Supplemental Digital Content is available in the text.
Keywords: angioplasty, drug-eluting balloon, femoropopliteal, intermittent claudication, paclitaxel, peripheral arterial disease
Abstract
Background:
Drug-coated balloons (DCBs) are a predominant revascularization therapy for symptomatic femoropopliteal artery disease. Because of the differences in excipients, paclitaxel dose, and coating morphologies, varying clinical outcomes have been observed with different DCBs. We report the results of 2 studies investigating the pharmacokinetic and clinical outcomes of a new DCB to treat femoropopliteal disease.
Methods:
In the ILLUMENATE Pivotal Study (Prospective, Randomized, Single-Blind, U.S. Multi-Center Study to Evaluate Treatment of Obstructive Superficial Femoral Artery or Popliteal Lesions With A Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon), 300 symptomatic patients (Rutherford class 2–4) were randomly assigned to DCB (n=200) or standard angioplasty (percutaneous transluminal angioplasty [PTA]) (n=100). The primary safety end point was freedom from device- and procedure-related death through 30 days, and freedom from target limb major amputation and clinically driven target lesion revascularization through 12 months. The primary effectiveness end point was primary patency through 12 months. In the ILLUMENATE PK study (Pharmacokinetic Study of the Stellarex Drug-Coated Angioplasty Balloon), paclitaxel plasma concentrations were measured after last DCB deployment and at prespecified times (at 1, 4, 24 hours and at 7 and 14 days postprocedure) until no longer detectable.
Results:
In the ILLUMENATE Pivotal Study, baseline characteristics were similar between groups: 50% had diabetes mellitus, 41% were women, mean lesion length was 8.3 cm, and 44% were severely calcified. The primary safety end point was met (92.1% for DCB versus 83.2% for PTA, P=0.025 for superiority) and the primary patency rate was significantly higher with DCB (76.3% for DCB versus 57.6% for PTA, P=0.003). Primary patency per Kaplan-Meier estimates at day 365 was 82.3% for DCB versus 70.9% for PTA (P=0.002). The rate of clinically driven target lesion revascularization was significantly lower in the DCB cohort (7.9% versus 16.8%, P=0.023). Improvements in ankle-brachial index, Rutherford class, and quality of life were comparable, but the PTA cohort required twice as many revascularizations. Pharmacokinetic outcomes showed that all patients had detectable paclitaxel levels after DCB deployment that declined within the first hour (54.4±116.9 ng/mL to 1.4±1.0 ng/mL).
Conclusions:
The data demonstrate superior safety and effectiveness of the Stellarex DCB in comparison with PTA, and plasma levels of paclitaxel fall to low levels within 1 hour.
Clinical Trial Registration:
URL: http://clinicaltrials.gov. Unique identifiers: NCT01858428 and NCT01912937.
Clinical Perspective.
What Is New?
The ILLUMENATE Pivotal randomized trial (Prospective, Randomized, Single-Blind, U.S. Multi-Center Study to Evaluate Treatment of Obstructive Superficial Femoral Artery or Popliteal Lesions With A Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon) demonstrates the safety and effectiveness of the Stellarex drug-coated balloon for treatment of peripheral artery disease in patients with claudication.
Both the primary safety and effectiveness end points were met and the superiority of Stellarex to percutaneous transluminal angioplasty was demonstrated in each case.
This is the second randomized trial of the Stellarex drug-coated balloon with similar outcomes, validating early conclusions and demonstrating consistency across cohorts and geographies.
What Is the Clinical Implication?
The strong safety profile and the superior patency in comparison with percutaneous transluminal angioplasty, and the low rate of clinically driven target lesion revascularizations (7.9% at 1 year) make this drug-coated balloon a valuable treatment option for patients with superficial femoral and popliteal artery disease.
Peripheral artery disease is estimated to affect >200 million people worldwide.1 Endovascular interventions for symptomatic lower extremity peripheral artery disease have become the primary treatment option in most clinical circumstances.2,3 Percutaneous transluminal angioplasty (PTA) remains highly effective for reducing symptomatic stenosis acutely. However, 1-year and longer-term effectiveness remains suboptimal as demonstrated in the control arm of modern stenting and drug-coated balloon (DCB) trials.4–7 The use of paclitaxel-coated balloons and drug-eluting stents has demonstrated improved patency and a reduction in clinically driven target lesion revascularization (CD-TLR) in comparison with PTA in randomized controlled trials.6–9 DCBs eliminate the presence of a mechanical scaffold, and thereby remove the technical burden of treating in-stent restenosis and the inherent risk of stent fracture.10–12
The 2 Food and Drug Administration–approved DCBs have important differences in both drug make-up (ie, excipient/paclitaxel dose and coating formulation) and effectiveness outcomes.6,7 The LEVANT 2 Study (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis)7 evaluated the Lutonix DCB (C.R. Bard/Lutonix Inc) with an excipient of polysorbate and sorbitol and a 2 μg/mm2 dose of paclitaxel with primary patency of 73.5% at 12 months. The IN.PACT SFA study (Randomized Trial of IN.PACT Admiral® Drug Coated Balloon vs Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease)6 evaluated the IN.PACT Admiral DCB (Medtronic Vascular) with urea as the excipient and a paclitaxel dose of 3.5 μg/mm2 with a primary patency of 86.6% at 12 months6,13; both DCBs were superior to their respective control PTA arms. The ILLUMENATE Pivotal Study (Prospective, Randomized, Single-Blind, U.S. Multi-Center Study to Evaluate Treatment of Obstructive Superficial Femoral Artery or Popliteal Lesions With A Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon) assessed the Stellarex DCB (Spectranetics Corp) with a 2 µg/mm2 paclitaxel dose and a novel excipient (polyethylene glycol).
The single-arm ILLUMENATE First-In-Human Study showed 12-month and 24-month patency rates of 89.5% and 80.3%, respectively.14 A 12-month patency rate of 89.0% was confirmed with the ILLUMENATE European Randomized Trial, with long-term follow-up underway.15 The ILLUMENATE Pivotal Study is the first randomized clinical study conducted in the United States to evaluate the safety and effectiveness of the Stellarex DCB for treatment of symptomatic lower extremity peripheral artery disease. To understand the clearance of paclitaxel with this new DCB, the concentration of circulating plasma paclitaxel following treatment with the Stellarex DCB was measured in the ILLUMENATE PK single-arm study (Pharmacokinetic Study of the Stellarex Drug-Coated Angioplasty Balloon).
Methods
Study Designs
The ILLUMENATE Pivotal Study was a multicenter, single-blind, randomized controlled study designed to assess the safety and effectiveness of the Stellarex DCB in comparison with noncoated balloon angioplasty (PTA) in patients with symptoms of claudication and rest pain attributable to femoropopliteal artery disease. The concentration of circulating plasma paclitaxel following treatment with the Stellarex DCB was measured in the ILLUMENATE PK single-arm study. Both studies were approved by the Institutional Review Board or local Competent Authority/Ethics Committee at each participating site and patients provided written informed consent before any study-related procedures were performed. The studies were prospectively registered at ClinicalTrials.gov (NCT01858428 and NCT01912937).
ILLUMENATE Pivotal Study
Patient Population
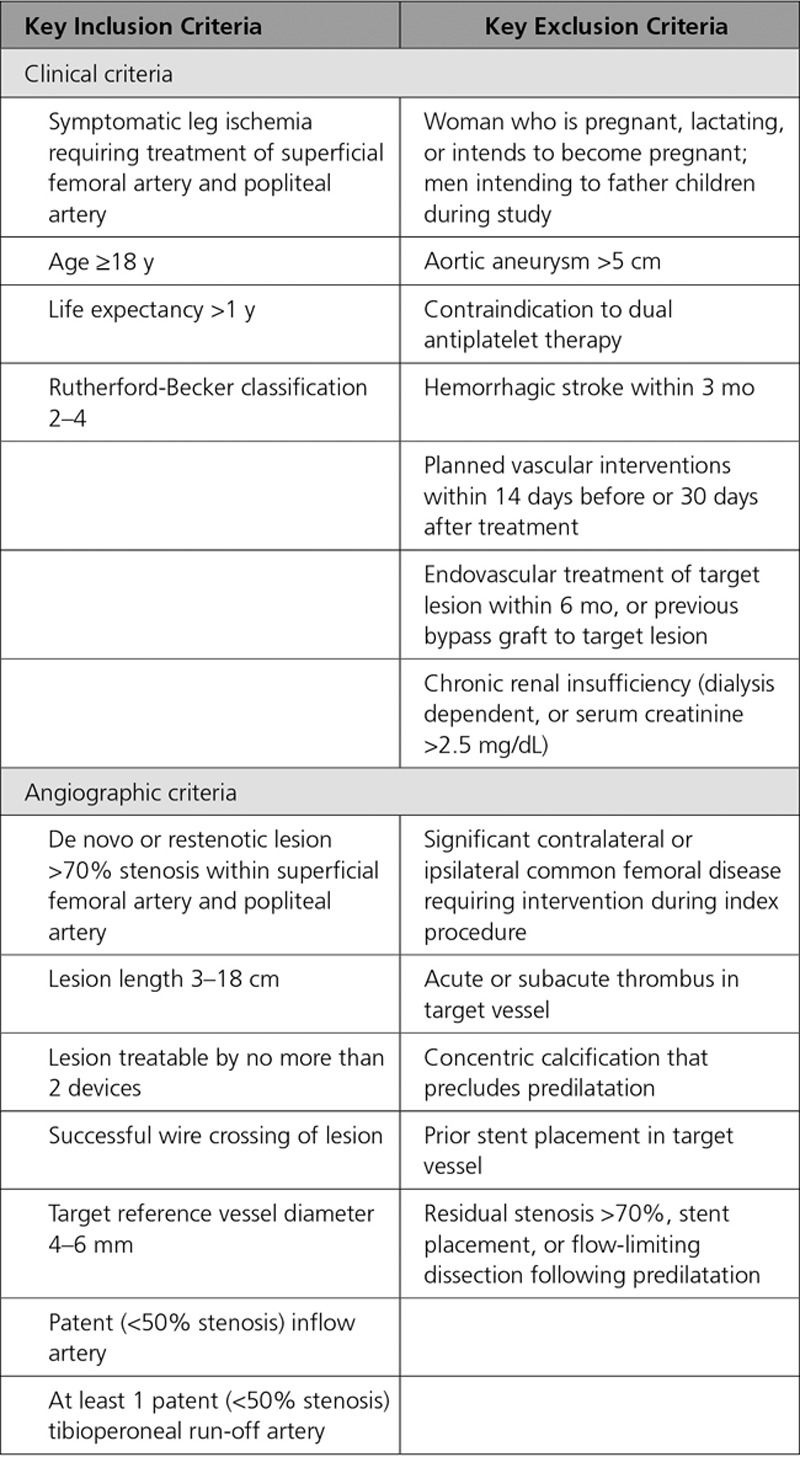
Eligible patients presented with symptomatic leg ischemia (Rutherford-Becker Clinical Category class 2–4), angiographic evidence of 70% to 99% stenosis or chronic total occlusions with lesion lengths between 30 and 180 mm, within the superficial femoral artery and/or popliteal artery (segments P1–P2). Key study inclusion/exclusion criteria are provided in Table 1.
Table 1.
Key Inclusion and Exclusion Criteria
Procedure and Follow-Up Requirements
Patients were screened for study eligibility based on the inclusion/exclusion criteria by clinical assessment, medical history, and angiography. Patients who met initial inclusion/exclusion criteria received acetylsalicylic acid (aspirin), clopidogrel, and/or ticlopidine per investigator standard of care. Appropriate anticoagulation was administered per physician discretion. Diagnostic angiography was performed to evaluate eligibility by angiographic criteria. If the patient was eligible, and the lesion was crossed, predilatation with a standard PTA balloon of 1 mm less than that of the reference vessel diameter was performed. The inflation times and pressures were conducted per physician preference. If a flow-limiting dissection or a residual stenosis >70% was noted, the patient was considered a screen failure, excluded from the study, and treated in accordance with accepted standard of care. If all general clinical and angiographic criteria were met, the patient was then randomly assigned in a 2:1 ratio. The patient was blinded to the treatment provided; however, the research team was unable to be blinded because of the visual differences between the DCB and standard balloon angioplasty catheters (uncoated). Research and treatment staff were educated and required to maintain the blinding status to patients. Following the procedure, patients were prescribed clopidogrel or ticlopidine for 30 days and aspirin for the duration of the study. The 1-month follow-up to review adverse events and medication compliance was conducted via office visit or telephone contact. Patients returned for clinical visits at 6 and 12 months, which included clinical assessment, functional status, adverse events, medication compliance, and duplex ultrasound (DUS). Follow-up is ongoing through 5 years.
End Points
The primary safety end point was a composite of freedom from device and procedure-related death through 30 days, and freedom from target limb major amputation and CD-TLR through 12 months. The primary effectiveness end point was primary patency through 12 months, defined as the absence of target lesion restenosis, measured by a duplex ultrasonography–derived peak systolic velocity ratio ≤2.5 and freedom from CD-TLR. CD-TLR was defined as reintervention of the target lesion because of percent diameter stenosis >50% by angiography, and Rutherford-Becker Clinical Category increase >1 or ankle-brachial index decrease >0.15. Primary end points were independently adjudicated for their clinical and imaging components by the Clinical Events Committee and core laboratories, respectively, both blinded to the patient’s assigned treatment. The duplex ultrasonography core laboratory was VasCore, Massachusetts General Hospital, and the angiography core laboratory was Beth Israel Deaconess Medical Center.
Secondary outcomes included CD-TLR, target limb major amputation, mortality, and changes in ankle-brachial index, walking impairment questionnaire score, walking distance per 6-minute walk test, Rutherford-Becker Clinical Category, and quality of life with the EuroQol 5 dimensions questionnaire, in comparison with baseline.
All data were 100% source verified. An independent, blinded Clinical Events Committee adjudicated adverse events, and an independent Data Safety and Monitoring Board monitored the study for safety.
Statistical Analysis
The primary safety hypothesis was that the composite safety end point at 12 months in patients treated with DCB would be noninferior to PTA. The primary effectiveness hypothesis was that the composite effectiveness outcome at 12 months in patients treated with DCB would be superior to PTA. Based on a 2:1 randomization, 90% power, 1-sided α=0.025, and estimated effectiveness rates of 65% for DCB and 45% for PTA, 288 patients were required to be evaluable at 12 months. The sample size calculations were derived from the χ2 test for the primary effectiveness end point and the Farrington-Manning score test for the noninferiority of the primary safety end point.
Outcomes are reported by using the intent-to-treat population, which included all randomly assigned patients regardless of actual treatment received. Continuous data are reported as mean and SD; categorical data are reported as frequencies and percentages. Comparisons of baseline characteristics were performed with independent samples t test, Wilcoxon signed rank test, χ2 test, or Fisher exact test, as appropriate. As secondary analyses of the primary end points, Kaplan-Meier curves through the 12-month window (day 410) are provided along with associated log-rank P values. Survival estimates at 12 months (365 days) are provided as well. A clinical imputation for missing patency data (no DUS at 12 months and no CD-TLR) included a success-carried-backward imputation of the earliest post-12 months DUS patency success in the absence of target lesion revascularization or a failure-carried-forward imputation of the earliest DUS patency failure which included DUS obtained at hospital discharge. Multiple-imputation analysis was used for the imputation of the remaining missing primary end point data. Full imputation of missing covariate values using the Markov Chain Monte Carlo method and logistic regression for imputation of missing end points was used to produce 10 imputed data sets. The logistic regression model included selected predictive baseline covariates, and available 6- and 12-month outcomes, as well. The differences in proportions and associated standard errors were computed within each imputed data set. The statistics were combined across imputations to calculate the overall differences in proportions, associated confidence limits, and P values. The poolability of the data across clinical centers was assessed using a Cochran-Mantel-Haenszel test on the outcome, treatment, and site, with a prespecified P value >0.15 indicating poolability. There was no statistical evidence of a treatment interaction across centers for either primary safety or efficacy; therefore the data were considered poolable.
All analyses were prespecified in a statistical analysis plan. Data were analyzed with SAS version 9.3 or higher. Statistical significance was set at P<0.05 for all comparisons unless otherwise stated.
ILLUMENATE PK Study
The ILLUMENATE PK Study was a prospective, single-arm, multicenter study. The purpose of the study was to describe the pharmacokinetics of paclitaxel in the blood delivered from the Stellarex DCB.
Inclusion and exclusion criteria were almost identical to those from the ILLUMENATE Pivotal Study (Table 1). Differences included the inclusion of up to 2 target lesions per patient, and the maximum allowable lesion length was 20 cm. No other DCB or drug-eluting stent could have been implanted or used within 12 months of the interventional procedure. There was also no exclusion criterion for chronic renal insufficiency in the ILLUMENATE PK Study because paclitaxel metabolism is primarily hepatic and renal function would not be expected to influence the circulating paclitaxel concentration.
Within the ILLUMENATE PK Study, blood samples assayed for circulating plasma paclitaxel concentration occurred immediately after last DCB deployment and at 1, 4, and 24 hours, and at 7 and 14 days (as applicable) postprocedure. Resampling occurred if there were detectable levels of paclitaxel in the patient’s sample at the prior evaluation or if the paclitaxel evaluation results of the previous time point were not available. Once the paclitaxel level was below quantifiable limits, the sampling stopped. Bioanalytical samples were evaluated by an independent core laboratory (Bioanalytical Systems, Inc. [BASi]).
Results
ILLUMENATE PK Study
The ILLUMENATE PK Study enrolled 25 patients (34 target lesions) at 2 sites in New Zealand between June 2013 and June 2015. The mean age was 73.0 years and 56.0% (14/25) were male. Target lesions comprised 73.5% (25/34) de novo lesions and 14.7% (5/34) were chronic total occlusions. The mean lesion length was 5.5 cm. A total of 5 lesions (14.7%) received treatment with 2 DCBs.
Mean paclitaxel plasma concentrations for the 25 patients assessed in the ILLUMENATE PK Study are shown in Figure 1. Paclitaxel clearance was as expected7; all patients had detectable levels of paclitaxel immediately after the last DCB deployment that declined rapidly within the first hour (54.4±116.9 ng/mL to 1.4±1.0 ng/mL), followed by a gradual decline to 0.3±0.1 ng/mL over 24 hours. Paclitaxel concentrations after 24 hours were below the lower limit of quantification (<0.100 ng/mL) in 96% (24/25) patients. Cmax (maximum observed concentration) was 54.4 ng/mL, area under the curve from t=0 until 24 hours was 37.2 ng×hours/mL, and T1/2 (terminal elimination half-life) was 10 hours.
Figure 1.
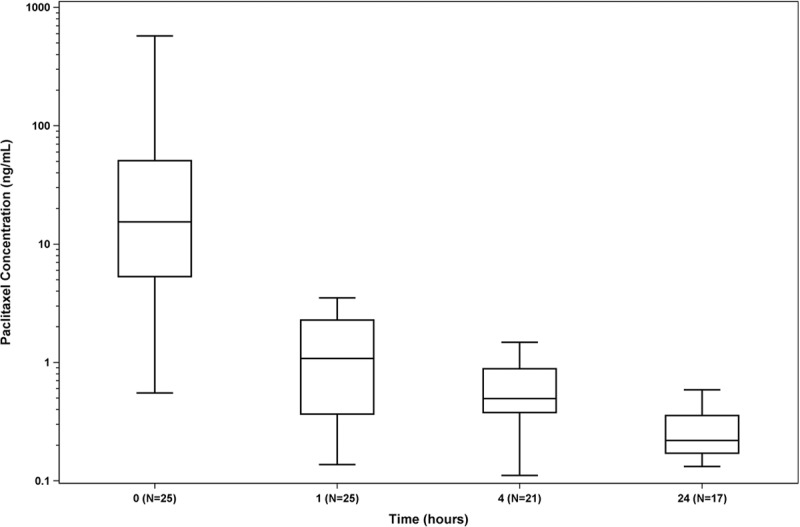
Serum paclitaxel concentrations from the ILLUMENATE PK study (Pharmacokinetic Study of the Stellarex Drug-Coated Angioplasty Balloon). Individual paclitaxel concentrations at each time point are shown. Mean paclitaxel concentrations declined rapidly within the first hour (54.4±116.9 ng/mL to 1.4±1.0 ng/mL), followed by a gradual decline to 0.3±0.1 ng/mL over 24 hours.
ILLUMENATE Pivotal Study
Baseline and Procedural Characteristics
Between June 2013 and July 2015, 300 patients (200 DCB, 100 PTA) were enrolled at 43 sites (41 in the United States and 2 in Austria). Patients were randomly assigned following successful predilatation. Eleven patients were screen failures because of residual stenosis of >70% or flow-limiting dissections requiring stent placement following predilatation. A study flow chart is provided in Figure 2. Baseline patient and angiographic lesion characteristics were similar between groups (Table 2 and Table 3). Mean reference vessel diameter was significantly greater in the PTA versus the DCB group (5.2 mm versus 4.9 mm, P=0.017), and there were more restenotic lesions in the PTA cohort (DCB: 9.5%, PTA: 18.0%, P=0.035); no other statistically significant differences were noted between groups. Of clinical importance, there was a high percentage of women (DCB: 44.0% versus PTA: 36.0%, P=0.185), patients with diabetes mellitus (DCB: 49.5% versus PTA: 52.0%, P=0.683), obesity (DCB: 39.5% versus PTA: 30.0%, P=0.107), and a high degree of severe calcification (defined by the core laboratory as calcific radio-opacities noted on both sides of the arterial wall and extending >1 cm of length from at least 2 views of 30 degrees difference from one another before contrast injection or digital subtraction) (DCB: 43.9% versus PTA: 43.0%, P=0.877). The mean lesion length was 8.0 cm in the DCB cohort and 8.9 cm in the PTA cohort (P=0.105).
Figure 2.
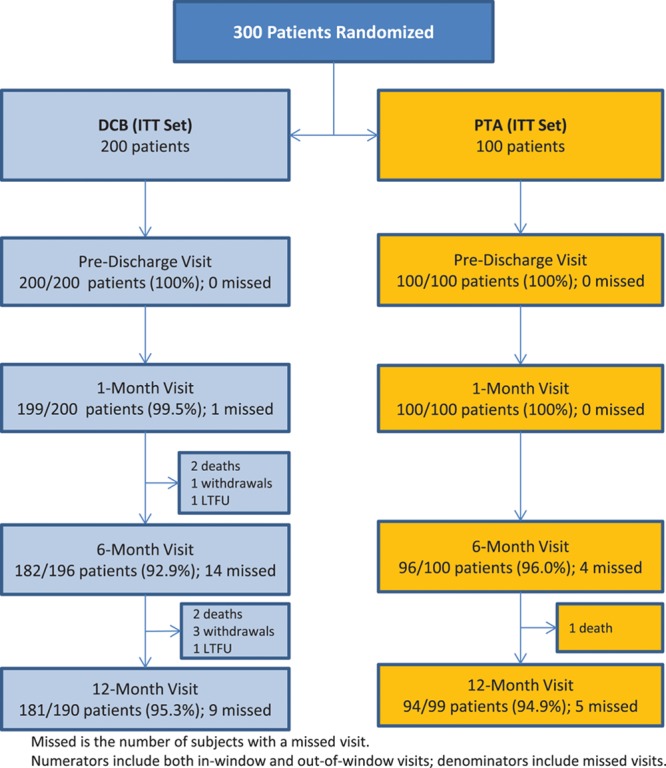
CONSORT patient flow diagram. DCB indicates drug-coated balloon; ITT, intent-to-treat; LTFU, lost to follow-up; and PTA, percutaneous transluminal angioplasty.
Table 2.
Baseline Patient Characteristics
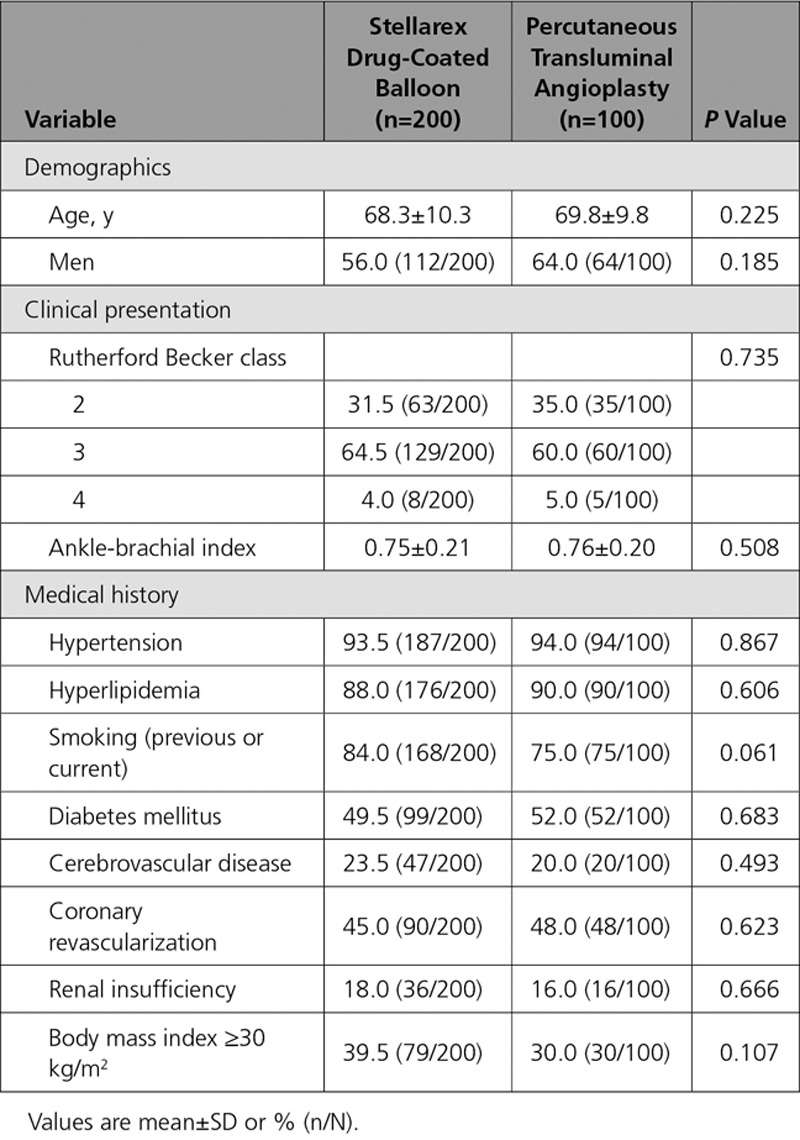
Table 3.
Baseline Lesion Characteristics (per Angiographic Core Laboratory Assessment)
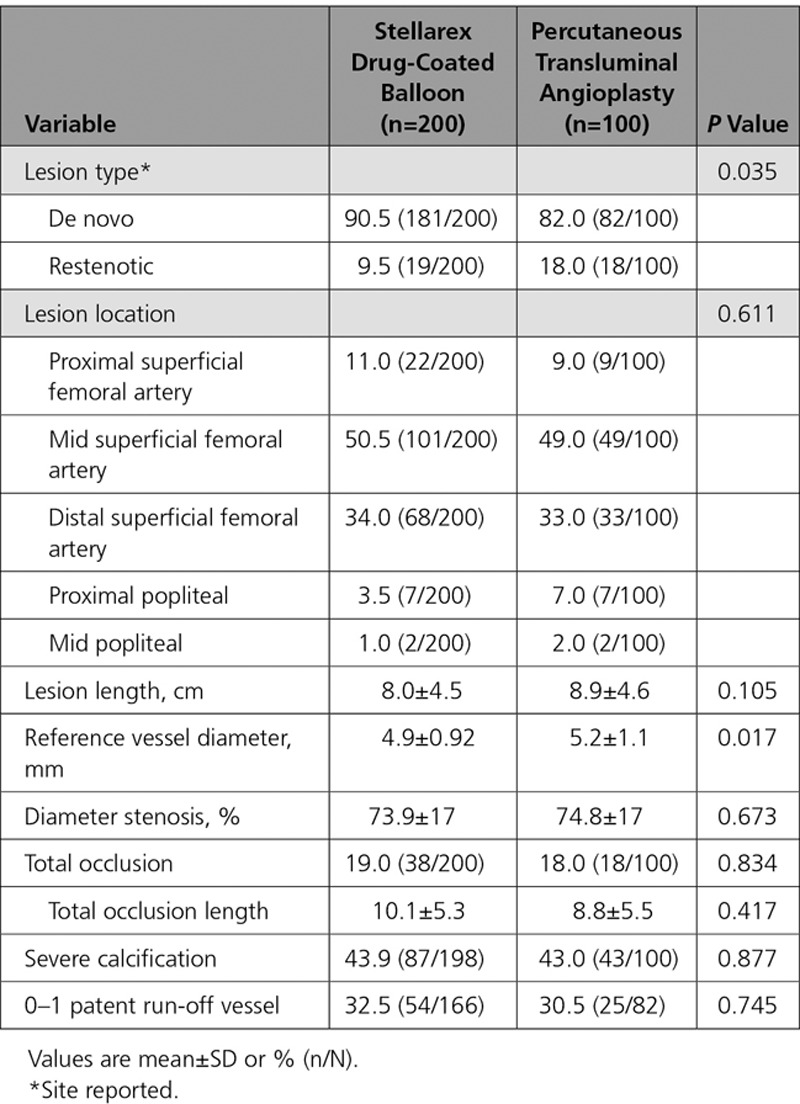
Procedural details and immediate outcomes were comparable between groups (Table 4). All patients underwent predilatation with a PTA balloon before treatment with DCB or PTA. Mean balloon inflation time per lesion was 3.9±2.0 minutes in the DCB cohort and 3.7±2.3 minutes with PTA (P=0.557). Provisional stent placement occurred in 6% of cases in each cohort. No flow-limiting dissections occurred in either group. One patient treated with DCB (0.5%) and no patients undergoing PTA had an ipsilateral embolic event of the target limb through 12 months. Procedural success, defined as final in-lesion residual diameter stenosis of ≤50% without the occurrence of a major adverse event during the procedure, was achieved in 98.5% in the DCB cohort and 98.0% in the PTA (P>0.999).
Table 4.
Procedural Characteristics and Outcomes
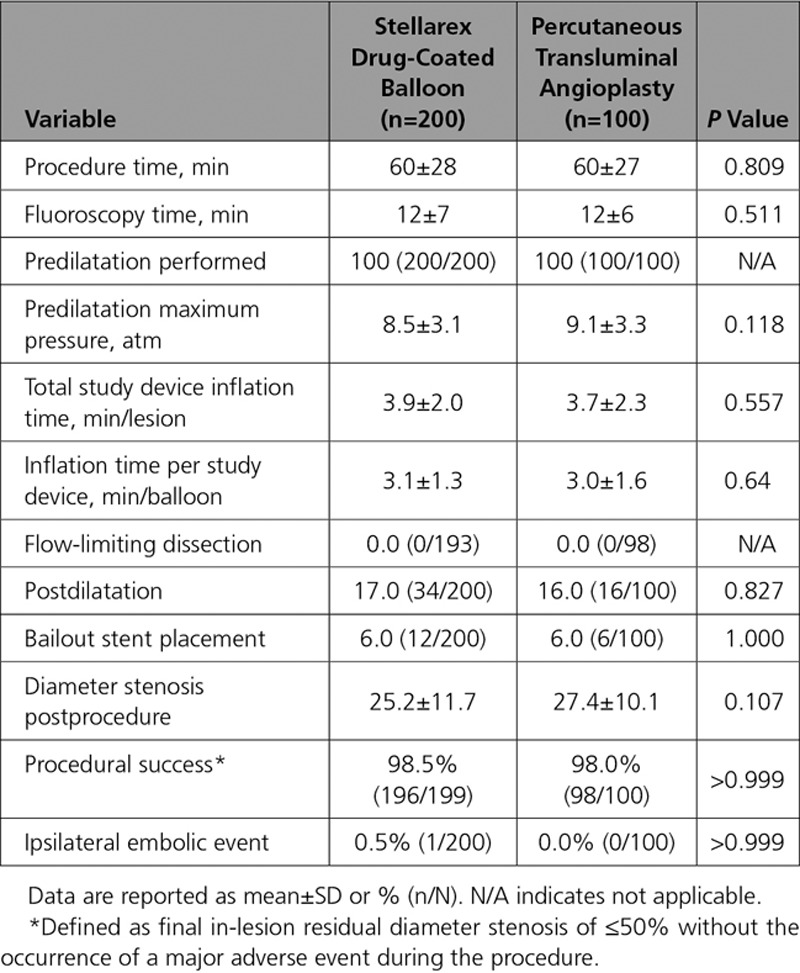
The composite primary safety objective demonstrating noninferiority was met (P=0.001). Moreover, superiority of DCB over PTA was demonstrated with a rate of 92.1% (174/189) in the DCB cohort and 83.2% (79/95) in the PTA cohort (P=0.025) through the full predefined 12-month follow-up window of 410 days. There were no device- or procedure-related deaths through 30 days and no target limb major amputations; therefore, the primary safety end point rate was driven solely by CD-TLR. The primary effectiveness end point, primary patency, was also met and superiority demonstrated with a significantly higher patency rate observed with DCB than with PTA (76.3% [135/177] versus 57.6% [53/92], P=0.003) through the full 12-month follow-up window of 410 days. Per Kaplan-Meier estimates (Figure 3) at day 365, the patency rates were 82.3% versus 70.9% (P=0.002 by log-rank test).
Figure 3.
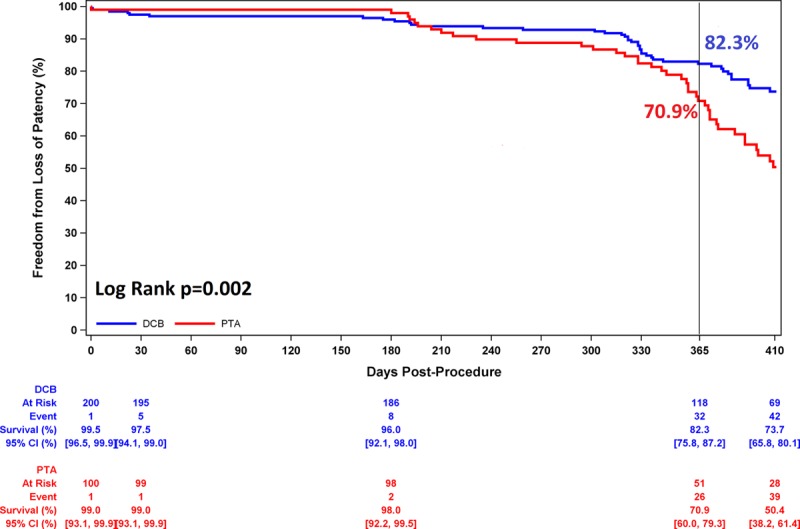
Primary patency through 1 year. Comparing DCB with PTA, primary patency was 82.3% versus 70.9% at day 365 (P=0.002 by log-rank test). CI indicates confidence interval; DCB, drug-coated balloon; and PTA, percutaneous transluminal angioplasty.
The CD-TLR rate through day 410 was significantly lower in the DCB cohort than the PTA cohort (7.9% [15/189] versus 16.8% [16/95]; P=0.023). Likewise, freedom from CD-TLR through 12 months per Kaplan-Meier estimates was significantly higher in the DCB cohort: 93.6% versus 87.3% (estimates at 365 days, log-rank P value= 0.025, Figure 4). No major amputations were reported in either group. All-cause mortality was comparable between groups through 12 months (2.6% DCB versus 2.1% PTA, P value >0.999). No deaths were determined by the Clinical Events Committee to be device or procedure related.
Figure 4.
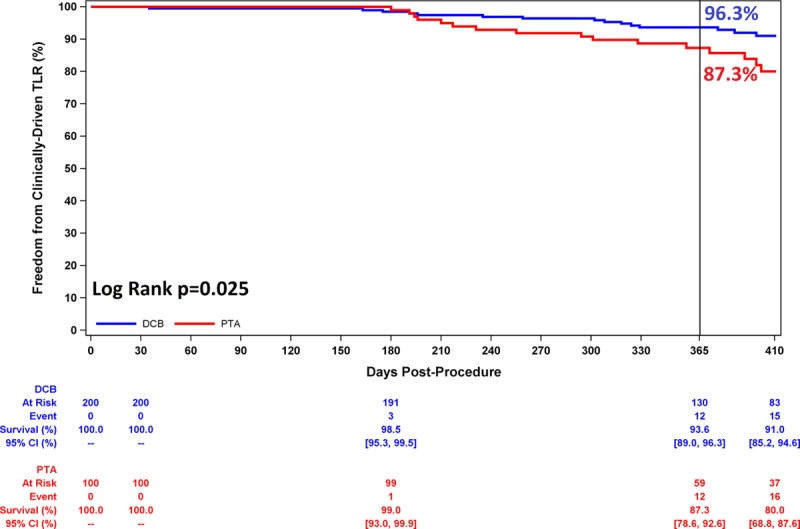
Freedom from clinically driven target lesion revascularization through 1 year. Comparing DCB with PTA, freedom from clinically driven target lesion revascularization was 93.6% versus 87.3% at day 365 (P=0.025 by log-rank test). CI indicates confidence interval; DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty; and TLR, target lesion revascularization.
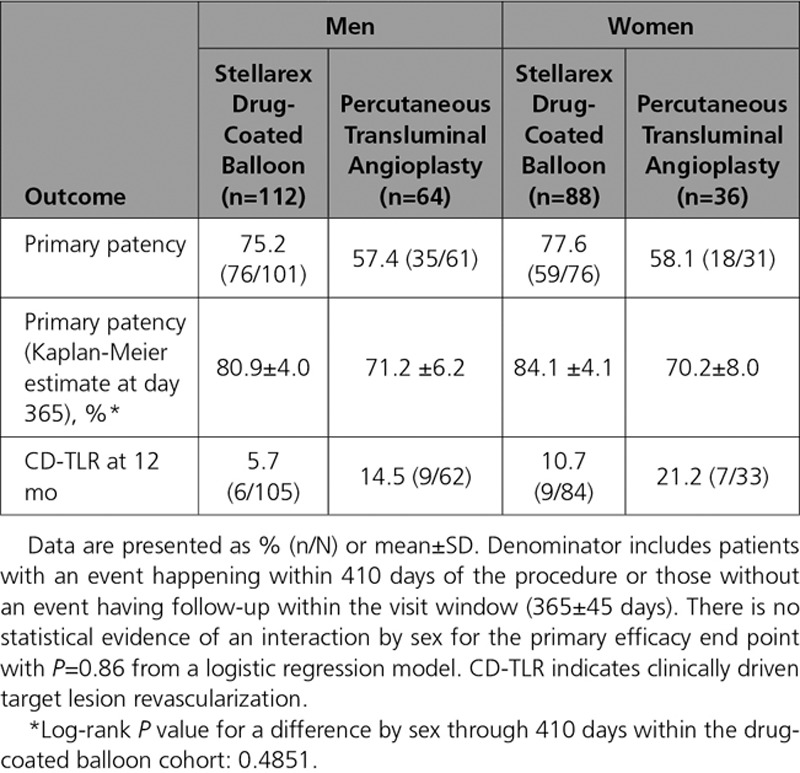
Walking impairment questionnaire composite scores were improved at 12 months in comparison with baseline in 78% of patients in both cohorts. Similarly, 73% of patients in both cohorts had an improvement in walking distance as assessed by the 6-minute walk test at 12 months in comparison with baseline. The mean change in EuroQol 5 dimensions questionnaire index scores was 0.10 (55.9% with improved scores) in the DCB cohort and 0.04 (53% with improved scores) in the PTA cohort. The DCB cohort achieved these comparable functional outcomes with a 46.9% lower TLR rate. Additional 12-month outcomes are provided in Tables 5 and 6. Outcomes were similar in women in comparison with men and are reported in Table 7. A logistic regression model was used to assess for interaction by sex for the primary effectiveness end point (Pinteraction=0.86).
Table 5.
Twelve-Month Outcomes
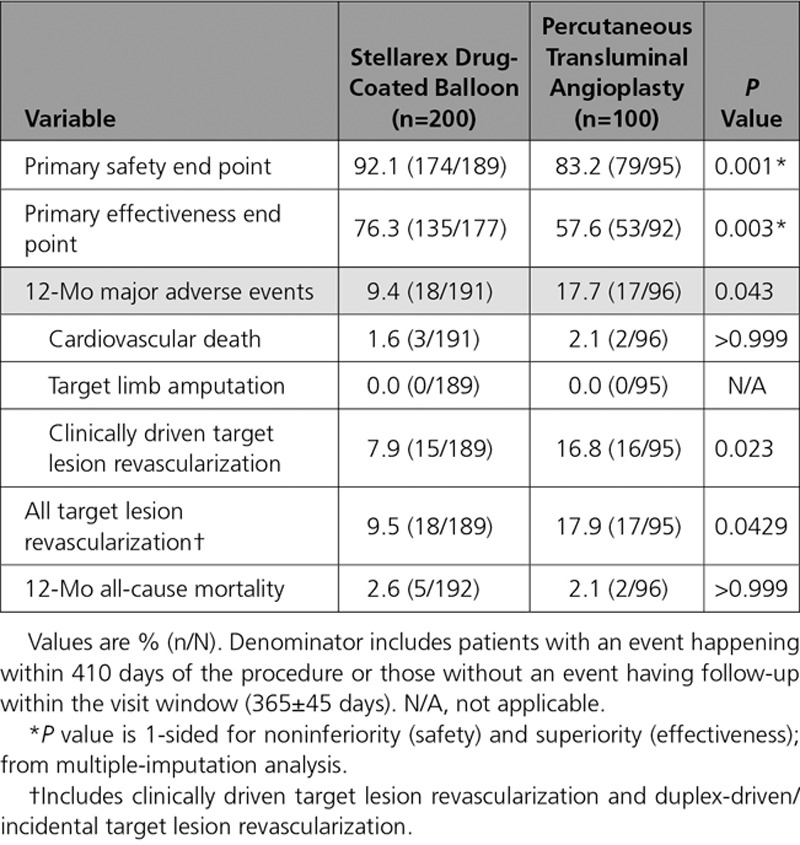
Table 6.
Twelve-Month Clinical and Functional Outcomes
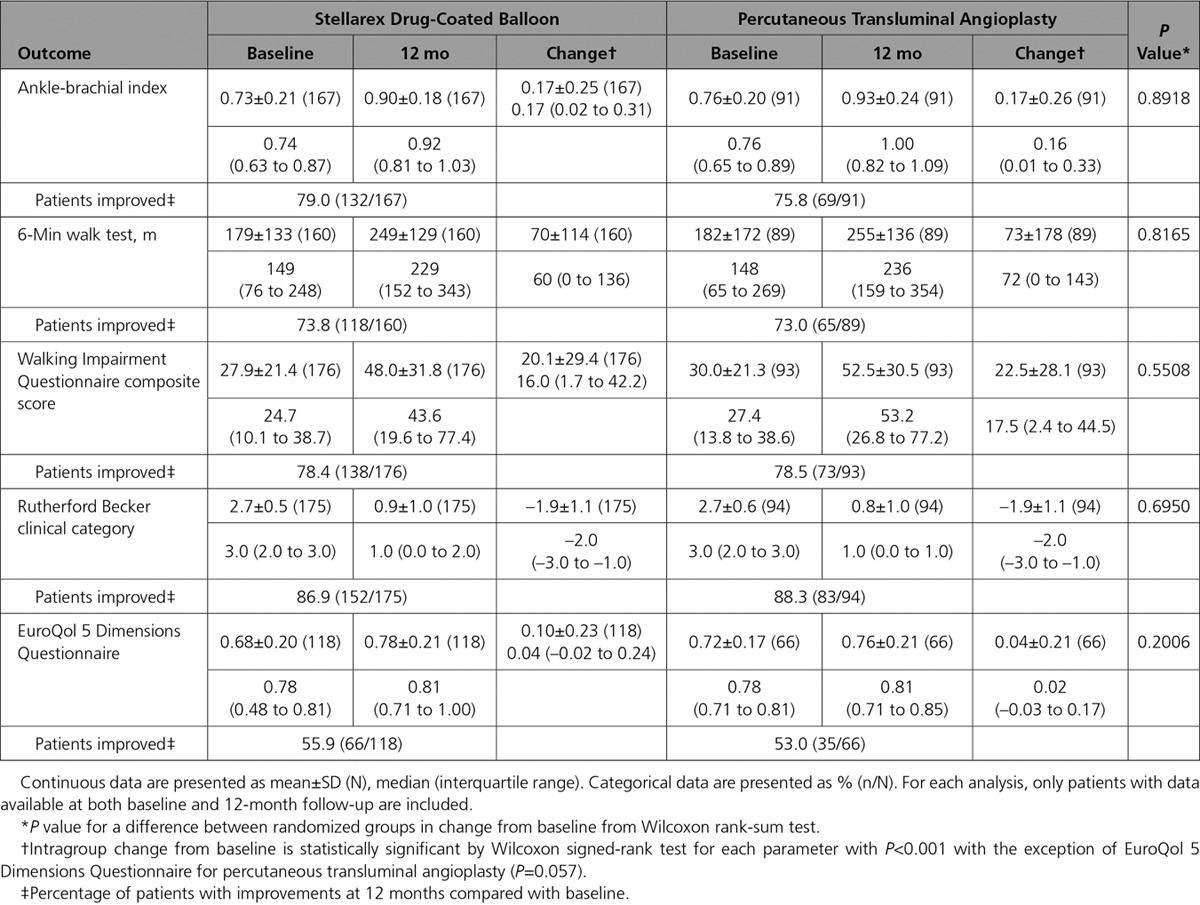
Table 7.
Key 12-Month Outcomes, by Sex
Discussion
The ILLUMENATE Pivotal trial demonstrated superior safety and effectiveness of the Stellarex DCB in comparison with the uncoated PTA for the treatment of symptomatic femoropopliteal artery disease. This study had a preponderance of patients who were women, who were obese, and who had diabetes mellitus. There was also a high percentage of lesions that were categorized as severely calcified.
Female sex has been associated with inconsistent outcomes after DCB use. In the women enrolled in the LEVANT 2 Trial, the primary effectiveness end point of primary patency was met in 56.4% (57/101) treated with the Lutonix DCB and 61.4% (27/44) treated with PTA.16 Although not powered to statistically examine differences in results between sexes, these outcomes raised concerns for reduced treatment effect in women. However, women in the IN.PACT SFA study did experience a significantly higher patency rate following treatment with the IN.PACT DCB than with PTA.9 In the ILLUMENATE Pivotal Study, a significant treatment effect was observed in women after use of the Stellarex DCB in comparison with PTA, with no significant difference in primary patency between men and women (80.9% versus 84.1%, log-rank P value= 0.4851).
Definitions of calcium severity differ. Circumferential calcium has been show to be a stronger predictor of failure than longitudinal calcification,17,18 with the former being the main component in all published definitions of severe calcium.17,19–21 Calcium may act as a barrier to the absorption of paclitaxel resulting in greater late lumen loss and lower primary patency rates. The definition or criteria for a lesion to be classified as severely calcified were not published in other DCB trials and likely vary. However, only 8% to 10% of lesions studied were designated as severely calcified in said DCB trials. The ILLUMENATE Pivotal Study did not exclude lesions based on the degree of calcification, and, per protocol, allowed randomization of any lesion that was successfully predilated with a plain balloon angioplasty.
The effectiveness of the Stellarex DCB despite the higher prevalence of core laboratory-defined calcified lesions, women, and patients with diabetes mellitus, was demonstrated in the ILLUMENATE Pivotal trial. It is important to note that results obtained from different DCB trials are not uniform or comparable and each DCB must be evaluated on the strength of the corresponding data. The demonstrated differences in effectiveness across DCBs may be explained by technical features such as drug dose, coating formulation, and type of excipient, which all affect the pharmacokinetic profile. These features impact drug tissue release timing and maintenance of therapeutic levels, as well as drug wash-out and downstream embolization.22 Procedural variables such as balloon sizing, inflation times, correct alignment (avoiding geographic miss), and inflation pressures impact the effectiveness of the DCB as well.
The Stellarex DCB uses a 2 μg/mm2 paclitaxel dose in a hybrid formulation made of both amorphous and crystalline constituents combined with a polyethylene glycol (PEG) excipient. The hybrid formulation helps maintain coating integrity in comparison with pure crystalline while allowing for sustained drug tissue release.22 PEG is a polymer characterized by high molecular weight that results in durability of the drug coating, enabling it to be resistant to balloon deformation such as flexion and elongation.23 Furthermore, PEG has shown a high affinity for hydroxyapatite, the primary structural mineral form found in calcified atherosclerotic lesions to which PEG forms ionic bonds.24 The combination of the hybrid coating and the durability of PEG is the basis of the drug transfer efficiency of the Stellarex DCB; the affinity of PEG for hydroxyapatite may maintain this transfer efficiency in the presence of calcified lesions and may explain the statistically superior primary patency of the Stellarex DCB over PTA despite the core laboratory–defined larger number of patients with severe calcification.
Despite the lack of physician blinding to patient treatment during follow-up evaluations, the CD-TLR rate in the PTA cohort of this trial (16.8%) is the same as the double-blinded LEVANT 2 Study.7 It is noteworthy that the Kaplan-Meier estimate of freedom from loss of patency at day 365 in the PTA cohort is high at 70.9%. However, the proportional rate, taking into account the full follow-up window, is 57.6% (53/92), and comparable trial results have had similar rates of 52.4% (54/103) for IN.PACT SFA6 and 52.6% (71/135) in LEVANT 2.7 In addition, the provisional stent rate of 6% in the LLUMENATE Pivotal Trial is in line with previous DCB investigational device exemption studies.6,7 These similar results, in a more complex study cohort, are likely attributable to angioplasty techniques including long inflation times (3.9 minutes).
Improvements in walking distance, Rutherford-Becker Clinical Category, and ankle-brachial index were similar between cohorts; however, the outcomes were achieved in the PTA cohort with twice as many revascularization procedures. The demonstrated effectiveness and the low stent rate illustrate that the Stellarex DCB can achieve sustained effectiveness with an excellent safety profile in a diverse and complex patient population.
Limitations
Although occurring despite randomization, the higher prevalence of restenotic lesions in the PTA cohort may impact the study results. Long-term follow-up is required in determining the extended durability of the intervention. Outcomes from this trial cannot be generalized to patients not included in this trial or to other DCBs. Future studies should encompass adjunctive therapeutic options, and optimal medical therapy and exercise, as well.
Conclusions
The ILLUMENATE Pivotal Trial is a prospective, multicenter randomized trial that confirms that the Stellarex DCB is superior to PTA for both safety and effectiveness for the treatment of symptomatic (Rutherford class 2–4) femoropopliteal disease. Pharmacokinetic data show that plasma concentrations are at low levels by 1 hour after use. The 12-month clinical outcomes demonstrate superior patency over standard PTA and superior freedom from CD-TLR, despite the complexity of patients studied. This trial establishes the Stellarex DCB as an important consideration in the treatment algorithm for patients with superficial femoral artery and popliteal disease.
Acknowledgments
The authors thank M. Schadow and A. Tarricone for medical writing assistance and T. Yurik and S. Verdoliva for statistical support. The authors also thank J. Farenbaugh for study management and all the investigators and clinical staff for their outstanding contributions.
Sources of Funding
This study was sponsored by the Spectranetics Corp., Colorado Springs, CO.
Disclosures
Dr Krishnan is a consultant for Medtronic, Spectranetics, C.R. Bard PV, and Abbott. Dr Mena-Hurtado is a consultant for Medtronic, Cook, and C.R. Bard PV. Dr Mustapha is a consultant to C.R Bard PV, Boston Scientific, Medtronic, Spectranetics, and Terumo. Dr Bachinsky is a consultant for Abbott Vascular, Abiomed, C.R. Bard PV, Medtronic, Rex Medical, and Spectranetics. Dr Werner has received speaking honoraria/consulting fees from Spectranetics. Dr Jaff is a noncompensated advisor to Abbott Vascular, Boston Scientific, Cordis, and Medtronic. He has equity investments in PQ Bypass, Vascular Therapies, Micell, and Primacea. He is a consultant for Philips/Volcano and Venarum. He is also a Board Member of VIVA Physicians, a 501(c)(3) nonprofit education and research organization. Dr Brodmann has received consulting fees and/or honoraria from Medtronic, C.R Bard PV, Spectranetics, Intact Vascular, Avinger, Soundbite Medical, Rexgenero, Biotronik, Bayer, Daiichi, Böhringer Ingelheim, and Astra Zeneca. Dr Lyden is a consultant for Biomet, Endologix, TVA Medical, and Spectranetics; he is also a Board Member of VIVA Physicians, a 501(c)(3) nonprofit education and research organization. Dr Niazi has received research funding from C.R Bard and Spectranetics and speaker honoraria from Medtronic. Dr Sachar has received consulting fees from Medtronic, Boston Scientific, and Spectranetics, and he is a shareholder for Contego Medical. Dr Cardenas has been a consultant to Endologix, Boston Scientific, Cordis, and Gore. Drs Faries, Holden, and Jain have no disclosures.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.028893/-/DC1.
Circulation is available at http://circ.ahajournals.org.
Contributor Information
Collaborators: Mark Mewissen, Barry Katzen, Aravinda Nanjundappa, Matheen A. Khuddus, Jason Ricci, Dennis Fry, Mehdi Shishehbor, Christopher Bosarge, Richard Kovach, Mark Goodwin, Mohammad Laiq Raja, Guy Mayeda, Jasvinder Sandhu, Oscar Rosales, William Crowder, David Paolini, John Henretta, Pratik Desai, Naim Farhat, Edward Kang, Gary Ansel, Mohammad Ghani, William Miller, Christopher Pollock, Ethan Korngold, John F. Angle, Greg Schultz, Todd Gensler, Louis Lopez, James Park, Georges Al-Khoury, Charles Joels, and Christopher Metzger
References
- 1.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Browner D, Fronek A, Klauber MR, Coughlin SS, Barrett-Connor E, Gabriel S. Peripheral arterial disease in large vessels is epidemiologically distinct from small vessel disease. An analysis of risk factors. Am J Epidemiol. 1989;129:1110–1119. doi: 10.1093/oxfordjournals.aje.a115233. [DOI] [PubMed] [Google Scholar]
- 3.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470. doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumura JS, Yamanouchi D, Goldstein JA, Pollock CW, Bosiers M, Schultz GA, Scheinert D, Rocha-Singh KJ. The United States study for evaluating endovascular treatments of lesions in the superficial femoral artery and proximal popliteal by using the Protégé EverfLex Nitinol Stent System II (DURABILITY II). J Vasc Surg. 2013;58:73–83.e1. doi: 10.1016/j.jvs.2012.12.066. doi: 10.1016/j.jvs.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 5.Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Cao AY, Jaff MR RESILIENT Investigators. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. doi: 10.1583/11-3627.1. doi: 10.1583/11-3627.1. [DOI] [PubMed] [Google Scholar]
- 6.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D LEVANT 2 Investigators. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 8.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 9.Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H, Hasenbank MS, Jaff MR IN.PACT SFA Trial Investigators. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338. doi: 10.1016/j.jacc.2015.09.063. doi: 10.1016/j.jacc.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 10.Iida O, Nanto S, Uematsu M, Ikeoka K, Okamoto S, Nagata S. Influence of stent fracture on the long-term patency in the femoro-popliteal artery: experience of 4 years. JACC Cardiovasc Interv. 2009;2:665–671. doi: 10.1016/j.jcin.2009.04.014. doi: 10.1016/j.jcin.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Scheinert D, Scheinert S, Sax J, Piorkowski C, Bräunlich S, Ulrich M, Biamino G, Schmidt A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–315. doi: 10.1016/j.jacc.2004.11.026. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Tosaka A, Soga Y, Iida O, Ishihara T, Hirano K, Suzuki K, Yokoi H, Nanto S, Nobuyoshi M. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16–23. doi: 10.1016/j.jacc.2011.09.036. doi: 10.1016/j.jacc.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Jaff MR. Drug-coated balloon treatment for patients with intermittent claudication: insights from the IN.PACT Global Full Clinical Cohort.. Paper presented at: Vascular InterVentional Advances (VIVA) 2016; September 19–20, 2016; Las Vegas, NV. [Google Scholar]
- 14.Schroeder H, Meyer DR, Lux B, Ruecker F, Martorana M, Duda S. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: outcomes from the ILLUMENATE first-in-human study. Catheter Cardiovasc Interv. 2015;86:278–286. doi: 10.1002/ccd.25900. doi: 10.1002/ccd.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder H, Werner M, Meyer DR, Reimer P, Krüger K, Jaff MR, Brodmann M ILLUMENATE EU RCT Investigators. Low-dose paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-year results of the ILLUMENATE European Randomized Clinical Trial (Randomized Trial of a Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon). Circulation. 2017;135:2227–2236. doi: 10.1161/CIRCULATIONAHA.116.026493. doi: 10.1161/CIRCULATIONAHA.116.026493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutonix 035 Drug Coated Balloon PTA Catheter Instructions for Use. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130024d.pdf. Accessed May 2017.
- 17.Fanelli F, Cannavale A, Gazzetti M, Lucatelli P, Wlderk A, Cirelli C, d’Adamo A, Salvatori FM. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37:898–907. doi: 10.1007/s00270-014-0904-3. doi: 10.1007/s00270-014-0904-3. [DOI] [PubMed] [Google Scholar]
- 18.Tepe G, Beschorner U, Ruether C, Fischer I, Pfaffinger P, Noory E, Zeller T. Drug-eluting balloon therapy for femoropopliteal occlusive disease: predictors of outcome with a special emphasis on calcium. J Endovasc Ther. 2015;22:727–733. doi: 10.1177/1526602815600156. doi: 10.1177/1526602815600156. [DOI] [PubMed] [Google Scholar]
- 19.Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;83:E212–E220. doi: 10.1002/ccd.25387. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26:355–360. [PubMed] [Google Scholar]
- 21.Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, Hiatt WR, Ho M, Ikeda K, Ikeno F, Jaff MR, Jones WS, Kawahara M, Lookstein RA, Mehran R, Misra S, Norgren L, Olin JW, Povsic TJ, Rosenfield K, Rundback J, Shamoun F, Tcheng J, Tsai TT, Suzuki Y, Vranckx P, Wiechmann BN, White CJ, Yokoi H, Krucoff MW. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015;65:931–941. doi: 10.1016/j.jacc.2014.12.036. doi: 10.1016/j.jacc.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granada JF, Stenoien M, Buszman PP, Tellez A, Langanki D, Kaluza GL, Leon MB, Gray W, Jaff MR, Schwartz RS. Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: impact on neointimal proliferation and healing. Open Heart. 2014;1:e000117. doi: 10.1136/openhrt-2014-000117. doi: 10.1136/openhrt-2014-000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mark J, Ngai K, Graessley WW, Mandelkern L, Samulski E, Koenig J, Wignall G. Physical Properties of Polymers. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 2004. [Google Scholar]
- 24.Venkatasubba GD, Ramasamy S, Avadhani GS, Ramakrishnan V, Kumar J. Surface modification and paclitaxel drug delivery of folic acid modified polyethylene glycol functionalized hydroxyapatite nanoparticles. Powder Technol. 2013;235:437–442. [Google Scholar]


