ABSTRACT
The French phase 3 trial (OS 2006) testing zoledronic acid, an osteoclast inhibitor, with chemotherapy and surgery did not improve the outcome of patients with osteosarcoma (OS). To understand this unexpected result, the presence of infiltrating immune cells was investigated in 124 pre-therapeutic biopsies of patients enrolled in the trial. The percentage of CD68/CD163 tumor-infiltrating macrophages (TAMs), CD8+ lymphocytes, osteoclasts, and the PD1/PDL-1 checkpoint were assessed by immunohistochemistry. M1/M2 macrophage polarization was characterized by pSTAT1/CMAF staining. The expression of these biomarkers was correlated with clinical outcome. No statistical correlations were found with response to chemotherapy. High CD163 levels (>50% of cells per core; 43.8% of patients) were associated with CMAF nuclear expression and significantly correlated with better overall survival (p = 0.0025) and longer metastasis progression-free survival (MPFS, p = 0.0315) independently of metastatic status (p = 0.002). Only a trend was observed for patients with high CD68-positive cells (p = 0.0582). CD8+ staining was positive in >50% of cases with a median staining of 1%. Lower CD8+ levels were associated with metastatic disease at diagnosis and the presence of CD8-positive cells significantly correlated with improved overall survival in zoledronate-treated patients (p = 0.0415). PD1/PDL-1 staining was negative in >80% of cases and was not correlated with outcome. Finally, CD163-positive TAMs and CD8 positive cells are crucial prognostic biomarkers in OS, whereas PD1/PDL-1 checkpoint plays a minor role. For the first time, we described a correlation between CD8 positive cells and survival in zoledronate-treated patients. The immunohistochemical analysis of the microenvironment in biopsies may represent a novel tool for therapeutic stratification.
KEYWORDS: CD8, CD163, macrophages, Osteosarcoma, PD1/PDL-1 Checkpoint
Introduction
Osteosarcoma (OS) is the most frequent primary bone malignancy with an annual incidence of around three cases per million in Europe, which is higher in adolescents (0.8–1.1/100,000/y for ages 15–19).1 The survival rates for OS patients increased dramatically with the introduction of chemotherapy but have since reached a plateau. Treatment consists of neoadjuvant chemotherapy followed by surgical resection and adjuvant chemotherapy.2 Today, 5-y overall survival rates for patients with localized disease are up to 70–75%, but this drops to 20–30% for those with metastatic disease.3
Whole genome sequencing of high-grade OS has confirmed that these cancers demonstrate significant chromosomal instability with high levels of somatic structural variations and copy number alterations.4,5 In addition, cancers with higher mutational loads and tumor-specific neoantigens have been associated with a higher level of immune infiltration.6 To date, the search for common molecular therapeutic targets in OS has been disappointing. Several pathways have been targeted in clinical trials with varying results but ultimately no significant improved outcome (for review see Ref. [7]).
The OS bone microenvironment is heterogeneous and consists of osteoclasts, osteoblasts and hematopoietic cells from which monocytes/macrophages derive. All of these cells release multiple growth factors and cytokines with contrasting effects that are not well documented in the context of OS. However, it is widely thought that this microenvironment plays an important role in tumor development. Indeed, intratumoral accumulation of Forkhead box P3 (FOXP3+) regulatory T-cells has been shown as a major immune escape mechanism of many tumors. In OSs, the ratio of intratumoral CD8+ T-cells to FOXP3+ cells in pretreatment biopsies was able to separate OS patients with prolonged survival from non-survivors.8 A recent study reported that the immune infiltrate in OS is mainly composed of tumor-associated macrophages (TAMs), but with a significant number of dendritic cells (DC), T lymphocytes and myeloid cells (MC).9 As for most other tumors, tumor infiltration by antigen presenting cells (APCs) including CD1a DCs and CD68 macrophages has been correlated with poorer prognosis, and tumor PDL-1 expression has been associated with a poorer 5-y event-free survival (EFS).10However, other studies have also associated TAMs with reduced metastasis and improved survival in high-grade OS.11,12
Zoledronic acid (ZA) is a bisphosphonate that exerts a direct antiproliferative effect on OS cell lines, reduces primary tumor growth, suppresses lung metastases and prolongs survival in preclinical studies.13,14 Thus, ZA was tested in combination with chemotherapy and surgery for OS patients in France in a randomized phase 3 study (OS2006). The trial was stopped for futility since, unexpectedly, the risk of treatment failure was not reduced and was even marginally higher in ZA-treated (Z+) compared with ZA non-treated (Z−) patients, with the results shown to be stable from sensitivity analyses and fairly homogeneous across the randomization strata.15 Here, we try to explain this lack of effects through the immunohistochemical analysis of the OS-infiltrating immune cells (T lymphocytes, macrophages) in 124 biopsies of patients enrolled in the OS2006 trial. To characterize the macrophage polarization in situ, we stained for the transcription factor pSTAT1 (to indicate T helper 1 responses and M1 polarization) and CMAF (for T helper 2 responses and M2 polarization).16 Our data provide important findings on the OS tumor microenvironment and show that CD163-positive M2-polarized macrophages and CD8-positive lymphocytes are strong biomarkers for the therapeutic stratification of OS patients at diagnosis.
Materials and methods
Patient and tumor characteristics
Biological samples have been collected prospectively in parallel with the therapeutic protocol approved for the OS2006 trial. A specific informed consent for blood and tumor samples was obtained from patients or their parents or guardians if patients were under 18 y of age upon enrolment. As part of the study, tissue microarrays (TMA) were prepared from the diagnostic biopsies of 124 patients from the 522 patients assessed for eligibility in the trial, and TMA analyses (triplicate sampling of 1 mm) were performed at two sites (Marseille, CB; Toulouse, AGB). For all cases, the TMA cores have been selected in the most cellular areas and for each case the mean of the percentages in the three core samples was performed. A double-blind examination by two pathologists, experts in bone sarcoma, was performed.
318 of the 522 patients assessed for eligibility, were enrolled in the trial. Only 124 biopsies from Lille, Marseille, Nantes, Nancy, Paris (Cochin, Curie and Gustave Roussy institutes), Toulouse and Strasbourg were available and interpretable by immunohistochemistry. In the other 398 cases, analysis was either not possible (due to microbiopsies, low cellularity, necrosis) or were unavailable, in spite of several requests with the concerned centers. All OS samples were reviewed and reclassified by the accredited pathologists (CB, SA, JMG, BM, FL, GdP, AGB) of the GFPO (French Group of Bone Pathologists), according to the WHO 2013 classification.
The TMAs of the patient samples were then stored at the certified NF 96–900 cancer biobank of Toulouse (BB-0033-00014) where the immunohistochemistry study was conducted. According to the French law, the biobank cancer collection was declared to the Ministry of High Education and Research (DC-2008-463) and a transfer agreement was obtained (AC-2013-1955) after approbation by ethical committees. All patient records and information were anonymized and de-identified before analysis.
The demographic, clinical and histological data of the 124 patients compared with the eligible patients population are summarized in Table 1. They all had biopsies for diagnosis followed by pre-surgical chemotherapy, then surgery of the primary tumor and post-surgical chemotherapy adapted to risk factors, as described in the OS2006 protocol.15 There were more chondroblastic samples in our study than in the excluded OS2006 population, and more patients treated with the MTX-based chemotherapy. 44 (35.5%) of these 124 patients were also randomly selected to receive ZA (Z+) and the other 80 received only chemotherapy (Z−). No statistical difference was shown between the two groups of patients (Z+ vs Z−) for all clinical parameters.
Table 1.
Patients characteristics.
| Population | |||||
|---|---|---|---|---|---|
| Eligible | Cohort | Cohort included |
|||
| patients | excluded | Total | ZA− | ZA+ | |
| OS2006 | N = 522 | N = 398 | N = 124 | N = 80 | N = 44 |
| Age (N = 522) | p = 0.4032 | ||||
| Median | 16 | 15 | 16 | 16 | 16 |
| (Range) | (4: 67) | (4: 67) | (6: 50) | (6: 49) | (9: 50) |
| p = 0.9505 | |||||
| Age < 18y | 359 (68.8%) | 274 (68.8%) | 85 (68.5%) | 58 (72.5%) | 27 (61.4%) |
| Age ≥ 18y | 163 (31.2%) | 124 (31.2%) | 39 (31.5%) | 22 (27.5%) | 17 (38.6%) |
| Sex (N = 522) | p = 0.5442 | ||||
| Male | 295 (56.5%) | 222 (55.8%) | 73 (58.9%) | 49 (61.3%) | 24 (54.5%) |
| Female | 227 (43.5%) | 176 (44.2%) | 51 (41.1%) | 31 (38.8%) | 20 (45.5%) |
| Limb vs Axial (N = 521) | p = 0.9132 | ||||
| Axial | 56 (10.7%) | 43 (10.8%) | 13 (10.5%) | 7 (8.8%) | 6 (13.6%) |
| Limb | 465 (89.3%) | 354 (89.2%) | 111 (89.5%) | 73 (91.3%) | 38 (86.4%) |
| Missing | 1 | 1 | 0 | ||
| Histological sub-type (N = 517) | p = 0.0091 | ||||
| Chondroblastic | 86 (16.6%) | 55 (14.0%) | 31 (25.0%) | 22 (27.5%) | 9 (20.5%) |
| Osteoblastic | 342 (66.2%) | 264 (67.2%) | 78 (62.9%) | 49 (61.3%) | 29 (65.9%) |
| Fibroblastic | 37 ( 7.2%) | 28 ( 7.1%) | 9 ( 7.3%) | 5 ( 6.3%) | 4 ( 9.1%) |
| Others | 52 (10.1%) | 46 (11.7%) | 6 ( 4.8%) | 4 (5.0%) | 2 (4.5%) |
| Missing | 5 | 5 | 0 | ||
| Initial staging (N = 521) | p = 0.5703 | ||||
| Localized disease | 429 (82.3%) | 329 (82.9%) | 100 (80.6%) | 64 (80.0%) | 36 (81.8%) |
| Metastases | 92 (17.7%) | 68 (17.1%) | 24 (19.4%) | 16 (20.0%) | 8 (18.2%) |
| Missing | 1 | 1 | 0 | ||
| Chemotherapy regimen(N = 522) | p = 0.0016 | ||||
| API-AI | 107 (20.5%) | 94 (23.6%) | 13 (10.5%) | 6 (7.5%) | 7 (15.9%) |
| MTX | 415 (79.5%) | 304 (76.4%) | 111 (89.5%) | 74 (92.5%) | 37 (84.1%) |
| Histological response(N = 116) | p = 0.0766 | ||||
| Good responders | 294 (61.1%) | 215 (58.9%) | 79 (68.1%) | 48 (62.3%) | 31 (79.5%) |
| Poor responders | 187 (38.9%) | 150 (41.1%) | 37 (31.9%) | 29 (37.7%) | 8 (20.5%) |
| Missing | 41 | 33 | 8 | 3 | 5 |
P*: p-value between excluded and included patients.
Immunohistochemistry
Immunostainings were performed with antibodies directed against CD68, CD163, CMAF, pSTAT1, CD8+ and PD1, using a DISCOVERY ULTRA automate (Ventana Medical Systems, Innovation Park Drive Tucson, Arizona 85755 USA, ROCHE) and against PDL-1 on the Autostainer link 48 from DAKO (Agilent USA, Denmark).
The steaming and deparaffinization steps programmed into the DISCOVERY ULTRA consist of heating the slides at 60°C for 8 min, followed by the application of a ready-to-use Tris acid solution (EZprep solution, Ventana) (three washes for 8 min) at 69°C. For CD68 staining, sections were pre-treated with protease 1 (Ventana) for 4 min at 37°C and for the other markers (CD163, CD8+, PD1, CMAF and pSTAT1), sections were pre-treated with the specific CC1 solution (Tris-EDTA pH 8–8.5, Ventana) for 64, 32, 64, 16, 32 and 40 min, respectively. Endogenous peroxidase activity was blocked using the CM inhibitor for 32 min at 37°C (Ventana). The primary ready-to use CD68 (PREKIT 168), CD163 (MRQ-26), CD8+ (clone SP57) and PD1 (NAT105) antibodies were incubated, respectively, for 20 min at 36°C, 32 min at 36°C, 20 min at 36°C and 16 min at 36°C. The primary pSTAT1 (sc-7988R) and CMAF (sc-7866) antibodies were both used at 1:25 dilutions and sections were incubated for 1 h at 37°C. Staining was performed with the Ventana kit (secondary antibody associated with HRP for 16 min at 37°C). Sections were revealed by incubation in a diaminobenzidine and H2O2 solution for 7 min at room temperature. Then, slides were stained with hematoxylin (Ventana), for 8 min followed by post-coloration by the Bluing reagent for 4 min at room temperature. Slides were then rinsed with water, dehydrated (ethanol and xylene) and mounted.
For PDL-1 staining, preparations were dried for 1 h at 58°C, then overnight at 37°C. Sections were deparaffinized with toluene and rehydrated in ethanol. They were then pre-treated with the high pH target retrieval solution (DAKO, EnVision Flex, Denmark), and a heat-based antigen retrieval method was used before incubation. Endogenous peroxidase activity was blocked using a 3% H2O2 incubation for 5 min. Primary antibodies were used at a 1:500 dilution (Clinisciences, Nanterre, France; clone E1L3N) for 20 min at 37°C. Stainings were performed with the Envision kit (DAKO, Carpinteria, CA, USA). Sections were revealed by incubation in a diaminobenzidine solution for 10 min then staining with hematoxylin for 5 min.
Immunoreactivity was considered positive if detected in >1% of cells per core of 1 mm, irrespective of staining intensity. Anti-CD68 and -CD163 were used to identify macrophages in tissue sections. Their staining was considered “high” when >50% positive cells per core were present. The macrophage polarization was determined in situ by pSTAT1 and CMAF staining, respectively, for the characterization of M1 and M2 subpopulations. Osteoclastic cells (also known as giant cells) were evaluated independently as giant multinucleated cells by CD68 staining. The presence of CD8+ (lymphocyte) checkpoint markers was analyzed with PD1 and PDL-1 antibodies. Tonsils and lymphoid nodes were used as positive controls for the CD8+, PD1 and PDL-1 antibodies, giant cell tumors for the CD68 and CD163 antibodies, and lymphoma samples were used for pSTAT1 and CMAF antibodies.
Statistical analysis
Data are summarized as the frequency and percentage for categorical variables and the median and range for continuous variables. Correlations between quantitative data were assessed using the Spearman's rank correlation coefficient. Links with diagnosis status or histological response were assessed with the Fisher's test for categorical covariates and the Mann–Whitney U test for quantitative covariates.
Overall survival was defined as the time from inclusion to death from any cause (event) or the last follow-up (censored data). Metastatic progression-free survival (MPFS) was defined as the time from inclusion to metastatic progression or death (event) or the last follow-up (censored data). Patients who locally relapsed as their first event were considered to be censored data, to avoid the bias related to the quality of the surgical resection margins. All survival rates were estimated by the Kaplan–Meier method with 95% confidence intervals (CI). Univariate analyses were performed using the log-rank test. Multivariate analysis with a backward selection was performed using the Cox proportional hazard model. Only covariates evaluable at the date of inclusion with p-values <0.10 from univariate analyses were included in the model.
Two-sided p-values <0.05 were considered statistically significant. All statistical analyses were performed using STATA 12.0 software.
Results
Immunohistochemical analyses
Patient biopsies were subjected to IHC biomarker analysis. The percentage of cells stained for all the markers studied are summarized in Table 2. Among patients for whom CD163 and CD68 stainings were available, 42/96 (43.8%) and 26/111 (23.4%) had staining greater than 50% per core, respectively, (Table 2A and Fig. 1A and B). To better define macrophage polarization between the M1 and M2 subtypes, the expression of pSTAT1 and CMAF was also tested, showing that high level of CD163 staining was associated with a high level of CMAF nuclear expression but not related to high pSTAT1 expression (Fig. 1C and D).
Table 2.
Biomarker staining results and correlations.
| A Biomarker staining results | ||||
|---|---|---|---|---|
| Antibody | Nb tested | Median cell positive (range) | Nb ≥1% positive cells (%) | Nb ≥50% positive cells (%) |
| PD1 | 110 | 0 (0:30) | 18 (16.4%) | |
| PDL1 | 116 | 0 (0:20) | 17 (14.7%) | |
| CD8+ | 109 | 1 (0:60) | 58 (53.2%) | |
| CD163 | 96 | 30 (0:80) | 42 (43.8%) | |
| CD68 |
111 |
20 (0:80) |
|
26 (23.4%) |
| B Correlations between biomarkers | ||||
| |
PD1 |
PDL1 |
CD8+ |
CD163 |
| PDL1 | 0.4030a | |||
| 0.0000b | ||||
| CD8+ | 0.4767a | 0.3417a | ||
| 0.0000b | 0.0004b | |||
| CD163 | 0.4757a | 0.3144a | 0.3575a | |
| 0.0000b | 0.0027b | 0.0007b | ||
| CD68 | 0.3152a | 0.2645a | 0.3462a | 0.7585a |
| 0.0013b | 0.0067b | 0.0004b | 0.0000b | |
Spearman's rank correlation coefficient.
Significance level.
Figure 1.
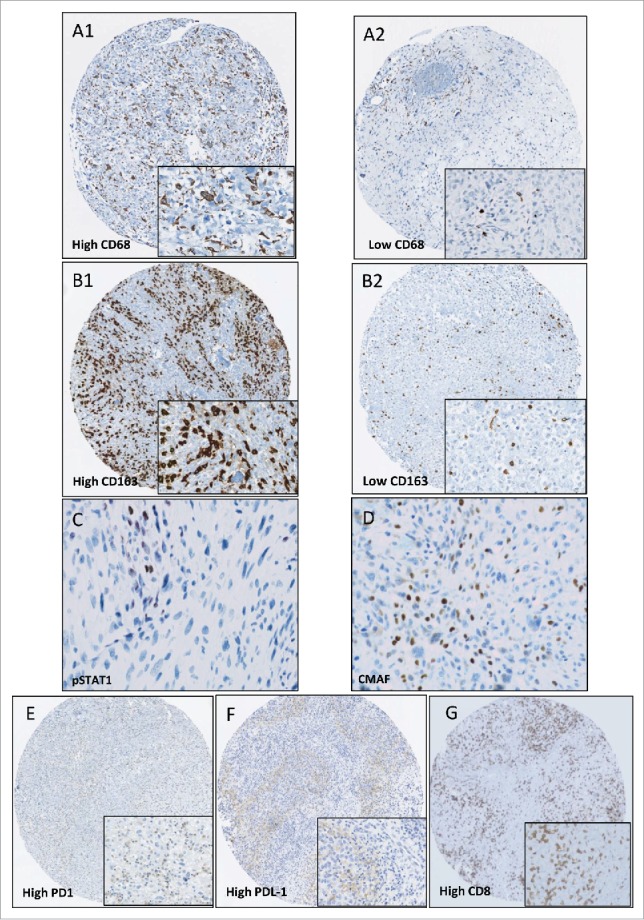
Immunohistochemical staining. Sample images of tissue microarrays prepared from patient biopsies and stained for CD68 (A1: high, A2 low); CD163 (B1: high, B2: low), pSTAT1 (C), CMAF (D), PD1 (E: high) and PDL-1 (F: high), CD8+ (G: high) (magnification X7). Frames correspond to the high power field of each picture (magnification X40). A high level of CD163 staining was associated with a high level of CMAF nuclear expression and not with pSTAT1 expression.
43 OS samples (38.7%) contained osteoclastic cells. CD8+ staining was positive in 58/109 (53.2%) cases but with a low median (1%). PD1 and PDL-1 staining had comparable results, with medians of 0 and no staining in more than 80% of cases (Table 2A and Fig. 1E, F and G). 87 of 124 patients presented a double CD163/ CD8+ staining (70%). Among them, high CD163 > 50% and CD8+ > 1% staining was observed in 25 cases (28.7%) (Data not shown).
Statistical analyses
Correlation between biological markers
Correlations between biomarker stainings are presented in Table 2B. All biomarkers were correlated together. CD68 and CD163 were highly correlated (CD68/CD163: ρ = 0.76, p<0.0001), as CD8+ and CD163 (CD8+/CD163: ρ = 0.357, p<0.001). Only CD68 staining was correlated with the presence of osteoclastic cells (median 15 versus 30, for absence versus presence of osteoclastic cells; p = 0.0141) (Data not shown).
Biomarkers and clinical parameters associated with diagnosis and histological response
Among the biomarkers tested, only CD8+ was associated with the presence of metastases at diagnosis (Table 3). Patients with metastases presented a lower CD8+ expression (median: 0; range: 0–5) compared with patients with localized disease (median: 1, 0–60; p = 0.0422). The combination of high CD163/CD8+ staining was not correlated with the presence of metastases at diagnosis whatever the group of patients (Z+ or Z−). No statistical correlation was found between immunohistochemical parameters and response to chemotherapy (data not shown).
Table 3.
Correlations between biomarker staining and diagnosis status.
| Diagnosis status |
|||
|---|---|---|---|
| Localized | Metastases | ||
| N = 100 | N = 24 | p-value | |
| PD1, N(%) | 0.1160 | ||
| <1 | 71 (80.7%) | 21 (95.5%) | |
| ≥1 | 17 (19.3%) | 1 (4.5%) | |
| Missing | 12 | 2 | |
| PDL1, N(%) | 0.5219 | ||
| <1 | 79 (84.0%) | 20 (90.9%) | |
| ≥1 | 15 (16.0%) | 2 (9.1%) | |
| Missing | 6 | 2 | |
| CD8+, N(%) | 0.0422 | ||
| <1 | 37 (42.0%) | 14 (66.7%) | |
| ≥1 | 51 (58.0%) | 7 (33.3%) | |
| Missing | 12 | 3 | |
| CD163, N(%) | 0.9475 | ||
| <50 | 44 (56.4%) | 10 (55.6%) | |
| ≥50 | 34 (43.6%) | 8 (44.4%) | |
| Missing | 22 | 6 | |
| CD68, N(%) | 0.3726 | ||
| <50 | 69 (78.4%) | 16 (69.6%) | |
| ≥50 | 19 (21.6%) | 7 (30.4%) | |
| Missing | 12 | 1 | |
| Osteoclast N(%) | 0.6003 | ||
| Absence | 55 (62.5%) | 13 (56.5%) | |
| Presence | 33 (37.5%) | 10 (43.5%) | |
| Missing | 12 | 1 | |
| CD163/CD8+N(%) | 0.1369 | ||
| Others | 48 (67.6%) | 14 (87.5%) | |
| CD163+ CD8+ high | 23 (32.4%) | 2 (12.5%) | |
| Missing | 29 | 8 | |
Clinical parameters and biomarkers associated with survival in global population
Univariate and multivariate analysis results are presented in Table 4. After a median follow-up of 64 mo, 40 patients (32.3%) had died. The 5-y overall survival rate was estimated at 71.2% (95% CI [61.5; 78.8]). Apart from clinical features (chondroblastic OS, metastatic disease and poor response to chemotherapy), a high (>50%) level of CD163-positive cells in biopsies was significantly correlated with a higher overall survival rate in univariate analysis (p = 0.0025, Fig. 2). A trend for a higher survival was also observed for patients with >50% CD68-positive cells (p = 0.0582; Fig. 2). Multivariate analysis showed that a high level of CD163 staining was the only significant prognostic factor in addition to the presence of metastases at diagnosis (p = 0.0025; Table 4).
Table 4.
Univariate and multivariate analysis.
| Univariate analysis |
Multivariate analysis |
Backward selection |
||||
|---|---|---|---|---|---|---|
| Overall survival | HR 95%CI | p-value | HR 95%CI | p-value | HR 95%CI | p-value |
| Age ≥18y | 1.40 [0.72; 2.69] | 0.3168 | — | — | — | — |
| Female vs male | 0.88 [0.46; 1.66] | 0.6856 | — | — | — | — |
| Limb vs Axial | 0.46 [0.20; 1.04] | 0.0569 | 0.47 [0.18; 1.23] | 0.125 | — | — |
| Histological sub-type | 0.0029 | |||||
| Osteo vs chondro | 0.41 [0.22; 0.78] | 0.60 [0.26; 1.39] | 0.234 | — | — | |
| Others vs chondro | 0.11 [0.02; 0.86] | 0.20 [0.02; 1.58] | 0.126 | — | — | |
| Metastatis vs Localized | 2.45 [1.24; 4.85] | 0.0078 | 1.92 [0.83; 4.40] | 0.125 | 2.47 [1.12; 5.47] | 0.026 |
| Z+ vs Z- | 1.29 [0.67; 2.50] | 0.4432 | — | — | — | — |
| PR vs GR | 2.51 [1.26; 4.98] | 0.0066 | — | — | — | — |
| PD1 ≥ 1 | 0.53 [0.16; 1.75] | 0.2902 | — | — | — | — |
| PDL1 ≥ 1 | 0.34 [0.08; 1.43] | 0.1246 | — | — | — | — |
| CD8+ ≥ 1 | 0.61 [0.31; 1.20] | 0.1464 | — | — | — | — |
| CD163 ≥ 50 | 0.28 [0.11; 0.67] | 0.0025 | 0.36 [0.10; 1.26] | 0.109 | 0.22 [0.09; 0.59] | 0.002 |
| CD68 ≥ 50 | 0.38 [0.13; 1.08] | 0.0582 | 0.66 [0.15; 2.96] | 0.588 | — | — |
| Osteoclastic cell |
0.84 [0.42; 1.70] |
0.6246 |
— |
— |
— |
— |
| Univariate analysis |
Multivariate analysis |
Backward selection |
||||
| MPFS |
HR 95%CI |
p-value |
HR 95%CI |
p-value |
HR 95%CI |
p-value |
| Age ≥ 18y | 1.27 [0.69; 2.33] | 0.4403 | — | — | — | — |
| Female vs male | 1.17 [0.66; 2.09] | 0.5937 | — | — | — | — |
| Limb vs Axial | 0.86 [0.34; 2.17] | 0.7412 | — | — | — | — |
| Histological sub-type | 0.0062 | |||||
| Osteo vs chondro | 0.41 [0.22; 0.75] | 0.44 [0.20; 0.97] | 0.041 | — | — | |
| Others vs chondro | 0.34 [0.11; 1.00] | 0.46 [0.13; 1.70] | 0.247 | — | ||
| Metastatis vs Localized | 2.48 [1.32; 4.67] | 0.0036 | 1.87 [0.85; 4.11] | 0.119 | — | — |
| Z+ vs Z- | 1.03 [0.56; 1.89] | 0.9262 | — | — | — | — |
| PR vs GR | 2.74 [1.50; 5.00] | 0.0006 | — | — | — | — |
| PD1 ≥ 1 | 0.48 [0.17; 1.36] | 0.1588 | — | — | — | — |
| PDL1 ≥ 1 | 0.38 [0.12; 1.22] | 0.0898 | 0.38 [0.09; 1.63] | 0.192 | — | — |
| CD8+ ≥ 1 | 0.62 [0.33; 1.15] | 0.1254 | — | — | — | — |
| CD163 ≥ 50 | 0.45 [0.21; 0.95] | 0.0315 | 0.58 [0.25; 1.34] | 0.202 | 0.40 [0.18; 0.86] | 0.019 |
| CD68 ≥ 50 | 0.66 [0.29; 1.49] | 0.3104 | — | — | — | — |
| Osteoclastic cell | 1.33 [0.70; 2.53] | 0.3758 | — | — | — | — |
Figure 2.
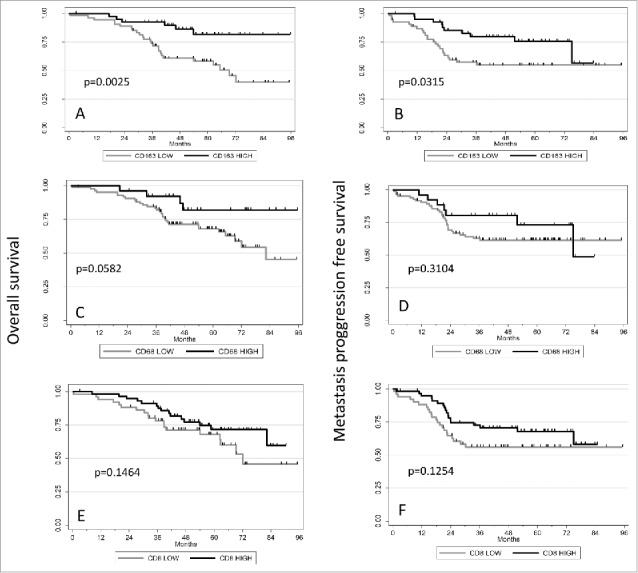
Correlations between CD68/CD163/CD8+ expression and patient outcomes. Kaplan–Meier curves showing the association between CD68 (A, B) or CD163 (C, D) or CD8+ (E, F) expression with overall survival (A, C) and metastatic progression-free survival (B, D). p-values are shown.
Metastatic progression-free survival
Post-treatment events occurred in 37.1% of patients (46/124) and the 5-y MPFS rate was estimated to be 61.23% (95%CI [51.58; 69.53]). Univariate analysis showed that high level of CD163 staining correlated with better MPFS (p = 0.0315) as metastasis at diagnosis and chondroblastic subtype (Table 4; Fig. 2). After backward selection, only CD163 remains statistically associated with MPFS (p = 0.019) (Table 4).
Correlations between ZA treatment, immunostaining analysis and patient survival
In the group of patients who did not received ZA (Z−), CD163 staining was correlated with overall survival (p = 0.0079), whereas in the group of patients treated with ZA, there was no statistical correlation between CD163 staining and survival (p = 0.1294; Table 5). On the contrary, the presence of CD8+ significantly correlated with a better overall survival in the group of patients treated with ZA (p = 0.0415; Table 5 and Fig. 3). However, no significant correlation was found between high levels of the CD163/CD8+ double staining and overall survival whatever the group of patients (ZA+ or ZA−) (data not shown). No correlation was found between any marker staining and MPFS (Table 5).
Table 5.
Univariate analysis according to Z+ or Z− treatment.
| Z− |
Z+ |
|||
|---|---|---|---|---|
| Overall survival | HR 95%CI | p-value | HR 95%CI | p-value |
| PD1 ≥ 1 | 0.59 [0.14; 2.53] | 0.4687 | 0.46 [0.06; 3.57] | 0.4460 |
| PDL1 ≥ 1 | 0.44 [0.10; 1.89] | 0.2580 | 0.00 [0.00; ] | 0.2405 |
| CD8+ ≥ 1 | 0.80 [0.33; 1.93] | 0.6245 | 0.31 [0.09; 1.02] | 0.0415 |
| CD163 ≥ 50 | 0.22 [0.06; 0.75] | 0.0079 | 0.38 [0.10; 1.40] | 0.1294 |
| CD68 ≥ 50 | 0.22 [0.05; 0.92] | 0.02312 | 1.24 [0.27; 5.73] | 0.7861 |
| Osteoclastic cell |
1.02 [0.43; 2.38] |
0.9725 |
0.58 [0.15; 2.17] |
0.4095 |
| Z− |
Z+ |
|||
| MPFS |
HR 95%CI |
p-value |
HR 95%CI |
p-value |
| PD1 ≥ 1 | 0.57 [0.17; 1.90] | 0.3532 | 0.33 [0.04; 2.51] | 0.2589 |
| PDL1 ≥ 1 | 0.32 [0.08; 1.34] | 0.090 | 0.56 [0.07; 4.23] | 0.5671 |
| CD8+ ≥ 1 | 0.60 [0.27; 1.33] | 0.2068 | 0.63 [0.23; 1.74] | 0.3693 |
| CD163 ≥ 50 | 0.44 [0.17; 1.13] | 0.0804 | 0.48 [0.14; 1.59] | 0.2195 |
| CD68 ≥ 50 | 0.43 [0.15; 1.24] | 0.1072 | 1.66 [0.46; 6.04] | 0.4372 |
| Osteoclastic cell | 1.59 [0.72; 3.48] | 0.2449 | 0.93 [0.30; 2.85] | 0.9012 |
Figure 3.
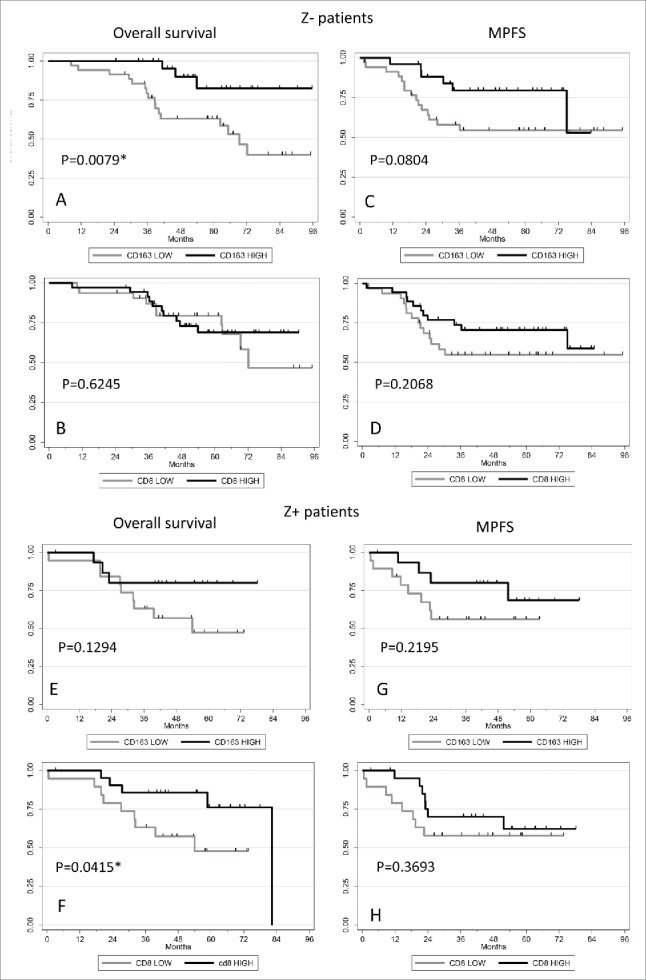
Correlations between CD163/ CD8+ expression and Z+/Z− patients outcomes. Kaplan–Meier curves showing the association between CD163 (A, C, E, G) or CD8+ (B, D, F, H) expression with overall survival (A, B, E, F) and metastatic progression-free survival (C, D, G, H) in Z− (A, B, C, D) and Z+ (E, F, G, H) patients. p-values are shown.
Effect of ZA on macrophages/lymphocytes population in resection specimens
We planned to analyze CD163 and CD68 staining in resection specimens, comparing ZA treated versus ZA untreated patients. The usable resection specimens only correspond to poor responders, with a variable proportion of viable cells ranging from 10% to 100% according to the grading of Huvos and Rosen.2 The CD163 and CD68 staining assessed in eight cases of poor responder patients confirmed the high heterogeneity between tumors and within the same tumor (Figs. 4 and 5), but did not allow us to conclude on the effect of ZA on macrophage populations.
Figure 4.
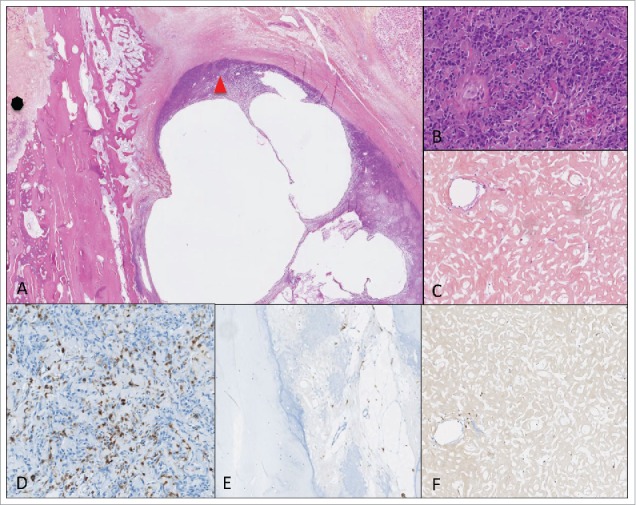
An example of poor responder patient treated with ZA with a viable isolated soft tissue nodule of osteosarcoma cells, next to intramedullary necrosis areas. (A) HE (magnification X0.4); on the left and (C) (magnification X8.9): necrosis areas; on the right and (B) (magnification X8.9): viable nodule (D, E, F) immunohistochemical staining with CD163 (magnification X9.5); (D) high CD163 staining; (E) low CD 163 staining, in necrosis areas.
Figure 5.
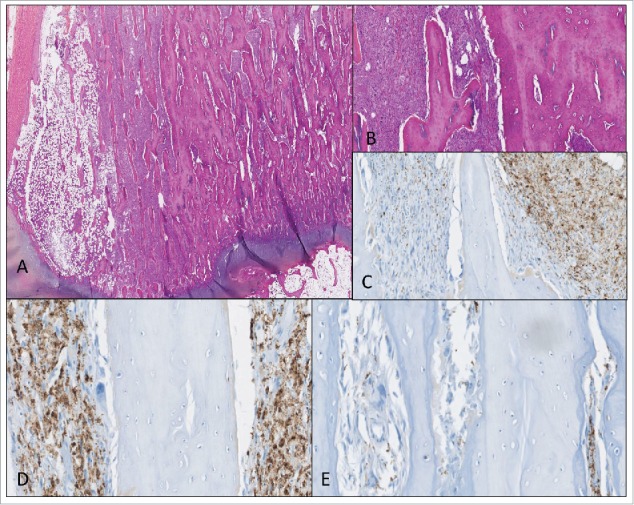
An example of poor responder patient treated with ZA, with relative homogeneous distribution of the tumoral cells (A, B); HE (magnification X0.72 and X2.84) (C, D, E) immunohistochemical staining with CD163; (C) heterogeneous areas (magnification X9.5); (D) high CD163 staining (magnification X15); (E) low CD163 staining (magnification X15).
Discussion
Over the past two decades the evolution of systemic treatment of OS has been disappointing and survival has not improved despite several clinical trials conducted worldwide.16,17,18 Although the recent OS2006 clinical trial also failed to provide a new treatment option (i.e. ZA added to chemotherapy), analyses of biopsies prospectively collected from the patients included in this trial are of main value in such a rare tumor, and emphasize the need of combined biological studies from the initial design of the clinical trial.
The immunohistochemistry analyses were performed on a cohort of 124 biopsies over the 522 eligible patients in the trial. The analyzed cohort and the excluded population differ significantly in two points: the proportion of patients with a chondroblastic subtype and the proportion of patients treated with a MTX-based chemotherapy were higher in the analyzed population as compared with the excluded cohort. However, both parameters could not explain the significant results obtained on the positive correlation between a high CD163 staining and overall survival or metastase-free progression survival.
Our results have identified that TAMs were present in the immune infiltrate in a high proportion of biopsies, and that an increased infiltration was associated with a better prognosis, as it has been previously reported.11,12 Among all the targets studied, we clearly identified that CD163 staining was the best prognostic biomarker to predict the outcome of OS2006 patients. Furthermore, we showed that the presence of CD68 and CD163 staining were highly correlated together, which suggests that a common subgroup of macrophages may be present. In agreement, our results clearly demonstrate that high levels of CD163 and CD68 were associated with better overall survival and MPFS; however, although this observation was significant for CD163, it was only a trend for CD68, suggesting that some CD68-positive macrophages have an opposite effect to CD163-positive cells. Differently polarized macrophages are known to coexist in tissues, M1 macrophages displaying a pro-inflammatory phenotype and tumoricidal activity. M1 macrophages have also been associated with non-metastatic OS,11,12 whereas M2 cells are thought to have an anti-inflammatory wound healing phenotype and favor tumor growth. The balance between the Th1- or Th2-predominant immune responses is thought to drive the shift between M1 versus M2 phenotypic macrophages.19 This classification of macrophages into two distinct subgroups must however be considered with caution since M2 sub-types are also described to include “non M1” macrophages which adopt heterogeneous activation states and play a wide range of roles in immunity. In addition, it has been demonstrated that CD163 is not an M2-specific macrophage biomarker and that CD163 staining in situ can be associated with Th1 responses, proinflammatory and tumoricidal activity.16 Furthermore, we found CD163 staining to be associated with high CMAF nuclear expression (a macrophage transcription factor associated with the Th2 immune response and M2 macrophage polarization) and low pSTAT1 expression (a transcription factor related to the Th1 immune response and M1-macrophage polarization) across the sample population. Thus, in the context of the bone microenvironment, the role and balance between M1- and M2-type functions appear to be variable with a ratio associated with extended survival in OS patients. The beneficial role of the macrophage infiltrate is in accordance with other studies11,12,20,21: the activation of M1-like macrophages in vitro with Liposomal-Muramyl TriPeptide–PhosphoEthanolamine (mifamurtide) and Interferon (IFN)γ was shown to inhibit OS cell growth, and IL-10-stimulated M2-like macrophages also inhibited OS cell growth when coated with the anti-EGFR antibody cetuximab.20 Further, the addition of mifamurtide (which promotes macrophage production) to chemotherapy significantly improved the 6-y overall survival in patients with localized OS and also, although not significant, in patients with metastatic OS in the INT trial.21 Because of methodological concerns in the design of this trial, there are still controversies about the place of mifamurtide in OS treatment. Our results thus consolidate previous data on the beneficial role of macrophage infiltration in OS and strongly support the need to better evaluate macrophage-activating drugs such as mifamurtide in OS patients.
Our results also begin to provide insights into the failure of the OS2006 trial. We showed that CD163 was significantly associated with better overall survival and MPFS in patients in the group without ZA, but not in patients treated with ZA. Based on a recent study from Junankar et al. suggesting that macrophages could represent the extraskeletal target for bisphosphonates,22 we propose that ZA could therefore disrupt the positive effects of CD163 infiltration. Therefore, we planned to analyze CD163 and CD68 staining in resection specimens, comparing ZA treated versus ZA untreated patients. Unfortunately this analysis was not informative and was probably not the good method to estimate the effect of the treatment on the infiltrating immune cells. The first limitation was linked to the fact that the usable resection specimens only correspond to poor responders, with a variable proportion of viable cells ranging from 10% to 100% according to the Huvos and Rosen's grading. The second pitfall was the average of the percentage of viable cells that did not reflect the distribution of cells on the histological section: the distinction between viable isolated nodules (of more than 10% of cells) within necrosis areas, and an homogeneous distribution of more than 10% of viable cells on the whole histological section were not possible with this grading. Therefore, we could not conclude on the effect of ZA on macrophage populations. We planned to answer to this question by another approach that consists in measuring the level of inflammatory cytokines relative to immune cells in the blood samples of OS2006 patients, and to complete this work at transcriptomic level, to determine the proportion of immune cell infiltrate both at diagnosis and also at surgery. The lack of association between CD163 and overall survival or MPFS in ZA treated patients may also be explained by a lack of power of the present statistical analysis due to the small number of ZA treated patients analyzed in our study, and should be validated in a larger series of OS patients.
In contrast to CD163, the level of CD8+ staining across the patient samples was low with a median staining of 1%, but CD8+ cells were detected in more than half of them and their presence was significantly associated with lower rate of metastasis at diagnosis. The use of a 1 mm TMA may have underestimated the number of CD8+ cells; however, we selected the most cellular areas of the biopsies for TMAs building, and the comparison of the mean of percentages of stained cells per whole slide was similar in the three core samples. This confirms the results of Frizsching et al. who showed that OS patients with increased intratumoral CD8+ T cell infiltration upon diagnosis have better outcomes.8 Together, this suggests that CD8+ T cells play a role in metastasis development in OS. In addition, the presence of CD8 positive cells significantly correlated with improved survival in patients treated with ZA. This could be related to an interaction between T lymphocytes and macrophages in the context of bone tumor microenvironment. One hypothesis is that zoledronate could sensitize OS cells to the Vγ9Vδ2 T cell cytotoxicity. Indeed, several studies in other cancer models report that tumor cell sensitivity to Vγ9Vδ2 T lymphocyte-mediated killing is increased by zoledronate.24,25 In OS, Liu et al.,23 described that combining the anti-HER-2 monoclonal antibody trastuzumab and ZA significantly increased the cytotoxic potential of Vγ9Vδ2 T cells. This hypothesis should be verified in a larger cohort to activate CD8+-TILs using ZA and/or other CD8+ TIL- activating drugs.
Finally, we found that more than 80% of samples were negative for PD1/PDL-1 staining: only one case presented a staining >10% for PD1 and two had a staining >10% for PDL-1. These cases also had high CD8+ staining (> 10%), suggesting that infiltrating CD8+ T cells might drive PDL-1 upregulation. Our results are concordant with those of the SARC 028 trial: one out of twenty relapsed OS patients responded to pembrolizumab, a PD1 inhibitor, whereas PDL-1 staining was detected in only 7% of 54 OS specimens.26 The authors also found PDL-1 expression to be significantly associated with a poorer 5-y EFS, but we did not found any correlation. Thus, taken together, these observations suggest that the role of the PD1/PDL-1 checkpoint is not predominant in the pathogenesis of OS. Other checkpoint candidates such as indoleamine 2,3-dioxygenase (IDO), which may explain the suppression of antitumor immunity in the tumor environment via CD8+ T cells, may be involved.27
In conclusion, our results support four main observations: (1) the presence of TAMs (CD163-positive M2-polarized macrophages) is crucial for the inhibition of OS progression, in contrast to what is observed in other solid tumors; (2) the PD1/PDL-1 checkpoint plays only a minor role in OS development; (3) CD8+-tumor infiltrating lymphocytes play a major role in delaying OS metastases; (4) for the first time, a relation could be established between the presence of CD8+ lymphocytes at diagnosis and a better overall survival in patients treated by ZA.
In view of these data, we propose that a systematic analysis of CD68, CD163, CD8+, PD1 and PDL-1 expression could be performed in OS biopsies at diagnosis (immunoscore) to stratify patients regarding their tumor microenvironment, and test a further therapeutic strategy targeting these immunological features (see algorithm in Fig. 6). This innovative approach, using the immune context of the tumor microenvironment for prognosis, could also be extended to other cancers with complex genomic instability.
Figure 6.
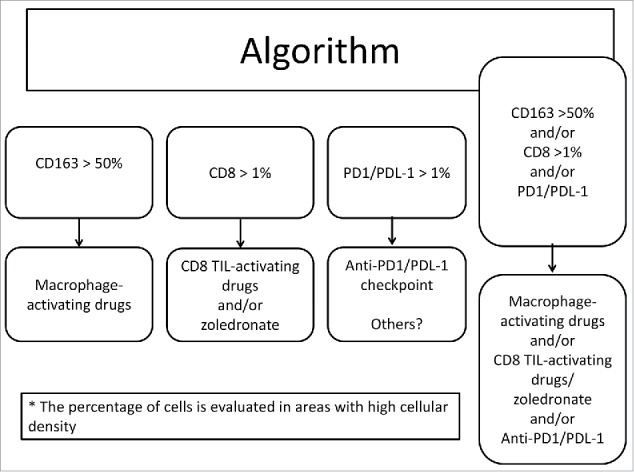
Algorithm to differentiate patients based on their immunoscore determined at diagnosis and the corresponding treatments.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Sophie Peries of the Cancer biobank of the Institute University of Cancer of Toulouse.
Funding
Funding was obtained from the French National Cancer Institute (INCa), Novartis, Chugai, Ligue Nationale contre le Cancer, Fédération Enfants et Santé, Société Française des Cancers et Leucémies de l'Enfant (SFCE) and the Association Etoile de Martin. The ancillary biological studies were approved by the Sarcoma Group of UNICANCER, the “Groupe des Sarcomes Français-Groupe d'Etude des Tumeurs Osseuses” (GSF-GETO), the SFCE and the InterSarc network.
References
- 1.ESMO/European Sarcoma Network Working Group Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3):iii113-23. [DOI] [PubMed] [Google Scholar]; Erratum in: Ann Oncol. 2015. September;26 Suppl 5:v174-7; PMID:25210081; https://doi.org/ 10.1093/annonc/mdu256 [DOI] [PubMed] [Google Scholar]
- 2.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 1982; 49(6):1221-30; PMID:6174200; https://doi.org/ 10.1002/1097-0142(19820315)49:6%3c1221::AID-CNCR2820490625%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 3.Osasan S, Zhang M, Shen F, Paul PJ, Persad S, Sergi C. Osteogenic sarcoma: a 21st century review. Anticancer Res 2016; 36(9):4391-8; PMID:27630274; https://doi.org/ 10.21873/anticanres.10982 [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L et al.. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep 2014; 7(1):104-12; PMID:24703847; https://doi.org/ 10.1016/j.celrep.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousquet M, Noirot C, Accadbled F, Sales de Gauzy J, Castex MP, Brousset P, Gomez-Brouchet A. Ann Oncol 2016;27(4):738-44 [DOI] [PubMed] [Google Scholar]
- 6.Champiat S, Ferté C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014; 3(1):e27817; PMID:24605269; https://doi.org/ 10.4161/onci.27817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer 2014; 14(11):722-35; PMID:25319867; https://doi.org/ 10.1038/nrc3838 [DOI] [PubMed] [Google Scholar]
- 8.Fritzsching B, Fellenberg J, Moskovszky L, Sápi Z, Krenacs T, Machado I, Poeschl J, Lehner B, Szendrõi M, Bosch AL et al.. CD8(+)/FOXP3(+)-ratio in osteosarcoma microenvironment separates survivors from non-survivors: a multicenter validated retrospective study. Oncoimmunology 2015; 4(3):e990800; PMID:25949908; https://doi.org/ 10.4161/2162402X.2014.990800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki Y, Hookway E, Williams KA, Hassan AB, Oppermann U, Tanaka Y, Soilleux E, Athanasou NA. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin Sarcoma Res 2016; 6:13; PMID:27482375; https://doi.org/ 10.1186/s13569-016-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koirala P, Roth ME, Gill J, Piperdi S, Chinai JM, Geller DS, Hoang BH, Park A, Fremed MA, Zang X et al.. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep 2016; 6:30093; PMID:27456063; https://doi.org/ 10.1038/srep30093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buddingh EP, Kuijjer ML, Duim RA, Bürger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PC, Lankester AC et al.. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res 2011; 17(8):2110-9; PMID:21372215; https://doi.org/ 10.1158/1078-0432.CCR-10-2047 [DOI] [PubMed] [Google Scholar]
- 12.Dumars C, Ngyuen JM, Gaultier A, Lanel R, Corradini N, Gouin F, Heymann D, Heymann MF. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016;7(48):78343-54; PMID:27823976; https://doi.org/ 10.18632/oncotarget.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer 2005; 104(11):2522-9; PMID:16270320; https://doi.org/ 10.1002/cncr.21530 [DOI] [PubMed] [Google Scholar]
- 14.Heymann D, Ory B, Blanchard F, Heymann MF, Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F, Redini F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone 2005; 37(1):74-86; PMID:15894525; https://doi.org/ 10.1016/j.bone.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 15.Piperno-Neumann S, Le Deley MC, Rédini F, Pacquement H, Marec-Bérard P, Petit P, Brisse H, Lervat C, Gentet JC, Entz-Werlé N et al.. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2016; 17(8):1070-80; PMID:27324280; https://doi.org/ 10.1016/S1470-2045(16)30096-1 [DOI] [PubMed] [Google Scholar]
- 16.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One 2013; 8(11):e80908; PMID:24260507; https://doi.org/ 10.1371/journal.pone.0080908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol 2015; 33(27):3029-35; PMID:26304877; https://doi.org/ 10.1200/JCO.2014.59.4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J et al.. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 2015; 33(20):2279-87; PMID:26033801; https://doi.org/ 10.1200/JCO.2014.60.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015; 2015:816460; PMID:26089604; https://doi.org/ 10.1155/2015/816460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahl JH, Kwappenberg KM, Varypataki EM, Santos SJ, Kuijjer ML, Mohamed S, Wijnen JT, van Tol MJ, Cleton-Jansen AM, Egeler RM et al.. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-γ. J Exp Clin Cancer Res 2014; 33:27; PMID:24612598; https://doi.org/ 10.1186/1756-9966-33-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M et al.. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival – a report from the Children's Oncology Group. J Clin Oncol 2008; 26(4):633-8; PMID:18235123; https://doi.org/ 10.1200/JCO.2008.14.0095 [DOI] [PubMed] [Google Scholar]
- 22.Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B et al.. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov 2015; 5(1):35-42; PMID:25312016; https://doi.org/ 10.1158/2159-8290.CD-14-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Sun LL, Li YJ, Li HY, Zhang J, Li BH, Ye ZM. Trastuzumab enhanced the cytotoxicity of Vγ9Vδ2 T cells against zoledronate-sensitized osteosarcoma cells. Int Immunopharmacol 2015; 28(1):160-7; PMID:26071219; https://doi.org/ 10.1016/j.intimp.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Sugai S, Yoshikawa T, Iwama T, Tsuchiya N, Ueda N, Fujinami N, Shimomura M, Zhang R, Kaneko S, Uemura Y et al.. Hepatocellular carcinoma cell sensitivity to Vγ9Vδ2 T lymphocyte-mediated killing is increased by zoledronate. Int J Oncol 2016; 48(5):1794-804; PMID:26936487; https://doi.org/ 10.3892/ijo.2016.3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HM, Cho HI, Shin CA, Shon HJ, Kim TG. Zoledronic acid induces dose-dependent increase of antigen-specific CD8 T-cell responses in combination with peptide/poly-IC vaccine. Vaccine 2016; 34(10):1275-81; PMID:26828454; https://doi.org/ 10.1016/j.vaccine.2016.01.026 [DOI] [PubMed] [Google Scholar]
- 26.Burgess MA, Crowley J, Reinke DK, Riedel RF, George S, Movva S, Van Tine BA, Davis LE, Schuetze S, Hu J et al.. SARC 028: a phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with advanced sarcomas. Paper presented at 2016 - American Society of Clinical Oncology (ASCO Annual Meeting); 2016, June «3–7 Chicago,US [Google Scholar]
- 27.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5(200):200ra116; PMID:23986400; https://doi.org/ 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]


