This is a prospective, nonrandomized, noncontrolled, two-center, Phase II clinical trial focused on patients with retinal vein occlusions. Intravitreal injections of conbercept significantly improved visual acuity and anatomical structure. Three injections for the first 3 months followed by pro re nata were enough for the patients with branch retinal vein occlusion, whereas patients with central retinal vein occlusion needed more monthly injections.
Key words: retinal vein occlusion, macular edema, conbercept, vascular endothelial growth factor, best-corrected visual acuity
Abstract
Purpose:
To assess the efficacy and safety of intravitreal conbercept injections in patients with macular edema secondary to retinal vein occlusion (RVO).
Methods:
A prospective, Phase II clinical trial was performed on 60 patients with macular edema secondary to RVO. Thirty patients had branch RVO (BRVO) and 30 had central RVO (CRVO). Each patient received intravitreal injections of conbercept monthly up to 3 months, followed by monthly evaluation and injection pro re nata to Month 9.
Results:
The average change of best-corrected visual acuity from baseline to Month 9 was 17.83 ± 10.89 letters in BRVO and 14.23 ± 11.74 letters in CRVO. The change in best-corrected visual acuity was not statistically different between the groups (P = 0.216). The mean reduction of central retina thickness from baseline to Month 9 was 289.97 ± 165.42 μm and 420.47 ± 235.89 μm in BRVO and CRVO, respectively. The mean numbers of injections was 7.14 ± 1.90 in BRVO and 7.59 ± 1.39 in CRVO from baseline to Month 9 (P = 0.4705). There were 7 serious adverse events (SAEs) in 5 patients (8.33%, 2 BRVO and 3 CRVO). All the SAEs were nonocular and were not related to the drug or the injection procedure.
Conclusion:
Intravitreal injections of conbercept demonstrated a generally favorable safety and tolerability profile as well as efficacy in the treatment of macular edema due to RVO.
Retinal vein occlusion (RVO) is an obstruction of the retinal venous system by thrombus formation and may involve the central (CRVO) or a branch (BRVO) retinal vein, of which the most common etiological factor is compression by adjacent atherosclerotic retinal arteries.1 Among the retinal vascular diseases, the prevalence of RVO is second only to that of diabetic retinopathy,2 which is 5.7 per 1,000 in Asia.3 The two main complications of RVO are macular edema (ME) and retinal ischemia leading to anterior segment and retinal neovascularization. The former is the most common cause of visual impairment in RVO. Thrombosis of the retinal veins causes an increase in retinal capillary pressure resulting in increased capillary permeability and leakage of fluid and blood into the retina. Increased production of vascular endothelial growth factor (VEGF) occurring early in RVO is a major contributor to the evolution and persistence of ME and hemorrhages.4 In addition, the high levels of VEGF promote the progression of retinal nonperfusion and ischemia, which may in turn increase the levels of VEGF,5 hence exacerbating ME and hemorrhages leading to visual impairment. Therefore, anti-VEGF therapy, including intravitreal ranibizumab (Lucentis; Genentech, Inc and Novartis International AG, Basel, Switzerland), aflibercept (Eylea; Regeneron, Tarrytown, NY and VEGF-Trap Eye;Bayer AG, Leverkusen, Germany), and bevacizumab (Avastin; Genentech, South San Francisco, CA) showed marked visual benefit in numerous studies, and superiority to laser when compared.2,6–10
Conbercept (Lumitin; Chengdu Kang Hong Biotech Co, Ltd, Sichuan, China) is a fusion protein composed of the extracellular domain 2 of VEGF receptor 1 and extracellular domains 3 and 4 of VEGF receptor 2 combined with the Fc portion of the human immunoglobulin G1. In preclinical trials, the binding affinity of conbercept for VEGF is substantially greater than that of bevacizumab,11 ranibizumab,12 or aflibercept.13 It also showed a very high affinity for placental growth factor, which can act as a mitogenic, chemotactic, and vascular permeability factor for endothelial cells. Phase I14 and II15 clinical studies have demonstrated good efficacy on ME resolution and acceptable safety, when administered intravitreally for up to 12 months in patients with neovascular age-related macular degeneration. The present study was to assess the efficacy and safety of conbercept in macular edema secondary to retinal vein occlusion (FALCON).
Methods
The FALCON study was a Phase II, nonrandomized, noncontrolled, 9-month trial assessing the efficacy and safety of intravitreal injection of conbercept (IVC) in macular edema secondary to retinal vein occlusion. The study was conducted at two sites in China (The Affiliated Eye Hospital of Wenzhou Medical University and Beijing Tongren hospital affiliated to Capital Medical University). The study protocol was approved by each institutional review board or ethics committee. The study was carried out in adherence with guidelines established by the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent to participate in this trial. The FALCON study was registered with ClinicalTrials.gov (identifier no. NCT01809236). Data described here were collected between September 2012 and May 2014.
Participants
Patients ≥18 years old with central macular edema secondary to BRVO or CRVO were eligible for enrollment if the occlusion occurred within 6 months, and best-corrected visual acuity (BCVA) was ≤73 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (20/40 Snellen equivalent) without lowest limit, and the central retina thickness (CRT) was ≥320 μm measured by spectral-domain optical coherence tomography (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Branch RVO was defined by the presence of retinal hemorrhages or other biomicroscopic evidence of RVO and a dilated venous system in ≤2 quadrants of the retina drained by the same vein. Central RVO was defined as an RVO that involved four retinal quadrants.14 Only one eye from each patient was included in this study.
The exclusion criteria included a relative afferent pupillary defect, a history of vitreoretinal surgery, anti-VEGF (such as ranibizumab or bevacizumab) injections in the study eye within 6 months or in the fellow eye within 3 months, systemic therapy of anti-VEGF within 6 months, intraocular or periocular steroid treatment in the study eye within the last 3 months or systemic steroids within 1 month, reductions in visual acuity from causes other than RVO, ocular inflammation in both eyes, uncontrolled glaucoma (intraocular pressure >25 mmHg or previous filtration surgery), and scatter or pan retinal laser, macular grid laser, or sector laser in the study eye.
Treatments
Sixty patients were included in the FALCON study. Thirty patients had BRVO and 30 had CRVO. In all 60 patients, intraocular injections of conbercept (0.5 mg/0.05 mL) were administered monthly in the loading phase of 3 months. During Month 3 to Month 8, patients were evaluated monthly and received injection as needed or pro re nata (PRN). Patients received rescue treatment in the following circumstances: 1) increase of ≥50 μm in CRT compared with the lowest previous measurement; 2) loss of ≥5 ETDRS letters compared with the last previous measurement; 3) presence of new or persistent cystic retinal changes, subretinal fluid, or neuroepithelial detachment; 4) presence of new macular hemorrhage, retinal neovascularization, or another new occlusion of a branch retinal vein; 5) and at the investigator's discretion. If ≥1 rescue treatment criterion was met, eyes received an injection. The final study visit was at Month 9 (Figure 1).
Fig. 1.
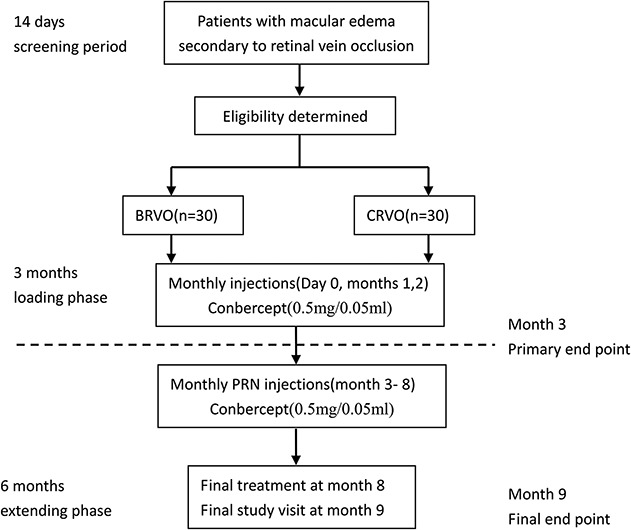
Study flow chart. Sixty patients included 30 patients with BRVO and 30 patients with CRVO. Intravitreal injection of conbercept (0.5 mg/0.05 mL) was administered monthly in the loading phase of 3 months. During Month 3 to 8, patients were evaluated monthly and received injection as needed or PRN. The rescue treatment criteria included 1) increase of ≥50 μm in CRT compared with the lowest previous measurement; 2) loss of ≥5 letters compared with the last previous measurement; 3) presence of new or persistent cystic retinal changes, subretinal fluid, or neuroepithelial detachment; 4) presence of new macular hemorrhage, retinal neovascularization, or another new occlusion of branch retinal vein; 5) the Investigator thought it needed. If ≥1 rescue treatment criterion was met, eyes received an injection. The final study visit was at Month 9.
Outcome Measurements
The primary efficacy outcome measure was a change from baseline in BCVA at Month 3. The secondary efficacy outcome measures were changes in BCVA and CRT monthly from baseline to Month 9, the mean injections in all patients during Month 3 to Month 9, and the change from baseline in macular volume (MV) at Month 3 and Month 9, respectively. Additional outcomes were the proportion of eyes that gained ≥15 ETDRS letters in BCVA from baseline to Month 3 and Month 9, respectively, as well as the comparison of the mean injections and the difference between the mean change in vision between BRVO and CRVO. Safety assessments included ocular and nonocular adverse events (AEs) and serious AEs (SAEs).
The BCVA, CRT, and MV were evaluated every month from baseline to Month 9. BCVA was assessed following the ETDRS protocol.19. The CRT and MV were evaluated with spectral-domain optical coherence tomography. Fundus photography (Topcon TRC.50-DX; Topcon, Japan) and fluorescein angiography (HRA-Ⅱ, Heidelberg, German) were performed at baseline, Months 3, 6 and 9, respectively. The CRT and MV data were measured and evaluated twice in a blinded manner by qualified readers from the two sites, respectively. The average values of the two data above were analyzed.
Statistical Analyses
The data of this study were analyzed by the Health Statistics Teaching and Research Section of the Fourth Military Medical University. All statistical tests were two-sided. A P-value of <0.05 were considered statistically significant. All the above analyses were performed using SAS9.1.3 (SAS Inc, Cary, NC).
Efficacy outcome measures were analyzed in the full analysis set, which comprised all eyes that received at least one injection and had a baseline and ≥1 postbaseline BCVA assessment. The changes of BCVA, CRT, and MV between the follow-up and the baseline were evaluated by paired t-test at a two-sided significance level of 5%. A between-group difference in the primary efficacy outcome measure was evaluated by the Cochran–Mantel–Haenszel test at a two-sided significance level of 5%. The differences in proportions of eyes that gained ≥15 ETDRS letters (post hoc analysis) and CRT reduced to ≤320 μm and numbers of injections between BRVO and CRVO were analyzed with the chi-square test. The differences in proportions of eyes that CRT reduced to ≤250 μm between BRVO and CRVO were analyzed with the Fisher exact test. The influence items on the change of BCVA from baseline to the last visit were assessed using the multivariable linear regression model. Missing data were imputed using the last-observation-carried-forward method. The safety analysis set included all patients who received any study treatment.
Results
Patient Disposition, Demographics, and Baseline Characteristics
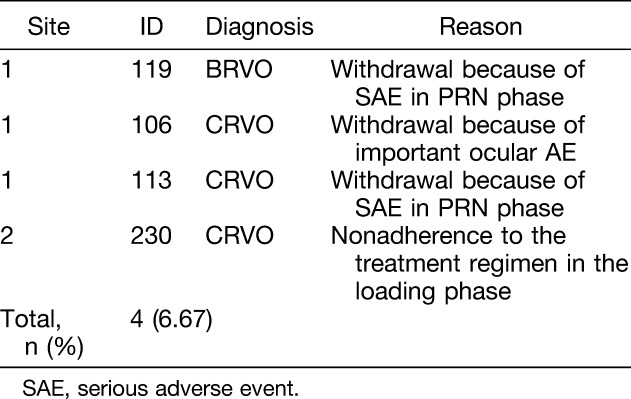
Between September 2012 and May 2014, 68 patients were screened; 8 patients were excluded because they either did not meet the inclusion/exclusion criteria (n = 7) or they withdrew consent (n = 1). Overall, 60 eyes were enrolled in the study. Patients were equally divided between BRVO (n = 30) and CRVO (n = 30), and all were included in the full analysis set (Table 1). Minor differences existed between the BRVO and CRVO patients, as seen in Table 1. Only 4 patients (6.67%) were excluded in the per protocol set. Three patients withdrew because of AEs and one patient due to a protocol deviation (Table 2). The patients' characteristics were nearly identical between the full analysis set and per protocol set. The safety analysis set included all 60 patients.
Table 1.
Patient Demographics and Baseline Characteristics

Table 2.
Patients Excluding the per protocol set
Efficacy
The mean change of BCVA from baseline was significantly improved. At Month 3, BCVA changed from 57.83 ± 13.42 to 72.40 ± 10.49 letters (20/63–20/32 Snellen equivalent) in BRVO, a mean increase of 14.57 ± 8.59 letters (t = 9.2892, P = 0.0000). The BCVA changed from 48.73 ± 15.91 to 60.23 ± 17.00 letters (20/100–20/50 Snellen equivalent) in CRVO, an increase of 11.50 ± 11.40 letters (t = 5.5248, P = 0.0000). The change in BCVA was not statistically different between the groups (P = 0.24).
At Month 9, BCVA changed to 75.67 ± 9.08 letters (20/25 Snellen equivalent) in BRVO, an improvement of 17.83 ± 10.89 letters (t = 8.9734, P = 0.0000). The BCVA changed to 62.97 ± 14.98 letters (20/50 Snellen equivalent) in CRVO, which improved to 14.23 ± 11.74 letters (t = 6.6385, P = 0.0000) (Figure 2A). The change in BCVA did not differ between the groups (P = 0.216).
Fig. 2.
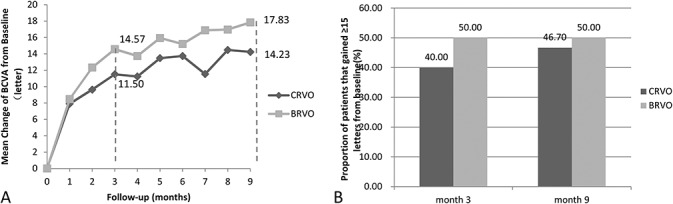
Visual outcomes. A. The mean change of BCVA from baseline. B. The proportion of patients that gained ≥15 letters from baseline to Month 3 and Month 9. Full analysis set.
There were 11 patients with baseline BCVA ≤35 letters (20/200 Snellen equivalent), of which three patients had lower baseline BCVA ≤23 letters (20/400 Snellen equivalent). The BCVA of these 11 patients improved to 18.18 ± 13.70 and 22.64 ± 15.33 letters at Month 3 and 9, respectively.
At Month 9, the proportion of patients that gained ≥15 letters from baseline to Month 3 did not differ between the groups: 50% for BRVO and 40% for CRVO (χ2 = 0.6061, P = 0.4363). The proportion of patients that gained ≥15 letters from baseline to Month 9 is 50% in BRVO and 46.67% in CRVO (Figure 2B). There was also no difference between the groups (χ2= 0.0667, P = 0.7961). The BCVA dropped ≥15 letters from baseline to Month 3 in only one patient with CRVO. At Month 9, none of the 60 patients had lost ≥15 letters.
In various kinds of baseline characteristics, the baseline BCVA had the greatest correlation with the change of BCVA from baseline to the last visits in all 60 patients (P < 0.001) (Table 3).
Table 3.
Correlation About Change of BCVA and Change of CRT From Baseline to Month 9 With Baseline Variables

The mean reduction of CRT from baseline to Month 3 was 295.53 ± 155.95 μm in BRVO (t = 10.3796, P = 0.0000) and was 383.63 ± 270.75 μm in CRVO (t = 7.7608, P = 0.0000). There was no difference between BRVO and CRVO (P = 0.129). At Month 9, the mean reduction was 289.97 ± 165.42 μm and 420.47 ± 235.89 μm in BRVO and CRVO, respectively (t = 9.6014, P = 0.0000; t = 9.7631, P = 0.0000) (Figure 3A). There was significant difference between BRVO and CRVO (P = 0.016). It was worth noting that there was a significant increase at Month 4 and Month 7 in CRVO, which corresponded to the decrease of BCVA. It also correlated with the change to PRN therapy at the previous month such as Month 3 and Month 6. The proportion of PRN injection in all 30 CRVO patients was 70.00%, 83.33%, and 56.67% at Months 3, 5, and 6, respectively.
Fig. 3.

Optical coherence tomography outcomes. A. The mean change of CRT from baseline monthly. B. The proportion of CRT that reduced to ≤250 μm and ≤320 μm from baseline to Month 3 and Month 9, respectively. Full analysis set.
At Month 3, CRT reduced to ≤320 μm in 27 patients (90%) of BRVO and in 18 patients (60%) of CRVO. There was significant difference between BRVO and CRVO (χ2 = 7.20, P = 0.0073). At Month 9, CRT reduced to ≤320 μm in 27 patients (90%) of BRVO and in 20 patients (66.67%) of CRVO with significant difference (χ2 = 4.8118, P = 0.0283) (Figure 3B). That was to say the CRT in three quarter of the patients was thinner than baseline after at least three injections.
At Month 3, CRT reduced to ≤250 μm in 14 patients (46.67%) of BRVO and in 6 patients (20%) of CRVO. There was no significant difference between BRVO and CRVO (P = 0.0539). At Month 9, CRT reduced to ≤250 μm in 14 patients (46.67%) of BRVO and in 8 patients (26.67%) of CRVO. There was also no significant difference between BRVO and CRVO (P = 0.1799) (Figure 3B). That was to say one third of the patients reduced to the normal thickness after at least three injections.
The mean reduction of MV from baseline in BRVO and CRVO was referred to in Figure 4. The trends of the MV changes were similar to that of the CRT changes.
Fig. 4.

The mean change of MV from baseline monthly. Full analysis set.
In the per protocol set, the efficacy was similar to the full analysis set analysis (data not shown). In the per protocol set, the mean number of injections was 7.14 ± 1.90 (median = 8; interquartile range = 6–9) in BRVO and 7.59 ± 1.39 (median = 8; interquartile range = 7–9) in CRVO from baseline to Month 9. There was not a significant difference between the groups regarding the mean number of injections (P = 0.4705). In BRVO, there were three patients who received only three injections (the loading phase only) and did not meet re-treatment criteria in the following 6 months. Most patients needed additional injections (Figure 5).
Fig. 5.
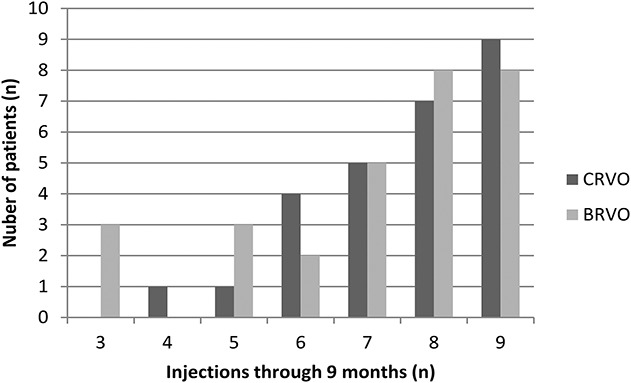
Distribution of the total number of injections of conbercept administered in macular edema secondary to RVO patients through 9 months according to the study criteria. The per protocol set.
Safety
All patients who received ≥1 injection of conbercept were evaluated for safety. From baseline to Month 9, the percentage of patients experiencing at least one ocular AE in the study eye was similar in BRVO and CRVO (90% and 86.67%, respectively). A retinal tear complicated with focal retinal detachment was detected in one patient of CRVO at Month 2 scheduled visit, who had received only one injection before without noted complication. After that, the patient withdrew from the study to accept the laser therapy. The retinal tear complicated with focal retinal detachment was considered possibly related to the injection procedure by the investigator. Retinal neovascularization developed in one patient with BRVO at Month 9; this patient had received only 3 injections in the first 3 months. Almost all the other AEs were common and mild, and were similar to those reported in other papers, such as conjunctival hemorrhage, vitreous opacity, temporary elevated intraocular pressure, and decreased visual sensitivity.2,7,15–18
Form baseline to Month 9, there were 7 SAEs in 5 patients (2 BRVO and 3 CRVO). The percentage was 8.33% in all 60 patients. All the SAEs were nonocular and were not related to the drug or the injection procedure.
Discussion
The Phase II FALCON study met the primary efficacy end point of the change of BCVA at Month 3 with conbercept treatment and all the secondary efficacy end points, including BCVA improvement and CRT decrease. Treatment with fixed monthly IVC over 3 months resulted in rapid and sustained improvements in visual acuity and anatomic end points. These improvements were largely maintained, and even increased, after PRN dosing with monthly evaluations through Month 9 was initiated. For patients with BRVO, at Month 9, a mean increase of 3.2 letters in BCVA and a mean decrease of 5.5 μm in CRT were gained compared with Month 3. For patients with CRVO, a mean increase of 2.7 letters in BCVA and decrease of 36.9 μm in CRT was gained compared with Month 3. Likewise, the percentage of patients gaining ≥15 letters and ≥30 letters at Month 9 was similar or even slightly higher to that at Month 3. Although the treatment regimen was monthly IVC for 3 months followed by IVC PRN (3 + PRN) in the FALCON study, the trend of improvement in visual acuity with conbercept is similar to that obtained with ranibizumab in the Ranibizumab for the Treatment of Macular Edema following BRAVO (BRAnch Retinal Vein Occlusion: Evaluation of Efficacy and Safety) study17 and Ranibizumab for the Treatment of Macular Edema after CRUISE (Central Retinal Vein OcclUsIon Study: Evaluation of Efficacy and Safety) trial18 as well as aflibercept in the VIBRANT study2 and the GALILEO study,19 all of which used a regimen of monthly intravitreal injection over 6 months followed by intravitreal injection as needed (6 + PRN). All the studies suggest that the effect of anti-VEGF agents on macular edema secondary to RVO occurs very soon after the initiation of treatment. In addition, 11 patients with BCVA ≤35 ETDRS letters (20/200 Snellen equivalent) were included in the FALCON study whose BCVA and CRT improved after treatment, demonstrating the benefit of conbercept on patients with vision worse than that typically enrolled in such trials.
Despite the shorter loading phase, visual acuity improvement in patients with BRVO at Month 3 in the FALCON study was comparable with that of patients in the BRAVO17 study and VIBRANT2 study, and the visual acuity benefits at Month 9 in the FALCON study was also similar to that in the BRAVO study. Moreover, in the 9-month whole study period, 27.6% of patients need ≤6 injections. It suggested that, for these patients, intravitreal conbercept injection monthly for 6 months would result in unnecessary treatment, and a shorter loading phase of three injections plus PRN treatment may provide similar outcomes as those seen in Phase 3 trials with other anti-VEGF agents. For patients with CRVO, the visual acuity benefits gained at Months 3, 6, and 9 were very close to that gained in the CRUISE18 study treated in which patients received 0.5 mg ranibizumab, but less than that in the GALILEO19 and COPERNICUS20 studies, both of which used aflibercept (also a VEGF receptor fusion protein like conbercept). A longer loading phase might result in more visual acuity improvement in patients with CRVO.
In the FALCON study, there was a trend for patients with BRVO gaining more visual acuity benefits than those with CRVO at Months 3 and 9 (14.6 vs. 11.5 and 17.8 vs. 14.2, respectively), but these differences were not statistically significant (P = 0.24 and P = 0.216, respectively), suggesting that intravitreal conbercept injection was effective in treating both BRVO and CRVO. In the Month 3 to Month 9 period, the change of BCVA from baseline of patients with CRVO showed a higher fluctuation than that of patients with BRVO. The pathological changes of CRVO, like superficial hemorrhages, cotton wool spots, retinal edema, and capillary nonperfusion, occurring in all four quadrants of the retina,1 is associated with more severe vision loss3 than that seen in patients with BRVO. The mean BCVA at baseline of CRVO was worse than that of BRVO in the current study (48.8 letters vs. 57.8 letters, 20/100 vs. 20/63 Snellen equivalent, P = 0.02), and so the CRT (768.4 μm vs. 563.8 μm, P = 0.0000) and MV (14.8 mm3 vs. 12.7 mm3, P = 0.015). Furthermore, the recurrence and severity of ME in CRVO was higher than that in BRVO. In this study, in parallel with the increases in BCVA, patients with BRVO or CRVO experienced a substantial reduction in CRT and MV, and subsequent decreases in BCVA were accompanied by increases in CRT and MV. After consecutive injections for 3 months, 100% of patients with BRVO and 90% of patients with BRVO gained ≥0 letters; more than half gained ≥15 letters. Accordingly, the proportion of CRT decreasing to 320 μm was 90% in patients with BRVO and 60% in patients with CRVO, and that decreasing to 250 μm was 47% and 27%, respectively. Almost half of the patients did not require an injection at Month 3. The CRT and MV increased immediately at Month 4, with a concomitant decrease in BCVA. After reinjection at Months 4 and 5, the visual and anatomical outcomes improved in patients with CRVO. Re-treatment was not indicated in almost half of the patients at Month 6, with resultant increases in CRT and MV at Month 7 and simultaneous decrease in BCVA. Unlike CRVO, patients with BRVO received conbercept injection at Month 4 to achieve stability and basically maintained the same benefits. The ME was identified as the main reason for the vision loss once again in the FALCON study, and anti-VEGF therapy was effective in ME resolution and concomitant improvement in visual acuity.
It was worth noting that no patient with BRVO experienced a loss of ≥15 letters at any point during the study, and no patient with CRVO had a loss of ≥15 letters at the end of the study. Several studies have investigated the efficacy of other anti-VEGF agents. The BRAVO study reported that 3 patients (2.3%) receiving monthly injections of 0.5 mg ranibizumab (n = 131) experienced a visual acuity loss from baseline BCVA of ≥15 letters at Month 12, whereas 1 patient (0.7%) in the 0.3 mg ranibizumab (n = 134) group experienced the same loss.17 The number of the patients who had a visual acuity loss of ≥15 letters was 3 (2.3%) and 5 (3.8%) in the 0.5 mg group (n = 130) and 0.3 mg group (n = 132) in the CRUISE study,18 1 (1.0%) and 6 (5.3%) in the group receiving aflibercept monthly from baseline to week 24 plus PRN treatment from weeks 24 to 52 (n = 103 and 114 respectively) in the GALILEO study19 and COPERNICUS study,20 respectively. These findings compare favorably with the results of the current study, and support previous studies with respect to anti-VEGF agents ability to maintain vision. The subjects were not excluded by poor vision or excessive retinal thickening as noted by BCVA and CRT in FALCON study. Allowing patients with worse vision and anatomical baseline characteristics more closely resembles the variety of patients who may require treatment in the real world.
No unexpected safety findings were reported. The safety outcomes were consistent with those reported in the Phase I and II studies of conbercept in wet AMD.15,16 The key AEs were either because of the injection procedure or the result of the underlying disease. An increase in the rates of macular edema and reduced visual acuity seen in patients receiving IVC after changing the treatment regimen from fixed dosing to PRN dosing suggests that some patients would have benefited from regular or additional monthly dosing rather than being treated in response to the recurrence of disease.
In conclusion, the FALCON study demonstrated efficacy of IVC in the treatment of macular edema due to RVO and was generally well tolerated. Intravitreal injection of conbercept offers the potential to manage patients with this sight-threatening condition. The results do suggest that while three monthly injections may be appropriate for the initial management of BRVO, a longer loading phase might be necessary for patients with CRVO. A longer loading phase in patients with BRVO may lead to unnecessary IVC injections. The results of the FALCON study are promising and certainly merit further study and progression to Phase III programs.
Appendix 1.
The FALCON study group investigators were Yingzi Li, Ying Huang, Weiwei Zheng, Tingye Zhou, Qianqian Zhu, Jirong Li, PengQu, Xiaoqiu Shen (Retina Department, The Affiliated Eye Hospital of Wenzhou Medical University). Liqin Gao, Yongpeng Zhang, Haixia Ji, Ying Xiong, Wei Yan, Shiqiang Zhao, Wei Zhang, Rong Shen (Department of Ophthalmology, Beijing Tongren hospital affiliated to Capital Medical University). Xiaojing Li, Fenglei Kuang, Zhili Niu, Biwei Zeng, Kun Luo (Chengdu Kanghong Biotechnology, Inc, Chengdu, China).
Footnotes
Supported by Chengdu Kanghong Biotech, Inc, Chengdu, Sichuan, China.
S. Tao and Q. Wu are employees of Chengdu Kanghong Biotech, Inc, Z. Ke is employee of Chengdu Kanghong Pharmaceutical Group, Chengdu Kanghong Biotech, Inc, and Chengdu Kanghong Pharmaceutical Group participated in the design of the study, data collection, data management, data analysis, preparation of the manuscript. The remaining authors have no financial/conflicting interests to disclose.
FALCON study group members listed in Appendix 1.
References
- 1.The Royal College of Ophthalmologists. Retinal Vein Occlusion (RVO) Guidelines. https://www.rcophth.ac.uk/standards-publications-research/clinical-guidelines/. Accessed July 15, 2015.
- 2.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 2015;122:538–544. [DOI] [PubMed] [Google Scholar]
- 3.Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:313–319 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther 2008;16:791–799. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013;120:795–802. [DOI] [PubMed] [Google Scholar]
- 6.Group CVOS. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 1995;102:1425–1433. [DOI] [PubMed] [Google Scholar]
- 7.Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein Occlusion (2 year). Ophthalmology 2014;121:1414–1420 e1. [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102–1112 e1. [DOI] [PubMed] [Google Scholar]
- 9.Regnier SA, Larsen M, Bezlyak V, Allen F. Comparative efficacy and safety of approved treatments for macular oedema secondary to branch retinal vein occlusion: a network meta-analysis. BMJ Open 2015;5:e007527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol 2009;93:452–456. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Li T, Wu Z, et al. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS One 2013;8:e70544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Yu D, Yang C, et al. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res 2009;26:204–210. [DOI] [PubMed] [Google Scholar]
- 13.Yu DC, Lee JS, Yoo JY, et al. Soluble vascular endothelial growth factor decoy receptor FP3 exerts potent antiangiogenic effects. Mol Ther 2012;20:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperduto RD, Hiller R, Chew E, et al. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the eye disease case-control study. Ophthalmology 1998;105:765–771. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Zhang J, Yan M, et al. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 2011;118:672–678. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Xu G, Wang Y, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 2014;121:1740–1747. [DOI] [PubMed] [Google Scholar]
- 17.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011;118:1594–1602. [DOI] [PubMed] [Google Scholar]
- 18.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 2011;118:2041–2049. [DOI] [PubMed] [Google Scholar]
- 19.Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology 2014;121:202–208. [DOI] [PubMed] [Google Scholar]
- 20.Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 2013;155:429–437 e7. [DOI] [PubMed] [Google Scholar]


