Abstract
Human bocaviruses (HBoVs), which were first identified in 2005 and are composed of genotypes 1–4, have been increasingly detected worldwide in pediatric patients with acute gastroenteritis. To investigate if HBoV infection is a risk factor of acute gastroenteritis in children younger than 5 years old, we searched PubMed, Embase (via Ovid), the Chinese Biomedical Literature Database (CBM), and the Cochrane Library for studies assessing the prevalence of HBoVs in individuals from Oct 25, 2005 to Oct 31, 2016. We included studies using PCR-based diagnostics for HBoVs from stool specimens of patients with or without acute gastroenteritis that carried out research for over 1 year on pediatric patients aged younger than 5 years old. The primary outcome was the HBoV prevalence among all cases with acute gastroenteritis. Pooled estimates of the HBoV prevalence were then generated by fitting linear mixed effect meta-regression models. Of the 36 studies included, the pooled HBoV prevalence in 20,591 patients with acute gastroenteritis was 6.90% (95% confidence interval (95% CI): 5.80–8.10%). In the ten studies with a control group, HBoVs were detected in 12.40% of the 3,620 cases with acute gastroenteritis and in 12.22% of the 2,030 control children (odds ratio (OR): 1.44; 95% CI: 0.95–2.19, p = 0.09 between case and control groups). HBoV1 and HBoV2 were detected in 3.49% and 8.59% of acute gastroenteritis cases, respectively, and in 2.22% and 5.09% of control children, respectively (OR: 1.40; 95% CI: 0.61–3.25; p = 0.43 and OR: 1.68; 95% CI: 1.21–2.32; p = 0.002, respectively). Current evidence suggests that the overall HBoV prevalence in children younger than 5 years old is not significantly different between groups with or without acute gastroenteritis. However, when HBoV1 was excluded, the HBoV2 prevalence was significantly different between these two groups, which may imply that HBoV2 is a risk factor of acute gastroenteritis in children younger than 5 years old.
Introduction
Acute gastroenteritis is one of the most common pediatric diseases. Its morbidity remains high among infants and young children, and acute gastroenteritis causes an estimated three million deaths annually as well as severe clinical problems and a high social burden. Although acute gastroenteritis is a common disease and a major public health problem worldwide, the etiological agent is not diagnosed in nearly 40% of cases [1–4].
On Oct 25, 2005, Allander et al. found that a novel virus, human bocavirus (family Parvoviridae, subfamily Parvovirinae, genus Bocaparvovirus), then named HBoV1, was associated with respiratory tract infection in children [5, 6]. In 2009–2010, three additional HBoV genotypes (HBoV2–4) were reported [7–13], inspiring interest in the role of HBoVs in children. A growing number of studies have subsequently shown that HBoV is frequently detected in the feces of children with acute gastroenteritis [14–16]. Some case-control studies reported that HBoV is just a bystander in acute gastroenteritis, but that finding disagrees with the results of other studies on this topic [17]. Given the conflicting reports, the field has not yet reached a consensus about the role of HBoVs in acute gastroenteritis. Thus, additional work is needed to determine if HBoVs have a pathogenic role in acute gastroenteritis. Here, we performed a systematic review and meta-analysis to investigate the role of HBoVs in pediatric patients younger than 5 years old who have acute gastroenteritis.
Materials and methods
Search strategy
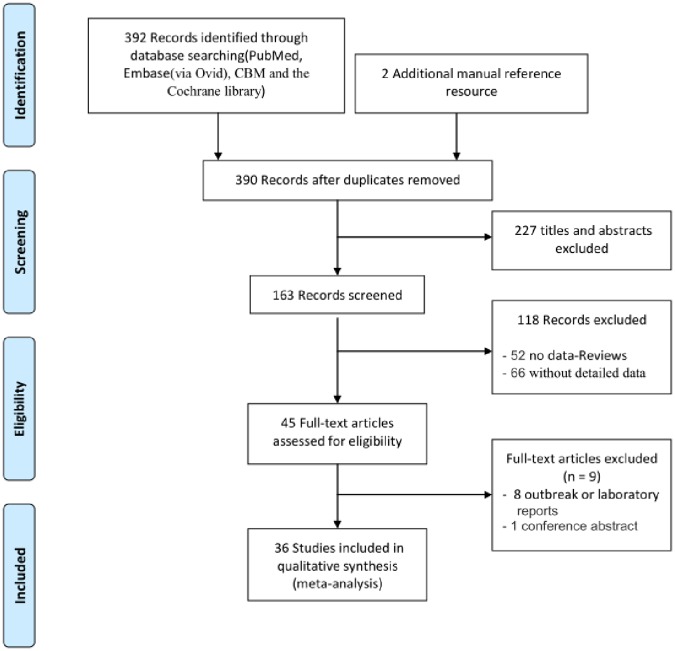
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [18], we performed a systematic, internet-based search using Pubmed, Embase (via Ovid), CBM(Chinese bibliographic database of biomedicine), and the Cochrane Library. The Boolean logic method was used to search all full articles. Fig 1 shows a list of the index or primary keywords used to search English and non-English studies. Since the initial evidence of HBoV infections was reported on Oct 25, 2005 by Allander et al. [5], our temporal limit for the search was set from Oct 25, 2005 to the date on which our study began, Oct 31, 2016.
Fig 1. Flow chart of the literature search.
The screening, and assessing of HBoV infection data for eligibility, and selecting articles for the meta-analysis.
Inclusion and exclusion criteria
After receiving training, two researchers independently reviewed the abstracts identified by keyword searches and selected articles for further detailed assessment according to the inclusion and exclusion criteria. When a study met the three requisite terms, 1) used PCR-based diagnostics for HBoV in stool specimens from patients with acute gastroenteritis infection or part of an asymptomatic control group, 2) carried out their research over at least 1 year in a specified area, and 3) studied pediatric patients aged under 5 years old, we downloaded the full manuscript text to organize or compress the qualified article in the next step. Studies were excluded if they were duplicate documents, had a lack of particular data, such as the number of HBoV-positive patients with acute gastroenteritis, had a lack of comparability between the case group and control group because they were collected from different hospitals or communities, or if they had low quality assessment scores.
Data extraction and stratification
The following information was extracted from each study when it was available: first author, year of publication, country, diagnostic method used, number of cases tested, number or rate of HBoV-positive cases, number of controls tested, and number or rate of positive controls. Data were stratified for descriptive analyses by the study type (with or without a control group), continent, diagnostic method, and HBoV prevalence level.
Statistical analysis
We generated pooled estimates of HBoV prevalence as the outcome in all pediatric patients with acute gastroenteritis who were under 5 years of age by using the software Stata 12.0. We used an unrestricted maximum likelihood linear random-effect meta-regression analysis that included global distribution (Asia, America, Europe, and Australia) and diagnostic methods (classical PCR, nested-PCR or semi-nested PCR, and real time PCR) as regressors for residual heterogeneity. The significant differences of the pooled HBoV prevalence were determined by using one-way ANOVA test of SPSS 20.0 (p < 0.05).
For studies with a control group, two of the authors of this manuscript assessed the quality of each by using the Newcastle-Ottawa Scale (NOS) in the three domains that are recommended by the Cochrane Collaboration [19]: the selection of study groups, the comparability of groups, and the exposure of groups.
Studies that received one star in every domain were judged as being of high quality. The data were then extracted from each study to calculate odds ratios (ORs) for risk effect estimates by a random effects model (DerSimonian and Laird) [20]. As a weighted average, an OR value that was located in the confidence region (p < 0.05) and greater than 1 suggested a higher risk factor. Cochrane’s Q statistic and the I2 index statistic, according to the method suggested by Higgins [21], were calculated for each analysis as a measure of the proportion of the overall variation that was attributable to between-study heterogeneity. An I2 of <25% indicates a low degree of heterogeneity, and an I2 of ≥75% suggests a high degree of heterogeneity. Egger’s test was used to evaluate the publication bias of studies with a control group, and a p-value of <0.05 was taken to indicate a significant publication bias. We performed several sensitivity analysis to test the robustness of our findings. All case-control studies were assessed with the software Stata 12.0.
Results
Search results and study selection
Based on the search strategy, we identified 392 studies from PubMed, Embase, CBM, and the Cochrane Library and 2 additional studies from the references of the identified studies. Of these, 4 duplicates, 227 with titles and abstracts only, 52 reviews, 66 without detailed data, 8 about outbreak or laboratory weekly reports, and 1 conference abstract were excluded (Fig 1 and S1 PRISMA Checklist). This left a final data base consists of 36 studies, including 10 studies with a control group.
Data extraction
We extracted data from the included 36 original publications and used it to form an Excel database (Table 1). The included studies were all published between Oct 25, 2005 and Oct 31, 2016, and they included data from 18 countries (Brazil, United States, Germany, Russia, Spain, Hungary, UK, Milan, Finland, China, Iranian, India, Pakistani, Japan, Korea, Thailand, Albania, and Australia). Overall, the systematic review included 20,591 children who were younger than 5 years of age. The studies were divided into four groups according to the continents on which the research had been performed (21 from Asia, 4 from America, 10 from Europe, and 1 from Australia) as well as into three groups based on the PCR method that had been used by the study (classical PCR group: 18 studies; real-time PCR group: 11 studies; and nested/semi-nested PCR group: 7 studies). Ten of these 36 studies contained control group data and met the criteria for NOS; these were applied to a meta-analysis composed of 5,650 cases (3,620 acute gastroenteritis patients and 2,030 healthy controls). The HBoV prevalence results of these studies were divided into different levels, ranging from <1% to >10%.
Table 1. Distribution of data among various strata from the 36 studies selected for meta-analysis.
| Number of studies (N = 36) | Median number of children tested per study (range) | Number of children tested (N = 20591) | |
|---|---|---|---|
| Types of studies | |||
| Studies with control-group | 10 | 276(96–878) | 3620 |
| Studies without control-group | 26 | 307(47–5250) | 16971 |
| Continents | |||
| Europe | 10 | 290(53–5250) | 10601 |
| America | 4 | 128(105–782) | 1565 |
| Asia | 21 | 365(47–1435) | 8239 |
| Australia | 1 | 186(-) | 186 |
| Diagnostic method | |||
| Classical PCR | 18 | 354(186–5250) | 12951 |
| Nested-/semi-nested PCR | 7 | 110(47–962) | 1946 |
| Real-time PCR | 11 | 381(200–1216) | 5694 |
| Level of HBoV prevalence (%) | |||
| <1 | 2 | 593(225–962) | 1187 |
| 1–2.5 | 6 | 1124(329–5250) | 10045 |
| 2.5–5 | 8 | 263(61–397) | 1970 |
| 5–7.5 | 3 | 418(273–1216) | 1907 |
| 7.5–10 | 10 | 200(45–527) | 3113 |
| >10 | 7 | 365(47–632) | 2369 |
Description analysis for the prevalence of HBoVs
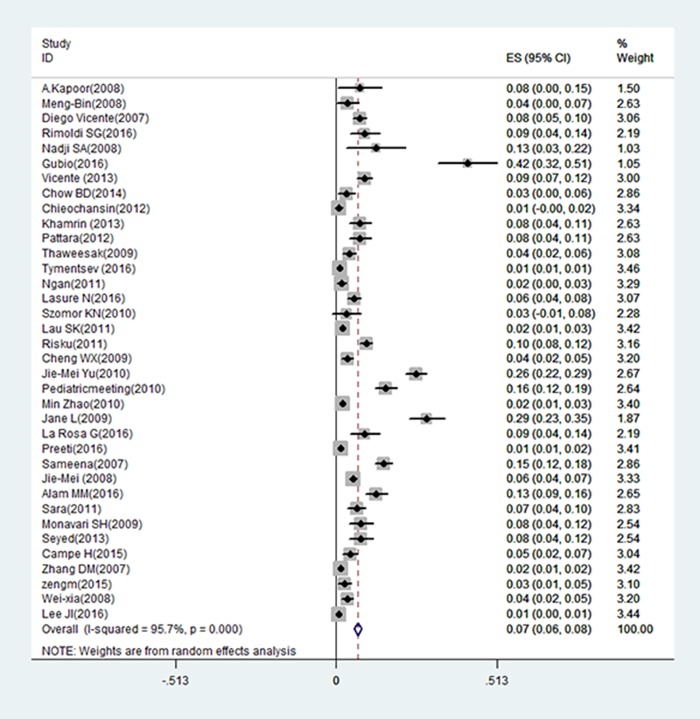
Among the included 36 studies, the overall HBoV prevalence in cases with acute gastroenteritis was 6.90% (95% confidence interval (95% CI): 5.80–8.10%) (Fig 2, S1 Text).
Fig 2. Forest plot showing pooled HBoV prevalence in patients <5 years old with acute gastroenteritis from 36 studies.
The ES (Expected shortfall) of pooled prevalence rate of HBoVs is 0.067 (95% CI: 0.056–0.078) using random effects modeled (D+L pool); I-squared (variation in ES attributable to heterogeneity) = 95.4%; estimate of between-study variance Tau2 = 0.0009.
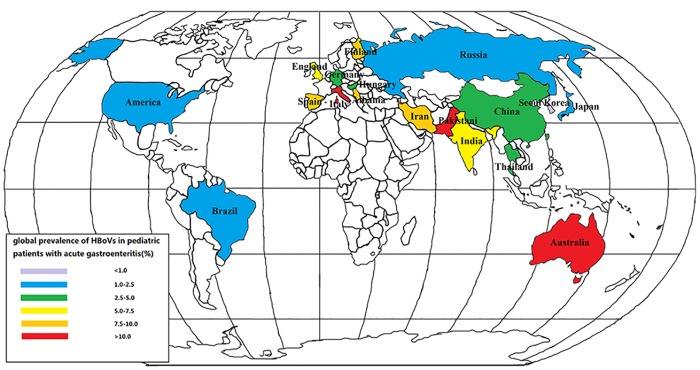
We mapped the global distribution of the HBoV incidences in pediatric patients younger than 5 years old with acute gastroenteritis that were reported from 18 countries during 2005 to 2016. The highest HBoV infection rate was reported in Australia (Fig 3). In China, the HBoV infection prevalence was 2.6–5%, as highlighted by the area shown with a green background in Fig 3, and most countries had 1.1–2.5% of their acute gastroenteritis population reported as positive for HBoV infection, as highlighted by the area with a blue background in Fig 3.
Fig 3. Reported global prevalence of HBoVs in pediatric patients <5 years old with acute gastroenteritis, from 2005 to 2016.
Red background indicates countries with >10% HBoVs population; orange background, 7.6–10%; yellow background, 5.1–7.5%; green background, 2.6–5%; blue background, 1.1–2.5%; and purple background, <1% population.
Based on data from 18 countries that were divided into four groups, the prevalence of HBoVs in children younger than 5 years old with acute gastroenteritis was 5.47% (95% CI: 5.04–5.90%; p < 0.05) in Asia, 6.90% (95% CI: 6.38–7.45%; p < 0.05) in America, 4.07% (95% CI: 2.82–5.33%; p < 0.05) in Europe, and 29.03% (only one report) in Australia. The distributions of HBoV prevalence among Asia, America, and Europe showed no statistical significant difference as determined by the ANOVA test.
We analyzed three groups based on the diagnostic methods used by the study. The HBoV prevalence was 4.96% (95% CI: 4.58–5.34%; p < 0.05) when assessed by classical PCR, 5.58% (95% CI: 4.98–6.18%; p < 0.05) when assessed by real-time PCR, and 6.10% (95% CI: 5.69–6.51%; p < 0.05) when assessed by nested- or semi-nested PCR. Although there was no significant difference in HBoV prevalence among these different diagnostic methods, as determined by the ANOVA test, this suggests there is a trend of HBoV prevalence being higher when nested or semi-nested PCR was used compared with when real-time PCR or classical PCR was used.
A meta-regression found that the test of heterogeneity I2 = 95.4%, Tau2 (Estimate of between-study variance) = 0.0009, adjusted R2 (Proportion of between-study variance explained) = 0.75% by variable of areas, and the adjusted R2 = 1.91% by variable of methods. These results suggest that the continents on which the study was conducted and the PCR methods used by the studies were both confounders and may be responsible for the detected heterogeneity between studies.
Meta-analysis for studies with a control group
The 10 of the 36 included studies that had control group data and met the criteria for NOS were applied to a meta-analysis. We performed a quality assessment for each of the individual studies based on the NOS recommended by the Cochrane Collaboration. A study can be awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars can be given for Comparability. Studies scoring 7–9, 4–6, and 0–3 are regarded as high-, moderate-, and low-quality, respectively. Of these ten studies, most were assigned over six stars in all three domains, which confirmed that they were of high quality (S2 Text).
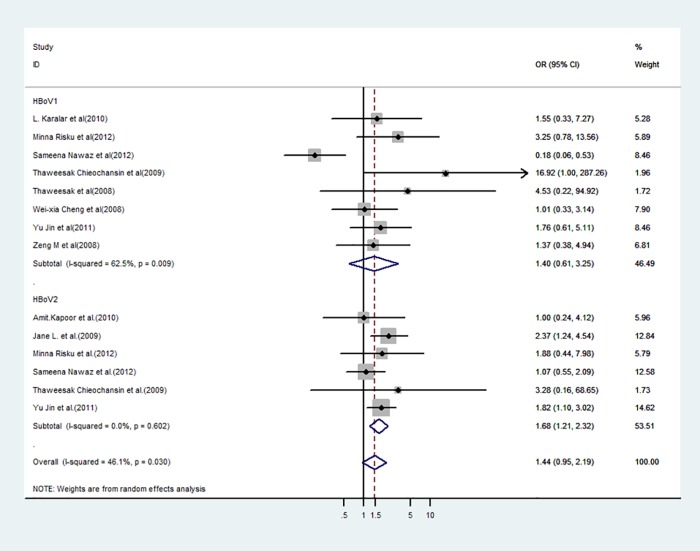
Among the 10 studies with a control group that were pooled in a meta-analysis (Table 2), HBoVs were detected in 12.40% (95% CI: 4.65–18.15%; p < 0.05) of cases with acute gastroenteritis and in 12.22% (95% CI: 4.39–17.01%; p < 0.05) of control children with an OR value of 1.44 (95% CI: 0.95–2.19 and the test of heterogeneity Tau2 = 0.25; I2 = 46%; p = 0.09) between the case and control groups, which revealed no statistical significance (Fig 4).
Table 2. HBoV prevalence in different groups among the 10 pooled studies with a control group.
| First author (year of publication) | City (Country) | Sample size(n) | In case-group with acute gastroenteritis | In control-group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases (n) | Number of HBoV positive (%) | Number of HBoV1 positive (%) | Number of HBoV2 positive (%) | Number of controls (n) | Number of HBoV positive (%) | Number of HBoV1 positive (%) | Number of HBoV2 positive (%) | |||
| Amit.Kapoor (2010) | San Francisco (USA) | 192 | 96 | 32 | 2 | 25 | 96 | 24 | 1 | 23 |
| Jane L (2009) | Adelaide (Australia) | 372 | 186 | 54 | 17 | 32 | 186 | 30 | 11- | 15 |
| L. Karalar (2010) | Regensburg (Germany) | 113 | 53 | 4 | 4 | - | 60 | 3 | 3 | - |
| Minna Risku (2012) | Tampere (Finland) | 990 | 878 | 85 | 49 | 29 | 112 | 6 | 1 | 0 |
| Sameena Nawaz (2012) | Liverpool (United Kingdom) | 1290 | 606 | 92 | 4 | 17 | 684 | 153 | 24 | 18 |
| Thaweesak Chieochansin (2009) | Bangkok (Tailand) | 540 | 327 | 14 | 12 | 2 | 213 | 0 | 0 | 0 |
| Thaweesak (2008) | Bangkok (Tailand) | 427 | 225 | 2 | 2 | - | 202 | 0 | 0 | - |
| Wei-xia Cheng (2008) | Lan zhou (China) | 512 | 397 | 14 | 14 | - | 115 | 4 | 4 | - |
| Yu Jin (2011) | Beijing (China) | 794 | 632 | 162 | 27 | 129 | 162 | 24 | 4 | 20 |
| Zeng M (2010) | Shanghai (China) | 420 | 220 | 6 | 6 | - | 200 | 4 | 4 | - |
| Total | 5650 | 3620 | 465(12.40) | 120(3.49) | 234(8.59) | 2030 | 248(12.22) | 41(2.22) | 76(5.09) | |
Fig 4. Forest plot of analysis on odds radio according to HBoVs infection and acute gastroenteritis.
CI: confidence interval, M–H: Mantel–Haenszel.
HBoV1 was detected in 3.49% (95% CI: 1.77–5.79%; p < 0.05) of cases with acute gastroenteritis and in 2.22% (95% CI: 1.32–3.80%; p < 0.05) of control children with an OR value of 1.40 (95% CI: 0.61–3.25) and a p-value of >0.05 as determined by the test of heterogeneity (Tau2 = 0.84; I2 = 63%; p = 0.43), which revealed no statistical significant difference. Additionally, the results of an Egger’s test did not suggest the presence of a publication bias (p = 0.464).
HBoV2 was detected in 8.59% (95% CI: 2.88–10.60%; p < 0.05) of cases with acute gastroenteritis and in 5.09% (95% CI: 1.76–8.72%; p < 0.05) of control group children with an OR value of 1.68 (95% CI: 1.21–2.32) and a p-value of <0.05 as determined by the test of heterogeneity (Tau2 = 0.00; I2 = 0%; p = 0.002), which revealed a statistical significant difference in the HBoV2-positive rates between the case and control groups. The results of an Egger’s test did not suggest the presence of a publication bias (p = 0.464).
We performed a sensitivity analysis to evaluate the effect of all included studies on the pooled ORs by omitting each HBoV group shown in Fig 4 in turn. The pooled ORs were not affected significantly by excluding any study, which revealed that all meta-analytic conclusions remained robust to this testing.
Discussion
Since HBoV1 was found in 2005, scientists worldwide have spent the last decade investigating the etiological role of HBoV1–4. HBoV1 is predominantly found in the respiratory tract, whereas HBoV2, HBoV3, and HBoV4 are mainly detected in stool [22]. However, many questions concerning HBoV still remain, especially regarding the role of HBoVs in acute gastroenteritis. In this study, we performed a systematic review and meta-analysis to assess if HBoV infection is a risk factor of acute gastroenteritis in children younger than 5 years of age.
Our results provide an updated estimate that HBoVs are associated with 6.90% (95% CI: 5.80–8.10%; p < 0.05) of all cases with acute gastroenteritis in children younger than 5 years of age. This finding supports previous descriptions of the human fecal virome in which Parvoviridae are frequently detected [17].
Most of the countries included in the present study had 1.1–2.5% of acute gastroenteritis patients reported as positive for HBoV. There is also a trend of decreasing prevalence from Australia (about 29.03%, based on only one study) to America (about 6.90%) to Asia (about 5.47%) to Europe (about 4.07%), as well as from nested- or semi-nested PCR (6.10%) to real-time PCR (5.58%) to PCR (4.96%) groups, but these differences were not statistically significant. The results of our meta-regression analysis show that the continents on which the studies were conducted and the PCR methods used by the studies were both confounders and may be responsible for the heterogeneity between studies. Therefore, it is possible that the differences of HBoV frequencies found among these countries may be related to the methodology used to detect the presence of HBoVs. This conclusion, which is in agreement with a report by Campos et al. [10], supports the importance of conducting an active HBoV surveillance over all continents in the world by using a uniform method.
Pooled estimates of HBoV prevalence were generated by fitting linear mixed effect meta-regression models. In the 10 studies with control groups (Table 2), HBoVs were detected in 12.40% of the 3,620 cases with acute gastroenteritis and in 12.22% of the 2,030 control children with an OR value of 1.44 (95% CI: 0.95–2.19; p = 0.09) between the case and control groups, which revealed that there is no statistical significant difference between these two groups (Fig 4). Despite the demonstrated low tropism of HBoVs for the human body, HBoVs were reported to show a high persistence in some sites, particularly in the lymphatic tissue [23], which may explain the high prevalence of HBoVs in the control group. However, when HBoV1, which was detected in 3.49% of acute gastroenteritis cases and in 2.22% of control children with an OR value of 1.40 (95% CI: 0.61–3.25; p = 0.43), was excluded, the prevalence of HBoV2, which was detected in 8.59% of acute gastroenteritis cases and in 5.09% of control children with an OR value of 1.68 (95% CI: 1.21–2.32; p = 0.002), was significantly different between these two groups of children. The results may imply that HBoV2 is a risk factor for acute gastroenteritis in children younger than 5 years of age, while HBoV1 detected in stools may be the product of viral discharge from the human body. In a review [17], Ong et al. reported the conclusion that HBoV2 was just a bystander in acute gastroenteritis, which is inconsistent with the findings in our work. These opposing conclusions may be due to the different inclusion and exclusion criteria and the selection of different studies in these analyses.
This systematic review has some shortcomings. First, age grouping was highly variable in published reports, which made it difficult to subgroup study populations into finite age groups. Second, not every included study had investigated all four HBoV genotypes. Due to the lower prevalence of some genotypes, it remains to be determined whether or not HBoV3–4 are also associated with acute gastroenteritis. Third, although we tried to reduce the causes of heterogeneity by performing subgroup analysis and regression analysis, the results heterogeneity tests show that the heterogeneity could not be completely eliminated. As such, we have discussed the residual heterogeneity in the clinical diversity and methodological diversity as well as the statistical heterogeneity. Another limitation is the lack of a large number of relevant research data. In particular, the two included case-control studies contain less than 200 samples each, which likely led to selection bias. More data should be accumulated to confirm the conclusion of this study.
Conclusion
In conclusion, current evidence suggests that even though HBoVs may be considered as a bystander of acute gastroenteritis, HBoV2 infection should be thought as a risk factor of acute gastroenteritis in children under 5 years old when HBoV1 has been excluded. However, this conclusion, which is based on the data provided by molecular methodology, should be treated with caution and should be confirmed with serology research.
Supporting information
(DOC)
(TXT)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Beijing Municipal Commission of Health and Family “The Special Fund for Capital Health Research and Development’’ (No. 2014-2-1132) and the Beijing Natural Science Foundation of China (No. 7152026).
References
- 1.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD,et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14: 725–730. doi: 10.1016/S1473-3099(14)70767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shang X, Fu X, Zhang P, Sheng M, Song J, He F, et al. An outbreak of norovirus associated acute gastroenteritis associated with contaminated barrelled water in many schools in Zhejiang, China. PLoS One 2017; 12: e0171307 doi: 10.1371/journal.pone.0171307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examinationof the English case-control Infectious Intestinal Disease Study (1993–1996). Eur J Clin Microbiol Infect Dis 2007; 26: 311–323. doi: 10.1007/s10096-007-0290-8 [DOI] [PubMed] [Google Scholar]
- 4.Amar CF, East CL, Grant KA, Gray J, Iturriza-Gomara M, Maclure EA, et al. Detection of viral, bacterial, and parasitological RNA or DNA of nine intestinal pathogens infecal samples archived as part of the english infectious intestinal disease study: assessment of the stability of target nucleic acid. Diagn Mol Pathol 2005; 14: 90–96. [DOI] [PubMed] [Google Scholar]
- 5.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 2005; 102: 12891–12896. doi: 10.1073/pnas.0504666102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broccolo F, Falcone V, Esposito S, Toniolo A. Human bocaviruses: possible etiologic role in respiratory infection. J Clin Virol 2015; 72: 75–81. [DOI] [PubMed] [Google Scholar]
- 7.Chow BD, Esper FP. The human bocaviruses: a review and discussion of their role in infection. Clin Lab Med 2009; 29: 695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam MM, Khurshid A, Shaukat S, Sharif S, Suleman RM, Angez M, et al. Human bocavirus in Pakistani children with gastroenteritis. J Med Virol 2015; 87: 656–663. doi: 10.1002/jmv.24090 [DOI] [PubMed] [Google Scholar]
- 9.Campe H, Hartberger C, Sing A. Role of Human Bocavirus infections in outbreaks of gastroenteritis. J Clin Virol 2008; 43: 340–342. doi: 10.1016/j.jcv.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 10.Campos GS, Silva Sampaio ML, Menezes AD, Tigre DM, Moura Costa LF, Chinalia FA, et al. Human bocavirus in acute gastroenteritis in children in Brazil. J Med Virol 2016; 88: 166–170. doi: 10.1002/jmv.24293 [DOI] [PubMed] [Google Scholar]
- 11.Cheng WX, Jin Y, Duan ZJ, Xu ZQ, Qi HM, Zhang Q, et al. Human bocavirus in children hospitalized for acute gastroenteritis: a case-control study. Clin Infect Dis 2008; 47: 161–167. doi: 10.1086/589244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhabra P, Payne DC, Szilagyi PG, Edwards KM, Staat MA, Shirley SH, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis 2013; 208: 790–800. doi: 10.1093/infdis/jit254 [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Cheng WX, Xu ZQ, Liu N, Yu JM, Li HY, et al. High prevalence of human bocavirus 2 andits role in childhood acute gastroenteritis in China. J Clin Virol 2011; 52: 251–253. doi: 10.1016/j.jcv.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 14.Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, et al. A newly identified bocavirus species in human stool. J Infect Dis 2009; 199: 196–200. doi: 10.1086/595831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, et al. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent inenteric infections. J Infect Dis 2010; 201: 1633–643. doi: 10.1086/652416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Zhu R, Qian Y, Deng J, Wang F, Sun Y, et al. Prevalence and phylogenetic analysis of human bocaviruses 1–4 in pediatric patients with various infectious diseases. PLoS One 2016; 11: e0160603 doi: 10.1371/journal.pone.0160603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong DS, Schuurman R, Heikens E. Human bocavirus in stool: A true pathogen or an innocent bystander? J Clin Virol 2016; 74:45–49. doi: 10.1016/j.jcv.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 18.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011; 39: 91–92. doi: 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015; 45: 139–145. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta- analyses. BMJ 2003; 327:557–560 doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund -Venermo M. Human bocavirus-the first 5 years. Rev Med Virol 2012; 22:46–64. doi: 10.1002/rmv.720 [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Gooding LR, Erdman DD. Human bocavirus in tonsillar lymphocytes. Emerg Infect Dis 2008; 14:1332–1334. doi: 10.3201/eid1408.080300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TXT)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.