Abstract
Abnormally high glycated hemoglobin (Hb) (HbA1c) is significantly associated with oxidative stress and an increased risk of cardiovascular disease (CVD). Serum total bilirubin (T-B) may have a beneficial role in preventing oxidative changes and be a negative risk factor of CVD. Limited information is available on whether serum T-B is an independent confounding factor of HbA1c. The study subjects were 633 men aged 70 ± 9 (mean ± standard deviation (SD)) years and 878 women aged 70 ± 8 years who were enrolled consecutively from among patients aged ≥40 years through a community-based annual check-up process. We evaluated the relationship between various confounding factors including serum T-B and HbA1c in each gender. Multiple linear regression analysis pertaining to HbA1c showed that in men, serum T-B (β = −0.139) as well as waist circumference (β = 0.099), exercise habit (β = 0.137), systolic blood pressure (SBP) (β = 0.076), triglycerides (β = 0.087), and uric acid (β = −0.123) were significantly and independently associated with HbA1c, and in women, serum T-B (β = −0.084) as well as body mass index (β = 0.090), smoking status (β = −0.077), SBP (β = 0.117), diastolic blood pressure (DBP) (β = −0.155), low-density lipoprotein cholesterol (β = 0.074), prevalence of antidyslipidemic medication (β = 0.174), and uric acid (β = 0.090) were also significantly and independently associated with HbA1c. Multivariate-adjusted serum HbA1c levels were significantly high in subjects with the lowest serum T-B levels in both genders. Serum T-B is an independent confounding factor for HbA1c among community-dwelling middle-aged and elderly persons.
Keywords: Serum total bilirubin, hemoglobin A1c, community-dwelling persons, confounding factor, middle-aged and elderly persons
Introduction
Glycated hemoglobin (HbA1c) is a reliable indicator of mean blood glucose concentrations over the preceding 3 months and an important test for the management of diabetes. Epidemiological studies have reported that high HbA1c was significantly associated with oxidative stress1 and an increased risk of chronic kidney disease (CKD),2,3 cardiovascular disease (CVD), and all-cause mortality regardless of diabetes status.4–8
Bilirubin, which consists of an open chain of four pyrrole-like rings (tetrapyrrole) and is a natural end product of heme catabolism, has been generally regarded as an important endogenous antioxidant9 and anti-inflammatory molecule.10 Current studies demonstrate that mildly elevated serum bilirubin may provide important protection against metabolic syndrome,11 diabetes,12 CVD, and all-cause mortality in adults.13,14 We also have demonstrated that low total bilirubin (T-B) was significantly associated with increased intima-media thickness and/or plaque formation of the carotid artery among nondiabetic15 and diabetic patients.16 Thus, some researchers hypothesize that the association of HbA1c with bilirubin may at least partly mediate the association between HbA1c and CVD and all-cause mortality.17 Oda and Kawai7 reported that bilirubin is negatively associated with HbA1c independent of other CVD risk factors in apparently healthy Japanese men and women. However, there are few reports on the relationship between serum T-B and HbA1c in Japanese middle-aged and elderly community-dwelling persons.
Firstly, this study investigated serum T-B and its relationship with potential confounding factors such as age, body mass index (BMI), habits, lipids, and glucose. Secondly, this study investigated whether there is an independent association of serum T-B with HbA1c. To examine these two issues, cross-sectional data from community-dwelling persons were used.
Methods
Subjects
The study population aged ≥40 years was selected through a community-based annual check-up process from the Nomura Health and Welfare Center in a rural town located in Ehime Prefecture, Japan. The physical activity level of subjects (e.g. exercise, drinking, and smoking habits), information on medical history, present conditions, and medications (e.g. antihypertensive, antidyslipidemic, and antidiabetic medication) were obtained by interview using a structured questionnaire. For all these individuals, overnight fasting plasma samples were made available. Participants with serum T-B ≥2.0 mg/dL or alanine transaminase (ALT) ≥100 IU/L or gamma glutamyl transpeptidase (GGT) ≥100 IU/L were excluded to avoid confounding factors due to the high possibility of potential Gilbert syndrome and hepatobiliary disease. Thus, 1511 (men 633 and women 878) patients were enrolled in the study. The study complies with the Declaration of Helsinki and was approved by the ethics committee of Ehime University School of Medicine with written informed consent obtained from each subject (Institutional Review Board: 1402009).
Methods
Information on demographic characteristics and risk factors was collected using clinical files. BMI was calculated by dividing weight (in kilograms) by the square of the height (in meters). Smoking status was defined as the number of cigarette packs per day multiplied by the number of years smoked (pack-year), and the participants were classified into never smokers, past smokers, light smokers (<20 pack-year), and heavy smokers (≥20 pack-year). Daily alcohol consumption was measured using the Japanese liquor unit in which a unit corresponds to 22.9 g of ethanol, and the participants were classified into never drinkers, occasional drinkers, daily light drinkers (<2 unit/day), and daily heavy drinkers (≥2 unit/day). Triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine (enzymatic method), uric acid, HbA1c, and serum T-B were measured when subjects were fasted. Estimate glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations modified by a Japanese coefficient (eGFRCKDEPI): male—Cr ≤ 0.9 mg/dl, 141 × (Cr/0.9)–0.411 × 0.993age × 0.813; Cr > 0.9 mg/dl, 141 × (Cr/0.9)–1.209 × 0.993age × 0.813; female—Cr ≤ 0.7 mg/dl, 144 × (Cr/0.7)–0.329 × 0.993age × 0.813; Cr > 0.7 mg/dl, 144 × (Cr/0.7)–1.209 × 0.993age × 0.813.18
Statistics
All values are expressed as the mean ± SD, unless otherwise specified, and for parameters with non-normal distribution (such as TG, HbA1c, and T-B), the data are shown as median (interquartile range) values. In all the analyses, parameters with non-normal distributions were used after log-transformation. Statistical analysis was performed using IBM SPSS Statistics version 21 (Statistical Package for Social Science Japan, Inc., Tokyo, Japan). Differences in means and prevalence among the groups were analyzed by Student’s t-test for continuous data and χ 2 test for categorical data, respectively. Pearson’s correlations were calculated in order to characterize the associations between various characteristics and serum T-B. Forced entry and stepwise multiple linear regression analysis (p value for entry was <0.05 and for exit was >0.10) were used to evaluate the contribution of each confounding factor to HbA1c. Analysis of covariance (ANCOVA) was performed using a general linear model approach to determine the association between confounding factors including that of serum T-B and HbA1c. In these analyses, HbA1c was the dependent variable, the four categories by quartile of serum T-B (men—first: 0.30–0.50; second: 0.51–0.70; third: 0.71–0.86; fourth: 0.87–1.90 and women—first: 0.20–0.49; second: 0.50–0.60; third: 0.61–0.79; fourth: 0.80–1.78) were the fixed variables, and all confounding factors in model 2 of Table 4 were added as covariates. A value of p < 0.05 was considered significant.
Results
Characteristics of subjects by gender
Table 1 shows the background characteristics by gender. The study subjects were 633 men aged 70 ± 9 (mean ± SD) years and 878 women aged 70 ± 8 years. Several characteristics differed between men and women. BMI, waist circumference, smoking status, alcohol consumption, history of CVD, DBP, TG, uric acid, and serum T-B were higher in men than in women. HDL-C, LDL-C, prevalence of antidyslipidemic medication, and eGFRCKDEPI were higher in women than in men. There were no intergroup differences regarding age, exercise habit, SBP, prevalence of antihypertensive medication, and HbA1c.
Table 1.
Characteristics of subjects within each gender.a
| Characteristics | Men, N = 633 | Women, N = 878 | p Value* |
|---|---|---|---|
| Age | 70 ± 9 | 70 ± 8 | 0.824 |
| Body mass index | 23.2 ± 3.0 | 22.6 ± 3.2 | <0.001 |
| Waist circumference | 82.4 ± 8.3 | 80.4 ± 9.0 | <0.001 |
| Smoking statusb (%) | 42.7/38.8/6.4/12.1 | 97.0/1.9/0.7/0.4 | <0.001 |
| Alcohol habitc (%) | 24.9/23.6/17.6/33.9 | 70.9/22.6/4.2/2.3 | <0.001 |
| Exercise habit (%) | 33.9 | 37.3 | 0.207 |
| History of CVD (%) | 9.2 | 4.3 | <0.001 |
| Systolic blood pressure (mmHg) | 135 ± 17 | 136 ± 17 | 0.220 |
| Diastolic blood pressure (mmHg) | 80 ± 10 | 77 ± 9 | <0.001 |
| Antihypertensive medication (%) | 43.2 | 42.6 | 0.824 |
| Triglycerides (mg/dL) | 89 (68–130) | 87 (65–117) | 0.004 |
| HDL cholesterol (mg/dL) | 61 ± 16 | 68 ± 17 | <0.001 |
| LDL cholesterol (mg/dL) | 115 ± 28 | 125 ± 29 | <0.001 |
| Antidyslipidemic medication (%) | 13.0 | 27.7 | <0.001 |
| eGFRCKDEPI (mL/min/1.73 m2) | 70.3 ± 11.5 | 72.2 ± 11.2 | 0.001 |
| Uric acid (mg/dL) | 5.9 ± 1.3 | 4.7 ± 1.1 | <0.001 |
| Serum total bilirubin (mg/dL) | 0.7 (0.5–0.9) | 0.6 (0.5–0.8) | <0.001 |
| HbA1c (%) | 5.7 (5.4–6.0) | 5.7 (5.5–5.9) | 0.112 |
CVD: cardiovascular disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Hb: hemoglobin.
aData are presented as means ± standard deviation. Data for triglycerides, hemoglobin A1c, and serum total bilirubin were skewed, presented as median (interquartile range) values, and log-transformed for analysis. Numbers in bold indicate significance (p < 0.05).
bSmoking status was classified as never smokers, past smokers, light smokers (<20 pack-year), and heavy smokers (≥ 20 pack-year).
cAlcohol habit was classified as never drinkers, occasional drinkers, daily light drinker (<2 unit/day), and daily heavy drinkers (≥2 unit/day).
*Student’s t-test was used for the continuous data and χ 2 test for the categorical data.
Simple relationships between confounding factors including serum T-B and HbA1c within each gender
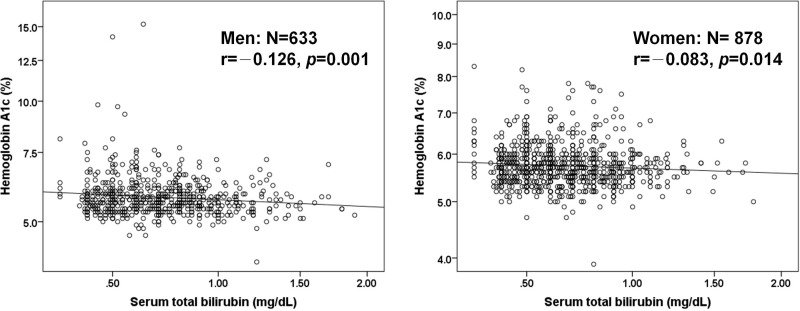
Table 2 shows the relationship between confounding factors including serum T-B and HbA1c within each gender. In men, BMI, waist circumference, exercise habit, SBP, TG, and LDL-C correlated positively with HbA1c, while HDL-C, uric acid, and serum T-B correlated negatively with HbA1c. In women, age, BMI, waist circumference, prevalence of antihypertensive medication, TG, prevalence of antidyslipidemic medication, and uric acid correlated positively with HbA1c, while smoking status, HDL-C, eGFRCKDEPI, and serum T-B correlated negatively with HbA1c. Figure 1 shows the correlation between serum T-B and HbA1c within each gender. The correlation coefficient between serum T-B and HbA1c was significant in both men (r = −0.126, p = 0.001) and women (r = −0.083, p = 0.014).
Table 2.
A simple relationship between variables including serum T-B and HbA1c within each gender.a
| Men, N = 633 | Women, N = 878 | |
|---|---|---|
| Characteristics | r (p value) | r (p value) |
| Age | 0.022 (0.585) | 0.086 (0.011) |
| Body mass index | 0.099 (0.013) | 0.161 (<0.001) |
| Waist circumference | 0.113 (0.005) | 0.157 (<0.001) |
| Smoking status | 0.047 (0.235) | −0.074 (0.028) |
| Alcohol habit | −0.046 (0.245) | −0.050 (0.136) |
| Exercise habit | 0.129 (0.001) | 0.007 (0.847) |
| History of CVD | 0.038 (0.334) | 0.042 (0.209) |
| Systolic blood pressure | 0.109 (0.006) | 0.064 (0.058) |
| Diastolic blood pressure | 0.047 (0.238) | −0.021 (0.536) |
| Antihypertensive medication | 0.048 (0.224) | 0.149 (<0.001) |
| Triglycerides | 0.126 (0.001) | 0.105 (0.002) |
| HDL cholesterol | −0.098 (0.014) | −0.100 (0.003) |
| LDL cholesterol | 0.086 (0.030) | 0.005 (0.873) |
| Antidyslipidemic medication | 0.078 (0.050) | 0.206 (<0.001) |
| eGFRCKDEPI | 0.049 (0.221) | −0.094 (0.005) |
| Uric acid | −0.109 (0.006) | 0.143 (<0.001) |
| Serum total bilirubin | −0.126 (0.001) | −0.083 (0.014) |
r: Pearson’s correlation coefficient.
aData for triglycerides, HbA1c, and serum total bilirubin were skewed and log-transformed for analysis. Numbers in bold indicate significance (p < 0.05).
Figure 1.
Relationship between serum total bilirubin and HbA1c within each gender.
Multivariate relationships between confounding factors including of serum T-B and HbA1c within each gender
As presented in Table 3, a multiple linear regression analysis performed to find independent confounding factors for HbA1c showed that in men, serum T-B (β = −0.139) as well as waist circumference (β = 0.099), exercise habit (β = 0.137), SBP (β = 0.076), TG (β = 0.087), and uric acid (β = −0.123) were significantly and independently associated with HbA1c, and in women, serum T-B (β = −0.084) as well as BMI (β = 0.090), smoking status (β = −0.077), SBP (β = 0,117), DBP (β = −0.155), LDL-C (β = 0.074), prevalence of antidyslipidemic medication (β = 0.174), and uric acid (β =0.090) were also significantly and independently associated with HbA1c.
Table 3.
Multiple linear regression analysis of variables including T-B for HbA1c within each gender.a
| Men, N = 633 | Women, N = 878 | |||
|---|---|---|---|---|
| Multiple linear regression analysis | Multiple linear regression analysis | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Characteristics | β (p value) | β (p value) | β (p value) | β (p value) |
| Age | 0.018 (0.743) | — | −0.006 (0.890) | — |
| Body mass index | −0.018 (0.825) | — | 0.067 (0.269) | 0.090 (0.011) |
| Waist circumference | 0.105 (0.208) | 0.099 (0.016) | 0.018 (0.771) | — |
| Smoking status | 0.036 (0.376) | — | −0.071 (0.033) | −0.077 (0.018) |
| Alcohol habit | −0.030 (0.479) | — | −0.034 (0.337) | — |
| Exercise habit | 0.139 (<0.001) | 0.137 (<0.001) | 0.012 (0.724) | — |
| History of CVD | 0.015 (0.705) | — | 0.004 (0.905) | — |
| Systolic blood pressure | 0.126 (0.040) | 0.076 (0.049) | 0.115 (0.032) | 0.117 (0.020) |
| Diastolic blood pressure | −0.056 (0.367) | — | −0.155 (0.003) | −0.155 (0.002) |
| Antihypertensive medication | 0.029 (0.504) | — | 0.066 (0.077) | 0.066 (0.063) |
| Triglycerides | 0.077 (0.077) | 0.087 (0.032) | 0.013 (0.737) | — |
| HDL cholesterol | −0.018 (0.691) | — | −0.015 (0.700) | — |
| LDL cholesterol | 0.078 (0.056) | 0.071 (0.071) | 0.068 (0.055) | 0.074 (0.030) |
| Antidyslipidemic medication | 0.047 (0.248) | — | 0.173 (<0.001) | 0.174 (<0.001) |
| eGFRCKDEPI | 0.090 (0.083) | — | 0.009 (0.834) | — |
| Uric acid | −0.088 (0.043) | −0.123 (0.002) | 0.095 (0.013) | 0.090 (0.008) |
| Serum total bilirubin | −0.128 (0.001) | −0.139 (<0.001) | −0.081 (0.014) | −0.084 (0.010) |
| R 2 | 0.097 (<0.001) | 0.075 (<0.001) | 0.099 (<0.001) | 0.097 (<0.001) |
β: standardized coefficient; R 2: multiple coefficient of determination. Model 1: forced entry method and model 2: stepwise method.
aData for triglycerides, HbA1c, and serum total bilirubin were skewed and log-transformed for analysis. Numbers in bold indicate significance (p < 0.05).
Mean (95% CI) HbA1c of the subjects categorized by quartile of serum T-B within each gender
Table 4 presents the levels of HbA1c after adjustment for all confounding factors in model 2 of Table 3. HbA1c levels were significantly low in subjects with a high serum T-B level in both genders.
Table 4.
Mean (95% CI) of HbA1c of the subjects categorized by serum T-B within each gender.a
| Characteristics | Men, N = 633 | Women, N = 878 | ||||
|---|---|---|---|---|---|---|
| N | Non-adjusted mean (95% CI) | Multivariate-adjusted mean (95%CI)b | N | Non-adjusted mean (95%CI) | Multivariate-adjusted mean (95%CI)b | |
| Quartile 1 | 153 | 5.86 (5.75–5.96) | 5.85 (5.75–5.95) | 259 | 5.80 (5.74–5.85) | 5.79 (5.74–5.84) |
| Quartile 2 | 201 | 5.83 (5.74–5.92) | 5.83 (5.74–5.92) | 175 | 5.75 (5.69–5.82) | 5.75 (5.69–5.82) |
| Quartile 3 | 125 | 5.78 (5.66–5.89)c | 5.78 (5.67–5.89)c | 211 | 5.70 (5.65–5.77)d | 5.70 (5.65–5.75)d |
| Quartile 4 | 154 | 5.66 (5.56–5.75)e | 5.66 (5.57–5.75)e | 233 | 5.70 (5.65–5.76)d | 5.71 (5.65–5.76)d |
CI: confidence interval.
aSubjects were divided into four groups based on quartile of serum total bilirubin within each gender (men—quartile 1, 0.30–0.50; quartile 2, 0.51–0.70; quartile 3, 0.71–0.86; quartile 4, 0.87–1.90 and women—quartile 1, 0.20–0.49; quartile 2, 0.50–0.60; quartile 3, 0.61–0.79; quartile 4, 0.80–1.78 mg/dL).
bAdjusted for all confounding factors in model 2 of Table 3.
c p < 0.02 versus quartile 2.
d p < 0.03 versus quartile 1.
e p < 0.01.
Discussion
To examine any possible contribution of serum T-B to HbA1c, we studied the relationship between confounding factors including serum T-B and HbA1c. We found that serum T-B was independently and negatively related to HbA1c. Increased serum T-B occurred in parallel with the decrease in HbA1c in both genders, and serum T-B quartiles were significantly and negatively associated with HbA1c, independent of other confounding factors in both genders. To our knowledge, this is the first study to indicate a negative relationship between mildly elevated serum T-B and HbA1c among Japanese middle-aged and elderly community-dwelling persons.
The precise mechanisms that lead to decreased HbA1c in individuals with increased serum T-B are not completely understood. HbA1c was significantly associated with an increase of oxidative stress.1 Numerous nonenzymatic antioxidants (e.g. vitamins C and E, glutathione (GSH), beta-carotene, ubiquinone, uric acid, bilirubin, etc.) exist in cells and relate to oxidative stress.19 Zelenka et al.20 demonstrated that mildly elevated serum bilirubin is generally associated with attenuation of oxidative stress and with better anthropometric parameters, decreased inflammatory status, increased glucose tolerance, fewer signs of cellular senescence, and enhanced mitochondrial function. Baranano et al.21 and Sedlak et al.22 showed that bilirubin is an antioxidant that protects cells from a 10,000-fold excess of oxidants, suggesting that the highly protective properties of HO-1 may be mediated predominantly through the action of bilirubin derived from HO-1 by inhibition of vascular endothelial activation and dysfunction in response to pro-inflammatory stress.23 An increase of oxidative stress and inflammation contribute as the most common causes of the pathogenesis of insulin resistance24 and atherosclerosis.25,26 Moreover, serum T-B correlated with several confounding risk factors for CVD, such as gender, age, smoking, alcohol, blood pressure, HDL-C, TG, LDL-C, diabetes, and obesity, and correlated directly with HDL-C.27 These contributions may appear to allow bilirubin to inhibit multiple steps in the pathogenesis of atherogenesis. In fact, current epidemiological studies have demonstrated that serum T-B is negatively correlated with risk of CVD.15,27–29 Therefore, mildly elevated serum T-B may inhibit the glycation of hemoglobin by reducing oxidative stress.
Serum bilirubin concentrations are affected by many factors including race, gender, age, smoking status, fasting, intake of numerous medications and/or plant products, and altitude.30 These factors are likely to influence biological impact of bilirubin production on human body. Since weight reduction is known to reduce several CVD risk factors, it is important to note that weight loss was associated with a linear increase in serum bilirubin level.31
There are some limitations to this study. Firstly, based on its cross-sectional study design, the present findings are inherently limited in the ability to eliminate causal relationships between confounding factors including serum T-B and HbA1c. Secondly, we could not eliminate the possible effects of underlying diseases (e.g. liver disease, gallstones, and excessive red cell destruction) and medications used for hypertension and dyslipidemia on the present findings. In this study, participants with serum T-B >2.0 mg/dL or ALT ≥ 100 IU/L or GGT ≥ 100 IU/L were excluded, but individuals with Gilbert’s syndrome could be included in the high T-B (1.00–2.00 mg/dL) group. Thirdly, secondary prevention interventions in obesity, hypertension, dyslipidemia, and diabetes mellitus may be successful in reducing confounding factors, thus attenuating the observed association of confounding factors with disease. Therefore, the demographics and referral source may limit generalizability.
In conclusions, the present study showed that serum T-B is strongly associated with HbA1c in both genders. The underlying mechanism behind this relationship is unclear but seems to be independent of confounding factors such as age, BMI, smoking status, drinking status, exercise habit, lipids, glucose, and medication. Further prospective population-based studies are needed to investigate the mechanism(s) underlying this association.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported, in part, by a grant-in-aid from the Foundation for the Development of the Community (2016). No additional external funding was received for this study. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ohara M, Fukui T, Ouchi M, et al. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res Clin Pract 2016; 122: 62–70. [DOI] [PubMed] [Google Scholar]
- 2. Bash LD, Selvin E, Steffes M, et al. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 2008; 168: 2440–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wadén J, Forsblom C, Thorn LM, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 2009; 58: 2649–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004; 141: 421–431. [DOI] [PubMed] [Google Scholar]
- 5. Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141: 413–420. [DOI] [PubMed] [Google Scholar]
- 6. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oda E, Kawai R. Bilirubin is negatively associated with hemoglobin A1c independently of other cardiovascular risk factors in apparently healthy Japanese men and women. Circ J 2011; 75: 190–19 5. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez D, Espejo-Gil A, Bernal-Lopez MR, et al. Association of HbA1c and cardiovascular and renal disease in an adult Mediterranean population. BMC Nephrol 2013; 14: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stocker R, Glazer AN,, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA 1987; 84: 5918–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008; 60: 79–127. [DOI] [PubMed] [Google Scholar]
- 11. Wu Y, Li M, Xu M, et al. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J Diabetes 2011; 3: 217–224. [DOI] [PubMed] [Google Scholar]
- 12. Jung CH, Lee MJ, Kang YM, et al. Higher serum bilirubin level as a protective factor for the development of diabetes in healthy Korean men: a 4 year retrospective longitudinal study. Metabolism 2014; 63: 87–93. [DOI] [PubMed] [Google Scholar]
- 13. Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Bio Med (Maywood) 2003; 228: 568–171. [DOI] [PubMed] [Google Scholar]
- 14. Ohnaka K, Kono S. Bilirubin, cardiovascular diseases and cancer: epidemiological perspectives. Expert Rev 2010; 5: 891–904. [DOI] [PubMed] [Google Scholar]
- 15. Kawamoto R, Ninomiya D, Hasegawa Y, et al. Mildly elevated serum bilirubin levels are negatively associated with carotid atherosclerosis among elderly persons. PLoS One 2014; 9: e114281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawamoto R, Ninomiya D, Hasegawa Y, et al. Mildly elevated serum total bilirubin levels are negatively associated with carotid atherosclerosis among elderly persons with type 2 diabetes. Clin Exp Hypertens 2016; 38: 107–112. [DOI] [PubMed] [Google Scholar]
- 17. Choi SW, Lee YH, Kweon SS, et al. Association between total bilirubin and hemoglobin A1c in Korean type 2 diabetic patients. J Korean Med Sci 2012; 27: 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horio M, Imai E, Yasuda Y, et al. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 2010; 56: 32–38. [DOI] [PubMed] [Google Scholar]
- 19. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008; 88: 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zelenka J, Dvořák A, Alán L, et al. Hyperbilirubinemia protects against aging-associated inflammation and metabolic deterioration. Oxid Med Cell Longev 2016; 2016: 6190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baranano DE, Rao M, Ferris CD, et al. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA 2002; 99: 16093–16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sedlak TW, Saleh M, Higginson DS, et al. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA 2009; 106: 5171–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawamura K, Ishikawa K, Wada Y, et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction Arterioscler Thromb Vasc Biol 2005; 25: 155–160. [DOI] [PubMed] [Google Scholar]
- 24. Vítek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem 2007; 43: 1–57. [DOI] [PubMed] [Google Scholar]
- 25. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction. A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 2003; 23: 168–175. [DOI] [PubMed] [Google Scholar]
- 26. Sela S, Shurtz-Swirski R, Awad J, et al. The involvement of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation among cigarette smokers. Israel Med Assoc J 2002; 4: 1015–1019. [PubMed] [Google Scholar]
- 27. Kimm H, Yun JE, Jo J, et al. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke 2009; 40: 3422–3427. [DOI] [PubMed] [Google Scholar]
- 28. Horsfall LJ, Nazareth I,, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation 2012; 126: 2556–2564. [DOI] [PubMed] [Google Scholar]
- 29. Kunutsor SK, Bakker SJ, Gansevoort RT, et al. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol 2015; 35: 716–724. [DOI] [PubMed] [Google Scholar]
- 30. Vítek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol 2012; 3: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson C, Weeke P, Fosbol EL, et al. Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: an analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism 2009; 58: 1109–1115 [DOI] [PubMed] [Google Scholar]