ch-BF Neurons Attenuated Systemic Inflammatory Responses in Septic mice
We induced repetitive bursts of action potentials in ch-BF neurons to analyze their neuromodulatory effects (experiment protocol in Fig. 1A and schematic drawing in Fig. 1B). When ch-BF neurons were photostimulated, serum levels of TNF-α were significantly lower after 3 (p < 0.01) and 12 (p < 0.001) hours in ChAT-lit septic mice than in ChAT-unlit septic mice (Fig. 1C). Serum levels of IL-6 were lower after 3 hours (p < 0.05) and further lowered after 12 hours (p < 0.001) in ChAT-lit mice (Fig. 1D). Our analysis of cytokine concentrations in spleen protein extracts produced similar results. Additionally, we found that TNF-α and IL-6 levels were lower at 3 and 12 hours after ch-BF neurons were photoactivated, whereas IL-10 levels were not significantly different between ChAT-lit and ChAT-unlit mice by CLP (Fig. 2, A–C). There was no significant difference in the concentrations of TNF-α, IL-6, and IL-10 between WT and ChAT septic mice that were not photostimulated. However, there was no significant difference in survival between ChAT-lit septic mice and ChAT-unlit septic mice (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CCM/C782). Collectively, these results indicate that photostimulating ch-BF neurons significantly alleviated systemic inflammatory responses, and especially the release of pro-inflammatory cytokines, following CLP surgery.
Figure 1.
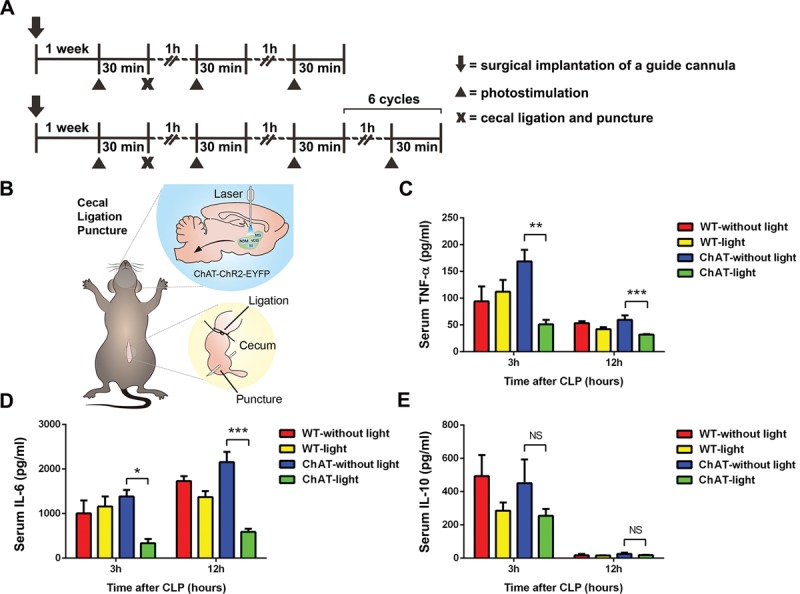
Selectively activating basal forebrain cholinergic neurons attenuated the systemic inflammatory response to cecal ligation and puncture (CLP)–induced sepsis. After photostimulation of basal forebrain cholinergic neurons and CLP surgery, blood was collected at 3 and 12 hr (n = 6–10 per group). A, The model of experiment protocol. B, Schematic drawing shows the detailed methods of photostimulation and CLP. C–D, The pro-inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6 were present at lower levels in photostimulated ChAT septic mice. E, There was no significant difference in the levels of anti-inflammatory cytokines (IL-10) between ChAT-lit septic mice and ChAT-unlit septic mice. These data are presented as the mean ± sem (*p < 0.05, **p < 0.01, ***p < 0.001). NS = no significant difference, WT = wild type.
Figure 2.
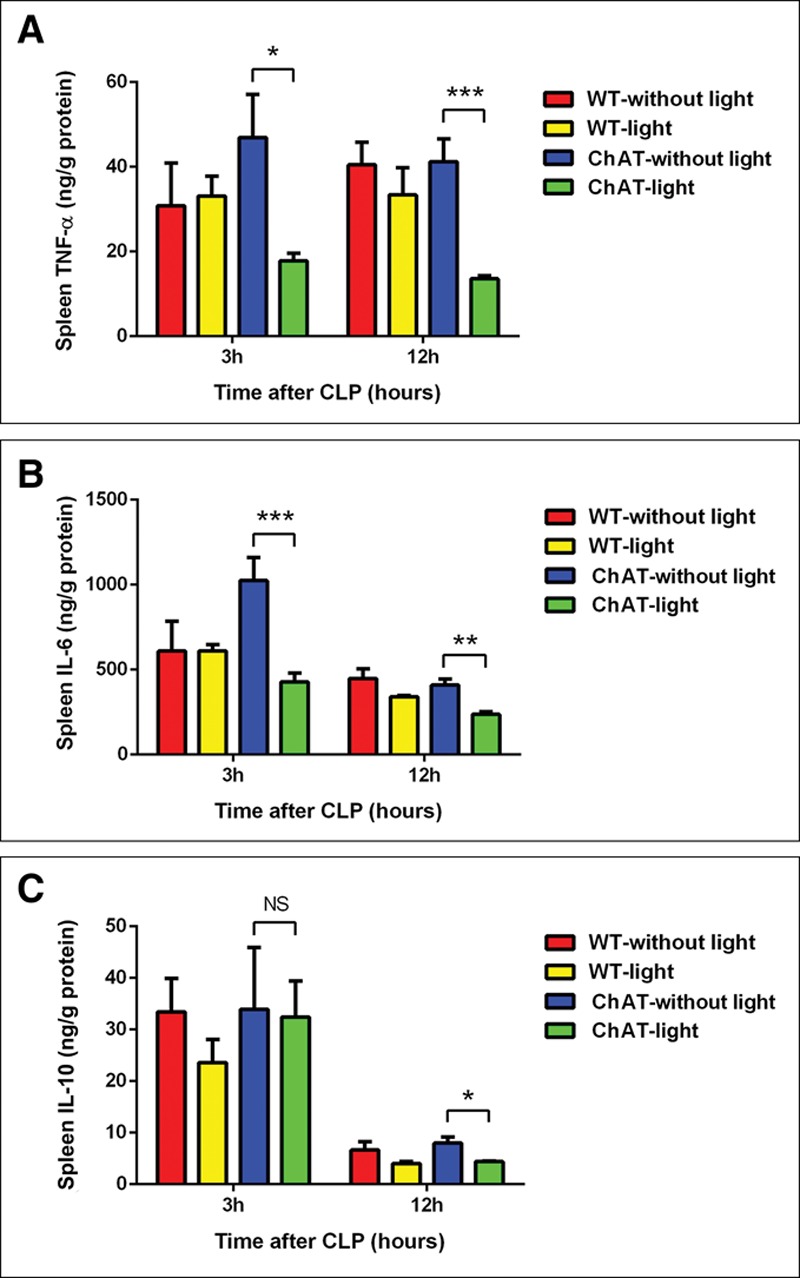
In cecal ligation and puncture (CLP)–induced sepsis, splenic inflammatory cytokines were regulated by the photostimulation of basal forebrain cholinergic neurons. Wild-type (WT) and ChAT mice were treated with photostimulation. Spleens were then collected at 3 and 12 hr after CLP (n = 6–10 per group). A–B, The levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were significantly lower in the spleens of photostimulated ChAT mice after CLP. C, The levels of IL-10 were not different between the spleens of ChAT mice that were treated or not treated with photostimulation after CLP. The data are presented as the mean ± sem (*p < 0.05, **p < 0.01, ***p < 0.001). NS = no significant difference.
The Attenuation of the Systemic Inflammatory Response in Sepsis Induced by Photostimulating ch-BF Neurons Is Nearly Abolished by Left Cervical Vagotomy
Previous studies have shown that animals subjected to unilateral vagotomy are abnormally sensitive to inflammatory challenge. To determine whether the vagus nerve is essential for the immunomodulatory function of ch-BF neurons during sepsis, we performed left cervical vagotomy before CLP surgery in photostimulated WT and ChAT mice. We observed that serum concentrations of TNF-α (p < 0.01) and IL-6 (p < 0.05) were lower in ChAT septic mice that did not undergo left cervical vagotomy, and this effect was nearly abolished after 12 hours in ChAT septic mice that underwent left cervical vagotomy (Fig. 3, A–B). Moreover, in ChAT mice, left cervical vagotomy restored IL-6 levels in spleen after 12 hours (p < 0.05) (Fig. 3, C–D). Taken together, these findings indicate that the vagus nerve is required for ch-BF neuronal photoactivation to modulate the systemic inflammatory response.
Figure 3.
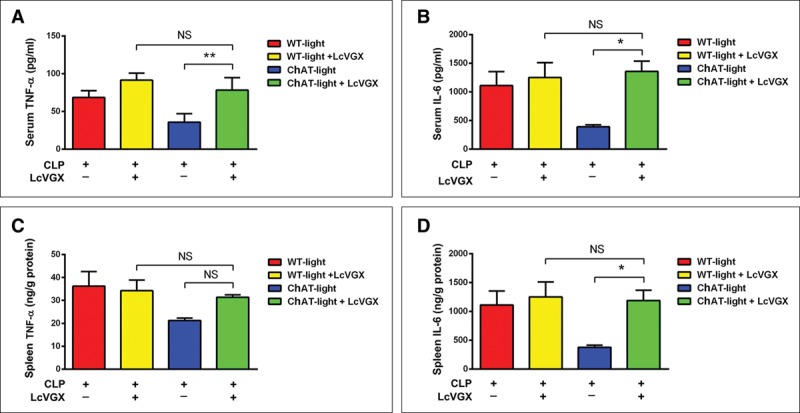
The vagus nerve was essential for basal forebrain cholinergic neurons to regulate the systemic inflammatory response. Wild-type (WT) and ChAT mice underwent left cervical vagotomy (LcVGX) or sham surgery (both groups of mice were implanted with a cannula 7 d prior to surgery). After 3 d, the mice underwent the cecal ligation and puncture (CLP) operation and were treated with photostimulation (n = 6 per group). A–B, Serum levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were lower in the ChAT septic mice that did not undergo LcVGX, and this effect was partly abolished in the ChAT LcVGX mice. C–D, TNF-α and IL-6 levels were restored in the spleens of ChAT LcVGX mice after CLP. These data are presented as the mean ± sem (*p < 0.05, ** p < 0.01). NS = no significant difference.
Stimulating ch-BF Neurons Significantly Induced c-Fos Expression in Both the BF and the Dorsal Motor Nucleus of the Vagus (DMN)/Ventral Part of the Solitary Nucleus (SolV)
To clarify the functional connection between ch-BF neurons and the peripheral immune response, we investigated neuronal activity in the nucleus of the vagus nerve after photoactivation of ChR2-expressing ch-BF neurons. We found that a large number of neurons in the BF region of photoactivated ChAT mice were c-Fos positive, and some of these c-Fos–positive neurons were also ChAT-positive (Fig. 4, A1–D1 and A2–D2), indicating that cholinergic neurons were activated by in vivo photostimulation. Interestingly, photoactivating ch-BF neurons also significantly activated c-Fos–positive neurons in the DMN/SolV (Fig. 4, A3–D3 and A4–D4). However, the number of c-Fos–positive neurons was significantly lower in the BF and DMN/SolV in ChAT mice not treated with photostimulation (Fig. S2, Supplemental Digital Content 1, http://links.lww.com/CCM/C782). Our analysis of the number of c-Fos–positive cells indicated that photostimulating ch-BF neurons in ChAT mice induced significantly more c-Fos expression in both the BF (p < 0.01) and the DMN/SolV (p < 0.001) than was observed in nonphotostimulated ChAT mice (Fig. 4E). These results collectively indicate that the neuronal connections between the BF and the DMN/SolV are involved in modulating the inflammatory response in sepsis.
Figure 4.
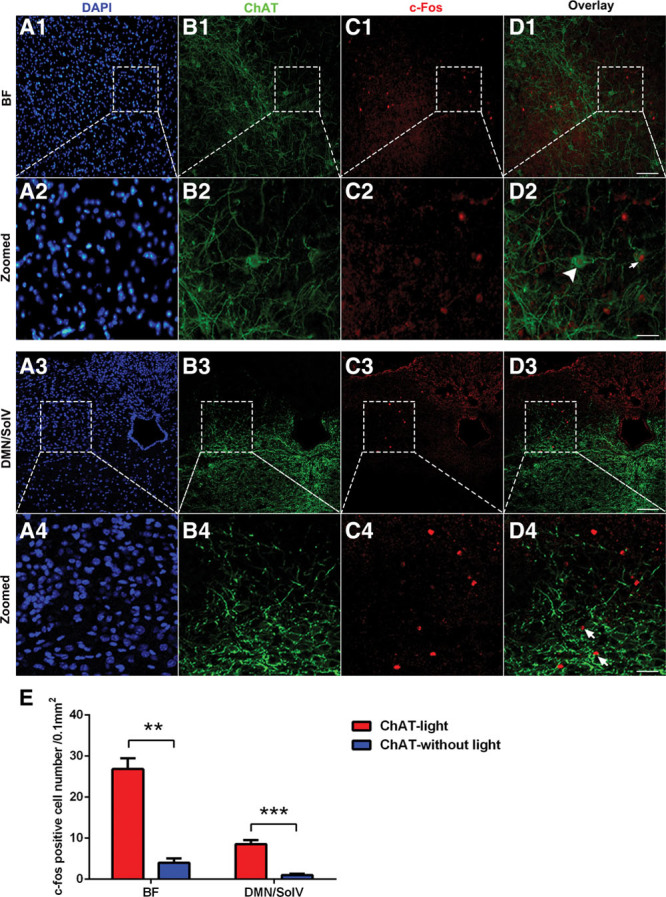
Photostimulating basal forebrain (BF) cholinergic neurons induced c-Fos expression in the BF and the dorsal motor nucleus of the vagus (DMN)/ventral part of solitary nucleus neurons (SolV). A1–D1, Immunofluorescent labeling showing that photostimulating BF cholinergic neurons induced c-Fos expression in a large number of BF neurons. A2–D2, Serial images projection. Some ChAT+ neurons are c-Fos+ (arrows in D2), and some neurons are ChAT– and c-Fos+ (arrowhead in D2). A3–D3, Increased c-Fos expression was observed in the DMN/SolV. A4–D4, Serial images projection. Most neurons were ChAT–/c-Fos+ (arrowhead in D4). E, Quantification of c-Fos–positive cells in ChAT mice that were or were not photostimulated. These data are presented as the mean ± sem (**p < 0.01, ***p < 0.001; scale bar = 50 μm). DAPI = 4′,6-diamidino-2-phenylindole.
Dopaminergic Neurons in the DMN/SolV When ch-BF Neurons Are Photoactivated and Mediated the Cholinergic Anti-Inflammatory Effect of Vagus Nerve
To determine what types of neurons activated by photostimulation of ch-BF neurons, we next examined the expression of tyrosine hydroxylase (TH), ChAT, and c-Fos in the DMN/SolV. We observed very few ChAT+ c-Fos+ cells (Fig. S3, Supplemental Digital Content 1, http://links.lww.com/CCM/C782) but a significant increase in the density of TH+ c-Fos+ cells in the DMN/SolV of the ChAT-lit mice (TH+ c-Fos+ cells vs ChAT+ c-Fos+ cells: 35.2% ± 4.6% vs 6.1% ± 3.9%) (Fig. 5, A1–D1 and A2–D2). Our results reveal that dopaminergic neurons, specifically labeled with TH, composed the main type of neurons in the DMN/SolV, which are activated by photostimulation of ch-BF neurons. Nerve fibers in the spleen that originate in the celiac ganglion use norepinephrine as their primary neurotransmitter. We next sought to determine whether the dopaminergic neurons in the DMN/SolV mediated such an anti-inflammatory effect by locally injecting 6-OHDA to destroy dopaminergic neurons in the spleen. Surprisingly, destroying dopaminergic transmission to the spleen almost completely reversed the reduction in serum (Fig. S4, Supplemental Digital Content 1, http://links.lww.com/CCM/C782) and spleen concentrations of TNF-α and IL-6 that was induced in photoactivated ChAT mice (Fig. 5E). Collectively, our data reveal that dopaminergic neurons in the DMN/SolV indeed mediated the modulation of the systemic inflammatory response of the vagus nerve.
Figure 5.
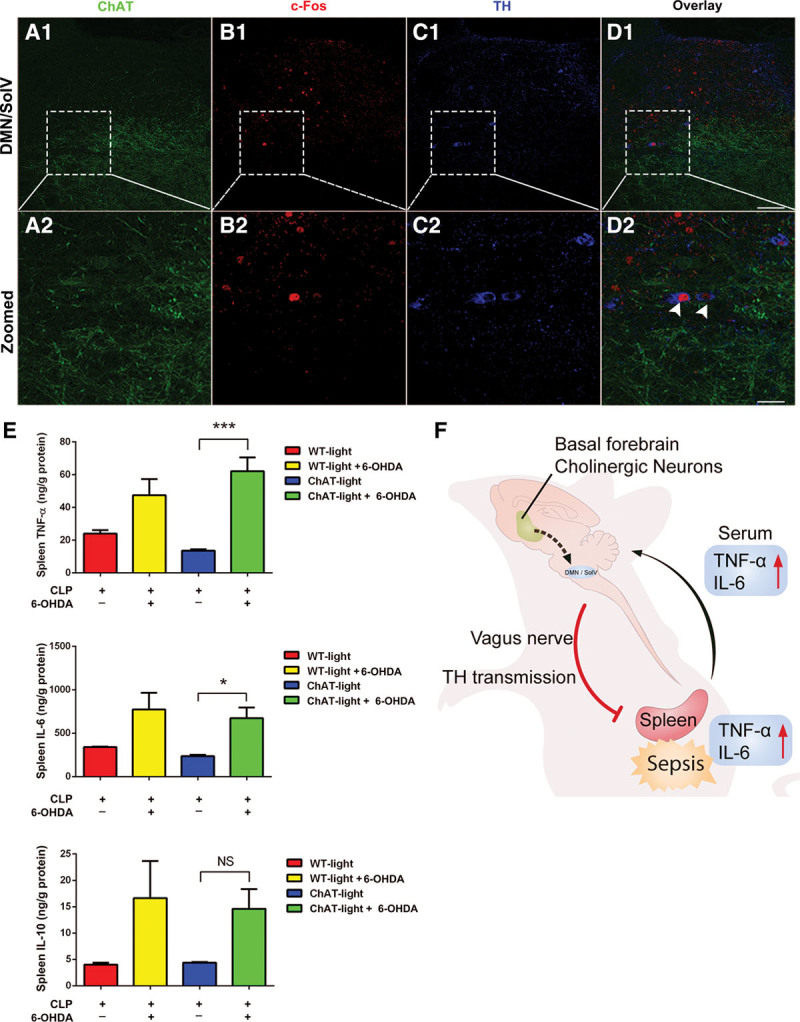
TH positive dopaminergic neurons occupy 35.2% of c-Fos–positive neurons in the dorsal motor nucleus of the vagus (DMN)/ventral part of solitary nucleus (SolV) activated by photostimulation of basal forebrain cholinergic neurons, and dopaminergic neurotransmission from the DMN/SolV to the spleen via vagus nerve is indispensable for the cholinergic activation–induced anti-inflammation. A1–D1, TH positive dopaminergic neurons occupy 35.2% of the c-Fos–positive neurons in the DMN/SolV. A2–D2, Serial image projection. Some c-Fos–positive neurons in the DMN/SolV were TH+ (arrows in D2). Two arrowheads indicated two example neurons expressing TH+ (blue) and c-Fos+ (red). Scale bar equals 50 μm. E, In ChAT mice, photoactivation reduced concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in the spleen, and this effect was nearly completely reversed by injecting 6-hydroxydopamine (6-OHDA). These data are presented as the mean ± sem (*p < 0.05, **p < 0.01, ***p < 0.001). F, Schematic model on the central and peripheral pathway of neuroimmune interaction: from activation of cholinergic neurons in basal forebrain to dopaminergic neurons in DMN/SolV via vagus nerve to the spleen in sepsis. CLP = cecal ligation and puncture, NS = no significant difference, WT = wild type.
DISCUSSION
The pathophysiology of neuromodulation in sepsis is complex; it is a substantial challenge to explore neuroimmune networks in sepsis (7, 23). Improving our understanding of the complicated interactions between the brain and peripheral inflammatory response may lead to the development of important potential therapeutic approaches for treating sepsis. A fundamental aim of this study was to explore the effect of ch-BF neurons on sepsis and identify the neuronal pathways potentially involved in the modulation of polymicrobial sepsis–induced inflammation. Our results strongly suggest that the specific activation of ch-BF neurons is pivotal to attenuating the systemic inflammatory response in septic mice and that this effect is mediated by the vagus nerve. Importantly, BF projections to the DMN/SolV substantially contribute to the role of cholinergic neurons in the cholinergic anti-inflammatory pathway. Here, we provide the first evidence showing that selectively activating ch-BF neurons dramatically attenuated systemic inflammation in sepsis via a mechanism involving the DMN/SolV neuronal pathway (Fig. 5F).
In recent decades, neurotransmitters, neuropeptides, neurohormones, and cytokines have been intensively investigated to increase our understanding of the coordinated interactions between the CNS and immune system (23). In particular, the unresolved question of how the immune system is innervated by the CNS prompted the uncovering neuronal circuits that regulate the innate and adaptive immune responses. It is generally accepted that the CNS dominates the inflammatory response in sepsis via two main mechanisms (24). The first involves the hypothalamic-pituitary adrenal axis, which is stimulated by vagal afferent fibers and induces to the release of glucocorticoids in a humoral manner (25). The second is termed the “inflammatory reflex,” which is a classic neural reflex uses negative feedback to inhibit the excessive release of inflammatory mediators in response to an immune challenge and provides real-time information to the brain regarding the body’s inflammatory status (26, 27). Our study is the first to show that photostimulating ch-BF neurons in ChAT mice decreased TNF-α and IL-6 levels but did not affect IL-10 levels. We further explored the effect of vagotomy on systemic inflammation in photostimulated WT and ChAT mice and found that the vagus nerve performed an essential role in the down-regulation of TNF-α and IL-6 in sepsis by selectively activating ch-BF neurons, which confirmed that ch-BF neurons signal through the efferent vagus nerve.
In the BF, cholinergic neurons comprise only 5% of the total cell population, whereas gamma-aminobutyric acid-ergic neurons account for 35% and glutamatergic neurons account for 55% (16, 29, 30). It was previously reported that the vagus nerve senses peripheral inflammation via afferent nerve fibers, transmits inflammatory signals from the periphery to the brain stem, and modulates immune responses in organs via reciprocal efferent fibers (9, 31). We therefore screened neuronal pathways in brain stem to identify the downstream neural targets of the BF that might mediate the peripheral inflammatory response in sepsis. Using c-Fos, a reliable marker of neuronal activity, we observed that photostimulating ch-BF neurons increased the number of c-Fos–positive neurons in the DMN/SolV, suggesting that ch-BF neurons might participate in regulating neuroimmune interactions via the DMN/SolV neuronal pathway. These results provide new insights into the specificity of cholinergic innervations and the topographical relationship between the BF and DMN/SolV.
So far, the research on the principal neurotransmitter in the vagus nerve has been focused on acetylcholine (32, 33). However, it remains unknown whether cholinergic neurons in the CNS directly modulate peripheral inflammatory responses through other neurotransmitters than acetylcholine in the vagus nerve. Our data showed that among the neurons in the DMN/SolV activated by photostimulation of ch-BF neurons, 35.2% were dopaminergic neurons, suggesting that dopaminergic neurotransmission to the spleen might mediated the cholinergic anti-inflammation. To clarity to what extent does the dopaminergic neurotransmission contribute to such an anti-inflammation, we destroyed dopaminergic neurons in the spleen by locally injection of 6-OHDA. Surprisingly, pharmacologically depletion of dopaminergic neurotransmission to the spleen almost completely reversed the ch-BF activation–induced anti-inflammation, and this effect is very similar to the surgical left vagotomy. These data showed that dopaminergic neurotransmission from DMN/SolV to the spleen via vagus nerve is necessary to the cholinergic activation–mediated anti-inflammation. Our novel finding indentified new therapeutic targets for developing new devices to reduce inflammation in sepsis.
CONCLUSIONS
In the present study, we provide the first evidence showing that selectively activating ch-BF neurons attenuates polymicrobial sepsis–induced inflammation. Such anti-inflammatory effect is mediated by neurotransmission from cholinergic in BF to dopaminergic in DMN/SolV through vagus nerve. These data suggest that developing novel therapeutic devices and drugs that target the central and peripheral pathway of neuroimmune interaction, especially on cholinergic-dopaminergic-vagus nerve, may potentially lead to novel treatments for sepsis and other inflammatory diseases.
ACKNOWLEDGMENT
We thank Prof. Shumin Duan for his generous support for this project, Yefei Li for her excellent graphic design, and Xiang Feng and Xiaoru Ma for their excellent technical assistance during the experiments.
Supplementary Material
Footnotes
This work was performed at The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the National Natural Science Foundation of China (grants 81130036, 81571170, and 31471021), Beijing, China, and the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period (grant 2012BAI11B05), Beijing, China.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NK, et al. ; International Forum of Acute Care Trialists: Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Marshall JC, Namendys-Silva SA, et al. ; ICON investigators: Assessment of the worldwide burden of critical illness: The Intensive Care Over Nations (ICON) audit. Lancet Respir Med 2014; 2:380–386. [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: A roadmap for future research. Lancet Infect Dis 2015; 15:581–614. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg BE, Sundman E, Terrando N, et al. Neural control of inflammation: Implications for perioperative and critical care. Anesthesiology 2016; 124:1174–1189. [DOI] [PubMed] [Google Scholar]
- 6.van Westerloo DJ, Giebelen IA, Florquin S, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis 2005; 191:2138–2148. [DOI] [PubMed] [Google Scholar]
- 7.Leentjens J, Kox M, van der Hoeven JG, et al. Immunotherapy for the adjunctive treatment of sepsis: From immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med 2013; 187:1287–1293. [DOI] [PubMed] [Google Scholar]
- 8.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov 2005; 4:673–684. [DOI] [PubMed] [Google Scholar]
- 9.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405:458–462. [DOI] [PubMed] [Google Scholar]
- 10.Czura CJ, Friedman SG, Tracey KJ. Neural inhibition of inflammation: The cholinergic anti-inflammatory pathway. J Endotoxin Res 2003; 9:409–413. [DOI] [PubMed] [Google Scholar]
- 11.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut 2013; 62:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romanovsky AA. Thermoregulatory manifestations of systemic inflammation: Lessons from vagotomy. Auton Neurosci 2000; 85:39–48. [DOI] [PubMed] [Google Scholar]
- 13.Lorton D, Lubahn CL, Estus C, et al. Bidirectional communication between the brain and the immune system: Implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation 2006; 13:357–374. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann J, Nagy JI, Atmadia S, et al. The nucleus basalis magnocellularis: The origin of a cholinergic projection to the neocortex of the rat. Neuroscience 1980; 5:1161–1174. [DOI] [PubMed] [Google Scholar]
- 15.Rye DB, Wainer BH, Mesulam MM, et al. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 1984; 13:627–643. [DOI] [PubMed] [Google Scholar]
- 16.Anaclet C, Pedersen NP, Ferrari LL, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun 2015; 6:8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci 2012; 32:10105–10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Shi YF, Xi W, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol 2014; 24:693–698. [DOI] [PubMed] [Google Scholar]
- 19.Ren J, Qin C, Hu F, et al. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 2011; 69:445–452. [DOI] [PubMed] [Google Scholar]
- 20.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 2003Second Edition: New York, Elsevier Science, December. [Google Scholar]
- 21.Rittirsch D, Huber-Lang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 2009; 4:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigliotti JC, Huang L, Bajwa A, et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J Am Soc Nephrol 2015; 26:2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riviello ED, Sugira V, Twagirumugabe T. Sepsis research and the poorest of the poor. Lancet Infect Dis 2015; 15:501–503. [DOI] [PubMed] [Google Scholar]
- 24.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun 2007; 21:727–735. [DOI] [PubMed] [Google Scholar]
- 25.Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859. [DOI] [PubMed] [Google Scholar]
- 26.Thayer JF. Vagal tone and the inflammatory reflex. Cleve Clin J Med 2009; 76(Suppl 2):S23–S26. [DOI] [PubMed] [Google Scholar]
- 27.Andersson J. The inflammatory reflex–introduction. J Intern Med 2005; 257:122–125. [DOI] [PubMed] [Google Scholar]
- 28.Oke SL, Tracey KJ. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann N Y Acad Sci 2009; 1172:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gritti I, Henny P, Galloni F, et al. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 2006; 143:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tingley D, Alexander AS, Quinn LK, et al. Cell assemblies of the basal forebrain. J Neurosci 2015; 35:2992–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 2005; 46:173–179. [DOI] [PubMed] [Google Scholar]
- 32.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012; 76:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler W, Traeger T, Westerholt A, et al. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch Surg 2006; 391:83–87. [DOI] [PubMed] [Google Scholar]


