ABSTRACT
Nivolumab, an anti PD-1 checkpoint inhibitor has demonstrated efficacy in metastatic non-small-cell lung cancer (NSCLC) patients after failure to standard chemotherapy. Standard chemotherapy agents could promote antitumor immune response. We thus examined whether the response to first line chemotherapy could impact on nivolumab benefit. One hundred and 15 patients with NSCLC were included in this retrospective study from 4 different French centers. Forty-three squamous cell carcinomas (SCC), and 72 non-SCC received nivolumab between 2015 and 2016 (3 mg/kg IV Q2W). Response to first-line chemotherapy and to nivolumab was retrospectively assessed on CT-scan by central review. The association between RECIST response to first-line chemotherapy and nivolumab efficacy were determined using Fisher's exact test and Cox proportional hazard model. Respectively 46 (40%), 44 (38%) and 25 (22%) patients experienced partial response (PR), stable disease (SD), or progressive disease (PD) in response to first-line platinum- based chemotherapy. Twenty 5 (21%), 34 (30%), 56 (49%) respectively experienced PR, SD and PD in response to nivolumab. 60% (54/90) of patients who experienced clinical benefit (PR + SD) after first-line chemotherapy also had clinical benefit after nivolumab, while only 20% (5/25) of patients with initial PD subsequently experienced clinical benefit with nivolumab (Fisher's exact test, P = 0.001). The type of first-line doublet chemotherapy did not influence the response rate to nivolumab. Univariate and multivariate analyses showed that patients with clinical benefit from first-line chemotherapy had higher second-line PFS (P = 0.003) (median PFS on nivolumab of 5, 3.3 and 1.9 months for patients with PR, SD and PD in response to first-line therapy, respectively). Similar results were obtained for OS. Thus this study suggests that the efficacy of first-line chemotherapy may be a valuable surrogate marker of the benefit of nivolumab in terms of PFS and OS.
Keywords: First-line chemotherapy, nivolumab, Non-small-cell lung cancer
Introduction
Lung cancer is a major public health issue and the leading cause of cancer-related death worldwide. In 2012, 1.8 million of new cases were diagnosed worldwide, representing around 13% of all detected cancers.1 Platinum-based doublet chemotherapy is the standard first-line treatment of patients with metastatic non-small-cell lung cancer (NSCLC) with no targetable oncogenic drivers, while tyrosine kinase inhibitors are the standard of care in first-line therapy for those harboring a genomic alteration of ALK, ROS1 or EGFR.2-4
Currently, it is well established that the immune system plays a key role in both the control of tumor induction, and tumor progression.5,6 Consequently, evasion of this immune surveillance mechanism by the tumor is a hallmark of cancer, and is a pre-requisite for tumor progression,7 especially in NSCLC. Overexpression of inhibitory receptors of the immune response, for example Programmed cell Death-Ligand 1 (PD-L1), is one of the mechanisms involved in tumor immunosubversion.6,8 Interaction between PD-L1 and its receptor PD-1 (on CD8 T cells) blunts T-cell antitumor functions, thus leading to immunoescape. In this context, the management of cancer and particularly of NSCLC, has taken a major step forward with the emergence of new therapies targeting immune checkpoints.9 Nivolumab, a highly selective, fully human IgG4 antibody directed against PD-1, can disrupt the engagement of PD-1 with its ligands (PD-L1 and 2) and prevent inhibitory signals in T cells, thus promoting the function of cytotoxic T cell effectors against tumor cells. Two randomized phase 3 trials have demonstrated the superiority of nivolumab over docetaxel as second-line treatment in advanced NSCLC patients,10,11 thus redefining the management of NSCLC patients. Anti-PD1 agents induce durable clinical responses regardless of the histological.12 However, only 20 to 25% of NSCLC patients experience a sustainable response to immune-checkpoint inhibitors.13-15 Therefore, finding biologic or clinical biomarkers that could help to select patients who respond to immune checkpoint blockade remains a key challenge.16
In addition to their capacity to directly kill tumor cells, cytotoxic agents may also affect the immune response. Notably, by killing tumor cells, cytotoxic agents could induce an antitumor immune response.17 These data provide a rationale to consider that effective standard chemotherapy inducing immunogenic cell death may improve antitumor immune response, whereas chemotherapy that is unable to kill a sufficient amount of cancer cells may not impact anticancer immune response. A previous meta-analysis has shown that response to the first-line regimen impacted modestly, but significantly, the response to second-line therapy.18 However, to the best of our knowledge, there are no data in the literature assessing the potential association between the response to the first-line chemotherapy doublet to the benefit of immunotherapy. Here, we examined in a cohort of 115 patients treated with nivolumab as second-line or third-line, whether response to first line platinum-based chemotherapy is associated with the benefit, in terms of response, Progression-Free Survival (PFS) and Overall survival (OS) of nivolumab.
Material and methods
Patients
This retrospective cohort included 115 patients with locally advanced (stage IIIB) or metastatic (stage IV) NSCLC experiencing disease progression during or after platinum-based doublet in 4 French thoracic oncology centers (Dijon University Hospital, Dijon Cancer Center, Center Léon Bérard and Nice University Hospital Biobank – BB-0033–00025). All consecutive patients who received nivolumab at the recommended dose (3mg/kg q2 weeks), regardless of whether patients received nivolumab in a clinical trial or after its EMA approval, were retrospectively included. We excluded from the analysis the patients who received nivolumab as first-line, or who did not receive platinum-based doublets as first-line treatment, as well as the patients for whom response to first-line chemotherapy could not be determined with RECIST criteria (see flowchart in Fig. S1). The following data were collected from each patients' medical records: age, sex, smoking status, EGFR and KRAS mutational status, ECOG performance status at the time of nivolumab initiation, nature of first-line platin-based doublet, best RECIST response to first-line chemotherapy, date of initiation of first-line chemotherapy, date of disease progression during or after first-line chemotherapy, number of treatment lines before nivolumab initiation, date of nivolumab initiation, best RECIST 1.1 response to nivolumab, date of progression during or after nivolumab and death from any cause or last follow-up. The database was closed on 1 February 2017. Three physicians (CK, JDF and AA) reviewed all CT-scans to validate response to first-line chemotherapy and to nivolumab. Approval from the local ethics committees was not necessary in accordance with French legislation governing strictly observational studies. The clinical database was declared and approved by the French authorities for data protection (CNIL, CCTIRS). PD-L1 expression was assessed by immunohistochemistry using SP142 mAb
Statistical analysis
All patients were followed up until death or the end of data recording (1 February 2017). Progression-free survival (PFS) was calculated (for first-line chemotherapy and for nivolumab treatment) as the time from the date of the treatment start to the date of disease progression by the RECIST criteria or death. A maintenance regimen was not considered as a second line. Overall survival (OS) was calculated as the time from the date of the first-line treatment start for metastatic disease, to the date of death. Median follow-up, with a 95% confidence interval (CI), was calculated using the reverse Kaplan-Meier method. Patient and disease characteristics were examined using the Fisher's exact test for qualitative variables, and the Kruskal-Wallis test for continuous variables, as appropriate, to compare groups of patients presenting either partial response (PR), stable disease (SD) or progressive disease (PD) to first line chemotherapy. Response to treatment was determined on CT scans using RECIST version 1.1.19 Response rates to nivolumab were compared with the response rate to the first-line regimen using the Fisher's exact test. Survival probabilities were estimated using the Kaplan-Meier method and survival curves were compared using the log-rank test. A multivariate Cox proportional hazard model was used to estimate the impact of response to first-line regimen on PFS and OS, after adjusting for clinical and pathologic parameters that were significant in univariate analysis. Statistical analyses were performed using the R software. All tests were 2 sided, and P values <0.05 were considered statistically significant.
Results
Patient characteristics
In the 4 participating centers, 151 patients were treated with second-line nivolumab monotherapy. When the database was closed, 101 patients were dead. Three patients who did not receive platinum-based chemotherapy as first-line and 3 patients who received nivolumab in first-line, were excluded from the analysis. Thirty patients additional were excluded because the radiological evaluation of response to first-line chemotherapy could not be performed (see flowchart of the study in Fig. S1). The final population included 115 patients, among whom 88 (77%) were men and 101 (87%) were smokers or former smokers. The most common histological type was adenocarcinoma (60%, n = 69), followed by squamous-cell carcinoma (36.4%, n = 43), large-cell neuroendocrine carcinoma (0.9%, n = 1), and large cell carcinomas (1.7%, n = 2). First-line regimen comprised carboplatin or cisplatin plus either gemcitabine (20 patients (17%)), docetaxel or paclitaxel [37 patients (32%)] and or pemetrexed [58 patients (50%)]. Only patients with adenocarcinoma received the platin-pemetrexed doublet. Only 9 patients received bevacizumab in addition to the platine-based first line doublet. The median age at diagnosis was 66.1 y. The clinical characteristics of the study population are listed in Table 1
Table 1.
Patient and tumor characteristics (n = 115).
| Response to first line |
||||||
|---|---|---|---|---|---|---|
| Partial response | Stable disease | Progressive disease | Overall | |||
| N=46 | N=44 | N=25 | N=115 | p-value | ||
| Age (year) | ||||||
| median (min;max) | 66 [36;85] | 63 [43;84] | 66 [55;80] | 66 [36;85] | 0.28 | |
| mean(sd) | 66 (11) | 63 (10) | 65 (8) | 65 (10) | ||
| Sex | ||||||
| Male | 36 (78%) | 33 (75%) | 19 (76%) | 88 (77%) | 0.93 | |
| Female | 10 (22%) | 11 (25%) | 6 (24%) | 27 (23%) | ||
| WHO PS | ||||||
| 0 | 18 (39%) | 15 (34%) | 7 (28%) | 40 (35%) | 0.64 | |
| 1 | 28 (61%) | 29 (66%) | 18 (72%) | 75 (65%) | ||
| Histology | Adenocarcinoma | 26 (57%) | 30 (68%) | 13 (52%) | 69 (60%) | |
| Epidermoid | 19 (41%) | 12 (27%) | 12 (48%) | 43 (37%) | 0.23 | |
| Other | 1 (2%) | 2 (5%) | 0 (0%) | 3 (3%) | ||
| EGFR | No | 26 (57%) | 30 (68%) | 13 (52%) | 69 (60%) | |
| Yes | 2 (4%) | 2 (5%) | 0 (0%) | 4 (3%) | 0.41 | |
| Unknown | 18 (39%) | 12 (27%) | 12 (48%) | 42 (37%) | ||
| KRAS | No | 15 (33%) | 24 (55%) | 10 (40%) | 49 (43%) | |
| Yes | 12 (26%) | 8 (18%) | 3 (12%) | 23 (20%) | 0.17 | |
| Unknown | 19 (41%) | 12 (27%) | 12 (48%) | 43 (37%) | ||
| PD-L1 | >1% | 11 (24%) | 14 (32%) | 8 (32%) | 33 (29%) | |
| negative | 20 (43%) | 9 (20%) | 4 (16%) | 33 (29%) | 0.08 | |
| Unknown | 15 (33%) | 21 (48%) | 13 (52%) | 49 (42%) | ||
| Smoking | Yes | 42 (91%) | 38 (86%) | 21 (84%) | 101 (88%) | |
| No | 4 (9%) | 6 (14%) | 4 (16%) | 14 (12%) | 0.62 | |
| First line doublet | gemcitabine | 7 (15%) | 9 (20%) | 4 (16%) | 20 (17%) | |
| taxane | 17 (37%) | 10 (23%) | 10 (40%) | 37 (32%) | 0.55 | |
| pemetrexed | 22 (48%) | 25 (57%) | 11 (44%) | 58 (51%) | ||
| Type of platin | Carboplatin | 20 (43%) | 18 (41%) | 12 (48%) | 50 (43%) | |
| Cisplatin | 26 (57%) | 26 (59%) | 13 (52%) | 65 (57%) | 0.85 | |
| Line of nivolumab | Second | 24 (52%) | 23 (52%) | 15 (60%) | 62 (54%) | |
| Third | 22 (48%) | 21 (48%) | 10 (40%) | 53 (46%) | 0.79 | |
Forty-six (40%) patients experienced radiological objective response, including 2 complete responses (CR) (1.7%); while 44 (38.3%) patients presented stable disease (SD) and 25 (21.7%) presented progressive disease (PD) as their best response during first-line chemotherapy. Sixty-two (54%) patients received nivolumab as second-line therapy and 53 (46%) as third-line. Best response to nivolumab was PR, SD and PD in 25 (21.7%), 34 (29.6%), 56 (48.7%) patients, respectively. Median PFS from start of nivolumab was 3 months (95%CI [2.1; .4.4]). Median OS after nivolumab initiation was 8.8 months (95%CI [7.2; NR]).
Association between nivolumab benefit and response to first line chemotherapy
We first investigated whether the type of chemotherapy received as first-line influenced the RECIST response rate (CR and PR) and PFS with nivolumab. We observed a similar response rate to nivolumab, irrespective of the type of doublet received as first-line (20%, 21.6% and 22.4% for gemcitabine, taxane, pemetrexed doublets, respectively, Fisher's exact test (P = 0.52)). Similarly, the type of doublet received in first-line did not influence nivolumab PFS (median PFS 4.4, 3.9 and 2.3 for gemcitabine, taxane, pemetrexed doublets, respectively; log-rank test P = 0.37) (Fig. S2). Similarly, we observed comparable response rates and PFS under nivolumab, irrespective of the use of cisplatin or carboplatin (18.5% and 26% of responders, respectively, P = 0.56; median PFS 2.4 and 4.1 months respectively, log-rank test P = 0.31) (Fig. S3).). The use of nivolumab as second- or third-line therapy did not influence the response rate or PFS (16.1% and 28.3% of responders in 2nd or 3rd line respectively, P = 0.28; median PFS of 2.8 and 3.3 months respectively, log-rank test P = 0.55) (Fig. S4). We then examined whether response to first-line therapy was associated with the response rate and PFS under nivolumab. Patients who had PR to first-line chemotherapy had an improved response rate to nivolumab. Interestingly, while 35% IC95% [0.21; 0.49] (16/46) of first line-responding patients (PR) obtained an objective response to nivolumab, the response rate was only 16% IC95% [0.05–0.27] (7/44) for patients with SD, and 8% IC95% [0.02–0.23] (2/25) for patients with PD during first-line chemotherapy, respectively (Fisher's exact test P = 0.001) (Fig. 1A). PFS from the initiation of nivolumab treatment was also significantly different according to best response to first-line treatment with a median PFS of 5, 3.3, and 1.9 months for patients with initial PR, SD or PD, respectively (P = 0.003, Fig. 1B). Response to first-line chemotherapy was significantly associated with the response rate and PFS in patients treated with nivolumab in the second-line setting. However, these results are not significant for patients treated with nivolumab in third-line setting Fig. S5). No significant differences were observed for other clinico-pathological features between the 3 groups of patients (Table 1).
Figure 1.
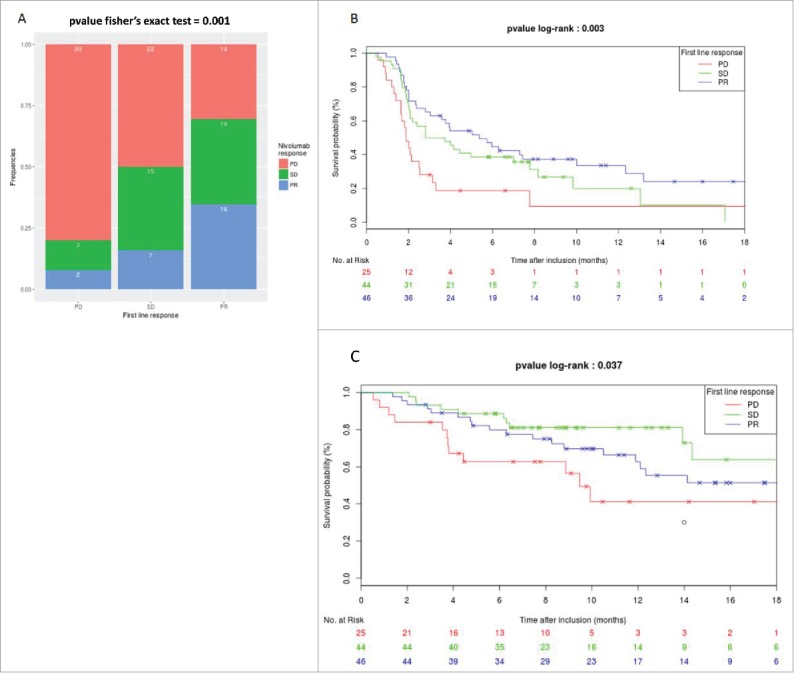
Association between response rate to first line regimen and efficacy of nivolumab. A. Repartition of response rate under nivolumab therapy in function of RECIST response to first line regimen therapy. B. Kaplan-meier curves of PFS under nivolumab therapy in function of RECIST response to first line regimen therapy. C. Kaplan-meier curves of OS under nivolumab therapy in function of RECIST response to first line regimen therapy.
Univariate Cox proportional hazard model indicated that an objective response to first-line therapy was significantly associated with improved PFS. In multivariate analyses, only gender and response to first-line were independently associated with improved PFS (Table 2). Similarly, a OS from the start of nivolumab treatment was significantly different in the 3 groups defined by best response to first-line treatment (P = 0.04), with median OS of 9.9, 8.6 and 6.6 months for patients with PR, SD and PD after first-line chemotherapy, respectively (Fig. 1C). Univariate and multivariate analyses also indicated that objective response to first-line therapy was significantly associated with an improvement of OS (Table 3).
Table 2.
Univariate and multivariate analysis (Cox regression) for factors associated with PFS.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | ||
| Age* | |||||||
| 0.98 | [0.96; 1.01] | 0.13 | 0.98 | [0.95; 1.01] | 0.18 | ||
| Sex | |||||||
| Female | 1 | 1 | |||||
| Male | 1.26 | [0.76; 2.1] | 0.36 | 1.81 | [1.02; 3.20] | 0.04 | |
| WHO PS | |||||||
| 0 | 1 | 1 | |||||
| 1 | 1.02 | [0.66; 1.59] | 0.92 | 1.05 | [0.62; 1.79] | 0.85 | |
| Histology | |||||||
| Adenocarcinoma | 1 | 1 | |||||
| Epidermoid | 0.87 | [0.56; 1.34] | 0.52 | 1.03 | [0.46; 2.31] | 0.94 | |
| Smoking | |||||||
| No | 1 | 1 | |||||
| Yes | 0.88 | [0.48; 1.63] | 0.70 | 1.06 | [0.55; 2.03] | 0.87 | |
| Stage | |||||||
| IIIB | 1 | 1 | |||||
| IV | 0.87 | [0.53; 1.79] | 0.93 | 0.63 | [0.28; 1.40] | 0.25 | |
| Line Nivolumab | |||||||
| 2 | 1 | 1 | |||||
| 3 | 0.88 | [0.57; 1.34] | 0.55 | 0.89 | [0.55; 1.44] | 0.63 | |
| Type of doublet | |||||||
| Pemetrexed | 1 | 1 | |||||
| Gemcitabine | 0.66 | [0.36; 1.22] | 0.18 | 0.58 | [0.24; 1.46] | 0.25 | |
| Taxane | 0.81 | [0.50; 1.30] | 0.38 | 0.70 | [0.0.29; 1.71] | 0.44 | |
| Type of platin | |||||||
| carboplatin | 1 | 1 | |||||
| cisplatin | 1.25 | [0.81; 1.92] | 0.31 | 1.13 | [0.64; 1.99] | 0.67 | |
| Response to first line | |||||||
| PD | 1 | 1 | |||||
| SD | 0.55 | [0.32; 0.95] | 0.03 | 0.43 | [0.23; 0.80] | 0.008 | |
| PR | 0.39 | [0.26; 0.69] | 0.001 | 0.32 | [0.17; 0.59] | 2.6e-4 | |
| KRAS | |||||||
| No | 1 | ||||||
| Yes | 0.65 | [0.35; 1.21] | 0.18 | ||||
| PD-L1 | |||||||
| Negative | 1 | ||||||
| >1% | 0.97 | [0.55; 1.69] | 0.90 | ||||
hazard ratio for continuous variable was calculated for one unit.
Table 3.
Univariate and multivariate analysis (Cox regression) for factors associated with OS:.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | ||
| Age* | |||||||
| 1 | [0.97; 1.04] | 0.91 | 1 | [0.96;1.04] | 0.80 | ||
| Sex | |||||||
| Female | 1 | 1 | |||||
| Male | 0.97 | [0.48; 2.00] | 0.94 | 118 | [0.55; 2.53] | 0.67 | |
| WHO PS | |||||||
| 0 | 1 | 1 | |||||
| 1 | 1.15 | [0.60; 2.22] | 0.66 | 1.23 | [0.57; 2.64] | 0.60 | |
| Histology | |||||||
| ADK | 1 | 1 | |||||
| CE | 0.87 | [0.46; 1.63] | 0.66 | 0.51 | [0.17; 1.59] | 0.25 | |
| Smoking | |||||||
| No | 1 | 1 | |||||
| Yes | 0.92 | [0.38; 2.19] | 0.84 | 0.82 | [0.32; 2.08] | 0.67 | |
| Stage | |||||||
| IIIB | 1 | 1 | |||||
| IV | 0.92 | [0.39; 2.20] | 0.85 | 0.63 | [0.57; 2.64] | 0.44 | |
| Line Nivolumab | |||||||
| 2 | 1 | 1 | |||||
| 3 | 1.32 | [0.71; 2.46] | 0.38 | 1.43 | [0.72; 2.83] | 0.30 | |
| Type of doublet | |||||||
| Pemetrexed | 1 | 1 | |||||
| Gemcitabine | 0.64 | [0.25; 1.62] | 0.35 | 0.91 | [0.27; 3.01] | 0.88 | |
| Taxane | 1.15 | [0.58; 2.27] | 0.68 | 1.59 | [0.48; 5.26] | 0.45 | |
| Type of platin | |||||||
| carboplatin | 1 | 1 | |||||
| cisplatin | 1.12 | [0.59; 2.09] | 0.73 | 1.12 | [0.51; 2.47] | 0.78 | |
| Response to first line | |||||||
| PD | 1 | 1 | |||||
| SD | 0.34 | [0.15; 0.80] | 0.01 | 0.30 | [0.12; 0.76] | 0.01 | |
| PR | 0.56 | [0.27; 1.17] | 0.13 | 0.48 | [0.21; 1.08] | 0.08 | |
| KRAS | |||||||
| No | 1 | ||||||
| Yes | 0.71 | [0.28; 1.80] | 0.47 | ||||
| PD-L1 | |||||||
| Negative | 1 | ||||||
| >1% | 1.40 | [0.63; 3.09] | 0.40 | ||||
hazard ratio for continuous variable was calculated for one unit.
Discussion
Monoclonal antibodies targeting PD-1 and PD-L1 have opened up a new field in thoracic oncology. Nivolumab, pembrolizumab and atezolizumab have demonstrated their superiority compared with chemotherapy in second-line setting, with median PFS of about 3 months, and median OS of approximately 12 months.10,11,20,21 The extent and durability of responses have revolutionized the conceptual approach to advanced lung cancer treatment. Pembrolizumab has demonstrated in first-line its superiority over platinum-based chemotherapy in patients with NSCLC with a high level of PD-L1 expression.22 However, less than a quarter of patients experience a clinical benefit from anti-PD-1 or anti-PD-L1 monoclonal antibodies. Therefore, biomarkers that can reliably predict the efficacy of immune checkpoint blockade are still lacking.
Here, we provide evidence supporting the fact that the quality of the tumor response obtained on first-line platinum-based chemotherapy may be a surrogate marker of subsequent benefit to nivolumab as second- or third-line therapy. In particular, in patients with PD after first-line chemotherapy, only 2 patients (8%) experienced PR to nivolumab (3 and 6.6 months of PFS for this 2 patients), with a median PFS of less than 2 months for this group of patients, raising questions about the clinical relevance of such treatment in this subset of patients.
Tumor labeling with anti PD-L1 antibody has been associated with better response to immune checkpoint inhibitors.23,24 However, among patients whose tumor was negative for PD-L1 expression by immunohistochemistry, about 10% exhibit a PR to anti PD-1/PD-L1 mAb, with a median PFS of 4 months.20 In addition, since PD-L1 expression is heterogeneous and variable of over time, PD-L1 is not considered a definitive biomarker.25,26 Thus, the identification of other markers remains a very active area of translational research.
First-line chemotherapy may affect patient's immune system. Recent evidence underlines that cell death induced by some cytotoxic agents could induce a process called “immunogenic cell death.” In mice and humans, some drugs, such as anthracyclines, cyclophosphamide and oxaliplatin induce this particular type of cell death, which induces the translocation of calreticulin to the cell surface, and release of HMGB1, ATP and annexin 1.27-29 These molecules promote phagocytosis of dying cells by dendritic cells, as well as optimal presentation of tumor antigen, and recruitment and optimal polarization of CD8+ T cells. This activation in turn promotes stimulation of patient's anti-tumor immune response, and a durable adaptive T cells response that can then be amplified by checkpoint inhibitors.30 Preclinical data in the context of spontaneous lung cancer reveals that the combination of cyclophosphamide and oxaliplatin is able to induce both immune response and tumor shrinkage, and may be synergic with checkpoint inhibitors.31
While carboplatin and cisplatin are usually considered to be non-immunogenic,32,33 it is currently unknown whether cytotoxics used in combination with cisplatin or carboplatin may be immunogenic. Indeed, while gemcitabine has been reported to induce immunogenic cell death,34 the immune properties of pemetrexed and taxane are not fully described. In addition to its capacity to induce immune cell death, chemotherapy may have other positive effects on the immune system. For example, gemcitabine can eliminate myeloid derived suppressor cells (MDSCs), a population of immune cells that blunt antitumor immune response.35,36 Furthermore, there is a strong rationale to consider that elimination of immunosuppressive cells may be a powerful tool to improve the efficacy of checkpoint inhibitors.37 Additional studies are required to determine the effects of pemetrexed and taxanes on immunosuppressive cells, such as regulatory T cells and myeloid derived suppressor cells.
Previous data suggest that tumors with a high mutational load have high neoantigen levels, and are thus more prone to induce T-cell immune response.38-40 These tumors are also considered to be the best candidates for immunotherapy.41,42 Such high levels of genetic instability could also lead to better response to cytotoxic agents, because high tumor genetic instability negatively impacts on the capacity of cancer cells to support additional damage induced by cytotoxic chemotherapies. This hypothesis should be tested in biomarker studies, using tumor exome sequencing.
Our data raise the hypothesis that clinical response to platinum-based doublet may trigger response and prime the efficacy of subsequent immune checkpoint blockers. However, previous data also suggest that response to the first-line regimen is also associated with better outcome of second line classical cytotoxic drugs.18 Thus, we cannot exclude the possibility that response to first-line chemotherapy is an intrinsic factor of better prognosis, regardless of the type of treatment used in second-line.
Our study has several limitations. First, the retrospective design implies that results must be interpreted with caution and must be validated in larger prospective trials and/or other retrospective cohorts of lung cancer patients. Similar results could also be easily validated in other cancers, where checkpoint blockers are currently used in second-line therapy, such as bladder or head and neck cancer. In addition, mixing patients treated by nivolumab as second- or third-line may have biased our results. However, similar results were obtained in both cohorts, suggesting that second-line therapy with cytotoxic agents did not impact on immunotherapy outcomes.
In conclusion, our study shows that objective response to first-line chemotherapy may be a valuable surrogate marker of benefit to the subsequent use of nivolumab as second or third line treatment. It also raises the hypothesis that cell death-induced by chemotherapy may promote the efficacy of nivolumab. Further biologic studies are required to determine whether clinically effective chemotherapy could positively affect in situ immune response. Our data may be rapidly validated by independent retrospective exploration of clinical trials using checkpoint inhibitors, such as pembrolizumab, atezolizumab and nivolumab in second-line therapy. Finally, our data also provide a rationale for the use of checkpoint inhibitor as maintenance therapy in case of good response to front-line chemotherapy.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2):87-108; PMID:25651787 [DOI] [PubMed] [Google Scholar]
- 2.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol 2012; 19(Suppl 1):S52-58; PMID:22787411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al.. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26(21):3543-51; PMID:18506025; https://doi.org/ 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group . Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Eng J Med 2002; 346(2):92-8; PMID:11784875; https://doi.org/ 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 5.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14(10):1014-22; PMID:24048123; https://doi.org/ 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331(6024):1565-70; PMID:21436444; https://doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3(11):991-8; PMID:12407406; https://doi.org/ 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 8.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Ann Rev Immunol 2011; 29:235-71; PMID:21219185; https://doi.org/ 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12(4):252-64; PMID:22437870; https://doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al.. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Eng J Med 2015; 373(17):1627-39; PMID:26412456; https://doi.org/ 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al.. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Eng J Med 2015; 373(2):123-35; PMID:26028407; https://doi.org/ 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27(4):450-61; PMID:25858804; https://doi.org/ 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettinger S, Herbst RS. B7-H1/PD-1 blockade therapy in non-small cell lung cancer: current status and future direction. Cancer J 2014; 20(4):281-9; https://doi.org/ 10.1097/PPO.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Eng J Med 2012; 366(26):2455-65; PMID:22658128; https://doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med 2012; 366(26):2443-54; PMID:22658127; https://doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al.. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515(7528):563-7; PMID:25428504; https://doi.org/ 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017; 17(2):97-111; PMID:27748397; https://doi.org/ 10.1038/nri.2016.107 [DOI] [PubMed] [Google Scholar]
- 18.Di Maio M, Lama N, Morabito A, Smit EF, Georgoulias V, Takeda K, Quoix E, Hatzidaki D, Wachters FM, Gebbia V, et al.. Clinical assessment of patients with advanced non-small-cell lung cancer eligible for second-line chemotherapy: a prognostic score from individual data of nine randomised trials. Euro J Cancer 2010; 46(4):735-43; PMID:20045311; https://doi.org/ 10.1016/j.ejca.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et al.. RECIST 1.1-Update and clarification: From the RECIST committee. Euro J Cancer 2016; 62:132-7; PMID:27189322; https://doi.org/ 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al.. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027):1540-50; PMID:26712084; https://doi.org/ 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 21.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al.. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387(10030):1837-46; PMID:26970723; https://doi.org/ 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 22.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al.. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Eng J Med 2016; 375(19):1823-33; PMID:27718847; https://doi.org/ 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 23.Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer 2016; 99:79-87; PMID:27565919; https://doi.org/ 10.1016/j.lungcan.2016.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer 2016; 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2016; 100:88-98; PMID:26895815; https://doi.org/ 10.1016/j.critrevonc.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, Committee IP. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thoracic Oncol 2015; 10(7):985-9 [DOI] [PubMed] [Google Scholar]
- 27.Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: A troika governing cancer and its treatment. Cell 2016; 166(2):288-98; PMID:27419869; https://doi.org/ 10.1016/j.cell.2016.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo M, Fu L. The effect of chemotherapy on programmed cell death 1/programmed cell death 1 ligand axis: some chemotherapeutical drugs may finally work through immune response. Oncotarget 2016; 7(20):29794-803; PMID:26919108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell Death Differ 2016; 23(6):938-51; PMID:26891691; https://doi.org/ 10.1038/cdd.2016.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015; 28(6):690-714; PMID:26678337; https://doi.org/ 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al.. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016; 44(2):343-54; PMID:26872698; https://doi.org/ 10.1016/j.immuni.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, et al.. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2011; 30(10):1147-58; PMID:21151176; https://doi.org/ 10.1038/onc.2010.500 [DOI] [PubMed] [Google Scholar]
- 33.Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 2014; 20(11):2831-7; PMID:24879823; https://doi.org/ 10.1158/1078-0432.CCR-13-3141 [DOI] [PubMed] [Google Scholar]
- 34.Gujar SA, Clements D, Lee PW. Two is better than one: Complementing oncolytic virotherapy with gemcitabine to potentiate antitumor immune responses. Oncoimmunology 2014; 3(1):e27622; PMID:24804161; https://doi.org/ 10.4161/onci.27622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70(8):3052-61; PMID:20388795; https://doi.org/ 10.1158/0008-5472.CAN-09-3690 [DOI] [PubMed] [Google Scholar]
- 36.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al.. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19(1):57-64; PMID:23202296; https://doi.org/ 10.1038/nm.2999 [DOI] [PubMed] [Google Scholar]
- 37.Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr, Bagala C, Colombi F, Cagnazzo C, Gioeni L, Wang E, et al.. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014; 25(9):1750-5; https://doi.org/ 10.1093/annonc/mdu205 [DOI] [PubMed] [Google Scholar]
- 38.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al.. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351(6280):1463-9; PMID:26940869; https://doi.org/ 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM, et al.. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015; 350(6257):207-11; PMID:26359337; https://doi.org/ 10.1126/science.aad0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun DA, Burke KP, Van Allen EM. Genomic approaches to understanding response and resistance to immunotherapy. Clin Cancer Res 2016; 22(23):5642-50; PMID:27698000; https://doi.org/ 10.1158/1078-0432.CCR-16-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348(6230):124-8; PMID:25765070; https://doi.org/ 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al.. PD-1 Blockade in tumors with mismatch-repair deficiency. N Eng J Med 2015; 372(26):2509-20; PMID:26028255; https://doi.org/ 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


