ABSTRACT
Myeloid-derived suppressor cells (MDSCs) are known to play a critical role in the suppression of T cell antitumor responses. Our preclinical data showed that the phosphodiesterase (PDE)-5 inhibitor sildenafil impaired MDSC functions, enhanced intratumoral T cell activity and prolonged survival of melanoma-bearing mice. In this study, we evaluated biologic effects, safety and efficacy of palliative treatment with the PDE-5 inhibitor tadalafil in metastatic melanoma patients. We conducted an open-label, dose de-escalation trial with tadalafil in pretreated metastatic melanoma patients. Tumor and peripheral blood samples were taken before and 4 weeks after the start of treatment. Samples were investigated by immunohistochemistry and FACS analysis, for different immune subsets with numbers of CD8+ tumor-infiltrating lymphocytes (TIL) as primary end point. Stable disease was achieved in 3/12 patients (25%). Median progression-free survival was 4.6 mo (range 0.7–7.1), median overall survival (OS) 8.5 mo (range 2.7–23.7). The treatment was well tolerated. Stable patients displayed significantly higher numbers of CD8+ TIL in the center of metastases before treatment as compared with progressive patients. Upon the therapy, they showed increased expression of ζ-chain (used as a marker of T cell activation) in CD8+ and CD4+TILs and CD8+T cells in the peripheral blood as compared with baseline. Our study suggests that the PDE-5 inhibitor tadalafil can improve clinical outcome of advanced melanoma patients by enhancing antitumor immunity and highlights its potential application in combined melanoma immunotherapy.
KEYWORDS: Immunotherapy, melanoma, myeloid suppressor cells, phosphodiesterase-5 inhibitor, tadalafil
Introduction
Cutaneous melanoma is an aggressive and often lethal skin cancer.1-3 Even though various immuno- and targeted-therapies are approved, the overall success for advanced melanoma is still limiting.4,5 Insufficient antitumor reactivity could be related to an impaired host T cell immunity leading to tumor-induced tolerance mediated by regulatory T cells, tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs).6-8 In the last decade, MDSCs have been reported to be enriched during the tumor progression and to display a strong inhibition of antitumor T cells immune responses by multiple mechanisms.9-13 MDSCs represent a heterogeneous population of immature myeloid cells that fail to finish terminal differentiation.9,14 In human, they can be characterized as HLA-DR−/lowCD11b+CD14−CD15+ polymorphonuclear MDSCs (PMN-MDSCs) and HLA-DR−/lowCD11b+CD14+CD15− monocytic MDSCs (Mo-MDSCs).9-14 It has been reported that different MDSC subpopulations may function in different types of tumors.8,12,13 We have recently demonstrated an accumulation of Mo-MDSCs in advanced melanoma patients that was negatively correlated with the progression-free survival of these patients.15 Immunosuppressive function of MDSCs has been shown to involve the activation of inducible nitric oxide (NO) synthase and arginase-1.9-14 These two pathways induced an impairment of T cell functions associated with a pronounced downregulation of the TCRζ-chain expression, which plays a key role in coupling the TCR-mediated antigen recognition to diverse signal transduction pathways.16,17 Therefore, a depletion of MDSCs or abrogation of their immunosuppressive functions may be a promising tool for melanoma therapy.
Recently, phosphodiesterase (PDE)-5 inhibitors like sildenafil or tadalafil have been shown to exert antitumor effects in various tumors. It has been found that tadalafil was capable to inhibit MDSCs and restore T cell function in head and neck squamous cell carcinoma patients.18,19 Using a ret transgenic murine melanoma model, we demonstrated that chronic administration of sildenafil led to a significant increase in the overall survival (OS) of tumor bearing mice.6 Moreover, we found no toxic side effects of the drug at least 6 weeks after start of therapy. Importantly, we demonstrated that all above-mentioned effects of sildenafil were strongly associated with an increase in TIL numbers and an enhancement of TCR ζ-chain expression in T cells from primary tumors and metastatic lymph nodes.20 Since ζ-chain levels in TILs have been reported as a prognostic and survival biomarker in cancer patients,21,22 it can be used to measure the biologic effect of a PDE-5 inhibitor therapy.
We conducted this pilot study to characterize immunological responses associated with the PDE-5 inhibitor therapy in metastatic melanoma patients and to explore its therapeutic potential in a palliative setting. We used tadalafil because of its longer half-live compared with sildenafil with a daily dose in the treatment of pulmonary hypertension. Based on our preclinical data, the main aim of this trial was to test the hypothesis that tadalafil could strengthen a T cell-mediated antitumor immune reactivity, improving thereby the clinical outcome of the patients.
Results
Demographics
Of 15 patients screened, 12 patients from the Department of Dermato-Oncology of the National Center for Tumor Diseases (NCT) were included from March 2012 to January 2015 and treated within the trial with tadalafil. Three patients had to be excluded (screen failures) because of fast deterioration of general health status based on progressive disease (two patients) or withdrawal of consent (one patient). Clinical characteristics of patients are given in Table 1. There were six female and six male patients with a median age of 72 y (range 33–75 y) at trial inclusion. Metastatic site stage was predominantly M1c (66.7%). Elevated lactate dehydrogenase (LDH) levels (>248 U/L) were seen in half of the patients (6 of 12; 50%). ECOG performance status was 0 in 10 patients (83.3%) and 1 in 2 patients (16.7%). 11 patients had a primary cutaneous melanoma and 1 patient a mucosal melanoma arising from the nasal sinus. 5/12 patients (41.7%) carried a BRAF mutation-positive tumor including one patient with an inactivating BRAF D594N mutation. In two patients a Q61 NRAS mutation was observed.
Table 1.
Patient characteristics and treatment outcome.
| ID | Stage | Mutation status | Age (years) | Gender | Tadalafil dosage | Elevated serum LDH (yes/no)1 | Best response (irRC) | Previous therapies | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 01 | M1c | wt | 75 | Male | 40 mg | yes | PD | DTIC, Ipi, P/C | 2.2 | 2.8 |
| 02 | M1b | wt | 64 | Male | 40 mg | no | SD | DTIC | 4.3 | 13.2 |
| 03 | M1c | wt | 55 | Male | 40 mg | yes | PD | DTIC, Ipi | 1.8 | 4.6 |
| 04 | M1c | V600E | 48 | Female | 20 mg | no | PD | Ipi | 1.8 | 15.9 |
| 05 | M1c | wt | 70 | Male | 20 mg | yes | PD | DTIC, Ipi | 1.8 | 8.8 |
| 06 | M1c | V600 | 33 | Male | 20 mg | yes | PD | DTIC, Ipi, BRAF-I | 0.7 | 5.0 |
| 07 | M1c | wt | 61 | Male | 10 mg | yes | PD | DTIC, Ipi, P/C, T/G, trofosfamide | 1.8 | 2.7 |
| 08 | M1c | V600E | 60 | Female | 10 mg | no | SD | Ipi | 3.0 | 6.3 |
| 10 | M1a | NRas | 70 | Female | 10 mg | no | PD | Ipi/Nivo | 1.8 | 23.7+ |
| 11 | M1c | D594N | 58 | Female | 5 mg | yes | PD | none (CI Ipi) | 1.9 | 22.2+ |
| 14 | M0 | V600E | 68 | Female | 5 mg | no | SD | ECT, Ipi | 7.1 | 20.8+ |
| 15 | M1a | NRas | 75 | Female | 5 mg | no | PD | DTIC | 2.1 | 8.2 |
| Median: 4.6 | Median: 8.5 |
Abbreviations (alphabetical order): BRAF-I = BRAF inhibitor, CI = contraindication, DTIC = dacarbazine, ECT = Electrochemotherapy, Ipi = Ipilimumab, irRC: immune-related response criteria, LDH: lactate dehydrogenase, Nivo = Nivolumab, OS = overall survival, P/C = Paclitaxel/Carboplatin, PFS = progression free survival, PD = progressive disease, SD = stable disease, T/C = Treosulfan/Gemcitabin, wt = wildtype;
at the start of treatment.
11 patients had progressed on at least one prior standard treatment, most frequently ipilimumab and/or chemotherapy with 50% of patients having received at least two pretreatments (Table 1). One patient was allowed to receive tadalafil as a firstline treatment inside the trial since she had multiple sclerosis, which is a contraindication for ipilimumab as the only approved treatment with efficacy at that time. Notably, the multiple sclerosis was asymptomatic and did not require chronic immunosuppressive therapy.
Tadalafil has clinical activity in patients with metastatic melanoma
Of 12 included patients, 3 (25%) achieved a stable disease as best response to treatment (Fig. 1A). All of them had a progressing melanoma under the pretreatment with new lesions developing under either dacarbazine (one patient) or ipilimumab (two patients). The latter patients received 4 cycles of ipilimumab without relevant toxicity. One of them revealed a stable disease for 11 mo and then progressed. Tadalafil was started 14 months (mo) after the last dose of ipilimumab. The second patient progressed on ipilimumab and received tadalafil after confirmation of progressive disease 3 mo after the last ipilimumab dose (Fig. 1A).
Figure 1.
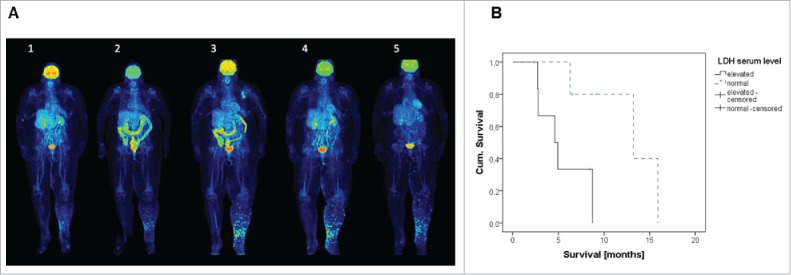
(A) PET-CT scans prior (1), after 4 cycles of ipilimumab (2), and 6 weeks later to exclude pseudo-progression (3); here progressive disease was confirmed and the patient included into the TaMe trial; after 8 weeks of treatment with tadalafil 5 mg daily p.o. (4) and after 5 mo (5). The scans show progressive disease under ipilimumab (1–3) and stable disease under tadalafil (3–5) – with a good regression of cutaneous metastases in the leg and a progressing left inguinal lymph node. (B) High LDH at treatment start was associated with poor prognosis under treatment with tadalafil.
Two out of the three stable patients carried a BRAF mutation-positive tumor. Remarkably, at trial inclusion, all of them had a low tumor load with serum LDH levels within normal limits. Statistically, a trend was detectable for LDH at treatment start as a marker for achieving a stable disease under tadalafil treatment (X2: p = 0.091). In addition, Kaplan–Meier analysis showed prognostic relevance for LDH regarding OS of patients (log-rank: p = 0.026; Fig. 1B) but not yet for progression-free survival (PFS; p = 0.086; data not shown). The effect of tadalafil was not related to dose because stable patients were found across different dose cohorts (5 mg, 10 mg and 40 mg).
PFS for all treated patients was 4.6 mo (range 0.7–7.1 mo). As of November 2016 median OS was 8.5 mo with 3/12 patients who were still alive (Table 1).
Tadalafil was safe in patients with metastatic melanoma
Overall, every patient in the study experienced one or more adverse events (AEs) with a median number of 7.5 (1–12) AEs per patient (Table S1). A total of 84 AEs were recorded during the study, 11 of 84 AEs (13.1%) of Grade 3–4. 6 of 84 (7.1%) severe AEs (SAEs) were registered in three patients. The most frequently reported AEs included vomiting/nausea (8.3%) and headache, fatigue and weight loss (3.6%) (Fig. 2). 15 of 84 (17.9%) AEs were thought to be treatment-related. One patient in the 10 mg dose-cohort experienced headaches that were resistant to pain medication and developed into a Grade 3 AE. The symptoms were thought to be related to the study drug and treatment was interrupted and dose held until toxicity returned to Grade 0–1. The administration of the study medication was then reduced by 50% to 5 mg tadalafil daily.
Figure 2.
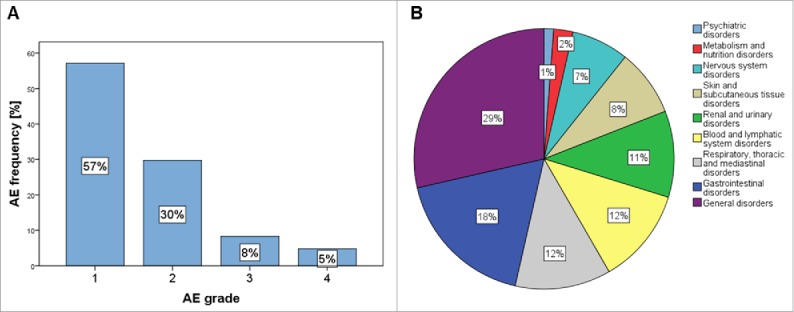
Adverse events: frequency of adverse events according to toxicity grades CTCAE4.0 criteria (A) and affected organ systems (B).
All six recorded SAEs were related to the underlying disease and disease progression, e.g., anasarca and ascites. There were no treatment-related deaths.
Treatment with tadalafil was well tolerated in patients with metastatic melanoma
Quality of life (QoL) was assessed using the SF-12™ Health Survey (SF-12) questionnaire at baseline, 4 and 8 weeks of treatment (Table S2). Data are complete except one missing score (last time point of patient 6), which could not be assessed due to rapid disease progression and death of the patient. SF-12 physical (PCS) and mental component scores (MCS) varied greatly among patients over the course of the trial. Comparing PCS and MCS at screening to the 4 week time point, we noted a minor decrease by a mean of 2.1 (range −11.0 to 19.1) points and 0.8 points (range −2.1 to 27.9), respectively (Fig. S1). There was no statistically significant difference in QoL between stable and progressive patients (Pearson correlation: p = 0.231 for PCS; p = 0.672 dor MCS) nor depending on the number of AE (Pearson correlation: p = 0.594 for PCS, p = 0.630 for MCS).
Modulation of MDSCs in melanoma patients by tadalafil
In this study, we investigated MDSCs in the peripheral blood and fresh tumor samples from melanoma patients before and after the treatment with tadalafil. Using flow cytometry, we analyzed the frequency of Mo- and PMN-MDSC subsets (that are defined as HLA−DR−/lowCD11b+CD14+CD15− and HLA−DR−/lowCD11b+CD14−CD15+ cells respectively) (11–14) (Fig. 3A). We found that frequencies of Mo-MDSCs from the peripheral blood of stable patients displayed a tendency to decrease after the treatment as compared with baseline (p = 0.071, Fig. 3B), whereas in progressive patients, the changes in Mo-MDSC frequencies after the treatment were not observed (Fig. 3C). Next, we tested the immunosuppressive pattern of MDSCs in treated patients. To address this question, we analyzed the production of NO known to be a molecule involved in MDSC functions by diaminofluorescein-2 diacetate using flow cytometry.8-14 In two of three stable patients, tadalafil induced a reduction of NO production by Mo-MDSCs infiltrating metastatic lesions as compared with the level before the therapy (Fig. 3D). In three of five progressive patients, the NO production was increased as compared with the level before the therapy (Fig. 3E). However, we failed to observe statistically significant differences in NO production by both MDSC subsets between stable and progressive patients (data not shown).
Figure 3.
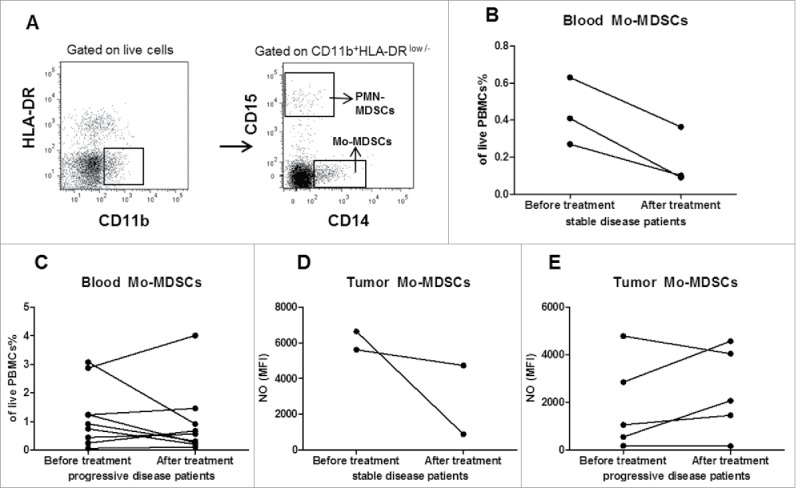
Evaluation of MDSCs in melanoma patients before and during tadalafil therapy. PBMCs and cells from melanoma metastases before and during tadalafil administration therapy were assessed by flow cytometry. (A) Representative dot plots with the gating strategy identifying Mo-MDSCs (HLA-DRlownegCD11b+CD15-CD14+ cells) and PMN-MDSCs (SSClowHLA-DRlownegCD11b+CD14-CD15+ cells). (B,C) The frequency of Mo-MDSCs in the peripheral blood of stable disease (B) or progressive disease patients (C) is presented as the percentage of viable PBMCs. (D,E) The intracellular concentration of NO in tumor-infiltrating Mo-MDSCs in stable disease (D) or progressive disease patients (E) before and during tadalafil treatment is expressed as mean fluorescence intensity (MFI).
Restoration of T cell activity upon treatment is associated with improved clinical outcome
Next, we examined whether tadalafil could modulate T cell frequency and activity. To this end, we first evaluated the distribution of CD4+ and CD8+T cells in the peripheral blood and freshly isolated tumor samples from melanoma patients treated with tadalafil. The number of CD8+ cells in progressive patients was significantly reduced upon treatment as compared with baseline (p = 0.039, Fig. 4A). We could not detect any changes in the number of CD8+ cells in patients with stable disease (Fig. 4B). To determine the impact of tadalafil on T cell activity, we tested the TCR ζ-chain expression. Upon therapy, stable patients displayed a significant elevation of ζ-chain expression in CD8+ TILs as compared with progressive patients (Fig. 4C). Moreover, we observed in stable patients an increase in the ζ-chain expression in tumor infiltrating and circulating CD8+ T cells during therapy as compared with the initial level (p = 0.0043 and 0.0283, respectively, Fig. 4D and E). In addition, we detected a strong elevation of the ζ-chain expression in CD4+ T cells in tumor samples taken from stable patients as compared with the baseline (p = 0.0261, Fig. 4F). However, circulating CD4+ T cells in these patients showed no statistically significant differences in the expression of ζ-chain (data not shown).
Figure 4.
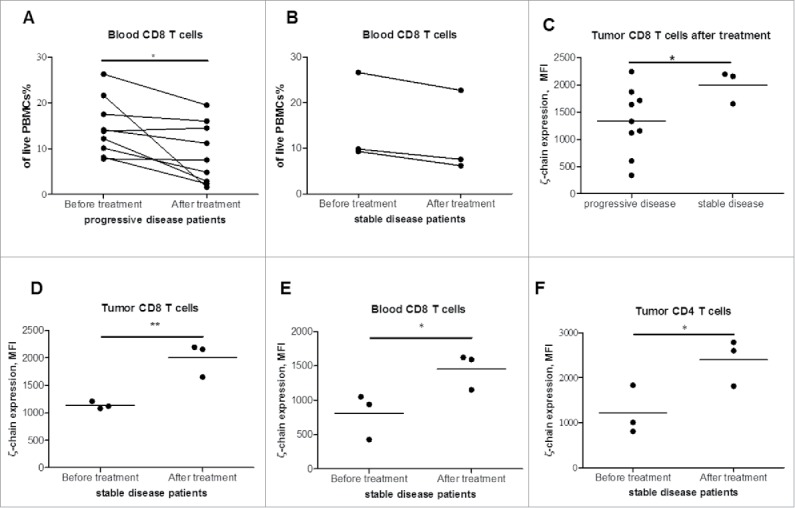
Analysis of T cells in melanoma patients treated with tadalafil. T cell frequency and activation status was assessed in the peripheral blood (PBMCs) and metastatic samples by flow cytometry. (A,B) The frequency of circulating CD8+ T cells of progressive disease (A) or stable disease patients (B) is shown as a percentage of viable PBMCs. (C) TCRζ-chain level is measured in CD8+ TILs in stable and progressive patients during tadalafil treatment and is expressed as MFI. (D,E,F) ζ-chain expression in CD8+ TILs (D) or circulating CD8+ T cells (E) or in CD4+ TILs (F) of stable patients before and during tadalafil therapy is presented as MFI (* p < 0.05, ** p < 0.01).
Clinically stable patients display accumulation of CD8+ T cells and reduction of regulatory T cells in melanoma metastases
Tumor tissue specimens were analyzed for their infiltration with T cells (CD3, CD8, FOXP3), B cells (CD20) and macrophages (CD163) by immunohistology. Computer-assisted analysis revealed a higher pretreatment level of infiltrating CD8+ T cells (p = 0.036; Fig. 5A) and a higher level of regulatory T cells (p = 0.057; Fig. 5B) in the center of the metastases from stable patients. Moreover, metastases of stable patients were characterized by a significant reduction of FOXP3+ regulatory T cells upon the treatment as compared with baseline (p = 0.044; Fig. 5B). No significant differences in numbers of B cells and macrophages were found between stable and progressive patients (data not shown). In addition, tumor samples were stained for PD-L1. Most tumors displayed PD-L1 expression. However, the treatment did not change it significantly (X2: p = 0.339). In addition, PD-L1 expression was not predictive for stabilization of the disease (X2: p = 0.513).
Figure 5.
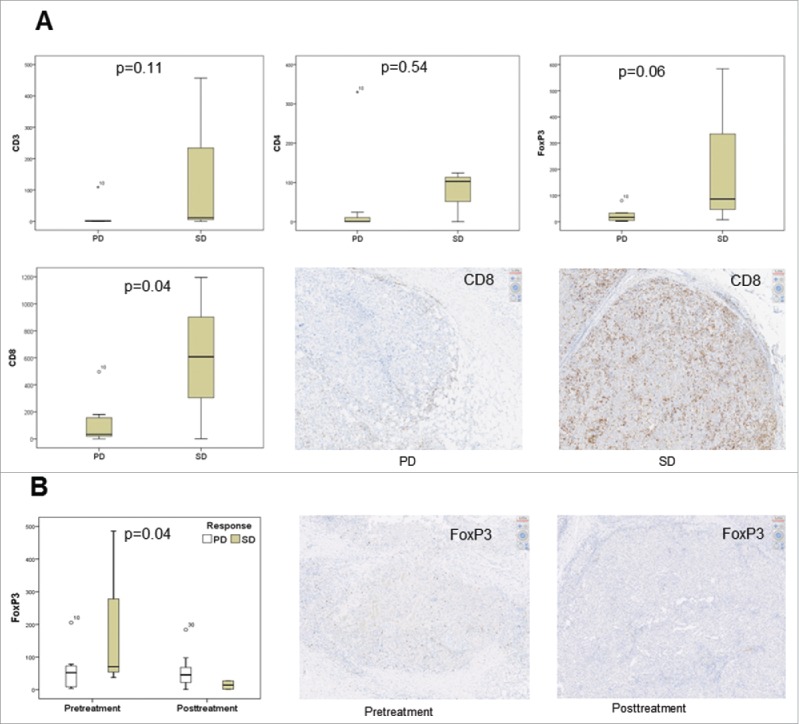
Tissue specimens before and after 4 weeks of treatment were immunohistochemically analyzed for their infiltration with T cells (CD3, CD8, FOXP3, PD-1), B cells (CD20) and macrophages (CD163). Computer-assisted analysis revealed a significantly higher number of infiltrating T cells, especially of the CD8+ phenotype, in stable patients pretreatment in the center of the metastases (p = 0.036) (A). After 4 weeks of treatment with tadalafil, no significant changes could be seen between stable and progressive patients with the exception of a drop in the infiltration with regulatory T cells (p = 0.044; Fig. 5B) (PD = progressive disease; SD = stable disease; y axis in box plots = mean cells/mm2).
Tadalafil concentration in melanoma metastases correlates with daily dose of tadalafil but not with clinical response
Even though patients with a stable disease were seen across the different dose cohorts, we quantified tadalafil to assess whether clinical benefit was concentration-dependent. Using a previously reported, validated tandem mass spectrometry method (UPLC-MS/MS),23 we quantified tadalafil in the 6 out of the 12 patients who had sufficient residual material for this analysis (Table S3). MS analysis revealed that tissue concentration in metastases taken under tadalafil treatment correlated well with the daily dosage of tadalafil (Table S3) also confirming good taking compliance. In contrast, plasma concentrations varied considerably, likely because they were taken on different time points of the dosing interval (between 8 and 10 a.m.), i.e., sometimes probably at the time of peak concentrations. Neither plasma nor tissue concentrations appeared to correlate with the clinical response. Interestingly, in the 5 mg dosage cohort tadalafil could not be detected in the melanoma tissue despite relevant plasma concentrations and even in patients with SD (i.e., patient 14).
Discussion
Within the past decade, a significant progress has been achieved in the treatment of metastatic melanoma especially by the application of immune checkpoint blockers.3,4 Nevertheless, about half of the patients do not respond to treatment probably due to the formation of a complex immunosuppressive network.5-8 One of the most important mechanisms of tumor progression mediated by immunosuppressive conditions is considered to be an inhibition of host antitumor T cell activity.6-8 Based on earlier results on the utilization of the PDE-5 inhibitor sildenafil in a ret transgenic mouse model,20 we investigated the immunomodulatory effect of tadalafil in patients with metastatic melanoma. Tadalafil led to a stabilization of the disease in 3 of 12 pretreated patients in the palliative setting. As expected, different daily doses of tadalafil lead to different tadalafil concentrations at the tumor site but patients with a clinical benefit were found across different dose cohorts including the 5 mg tadalafil low-dose group. Interestingly, tadalafil could not be detected intratumorally in the 5 mg dose cohort; hence, tadalafil seems to act especially on MDSC in the peripheral blood. Clinical analysis revealed that patients with a stable disease were characterized by a low tumor load (LDH values were within normal limits). LDH levels of the patients correlated not only with clinical responses but also patients' OS. This is in line with results on the treatment the immune checkpoint inhibitors such as ipilimumab and pembrolizumab, which demonstrated that patients with a high tumor load do not benefit from the treatment.24-26 A possible explanation for this is that tumor-reactive T cells are less efficient in accessing rapidly growing tumors. Of note, as seen in patient 14 (Fig. 1), tadalafil treatment led to a decrease in tumor burden in the patient's leg, an effect previously not achieved with ipilimumab. Hence, stable disease patients of our cohort did not just have a slowly growing melanoma.
In parallel to our trial, tadalafil had been used to treat head and neck cancer (HNSCC) patients.18,19 Tadalafil was given in an adjuvant or neoadjuvant setting to investigate its effects on immune subsets in the patients' peripheral blood. In contrast to our study, clinical efficacy could not be evaluated since patients had no measurable disease and treatment duration was short. However, the therapy with tadalafil was well tolerated in HNSCC patients.19 As in our study, the few severe AE were found to be unrelated to tadalafil. Three patients withdrew from the study because of severe pain (back, myalgias), which resolved after treatment cessation. We observed severe headache in one patient, which was reversible as well. In contrast to our patients, the patients with HNSCC did not experience nausea or vomiting.18,19 In our study, this was the most frequent AE considered to be drug-related. Nevertheless, the treatment was well tolerated having no impact on QoL of the patients as measured by the general health questionnaire SF-12.
Antitumor activity of PDE-5 inhibitors is considered to be induced by inhibition of MDSCs which play a major role in immunosuppression.8-14 In melanoma patients, numerous publications have been demonstrated that an increase in the frequency of MDSCs in the peripheral blood correlates with tumor progression and poor response to immunotherapy and targeted therapy.11-13,15,24,27-30 Therefore, several therapies aimed at reducing their number and abrogating their function.9-11,30-32 Another strategy for melanoma treatment involved the stimulation of MDSC differentiation to macrophages or dendritic cells, thereby reducing MDSCs expansion.33 Application of the PDE-5 inhibitor tadalafil represents a new approach to treat melanoma, leading to the impairment of MDSC functions and supporting thereby an antitumor T cell reactivity.18-20
We have previously shown that long-term administration of the PDE-5 inhibitor sildenafil resulted in a reduction of MDSC immunosuppressive functions and in an increase in CD8+ TIL numbers and activity (reflected by an elevation of TCR ζ-chain expression)in a spontaneous melanoma mouse model.20 Consistent with these findings, we found an upregulation of the TCR ζ-chain expression in CD4+ and CD8+ TILs in stable disease patients as compared with baseline. In addition, these patients showed an accumulation of CD8+ TILs in the center of metastases that could predict a beneficial response. Interestingly, also the number of regulatory T cells was higher in patients who then achieved a stable disease. After 4 weeks of tadalafil treatment they revealed a significant decrease of regulatory T cells compared with baseline as another possible indirect sign for MDSC impairment. MDSC have been demonstrated to promote regulatory T cell development in patients with hepatitis C or HIV-1 virus infection.34-36 Moreover, MDSC were shown to maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25− T cells and hence a regulatory T cell phenotype.37 It might be that tadalafil exhibited clinical efficacy especially in patients with a high number of intratumoral regulatory T cells. On the other side, compared with baseline, progressive patients displayed a decrease in the frequency of CD8+ T cells in the peripheral blood samples under tadalafil treatment. In the peripheral blood, we observed a trend to reduced Mo-MDSC numbers. A decrease in MDSC and an increase in tumor-reactive CD8+ T cell numbers was also observed in the patients with HNSCC.18,19 Hence, in both cancer types, tadalafil revealed biologic activity in the patients.
Notably, it has been recently reported that PDE-5 inhibitors may promote melanomas since in a large prospective study in the US sildenafil use was associated with an increased melanoma risk.38 A later large Swedish case-control study found that melanoma patients had significantly more single prescriptions of PDE-5 inhibitors.39 This effect was small and not seen with multiple prescriptions. Hence, the causality here is doubtful and might be biased by life-style of the people, e.g., an increased sun exposure. This is underlined by the fact a higher annual income was associated with melanoma risk as well. In addition, we found that at a daily dose of 5 mg tadalafil, which is the approved dose to treat erectile dysfunction, tadalafil could not be detected in the tumor tissue. However, in vitro studies found that PDE-5 inhibitors promoted the growth of human melanoma cell lines via a cGMP-dependent pathway especially but not exclusively in BRAF wildtype cell lines.40 PDE-5 was identified as one to be downregulated in response to BRAF activation, leading to increased cellular invasiveness.40 Furthermore, in a xenograft mouse model, an expression of PDE-5 in a human melanoma cell line led to reduced metastases in the lung.41 However, in mice sildenafil did not promote metastases. Hence, clinically the direct effect on melanoma cells seems to be negligible. In general, PDE-5 expression in melanoma metastases is low and further reduction might not be meaningful.41 This is underlined by the fact that melanoma stage was not associated with sildenafil treatment in the Swedish patient cohort.39
In conclusion, PDE-5 inhibition represents a potential new treatment option for patients with metastatic melanoma. Although tadalafil seems to have a limited efficacy as a monotherapy, tadalafil could be combined with immune checkpoint inhibitors and targeted treatments to potentially increase efficacy.
Patients and methods
Patients
Eligible patients (between 18–75 y of age) with evaluable unresectable stage III or IV melanoma (according to AJCC 2010)42 who had progressed on at least one prior therapy were treated with tadalafil. For inclusion in the trial an accessible metastasis on skin or lymph nodes for biopsy was mandatory. Major exclusion criteria were evidence of brain metastases, severe heart disease (e.g., NYHA functional class II–IV, myocardial infarction within 6 mo, ventricular tachyarrhythmia requiring ongoing treatment, unstable angina pectoris, sinusbradycardia) and other contraindications for tadalafil therapy. Prohibited concomitant medication were nitrates, α-blockers, e.g., doxazosin, and other interacting medications (CYP3A4 inhibitors and CYP3A4 inductors).
Study design and treatment schedule
This open label, monocenter phase I clinical pilot trial (EudraCT-No: 2011-003273-28) was designed to establish the biologic effect, optimal biologic dose, efficacy and tolerability of tadalafil as a palliative treatment of metastatic melanoma. The trial has been approved by the responsible ethics committee of the Medical Faculty of Heidelberg University and investigations on human samples have been done according to the Declaration of Helsinki. After obtaining written informed consent, patients were treated subsequently in cohorts of three in a dose deescalating design (40 mg–20 mg–10 mg–5 mg) starting with the highest approved dose (40 mg for pulmonary hypertension) and ending with the lowest approved dose (5 mg for erectile dysfunction). After the treatment of each cohort, the number of CD8+ TILs was evaluated by flow cytometry to decide if the dose could be lowered. Further cohorts were only included in the trial if the difference in the number of TILs between the higher and the lower dose did not exceed 20%. In addition, in case of no TIL increase of at least 10% after 4 weeks of tadalafil treatment in at least one patient in a cohort, the effect would have been considered as clinically not significant and no further patients would have been enrolled into the trial.
Study endpoints
The primary outcome parameter was the immune response as assessed by numbers of CD8+ and CD4+ T cells in the tumor tissue and the peripheral blood before and under treatment.
Secondary outcome parameters were (i) other immune response values such as number of MDSCs in tumor tissue and peripheral blood before and under treatment, (ii) number and distribution of T cell subtypes and macrophages in tumor tissue, (iii) response rate (CR + PR) and disease control rate (CR + PR + SD) according to immune-related response criteria (irRC)43 and the standard RECIST criteria 1.1, (iv) tolerability, (v) optimal dose for tadalafil, (vi) progression-free survival at 8 weeks of treatment, and (vii) QoL measured by the SF-12 questionnaire.
Assessment of clinical effect
Clinical efficacy
Whole body tumor assessments were performed as done in clinical routine before the start of treatment (baseline) and after 8 weeks. Response evaluation was performed according to irRC and the standard RECIST criteria 1.1. Responses were classified as immune-related complete response, immune-related partial response, immune-related stable disease or immune-related progressive disease.
Safety
All patients were monitored throughout the study for AEs, including immune-related AEs (irAEs). AEs were managed using protocol-specific guidelines and graded according to NCI CTCAE v4.0.
Modification of tadalafil doses
Patients had to be excluded from the study for any Grade 4 toxicity (according to NCI CTCAE v4.0).41 In case of Grade 3 toxicity, tadalafil was interrupted and doses held until toxicity returned to Grade 0–1. Subsequent were then reduced by 50%. If Grade 3 toxicity recurred at the reduced dose level, another 50% reduction was performed. Recurrent Grade 3 at this dose level required exclusion of the patient. No subsequent dose escalations were permitted after dose reduction for toxicity. Depending on the study cohort, the lowest possible dose was 2.5 mg per day. In addition, we defined further dose modifications and stopping rules for the management of specific AEs, e.g., hypotension.
Quality of life
The SF-12™ Health Survey (SF-12) questionnaire was used for evaluation of QoL. It has previously been developed and tested on the German general population.44,45 Time points for evaluation were at baseline, 4 and 8 weeks after the start of treatment.
Assessments of biologic effect
Patient samples
Prior and after 4 weeks of treatment with tadalafil, a biopsy from a cutaneous or lymph node metastasis was taken to evaluate tumor-infiltrating lymphocytes (TIL) by flow cytometry and histology using a high-resolution automated microscopy on complete tissue sections. At the same time point, 30 mL of blood was drawn for collection of peripheral blood mononuclear cells (PBMCs).
Flow cytometry analysis
PBMCs were obtained from heparinized venous blood by density gradient centrifugation centrifuged using Biocoll (Biochrom, #L6115), fresh tumor samples were harvested and mashed through cell strainer with a 5 mL syringe plunger. After depletion of red blood cells, the cell pellet was resuspended in FACS buffer at 107 cells/mL, analysis was done on the same day. The following fluorescent-labeled monoclonal antibodies (mAbs) were used: anti-CD11b-APC, anti-CD14-PerCP-Cy5.5, anti-CD15-PE, anti-HLA-DR-APC-Cy7, anti-CD3-APC-Cy7, anti-CD4-PE-Cy7, anti-CD8-APC, anti-CD45-PE-Cy7, anti-CD274-PE-Cy7 (BD Biosciences, # 550019, #550787, #555402, #561358, #557832, #557852, #555369, #557748, #558017, respectively), anti-FOXP3-PE (eBioscience, #12–4777), anti-CD45-PerCP-Cy5.5 and anti-CD247-FITC (Biolegend, #368504, #644104). PBMCs were treated with human FcR Blocking Reagent (Miltenyi Biotec, #130-059-901) for 15 min at 4 °C followed by the incubation with mAbs for 30 min at 4 °C. For intracellular staining of FoxP3, samples were pre-incubated with the FOXP3 fixation/permeabilization kit (eBioscience, #00-5521-00) according to the manufacturer's instruction. Acquisition was performed by six-color flow cytometry using FACSCanto II with FACSDiva software (both from BD Biosciences) with dead cell exclusion based on scatter profile or 7-AAD (Biolegend, #420204). The compensation control was performed with BD CompBeads set (BD Biosciences, #552843) using the manufacturer's instruction. FlowJo software (Tree Star, 7/9 Dongle) was used to analyze at least 100,000 events. Data were expressed as dot plots.
Immunohistochemical analysis
Tissue specimens were formalin fixed, paraffin embedded and cut to 4 µm slices. After deparaffinization and rehydration, the slides were boiled in 10 mmol/L citrate buffer (pH = 6) for 15 min to retrieve the antigens. The endogenous peroxidase activity was blocked by incubation with 0.6% H2O2 in methanol for 20 min. The sections were blocked with 10% normal horse serum (VECTASTAIN Elite ABC kit; Vector Laboratories). Mouse monoclonal antibodies recognizing human CD3ε (1:50 dilution; clone PS1; Acris, #DM112-05), CD8 (1:40 dilution; clone 4B11; Novocastra, #NCL-L-CD8-4B11), CD4 (1:100 dilution; clone NCL-L-368; Novocastra, #NCL-L-CD4-368), CD163 (1:500 dilution; clone EDHu-1; AbDSerotec, #MCA1853), PDL1 (1:100 dilution, clone 29E.2A3; BioLegend, #329710)and FOXP3 (1:100 dilution; clone 236A/E7; Abcam, #20034) were applied as primary antibodies at room temperature for 2 h. Slides were then incubated with the secondary antibodies and subsequent visualization was performed according to the manufacturer's instructions (Bond Polymer Refine Detection Kit, Leica #DS9800). Antigen detection was performed by a color reaction with 3,3-di-amino-benzidine. The sections were counterstained with hematoxylin and mounted with Aquatex (Merck, #1.08562.0050). Controls without primary antibody and isotype controls were used for all antibodies. Positive controls for the presence of T cells and macrophages consisted of adjacent normal tissue. Digital whole slide images of tissues with stained cells were quantified as described previously,46,47 using the Visiopharm software package for analysis and cell counting.
Tandem mass spectrometry (LC-MS/MS)
Tadalafil plasma concentrations were measured using a LC-MS/MS technique validated according to the Guidelines of the FDA and EMA on Bioanalytical Method Validation.23,48,49 The method fulfilled all validation criteria. For tadalafil quantification in metastases, this methodology was adapted to solid tissue analysis. The LLOQ of tadalafil in tissue was 1.26 ng/g and the calibrated concentration range was linear (1.26–1125 ng/g) with correlation coefficients >0.995. Within-batch accuracy and precision in the calibrated range was 91.5% and 1.8%, respectively.
Statistical analysis
Because this is a hypothesis-generating pilot trial, a patient sample size could not be estimated and has been selected for pragmatic reasons. Explorative analyses were performed for stable (SD) and progressive disease (PD) patients and statistical analysis was done with descriptive measures (X2, student t-test) using SPSS version 21. Kaplan–Meier analysis for survival was calculated from the start of treatment with tadalafil to progression (PFS) or death (OS) or patients were censored at last contact. Groups were compared by log rank testing. Results for MDSC and T cells were assessed with unpaired two-tailed Student's t test using GraphPad Prism software.
Supplementary Material
Disclosure of potential conflicts of interest
The authors state that there is no conflict of interests.
Funding
The trial was supported by the German Research Foundation (DFG). J.C.H. was supported by the Olympia Morata Grant of the Medical Faculty of the University Heidelberg. A.E., J.U. and V.U. are supported by the German Research Council (RTG2099).
References
- 1.Erdmann F, Lortet-Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, Bray F. International trends in the incidence of malignant melanoma 1953-2008–are recent generations at higher or lower risk? Int J Cancer 2013; 132(2):385-400; PMID:22532371; https://doi.org/ 10.1002/ijc.27616 [DOI] [PubMed] [Google Scholar]
- 2.Pflugfelder A, Kochs C, Blum A, Capellaro M, Czeschik C, Dettenborn T, Dill D, Dippel E, Eigentler T, Feyer P et al.. Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma.” J Dtsch Dermatol Ges 2013; 11 Suppl 6:1-116, 1-126; PMID:24028775; https://doi.org/ 10.1111/ddg.12113_suppl [DOI] [PubMed] [Google Scholar]
- 3.Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet 2014; 383(9919):816-27; PMID:24054424; https://doi.org/ 10.1016/S0140-6736(13)60802-8 [DOI] [PubMed] [Google Scholar]
- 4.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013; 25(2):268-76; PMID:23579075; https://doi.org/ 10.1016/j.coi.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33(17):1974-82; PMID:25605845; https://doi.org/ 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27:1-7; PMID:24413387; https://doi.org/ 10.1016/j.coi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol 2013; 35(5):585-600; PMID:23657835; https://doi.org/ 10.1007/s00281-013-0367-7 [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12(4):253-68; PMID:22437938; https://doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res 2015; 128:95-139; PMID:26216631; https://doi.org/ 10.1016/bs.acr.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 2016; 37(3):208-20; PMID:26858199; https://doi.org/ 10.1016/j.it.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann NY Acad Sci 2014; 1319:47-65; PMID:24965257; https://doi.org/ 10.1111/nyas.12469 [DOI] [PubMed] [Google Scholar]
- 12.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother 2012; 61(2):255-63; PMID:22120756; https://doi.org/ 10.1007/s00262-011-1161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol 2012; 144(3):250-68; PMID:22858650; https://doi.org/ 10.1016/j.clim.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 14.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S et al.. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7:12150; PMID:27381735; https://doi.org/ 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 2015; 136(10):2352-60; PMID:25353097; https://doi.org/ 10.1002/ijc.29297 [DOI] [PubMed] [Google Scholar]
- 16.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol 2003; 3(12):973-83; PMID:14647479; https://doi.org/ 10.1038/nri1245 [DOI] [PubMed] [Google Scholar]
- 17.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 2004; 4(9):675-87;PMID:22858650; https://doi.org/ 10.1038/nri1434 [DOI] [PubMed] [Google Scholar]
- 18.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C et al.. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015; 21(1):30-8; PMID:25564570; https://doi.org/ 10.1158/1078-0432.CCR-14-1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, Nazarian R, Califano J, Borrello I, Serafini P. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015; 21(1):39-48; PMID:25320361; https://doi.org/ 10.1158/1078-0432.CCR-14-1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M et al.. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A 2011; 108(41):17111-6; PMID:21969559; https://doi.org/ 10.1073/pnas.1108121108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichert TE, Day R, Wagner EM, Whiteside TL. Absent or low expression of the zeta chain in T cells at the tumor site correlates with poor survival in patients with oral carcinoma. Cancer Res 1998; 58(23):5344-7; PMID:9850063 [PubMed] [Google Scholar]
- 22.Boniface JD, Poschke I, Mao Y, Kiessling R. Tumor-dependent down-regulation of the ζ-chain in T-cells is detectable in early breast cancer and correlates with immune cell function. Int J Cancer 2012; 131(1):129-39; PMID:21823123; https://doi.org/ 10.1002/ijc.26355 [DOI] [PubMed] [Google Scholar]
- 23.Enderle Y, Meid AD, Friedrich J, Grünig E, Wilkens H, Haefeli WE, Burhenne J. Dried blood spot technique for the monitoring of ambrisentan, bosentan, sildenafil, and tadalafil in patients with pulmonary arterial hypertension. Anal Chem 2015; 87(24):12112-20; PMID:26583764; https://doi.org/ 10.1021/acs.analchem.5b03077 [DOI] [PubMed] [Google Scholar]
- 24.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D et al.. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res 2014; 20(6):1601-9; PMID:24323899; https://doi.org/ 10.1158/1078-0432.CCR-13-2508 [DOI] [PubMed] [Google Scholar]
- 25.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, Kapiteijn EW, de Groot JW, Soetekouw P, Jansen RL et al.. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63(5):449-58; PMID:24609989; https://doi.org/ 10.1007/s00262-014-1528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dick J, Lang N, Slynko A, Kopp-Schneider A, Schulz C, Dimitrakopoulou-Strauss A, Enk AH, Hassel JC. Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy 2016; 8(9):1033-44; PMID:27485076; https://doi.org/ 10.2217/imt-2016-0083 [DOI] [PubMed] [Google Scholar]
- 27.Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Görgens A, Giebel B, Schadendorf D, Paschen A. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer 2013; 133(7):1653-63; PMID:23526263; https://doi.org/ 10.1002/ijc.28168 [DOI] [PubMed] [Google Scholar]
- 28.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol. Immunother 2013; 62(11):1711-22; PMID:24072401; https://doi.org/ 10.1007/s00262-013-1475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pico de Coaña Y, Poschke I, Gentilcore G, Mao Y, Nyström M, Hansson J, Masucci GV, Kiessling R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res 2013; 1(6):438; PMID:24777678; https://doi.org/ 10.1158/2326-6066.CIR-13-0184 [DOI] [PubMed] [Google Scholar]
- 30.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A et al.. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res 2015; 21(24):5453-9; PMID:26289067; https://doi.org/ 10.1158/1078-0432.CCR-15-0676 [DOI] [PubMed] [Google Scholar]
- 31.Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol 2012; 22:319-26; PMID:22349515; https://doi.org/ 10.1016/j.semcancer.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol 2011; 77(1):12-9; PMID:20304669; https://doi.org/ 10.1016/j.critrevonc.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev Immunol 2009; 9(3):162-74; PMID:19197294; https://doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren JP, Zhao J, Dai J, Griffin JW, Wang L, Wu XY, Morrison ZD, Li GY, El Gazzar M, Ning SB et al.. Hepatitis C virus-induced myeloid-derived suppressor cells regulate T-cell differentiation and function via the signal transducer and activator of transcription 3 pathway. Immunology 2016; 148(4):377-86; PMID:27149428; https://doi.org/ 10.1111/imm.12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren JP, Wang L, Zhao J, Wang L, Ning SB, El Gazzar M, Moorman JP, Yao ZQ. Decline of miR-124 in myeloid cells promotes regulatory T-cell development in hepatitis C virus infection. Immunology 2017; 150(2):213-220; PMID:27753084; https://doi.org/ 10.1111/imm.12680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Zhao J, Ren JP, Wu XY, Morrison ZD, El Gazzar M, Ning SB, Moorman JP, Yao ZQ. Expansion of myeloid-derived suppressor cells promotes differentiation of regulatory T cells in HIV-1+ individuals. AIDS 2016; 30(10):1521-31; PMID:26959508; https://doi.org/ 10.1097/QAD.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang X, Zhang X, Liu Z, Xu H, Wang T, He L, Zhao A. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-β/β-catenin pathway. Mol Hum Reprod 2016; 22(7):499-511; PMID:27016139; https://doi.org/ 10.1093/molehr/gaw026 [DOI] [PubMed] [Google Scholar]
- 38.Li WQ, Qureshi AA, Robinson KC, Han J. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA Intern Med 2014; 174(6):964-70; PMID:24710960; https://doi.org/ 10.1001/jamainternmed.2014.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loeb S, Folkvaljon Y, Lambe M, Robinson D, Garmo H, Ingvar C, Stattin P. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA 2015; 313(24):2449-55; PMID:26103029; https://doi.org/ 10.1001/jama.2015.6604 [DOI] [PubMed] [Google Scholar]
- 40.Dhayade S, Kaesler S, Sinnberg T, Dobrowinski H, Peters S, Naumann U, Liu H, Hunger RE, Thunemann M, Biedermann T et al.. Sildenafil Potentiates a cGMP-Dependent Pathway to Promote Melanoma Growth. Cell Rep 2016; 14(11):2599-610; PMID:26971999; https://doi.org/ 10.1016/j.celrep.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 41.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell 2011; 19(1):45-57; PMID:21215707; https://doi.org/ 10.1016/j.ccr.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 42.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S et al.. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27(36):6199-206; PMID:19917835; https://doi.org/ 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G et al.. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res: Off J Am Assoc Cancer Res 2009; 15(23):7412-20; PMID:19934295; https://doi.org/ 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 44.Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34(3):220-33; PMID:8628042; https://doi.org/ 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 45.Bullinger M, Kirchberger I, Ware J. Der deutsche SF-36 Health Survey Übersetzung und psychometrische Testung eines krankheitsübergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität. Z f Gesundheitswiss 1995; 3:21-36; https://doi.org/ 10.1007/BF02959944 [DOI] [Google Scholar]
- 46.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B et al.. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res 2011; 71(17):5670-7; PMID:21846824; https://doi.org/ 10.1158/0008-5472.CAN-11-0268 [DOI] [PubMed] [Google Scholar]
- 47.Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, Suetterlin T, Brand K, Krauss J, Lasitschka F et al.. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-ccr5 therapy in cancer patients. Cancer Cell 2016; 29(4):587-601; PMID:27070705; https://doi.org/ 10.1016/j.ccell.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 48.US FDA Center for Drug Evaluation and Research Guidance for Industry. Bioanalytical Method Validation. 2001. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070107.pdf&usg=AFQjCNH9_-SV60XEIRd0uaX4tAN2plExQw&cad=rja [Google Scholar]
- 49.EMA Guideline on Bioanalystical Method Validation. 2012. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidline/2011/08/WC500109686.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


