ABSTRACT
Purpose: DNA demethylating agents have shown clinical effectiveness in hematological and solid tumors. This trial tested the safety, efficacy, and treatment outcomes of decitabine-based chemotherapy or combined with immunotherapy in recurrent ovarian cancer patients.
Patients and methods: Fifty-five patients with recurrent ovarian cancer were enrolled and 52 were assessable for clinical response and survival. Patients either received 5-d decitabine treatment, followed by reduced-dose of paclitaxel/carboplatin administration (DTC cohort), or the aforementioned regimen combined with cytokine-induced killer cells therapy (DTC+CIK cohort). The primary end point was clinical response rate and progression-free survival (PFS). Secondary evaluation included safety assessment and overall survival (OS).
Results: Disease control rate (DCR) and objective response rate (ORR) were 73.91% and 23.91% in disease measurable patients by RECIST criteria, totally 76.92% and 30.77%, including disease non-measurable patients, which were higher in platinum-resistant/refractory patients. Clinical benefits could be associated with the number of DAC treatment cycles and the inclusion of CIK immunotherapy. In DTC+CIK cohort, DCR and ORR reached 100% and 58.30%, respectively. Notably, DTC+CIK treatment in platinum-resistant/refractory patients had an ORR of 87.50%. Consistently, PFS was longer in platinum-resistant/refractory patients comparing with that of platinum-sensitive patients. PFS and OS were 8 and 19 mo in platinum-resistant/refractory patients with DTC+CIK therapy. The most common toxicities were nausea, anorexia, fatigue, neutropenia, and anemia; many of which were grade 1–2.
Conclusion: Low-dose DAC/paclitaxel/carboplatin regimen demonstrates disease benefit, especially in patients with platinum-resistant/refractory ovarian cancer, and might show remarkable clinical response when combined with adoptive immunotherapy in platinum-resistant/refractory ovarian cancer patients.
KEYWORDS: CIK therapy, decitabine, epigenetic therapy, platinum sensitivity, recurrent ovarian cancer
Introduction
Ovarian cancer is the most lethal gynecologic malignancy, with over 50% ovarian cancer patients being diagnosed at an advanced stage. The current standard treatment of advanced ovarian cancer is both surgical cytoreduction and chemotherapy with a two-drug combination of paclitaxel (175 mg/m2) and carboplatin (area under the curve 5–6). Initially, most patients are responsive to first-line platinum-based therapy with a complete remission rate of about 70%. However, many patients will ultimately experience disease progression and resistance to the first-line regimen. Patients who have a relapse of more than 6 mo after the last platinum-based therapy were traditionally termed as having “platinum-sensitive disease,“1,2 with ORR of 30% or more to second-line platinum.3,4 On the other hand, patients recurring within 6 mo of last platinum-based therapy were called “platinum-resistant” or having “refractory disease.”1,2 For these latter patients, distinct strategies devoid of platinum have been suggested, such as maintenance therapy, new drug combinations, molecular targeted therapy, and immunotherapies.5 Clinical trials using various agents, such as pegylated liposomal doxorubicin (PLD), topotecan, paclitaxel, or gemicitabine, have been performed with ORR ranging between 10% and 20%, and the median PFS being about 3.7–4.0 mo.6-8 Although new drugs have shown some advantages in selected cases, the OS in platinum-resistant/refractory patients has not improved. Therefore, exploration of novel drugs or treatment strategies for patients with recurrent platinum-resistant/refractory ovarian cancer is urgently needed.
Reversal of drug-resistance, especially to platinum drugs, is a central issue in ovarian cancer therapy. Aberrant DNA hypermethylation contributes to the development and progression of ovarian cancer, and increasingly, preclinical and clinical studies have shown that non-toxic low-dose DNA methyltransferase (DNMT) inhibitors can increase the expression of hypermethylation-silenced tumor suppressor genes and thus enhance the sensitivity of ovarian cancer cells to chemotherapeutic drugs.9-11 Matei, et al. reported that in 17 platinum-resistant ovarian cancer patients, low-dose DNA demethylating agent, decitabine (DAC), in combination with carboplatin induced an ORR of 35%, with PFS and OS being 10.2 and 13.8 mo, respectively.9 Although this degree of clinical activity with DAC and carboplatin combinatory regimens is encouraging, the ORR and OS are still limited, and exploring superior DAC-based strategies in a larger patient cohort with platinum-resistant/refractory ovarian cancers is warranted.
We hypothesized that since accumulating evidence suggests that ovarian cancer is immunogenic, that epigenetic therapy may be synergistic with immunotherapy and that the presence of tumor-infiltrating T cells correlates with favorable clinical outcome in advanced ovarian carcinoma,12-14 the combination of epigenetic therapy and adoptive immunotherapy would show improved clinical activity. Cancer adoptive immunotherapy, such as the use of cytokine-induced killer cells (CIKs), has shown objective clinical responses in many solid tumors, including renal cell cancer, non-small-cell lung cancer, and hepatocellular cancer.15-17 Although potential efficacy of CIK cells as maintenance therapy and significant antitumor capacity of CIK cells against ovarian cancer in vitro, the clinical effect of CIK monotherapy in ovarian cancer patients has not translated satisfactorily to the bedside.18,19
Here, in this phase II clinical trial, based on the results of our phase I study, which tested the safety of low-dose decitabine monotherapy or combined therapy in solid tumor patients, we tested the clinical efficacy of three-drug combination of DAC, paclitaxel and carboplatin (DTC) regimen in a large population with ovarian cancer. Our aim was to determine the optimal DAC-based treatment strategy in ovarian cancer with an emphasis on patients with recurrent platinum-resistant/refractory ovarian cancer.
Methods
Patient population
Patients were enrolled from April 12, 2012 to April 12, 2016. Women were eligible if they had a histologically or cytologically confirmed epithelial ovarian or fallopian tube cancer, which had either (1) progressed while receiving or within 4 weeks of receiving at least four cycles of platinum-based chemotherapy (defined as platinum-refractory), (2) recurred during 1–6 mo after completion of the aforementioned platinum therapy (defined as platinum-resistant recurrence), (3) recurred more than 6 mo after completion of the platinum-based therapy (defined as platinum-sensitive recurrence).2 Women also had to be over 18 y old; have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and have adequate renal, hepatic, and hematologic functions. For patients with non-measurable disease, either clinically or radiographically, the cancer antigen (CA-125) levels had to be more than twice the upper limit of normal within 2 weeks of starting the trial. Women were excluded if they were pregnant or lactating; had an ECOG performance status >3; had uncontrolled infectious disease; had other serious diseases, such as HIV, active hepatitis, systemic autoimmune disease, or immunodeficiency disease; or had received prior treatment with immunosuppression agents. All patients provided written informed consent and the protocol was approved by the institutional review board of PLA General Hospital (2012–062).
Generation of CIK cells
CIK cells were prepared from PBMCs, which were isolated by standard Ficoll separation with CIK medium (Takara, Japan) supplemented with 0.6% autogeneic serum. The generation of CIK cells was primed by adding 1,000 U/mL recombinant human IFNγ, 1,000 U/mL recombinant human IL-2 (rhIL-2), and 5 μg/mL anti-CD3 antibody on day 0. Every 3 d, fresh CIK medium and 1,000 U/mL rhIL-2 were added. After 14-d culture, approximately 1 × 109 CIK cells were harvested, with a survival rate of >95%. The phenotype of CIK cells was detected by Fluorescence-activated cell sorting (FACS) analysis by using antibodies against CD3/4/8/56. The phenotype of PBMCs was analyzed as a control.
Study design and treatment
This was a single institutional, open-labeled, phase II study at Chinese PLA general hospital in Beijing (www.clinicaltrials.gov: NCT01799083). This study was conducted in accordance with the principles of good clinical practice, approved by the institutional review board as well as the appropriate regulatory agencies. Considering the funding capacity, 55 patients were randomly assigned 3:1 to receive either DTC or DTC+CIK treatment. A blocked random assignment approach was used to assign patients to each treatment groups in a 3:1 ratio. Patients were stratified according to platinum sensitivity.
DAC was given 7 mg/m2/d over five consecutive days. A reduced-dose of paclitaxel (135 mg/m2)/carboplatin (AUC = 5) was given on day 6 for a 28-d cycle. For the DTC+CIK cohort, patients were given 5-d of DAC and paclitaxel/carboplatin chemotherapy, followed by CIK cells at 1.0–5.0 × 109/L for 2 d on day 14 and 15 in a 28-d cycle. Treatment lasted at least two cycles or continued until disease progression or until intolerable toxicity was reached. All patients underwent a complete medical interview and a physical examination that included a blood profile as well as a CT scan or MRI of the lesions. All patients were restaged by CT scan or MRI every two cycles. For patients with grade 4 hematological or other non-hematological adverse events related to decitabine or chemotherapy, treatment was suspended for 2 weeks to resolve the event until it subsided to below grade 1 or returned to baseline levels. If more than 2 weeks was required for an adverse effect to resolve, the patient was removed from the study. For each cycle, therapy was continued until PD or unacceptable toxicity occurred, or other illness prevented further treatment. In addition, treatment was suspended if patients exhibited white blood cell counts fewer than 3.0 × 109/L or platelet counts fewer than 100 × 109/L.
Outcomes/efficacy and safety assessment
Safety data were evaluated on day 0 (the day before treatment on day 1) of each cycle and graded according to the Common Terminology Criteria for Adverse Events (CTAEs) (version 3.0). Tumor burden was evaluated radiographically by CT scan or MRI at baseline and every 8 weeks during treatment. Serum CA-125 was detected at day 0 of each cycle. For patients with measurable disease, efficacy was assessed using the Response Evaluation Criteria in Solid Tumors assessment (RECIST, v1.1). For non-measurable disease, efficacy was evaluated using the Gynecologic Cancer Intergroup (GCIG) criteria defined as a 50% reduction in CA-125 levels response to treatment maintained for at least 28 d.20,21 A quarterly follow-up evaluated survival until the patient either died or withdrew from the trial. Platinum-free interval (PFI) referred to the interval from the last date of platinum-based therapy until disease progression.22
Translational Research
Peripheral blood mononuclear cells (PBMCs) were harvested at baseline (day 0) and day 8 of first cycle by Ficoll-Hypaque density-gradient centrifugation, and viably cryopreserved in liquid nitrogen for the subsequent assays. DNA was extracted using QIAmp DNA Blood Mini Kits (Qiagen, USA). Global methylation was analyzed using Global DNA Methylation LINE-1 kit (Active Motif, USA) according to the manufacturer's instructions.23
Statistical analysis
The objective response rate (ORR) was defined as the ratio of patients with a complete response (CR) or a partial response (PR), by either RECIST (for measurable disease) or GCIG criteria (for non-measurable disease). The disease control rate (DCR) was defined as the ratio of patients with a CR, PR, or stable disease (SD). All efficacy and safety analyses were conducted on an intent to-treat basis. The median time of progression-free survival (PFS) and overall survival (OS) were calculated from the date of first decitabine treatment and assessed using the Kaplan–Meier method and log-rank test. For PFS, patients who were alive without progressive disease (PD) were censored at the start of subsequent antitumor therapy. For OS, patients who were alive through the November 12, 2016 data cut-off were censored at the time of last contact. Hazard ratios (HRs) with 95% CI were estimated using a Cox regression model.
Results
Enrollment and demographics
Between April 12, 2012, and April 12, 2016, 55 patients with recurrent ovarian cancer were recruited, 3:1 randomly assigned, and given the decitabine-based therapy, among which 52 patients were assessable for primary and long-term analysis. These ovarian cancer patients included 31 with high-grade serous, 8 with low-grade serous, 8 with endometrioid, 2 with clear cell, 1 with mucinous and 1 with mixed epithelial ovarian cancer, as well as 1 with fallopian tube cancer. Demographics and baseline characteristics are shown in Table 1 and Table S1. The median age was 53 y with 19.23% of patients’ age ≥65 y and 88.46% of patients had measurable disease. The median PFI was 6 mo, and 51.92% of patients with platinum-resistant/refractory ovarian cancer (20 platinum-resistant and 7 platinum-refractory).
Table 1.
Baseline characteristics, by treatment.
| DTC (n = 40) |
DTC ± CIK (n = 12) |
Total (n = 52) |
||||
|---|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | No. | % |
| Ages (y) | ||||||
| Median (range) | 51 (32–75) | 56 (36–73) | 53 (32–75) | |||
| ≥ 50 y | 23 | 57.50 | 11 | 91.67 | 34 | 65.38 |
| ≥ 65 y | 7 | 17.50 | 3 | 25.00 | 10 | 19.23 |
| ECOG performance status | ||||||
| 0 | 18 | 45.00 | 7 | 58.33 | 25 | 48.08 |
| 1 | 13 | 32.50 | 3 | 25.00 | 16 | 30.77 |
| 2 | 8 | 20.00 | 2 | 16.67 | 10 | 15.38 |
| 3 | 1 | 2.50 | 0 | 0 | 1 | 1.92 |
| FIGO stage | ||||||
| II–IIIB | 2 | 5.00 | 2 | 16.67 | 4 | 7.69 |
| III (not further classified) | 2 | 5.00 | 0 | 0 | 2 | 3.85 |
| IIIC | 35 | 87.50 | 10 | 83.33 | 45 | 86.54 |
| IV | 1 | 2.50 | 0 | 0 | 1 | 1.92 |
| Measurable disease | 36 | 90.00 | 10 | 83.33 | 46 | 88.46 |
| Non-measurable disease | 4 | 10.00 | 2 | 16.67 | 6 | 11.54 |
| Histology | ||||||
| Serous | 28 | 70.00 | 11 | 91.67 | 39 | 75.00 |
| High-grade serous | 20 | 71.43 | 11 | 100 | 31 | 79.49 |
| Low-grade serous | 8 | 28.57 | 0 | 0 | 8 | 20.51 |
| Endometrioid | 7 | 17.50 | 1 | 8.33 | 8 | 15.38 |
| Clear-cell | 2 | 5.00 | 0 | 0 | 2 | 3.85 |
| Other | 3 | 7.50 | 0 | 0 | 3 | 5.77 |
| Grade | ||||||
| 1 | 6 | 15.00 | 0 | 0 | 6 | 11.54 |
| 2 | 20 | 50.00 | 4 | 33.33 | 24 | 46.15 |
| 3 | 14 | 35.00 | 8 | 66.67 | 22 | 42.31 |
| Outcome of debulking surgery | ||||||
| No debulking surgery | 2 | 5.00 | 1 | 8.33 | 3 | 5.77 |
| Optimal microscopic (0 cm) | 2 | 5.00 | 1 | 8.33 | 3 | 5.77 |
| Optimal macroscopic (0–1cm) | 30 | 75.00 | 9 | 75.00 | 39 | 75.00 |
| Optimal unknown | 1 | 2.50 | 0 | 0 | 1 | 1.92 |
| Suboptimal (>1 cm) | 5 | 12.50 | 1 | 8.33 | 6 | 11.54 |
| Numbers of prior systemic therapies (median, range) | 12 (1–30) | 9.5 (1–27) | 10 (1–30) | |||
| Recurrent platinum-resistant/refractory disease | 19 | 47.50 | 8 | 66.67 | 27 | 51.92 |
| Recurrent platinum-sensitive disease | 21 | 52.50 | 4 | 33.33 | 25 | 48.08 |
DTC, decitabine+paclitaxel+carboplatin; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics.
Efficacy
Patient disposition is shown in trial profile (Fig. 1). Totally, 55 patients were enrolled in this study, with three patients excluded because of cerebral infarction (n = 1) or intestinal obstruction (n = 2).
Figure 1.
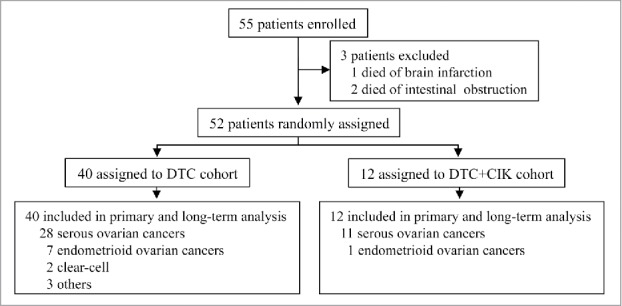
Trial profile DAC, 7 mg/m2/day for 5 d; T, paclitaxel 135 mg/m2; C, carboplatin AUC = 5.
The waterfall plot of RECIST-defined tumor responses indicated that 11 patients had PR, 23 patients had SD, and 12 patients experienced PD (Fig. 2A). In patient UPN23, for example, a 56-y old woman with recurrent platinum-resistant high-grade serous ovarian cancer, the tumor lesions were dramatically reduced after six cycles of DTC + CIK treatment as analyzed by PET-CT scanning. This patient completed a 19-cycle trial and continued to experience a PR for 25 mo with CA-125 level decreasing to normal range after five cycles (Fig. 2B, 2C and Fig. S1A). Another representative patient UPN33 was a 52-y-old woman with recurrent high-grade serous ovarian cancer. After four courses of DTC + CIK treatment, these lesions were largely reduced and her CA-125 levels decreased to normal range, achieving a PR at 8 mo after initiation of treatment (Fig. 2D and 2E). For patient UPN20, a 73-y-old woman who had recurrent high-grade serous ovarian cancer characterized merely by an incremental rise in her CA-125 levels (no tumor lesions observed on imaging), experienced a CR. After three cycles of DAC+TC+CIK treatment, her CA-125 levels decreased to the normal range (Fig. S1B).
Figure 2.
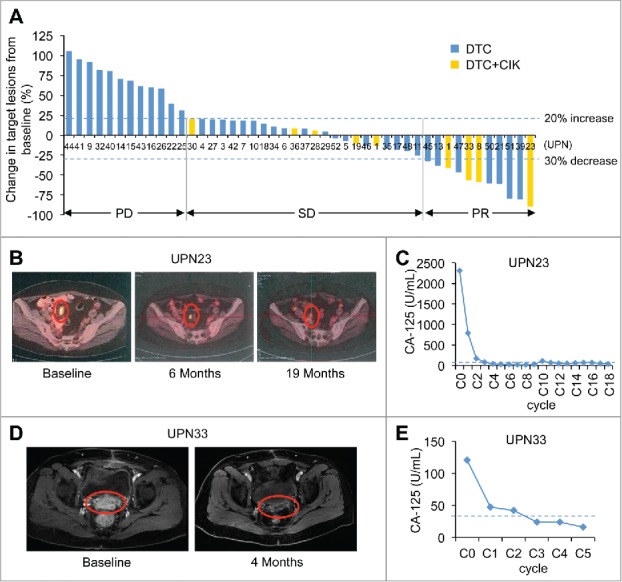
(A) Maximum change by RECIST (version 1.1) in ovarian cancer patients from baseline. Changes in target lesions from baseline in 46 patients with measurable disease who received DTC or DTC+CIK treatment. Each bar represents an individual patient in the clinical trial. The two horizontal dashed lines mark the thresholds for objective response (lower line, −30%) and PD (higher line, 20%), according to RECIST (version 1.1). (B) Partial response of platinum-resistant/refractory and recurrent high-grade serous ovarian cancer in a 56-y-old patient (UPN23), who received DTC+CIK treatment of 19 cycles. This patient had previously undergone primary surgery, and progressive disease had developed after treatment with systemic chemotherapies (i.e., cisplatin and docetaxel). The pelvic mesenteric lymph node metastases were observed on a baseline PET-CT image (left), these lesions were reduced at 6 mo (middle) and maintained for 19 mo (right) after the start of this treatment. The red circles show regression of recurrent lymph node metastases. (C) Tumor marker CA-125 decreased to normal range after five course of DAC-based therapy in patient UPN23. C0, baseline; C2, day 28 in cycle 2. The horizontal dashed line represents the cut-off level for CA-125 (35 U/mL). (D) Partial response of platinum-resistant and recurrent high-grade serous ovarian cancer in a 52-year-old patient (UPN33), who received DAC+TC+CIK treatment of five cycles, and PR maintained for 8 mo until disease progressed. The tumor lesions reduced after four cycles of treatment (right) as compared with the baseline (left) as analyzed by the MRI examination. (E) In UPN33, CA-125 level decreased to normal range after two cycles. C0, baseline; C1, day 28 in cycle 1. The horizontal dashed line represents the cut-off level for CA-125 (35 U/mL).
In the 46 patients with measurable disease, 11 patients achieved a confirmed PR per RECIST (v1.1), with 18.92% (7 of 37) in the DTC cohort and 44.4% (4 of 9) in the DTC+CIK cohort. The DCR and ORR for disease measurable ovarian cancer patients were 23.91% and 73.91%, respectively. For the 6 patients with non-measurable disease, 2 CR, 3 PR and 1 SD were confirmed per GCIG criteria (1 CR and 1 PR in DTC cohort, 1 CR and 2 PR in DTC+CIK cohort). Overall, DCR was 76.92% and ORR was 30.77% (Table 2). Notably, the DCR and ORR reached 100% and 58.3%, respectively, in the DTC+CIK cohort.
Table 2.
Clinical responses.
| DTC |
DTC ± CIK |
Total |
||||
|---|---|---|---|---|---|---|
| Response | No. | % | No. | % | No. | % |
| Total (n = 52) | 40 | 12 | 52 | |||
| Confirmed DCR | 28 | 70.00 | 12 | 100 | 40 | 76.92 |
| ORR | 9 | 22.50 | 7 | 58.30 | 16 | 30.77 |
| CR | 1 | 2.50 | 1 | 8.30 | 2 | 3.85 |
| PR | 8 | 20.00 | 6 | 50.00 | 14 | 26.92 |
| SD | 19 | 47.50 | 5 | 41.67 | 24 | 46.15 |
| PD | 12 | 30.00 | 0 | 0 | 12 | 23.08 |
| RECIST criteria (n = 46) | 37 | 9 | 46 | |||
| Confirmed DCR | 25 | 67.57 | 9 | 100 | 34 | 73.91 |
| ORR | 7 | 18.92 | 4 | 44.4 | 11 | 23.91 |
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 7 | 18.92 | 4 | 44.4 | 11 | 23.91 |
| SD | 18 | 48.65 | 5 | 55.6 | 23 | 50.00 |
| PD | 12 | 32.43 | 0 | 0 | 12 | 26.09 |
| CA-125 criteria (n = 6) | 3 | 3 | 6 | |||
| CR | 1 | 33.33 | 1 | 33.33 | 2 | 33.33 |
| PR | 1 | 33.33 | 2 | 66.67 | 3 | 50 |
| SD | 1 | 33.33 | 0 | 0 | 1 | 16.67 |
| PD | 0 | 0 | 0 | 0 | 0 | 0 |
| PFI confirmed ORR (totally n = 52) | ||||||
| ≤6 m (resistant/refractory) | 4 of 19 | 21.05 | 7 of 8 | 87.50 | 11 of 27 | 40.74 |
| >6 m (sensitive) | 5 of 21 | 23.80 | 0 of 4 | 0 | 5 of 25 | 20.0 |
| PFI confirmed DCR (totally n = 52) | ||||||
| ≤6 m (resistant/refractory) | 15 of 19 | 78.95 | 8 of 8 | 100 | 23 of 27 | 85.19 |
| >6 m (sensitive) | 13 of 21 | 61.90 | 4 of 4 | 100 | 17 of 25 | 68.0 |
| PFI confirmed ORR (Measurable disease n = 46) | ||||||
| ≤6 m (resistant/refractory) | 3 of 17 | 17.65 | 4 of 5 | 80.0 | 7 of 22 | 31.82 |
| >6 m (sensitive) | 4 of 20 | 20.00 | 0 of 4 | 0 | 4 of 24 | 16.67 |
| PFI confirmed DCR (Measurable disease n = 46) | ||||||
| ≤6 m (resistant/refractory) | 13 of 17 | 76.47 | 5 of 5 | 100 | 18 of 22 | 81.82 |
| >6 m (sensitive) | 12 of 20 | 60.00 | 4 of 4 | 100 | 16 of 24 | 66.67 |
m, month; PFI, platinum-free interval.
Furthermore, we evaluated the correlation between clinical responses and patients’ characteristics. There was an association toward having more objective clinical responses if given CIK treatment (p = 0.018), or not heavily pretreated (p = 0.016), or with non-measurable disease (p = 0.003) (Table S2). Additionally, in clinical benefit patients (n = 39), increased cycles (>4) of DAC-based therapy could be associated with improved therapeutic effects (p = 0.029).
Progression free and overall survival rates
For all the 52 patients evaluated, the median PFS and OS time were 4 and 14 mo, respectively. Notably, PFS in patients with platinum-resistant/refractory ovarian cancer (6 mo, 95% CI, 5.03–6.97 mo) was significant longer than that with platinum-sensitive cancer (3 mo, 95%, 3.38–4.62 mo; HR, 0.52; 95% CI, 0.29–0.94; p = 0.030), while the OS was not statistically significant (Table 3, Fig. 3A and 3B). Additionally, in patients with platinum-resistant/refractory ovarian cancer, a prominent longer PFS with DTC+CIK treatment comparing with DTC treatment was observed (8 vs. 4 mo; HR, 0.38; 95% CI, 0.15–0.97; p = 0.043). No statistically significant difference was observed in OS (19 vs. 12 mo; p = 0.14; Table 3, Fig. 3C and 3D). For patients with measurable disease, the median PFS and OS time were 4 and 14 mo, respectively. Although no significant differences of PFS and OS were detected between platinum-resistant/refractory and platinum-sensitive ovarian cancer patients, the PFS was dramatically longer in platinum-resistant/refractory ovarian cancer patients with DTC+CIK treatment than with DTC treatment (8 vs. 4 mo; HR, 0.24; 95% CI, 0.067–0.87; p = 0.03; Fig. S3A, S3B, 3E and Table 3). No statistically significant difference was observed in OS (19 vs. 10 mo; p = 0.13; Table 3 and Fig. 3F).
Table 3.
PFS and OS.
| Survival | DTC | DTC + CIK | Total | |
|---|---|---|---|---|
| Total patients (No.) | 40 | 12 | 52 | |
| PFS, months | Median | 4 | 6 | 4 |
| 95% CI | 3.37–4.63 | 2.61–9.40 | 3.33–4.67 | |
| OS, months | Median | 13 | 16 | 14 |
| 95% CI | 8.78–17.22 | 10.34–21.66 | 11.53–16.47 | |
| Patients with measurable disease (No.) | 37 | 9 | 46 | |
| PFS, months | Median | 4 | 5 | 4 |
| 95% CI | 3.43–4.57 | 2.08–7.92 | 3.39–4.61 | |
| OS, months | Median | 12 | 16 | 14 |
| 95% CI | 8.40–15.60 | 13.08–18.92 | 10.04–17.96 | |
| Total platinum-resistant/refractory patients (No.) | 19 | 8 | 27 | |
| PFS, months | Median | 4 | 8 | 6 |
| 95% CI | 2.64–5.36 | 5.23–10.77 | 5.03–6.97 | |
| OS, months | Median | 12 | 19 | 15 |
| 95% CI | 8.21–15.79 | 13.46–24.54 | 10.35–19.65 | |
| Total platinum-sensitive patients (No.) | 21 | 4 | 25 | |
| PFS, months | Median | 3 | 2 | 3 |
| 95% CI | 1.80–4.20 | – | 3.38–4.62 | |
| OS, months | Median | 14 | 8 | 14 |
| 95% CI | 8.86–19.14 | 0–20.74 | 8.25–19.75 | |
| Disease measurable platinum-resistant/refractory (No.) | 17 | 5 | 22 | |
| PFS, months | Median | 4 | 8 | 5 |
| 95% CI | 3.25–4.75 | 4.78–11.22 | 3.52–6.50 | |
| OS, months | Median | 10 | 19 | 14 |
| 95% CI | 6.26–13.74 | 12.56–25.44 | 9.79–18.22 | |
| Disease measurable platinum-sensitive (No.) | 20 | 4 | 24 | |
| PFS, months | Median | 3 | 2 | 3 |
| 95% CI | 1.64–4.36 | — | 1.02–4.99 | |
| OS, months | Median | 14 | 8 | 14 |
| 95% CI | 9.11–18.89 | 0–20.74 | 8.46–19.54 | |
CI, confidence interval.
Figure 3.
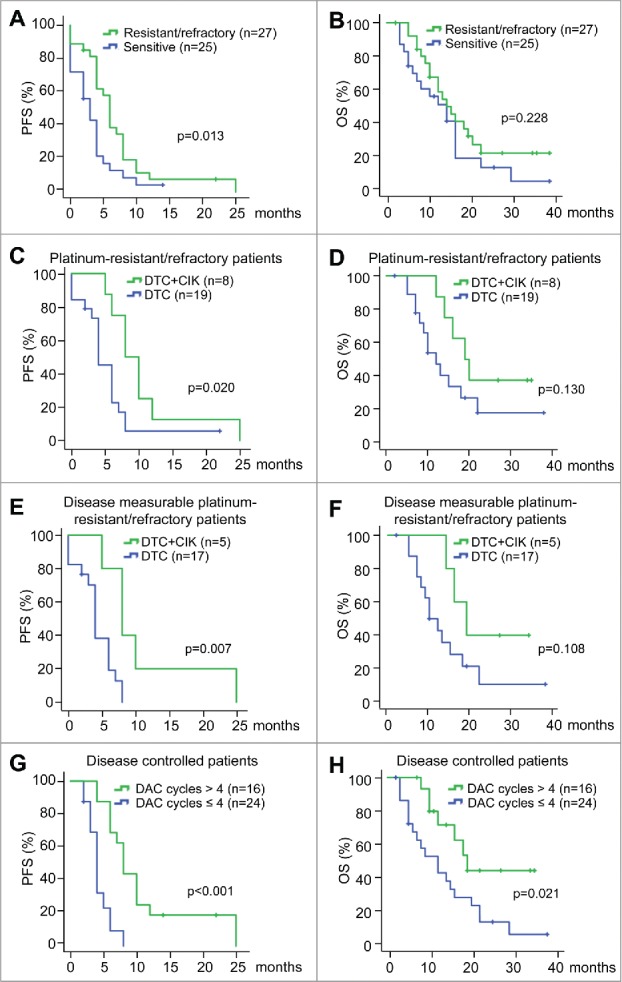
Kaplan–Meier analysis of PFS and OS with different platinum sensitivity and treatments (A and B) Kaplan–Meier survival curves of progression-free survival (A) and overall survival (B) for the platinum-resistant/refractory patients and platinum-sensitive patients, using the log-rank test to evaluate significance. (C and D) Kaplan–Meier survival curves of progression-free survival (C) and overall survival (D) in platinum-resistant/refractory patients based on different treatments (DTC or DTC+CIK treatment), using the log-rank test to evaluate significance. (E and F) Kaplan–Meier survival curves of progression-free survival (E) and overall survival (F) in disease measurable platinum-resistant/refractory patients based on different treatments (DTC or DTC+CIK treatment), using the log-rank test to evaluate significance. (G and H) Kaplan–Meier survival curves of progression-free survival (G) and overall survival (H) in disease controlled patients based on the number of cycles of DAC treatment, using the log-rank test to evaluate significance. PFS, progression-free survival. OS, overall survival.
In this trial, the median number of prior treatments was 10 (1–30). There were statistically significant differences in PFS but not OS between heavily pretreatment patients (number of prior treatment ≥10) and those with fewer treatments (<10) (HR, 2.18; 95% CI, 1.19–4.00; p = 0.011; Table S3), as well as platinum-resistant/refractory (PFI≤6) and platinum-sensitive (PFI>6) patients (HR, 0.52; 95% CI, 0.29–0.94; p = 0.03; Table S3 and S4). The detailed prior treatment regimens were listed in Table S5. However, these indexes could not independently predict the survival. Still, in disease controlled patients (n = 39), increased cycles of DAC treatment (>4) might be associated with longer PFS and OS, suggesting that at least four cycles of DAC-based therapy should be recommended in recurrent ovarian cancer (Fig. 3G and 3H).
Safety
The hematological and non-hematological adverse events to the DAC-based therapy are listed in Table 4. The common toxicities were nausea (26.9%), anorexia (23.1%), fatigue (19.2%), and hematologic toxicity, which included neutropenia (30.8%), anemia (21.2%), and thrombocytopenia (15.4%), without pneumonia or toxic deaths. The most common grade 3–4 adverse effects were neutropenia (5.8%), anorexia (3.8%), anemia (3.8%), and thrombocytopenia (3.8%).
Table 4.
Adverse events.
| All Grades |
Garde1/2 |
Garde3/4 |
||||
|---|---|---|---|---|---|---|
| Adverse events | n | % | n | % | n | % |
| Nausea | 14 | 26.9 | 14 | 26.9 | 0 | 0 |
| Vomiting | 3 | 5.8 | 3 | 5.8 | 0 | 0 |
| Constipation | 8 | 15.4 | 7 | 13.5 | 1 | 1.9 |
| Diarrhea | 3 | 5.8 | 3 | 5.8 | 0 | 0 |
| Anorexia | 12 | 23.1 | 10 | 19.2 | 2 | 3.8 |
| Allergic reaction | 2 | 3.8 | 2 | 3.8 | 0 | 0 |
| Neutropenia | 16 | 30.8 | 13 | 25.0 | 3 | 5.8 |
| Anemia | 11 | 21.2 | 9 | 17.3 | 2 | 3.8 |
| Thrombocytopenia | 8 | 15.4 | 6 | 11.5 | 2 | 3.8 |
| Abdominal distension | 2 | 3.8 | 1 | 1.9 | 1 | 1.9 |
| Fatigue | 10 | 19.2 | 9 | 17.3 | 1 | 1.9 |
| Dizziness | 3 | 5.8 | 3 | 5.8 | 0 | 0 |
| Fever | 1 | 1.9 | 1 | 1.9 | 0 | 0 |
| Febrile neutropenia | 1 | 1.9 | 1 | 1.9 | 0 | 0 |
| Mucositis, oral cavity | 1 | 1.9 | 1 | 1.9 | 0 | 0 |
Biological evaluation
To evaluate the biologic effects of the DAC-based therapy, its DNA demethylating activity was assessed. Due to the tumor specimens being unavailable, PBMCs were collected on days 0 (baseline) and day 8 of the first cycle, DNA extracted, and methylation of LINE-1 analyzed. As shown in Fig. S2, DAC-induced global DNA methylation inhibition was confirmed in all 25 patients tested. However, the changes in LINE-1 methylation in responders were not significantly different from non-responders, indicating that global DNA demethylation may not be a putative predictive marker for DAC therapy.
Discussion
Our study reports on a novel regimen of a three-drug combination (low-dose DAC pretreatment and decreased-dose of carboplatin/paclitaxel), which is tolerable and shows remarkable clinical activity in recurrent ovarian cancer, especially in platinum-resistant/refractory ovarian cancer with an ORR of 40.74% (31.82% for measurable disease). Furthermore, we show that this DAC-based chemotherapy is enhanced when combined with CIK, giving a statistically significantly increase in clinical efficacy in recurrent platinum-resistant/refractory ovarian cancer patients with an ORR of 87.5% (80% for measurable disease).
It is now widely accepted in platinum-based clinical trials in ovarian cancer that the PFI after first-line platinum-based therapy acts as a crucial prognostic indicator of survival and a marker of the subsequent therapy.2,8,9 Patients with recurrent platinum-resistant/refractory ovarian cancer (PFI <6 mo) have a reduced survival and, such a short PFI, suggests that a non-platinum-based therapy, for example, PLD, topotecan, docetaxel, or gemcitabine may be warranted. However, the ORRs of these drugs are generally only 20% in platinum-resistant ovarian cancer.24-26 For example, the ORR for topotecan and paclitaxel in platinum-resistant ovarian cancer patients were 13.3% vs. 6.7%, being 28.8% vs. 20.0% in platinum-sensitive patients.6 Previously, high dose of DNMT inhibitors AZA and DAC were used and regarded as cytotoxic drugs, but the intolerable toxicity limited the therapeutic application in solid tumors. Based on its DNA demethylation capacity, a low-dose regimen of DAC (100–135 mg/m2/cycle) was determined in hematological malignancies and an even lower dose (50–90 mg/m2/cycle) was suggested as the “optimal dose” for the treatment of solid tumors.9,27,28 In this present study, we demonstrated that low-dose DAC-based therapy was well tolerated and showed higher DCR in patients with recurrent platinum-resistant/refractory ovarian cancer as compared with patients with platinum-sensitivity (ORR and DCR: 40.74% vs. 20% and 85.19% vs. 68%, respectively).
In a trial by Matei et al. of 17 patients with refractory/resistant ovarian cancer, it was demonstrated that low-dose DAC could increase carboplatin sensitivity with a DCR of 70% by using the two-drug regimen (DAC+carboplatin).9 In our study, low-dose DAC combined with carboplatin/paclitaxel treatment had a slightly higher DCR of 78.95% in 19 recurrent platinum-resistant/refractory ovarian cancer patients, while the ORR in our trial was lower than that of Matei et al. study (21.05% vs. 35%).9 Furthermore, we found that the number of prior systemic therapy was negatively correlated with later clinical effects, and the median prior treatment number of platinum-resistant/refractory ovarian cancer patients in our study was 10 (range 2–17), which was much higher than that of the Matei et al. trial (median, 5; range 1–10). Notably, in our heavily pretreated platinum-resistant/refractory ovarian cancer patients, the ORR was as high as 87.5% in DTC plus CIK cohort. Moreover, the PFS (8 mo) and OS (19 mo) observed were longer when low-dose DAC-based chemotherapy was combined with CIK immunotherapy. In addition, we detected that patients treated with more than four cycles of DAC-based therapy were likely to obtain more survival benefits compared with those treated with fewer DAC therapy cycles among the disease controlled patients.
Evidences have indicated that low-dose DAC treatment can increase the expression of hypermethylation-silenced tumor suppressor genes, thus promoting cell apoptosis, inhibiting cell growth, and restoring the sensitivity of cytotoxic chemotherapeutic drugs, especially in chemoresistant cancer cells. Therefore, we observed a significant clinical improvement in platinum-resistant or refractory ovarian cancer patients. However, based on our data, the platinum-sensitive patients did not achieve remarkable clinical benefits from DAC combination therapy, as compared with other carboplatin-based treatments.29 Another group reported that the addition of decitabine reduced but not augmented the efficacy of carboplatin in platinum-sensitive ovarian cancer.30 With the small cases in our trial, we could not make the conclusion that decitabine showed negative effects in patients with platinum-sensitive ovarian cancers, yet these patients may be not suitable for this DAC-based therapy, and we would further readjust the therapeutic schedule in platinum-sensitive ovarian cancer patients. Moreover, low-grade serous ovarian cancer is uncommon and less sensitive to conventional chemotherapy than high-grade ovarian cancers, and hormonal therapy might show a benefit in patients with recurrent low-grade serous ovarian carcinoma.31 In these low-grade epithelial ovarian cancer patients, an overall response of 9% and SD rate of 62% were assessed.32 In our study, the ORR and DCR were 12.5% (1/8) and 87.5% (7/8), respectively, while the small number of patients may cause some deviation. The differences of clinical response toward DAC combination therapy might approach between high-grade and low-grade serous ovarian carcinoma but did not reach statistical significance (p = 0.122, Table S2).
Low-dose DAC-based therapy may be a promising strategy in patients with recurrent platinum-resistant/refractory ovarian cancer, but due to the limited sample size, we could not determine whether the three-drug combination (DAC + paclitaxel + carboplatin) was superior to two-drug combination (DAC + carboplatin). It is one important limitation that in our clinical trials, we were unable to adopt the 1:1 randomization approach; however, the addition of immunotherapy with low-dose DAC-based chemotherapy has shown significant clinical improvement in recurrent platinum-resistant/refractory ovarian cancer patients. Further assessment in a larger cohort is needed. Nevertheless, regardless of the type of treatment administered, drug-resistance might occur during repeated therapies, contributing to tumor progression. The need for predictive biomarkers became apparent since some patients had durable clinical responses, but many other patients eventually relapsed. In addition, some patients became re-sensitized again to platinum while others remained resistant to the repeated DAC-based therapy. Many important clinical issues remain unsolved such as when to stop dosing the responders who have a CR or PR, or is it even appropriate to cease therapy in responders? What's the best maintenance therapy regimen? Are there putative biomarkers for responses to low-dose DAC-based therapy? Although the biologic mechanism underlying the antitumor effect of DAC therapy is unclear, ovarian cancer is an immunogenic tumor, and low-dose DAC has been shown to induce the expression of tumor antigens, MHC-I molecules and Th1 cytokines in tumor cells, and function as a crucial modulator of the tumor microenvironment.33-35
In summary, we report the clinical outcomes of combining DAC-based chemotherapy with adoptive immunotherapy, and surprisingly found that this treatment strategy displayed outstanding clinical outcomes in recurrent platinum-resistant/refractory ovarian cancer patients. Although the detailed mechanism and with a larger cohort continue to be investigated, the combination of epigenetic therapy with immunotherapy may be a promising new weapon in our armamentarium against recurrent ovarian cancer.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The academic advisements of Zhiqiang Wu and Xiaolei Li, and technical assistance of Chao Jia and Wei Li, are gratefully acknowledged.
Funding
The study was supported by State's Key Project of Research and Development Plan of China (2016YFC1303504), Science and Technology Planning Project of Beijing City (Z151100003915076) and National Natural Science Foundation of China (31671338, 81472838, 81572914, 81650025, and 81230061).
Author contributions
Conception and design: Y. Zhang, Q. Mei, J. Nie, W. Han. Development of methodology: Q. Mei, X. Li, L. Dong, L. Shi. Acquisition of data: Y. Zhang, Q. Mei, Y. Liu, X. Li, M. Chen, Y. Wang, J. Nie. Analysis and interpretation of data: Y. Zhang, Q. Mei, Y. Liu, X. Li, M. Chen, Y. Nie, W. Han. Writing, review, and/or revision of the manuscript: Y. Zhang, Q. Mei, M.V. Brock, J. Nie, W. Han. Administrative, technical, or material support: Y. Zhang, Q. Mei, X. Li, L. Dong, L. Shi, J. Nie. Study supervision: M. Guo, M.V. Brock, J. Nie, W. Han
References
- 1.Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: A gynecologic oncology group study. J Clin Oncol 1994; 12:1748-53; PMID:7916038; https://doi.org/ 10.1200/JCO.1994.12.9.1748 [DOI] [PubMed] [Google Scholar]
- 2.Pujade-Lauraine E. How to approach patients in relapse. Ann Oncol 2012; 23(Suppl 10):x128-31; PMID:22987947; https://doi.org/ 10.1093/annonc/mds358 [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991; 9:389-93; PMID:1999708; https://doi.org/ 10.1200/JCO.1991.9.3.389 [DOI] [PubMed] [Google Scholar]
- 4.Cannistra SA. Is there a “best” choice of second-line agent in the treatment of recurrent, potentially platinum-sensitive ovarian cancer? J Clin Oncol 2002; 20:1158-60; PMID:11870154; https://doi.org/ 10.1200/JCO.2002.20.5.1158 [DOI] [PubMed] [Google Scholar]
- 5.Vaughan S, Coward JI, Bast RC Jr., Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D et al.. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat Rev Cancer 2011; 11:719-25; PMID:21941283; https://doi.org/ 10.1038/nrc3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson N, Spanczynski M et al.. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 1997; 15:2183-93; PMID:9196130; https://doi.org/ 10.1200/JCO.1997.15.6.2183 [DOI] [PubMed] [Google Scholar]
- 7.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001; 19:3312-22; PMID:11454878; https://doi.org/ 10.1200/JCO.2001.19.14.3312 [DOI] [PubMed] [Google Scholar]
- 8.Cannistra SA. Cancer of the ovary. N Engl J Med 2004; 351:2519-29; PMID:15590954; https://doi.org/ 10.1056/NEJMra041842 [DOI] [PubMed] [Google Scholar]
- 9.Matei D, Fang F, Shen C, Schilder J, Arnold A, Zeng Y, Berry WA, Huang T, Nephew KP. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res 2012; 72:2197-205; PMID:22549947; https://doi.org/ 10.1158/0008-5472.CAN-11-3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang F, Zuo Q, Pilrose J, Wang Y, Shen C, Li M, Wulfridge P, Matei D, Nephew KP. Decitabine reactivated pathways in platinum resistant ovarian cancer. Oncotarget 2014; 5:3579-89; PMID:25003579; https://doi.org/ 10.18632/oncotarget.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeller C, Dai W, Steele NL, Siddiq A, Walley AJ, Wilhelm-Benartzi CS, Rizzo S, van der Zee A, Plumb JA, Brown R. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene 2012; 31:4567-76; PMID:22249249; https://doi.org/ 10.1038/onc.2011.611 [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN et al.. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl JMed 2003; 348:203-13; PMID:12529460; https://doi.org/ 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 13.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol Oncol 2012; 124:192-8; PMID:22040834; https://doi.org/ 10.1016/j.ygyno.2011.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffmann I, Greve G, Jung M, Lubbert M. Epigenetic therapy approaches in non-small cell lung cancer: Update and perspectives. Epigenetics 2016; 11:858-70; PMID:27846368; https://doi.org/ 10.1080/15592294.2016.1237345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, Hao X, Ren X. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res 2012; 18:1751-9; PMID:22275504; https://doi.org/ 10.1158/1078-0432.CCR-11-2442 [DOI] [PubMed] [Google Scholar]
- 16.Li R, Wang C, Liu L, Du C, Cao S, Yu J, Wang SE, Hao X, Ren X, Li H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: A phase II clinical study. Cancer Immunol Immunother 2012; 61:2125-33; PMID:22581306; https://doi.org/ 10.1007/s00262-012-1260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW et al.. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148:1383-91 e6; PMID:25747273; https://doi.org/ 10.1053/j.gastro.2015.02.055 [DOI] [PubMed] [Google Scholar]
- 18.Kim HM, Kang JS, Lim J, Park SK, Lee K, Yoon YD, Lee CW, Lee KH, Han G, Yang KH et al.. Inhibition of human ovarian tumor growth by cytokine-induced killer cells. Arch Pharm Res 2007; 30:1464-70; PMID:18087816; https://doi.org/ 10.1007/BF02977372 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, An X, Yu W, Ren X, Hao X. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J Immunother 2014; 37:115-22; PMID:24509174; https://doi.org/ 10.1097/CJI.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 20.Rustin GJ. Can we now agree to use the same definition to measure response according to CA-125? J Clin Oncol 2004; 22:4035-6; PMID:15364965; https://doi.org/ 10.1200/JCO.2004.06.628 [DOI] [PubMed] [Google Scholar]
- 21.Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, Levenback CF, Sood AK, Wolf JK, Gershenson DM, Markman M et al.. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer 2011; 117:1661-9; PMID:21472713; https://doi.org/ 10.1002/cncr.25701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S, Pujade-Lauraine E, Sehouli J et al.. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer 2011; 21:771-5; PMID:21543939; https://doi.org/ 10.1097/IGC.0b013e31821bb8aa [DOI] [PubMed] [Google Scholar]
- 23.Mei Q, Chen M, Lu X, Li X, Duan F, Wang M, Luo G, Han W. An open-label, single-arm, phase I/II study of lower-dose decitabine based therapy in patients with advanced hepatocellular carcinoma. Oncotarget 2015; 6:16698-711; PMID:25895027; https://doi.org/ 10.18632/oncotarget.3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, Rosales R, Sharpington T. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol 2000; 18:3093-100; PMID:10963637; https://doi.org/ 10.1200/JCO.2000.18.17.3093 [DOI] [PubMed] [Google Scholar]
- 25.Vergote IB, Garcia A, Micha J, Pippitt C, Bendell J, Spitz D, Reed N, Dark G, Fracasso PM, Ibrahim EN et al.. Randomized multicenter phase II trial comparing two schedules of etirinotecan pegol (NKTR-102) in women with recurrent platinum-resistant/refractory epithelial ovarian cancer. J Clin Oncol 2013; 31:4060-6; PMID:24081946; https://doi.org/ 10.1200/JCO.2012.45.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, Del Medico P, Scaltriti L, Katsaros D, Priolo D et al.. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 2008; 26:890-6; PMID:18281662; https://doi.org/ 10.1200/JCO.2007.13.6606 [DOI] [PubMed] [Google Scholar]
- 27.Wijermans P, Lubbert M, Verhoef G, Bosly A, Ravoet C, Andre M, Ferrant A. Low-dose 5-aza-2'-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: A multicenter phase II study in elderly patients. J Clin Oncol 2000; 18:956-62; PMID:10694544; https://doi.org/ 10.1200/JCO.2000.18.5.956 [DOI] [PubMed] [Google Scholar]
- 28.Garrido-Laguna I, McGregor KA, Wade M, Weis J, Gilcrease W, Burr L, Soldi R, Jakubowski L, Davidson C, Morrell G et al.. A phase I/II study of decitabine in combination with panitumumab in patients with wild-type (wt) KRAS metastatic colorectal cancer. Invest New Drugs 2013; 31:1257-64; PMID:23504398; https://doi.org/ 10.1007/s10637-013-9947-6 [DOI] [PubMed] [Google Scholar]
- 29.Mahner S, Meier W, du Bois A, Brown C, Lorusso D, Dell'Anna T, Cretin J, Havsteen H, Bessette P, Zeimet AG et al.. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: Results from a subset analysis of the CALYPSO phase III trial. Eur J Cancer 2015; 51:352-8; PMID:25534295; https://doi.org/ 10.1016/j.ejca.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 30.Glasspool RM, Brown R, Gore ME, Rustin GJ, McNeish IA, Wilson RH, Pledge S, Paul J, Mackean M, Hall GD et al.. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in combination with carboplatin vs carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br J Cancer 2014; 110:1923-9; PMID:24642620; https://doi.org/ 10.1038/bjc.2014.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacker KE, Uppal S, Johnston C. Principles of Treatment for borderline, micropapillary serous, and low-grade ovarian cancer. J Natl Compr Canc Netw 2016; 14:1175-82; PMID:27587627; https://doi.org/ 10.6004/jnccn.2016.0124 [DOI] [PubMed] [Google Scholar]
- 32.Gershenson DM, Sun CC, Iyer RB, Malpica AL, Kavanagh JJ, Bodurka DC, Schmeler K, Deavers M. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 2012; 125:661-6; PMID:22406638; https://doi.org/ 10.1016/j.ygyno.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W et al.. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015; 527:249-53; PMID:26503055; https://doi.org/ 10.1038/nature15520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, Han H, Liang G, Jones PA, Pugh TJ et al.. DNA-Demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 2015; 162:961-73; PMID:26317465; https://doi.org/ 10.1016/j.cell.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heninger E, Krueger TE, Lang JM. Augmenting antitumor immune responses with epigenetic modifying agents. Front Immunol 2015; 6:29; PMID:25699047; https://doi.org/ 10.3389/fimmu.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


