ABSTRACT
Elotuzumab is a humanized therapeutic monoclonal antibody directed to the surface glycoprotein SLAMF7 (CS1, CRACC, CD319), which is highly expressed on multiple myeloma (MM) tumor cells. Improved clinical outcomes have been observed following treatment of MM patients with elotuzumab in combination with lenalidomide or bortezomib. Previous work showed that elotuzumab stimulates NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC), via Fc-domain engagement with FcγRIIIa (CD16). SLAMF7 is also expressed on NK cells, where it can transmit stimulatory signals. We tested whether elotuzumab can directly activate NK cells via ligation with SLAMF7 on NK cells in addition to targeting ADCC through CD16. We show that elotuzumab strongly promoted degranulation and activation of NK cells in a CD16-dependent manner, and a non-fucosylated form of elotuzumab with higher affinity to CD16 exhibited enhanced potency. Using F(ab')2 or Fc-mutant forms of the antibody, the direct binding of elotuzumab to SLAMF7 alone could not stimulate measurable CD69 expression or degranulation of NK cells. However, the addition of soluble elotuzumab could costimulate calcium signaling responses triggered by multimeric engagement of NKp46 and NKG2D in a CD16-independent manner. Thus, while elotuzumab primarily stimulates NK cells through CD16, it can also transduce effective “trans”-costimulatory signals upon direct engagement with SLAMF7, since these responses did not require direct co-engagement with the activating receptors. Trans-costimulation by elotuzumab has potential to reduce activation thresholds of other NK cell receptors engaging with their ligands on myeloma target cell surfaces, thereby potentially further increasing NK cell responsiveness in patients.
KEYWORDS: ADCC, elotuzumab, multiple myeloma, NK cells, SLAMF7
Introduction
Multiple myeloma (MM) is a neoplastic disorder characterized by expansion of malignant plasma cells in bone marrow, accumulation of monoclonal Ig protein in blood and urine, and end-organ damage, such as anemia, bone lesions, renal failure and hypercalcemia.1 About 30,000 cases of MM were predicted to be diagnosed in the US in 2016, with approximately 13,000 fatalities.2 Over the past decade, new therapeutic regimens have entered clinical practice to treat MM, including immunomodulatory drugs (lenalidomide, pomalidomide) and proteasome inhibitors (bortezomib, carfilzomib). These therapies, in combination with conventional treatments, have significantly improved overall survival of MM patients from a median of approximately 3–4 y to about 5–7 y. However, despite progress in therapeutic options, nearly all MM patients become refractory to treatment and ultimately die of their disease.3 Therefore, new therapies are needed to prolong remissions and control disease development.
Therapeutic monoclonal antibodies are demonstrating increased efficacy for treatment of human malignancies, including MM. They can mediate anti-tumor responses through multiple mechanisms, including directly blocking or engaging receptors on tumors and immune cells in the tumor microenvironment, promoting antibody-dependent cell-mediated cytotoxicity (ADCC), supporting antibody-dependent phagocytosis, and initiating complement-dependent cytotoxicity.4 Well-characterized myeloma surface antigens can be targeted with monoclonal antibodies, including signaling lymphocytic activation molecule F7 (SLAMF7),5 CD38,6 CD138,7 CD56,8 CD200,9 CD40,10 BCMA,11 and CD74.12
The humanized IgG1 monoclonal antibody elotuzumab (HuLuc63) was developed to target SLAMF7 (CS1, CRACC, CD319), which is highly expressed on myeloma cells and normal plasma cells. SLAMF7 is also found on most NK cells and activated subsets of T cells, monocytes, dendritic cells, and B cells.13 The receptor is a type I transmembrane glycoprotein with 2 extracellular Ig-like domains and an intracellular immunoreceptor tyrosine-based switch motif (ITSM). SLAMF7 mediates homotypic interactions with itself on adjacent cell surfaces. In NK cells, it functions as an activating receptor through ITSM-mediated interaction with the EAT-2 adaptor to trigger activation signaling.14 Elotuzumab promotes the in vitro cytolysis of myeloma cell lines or patient myeloma tumor cells via NK cell-mediated ADCC, as well as regression of MM xenografts in vivo.13,15,16 Preclinically, elotuzumab showed enhanced anti-myeloma activity in combination with bortezomib16 or lenalidomide,17 which both also promote NK cell functional responses.18 A recent study in mice also found enhanced anti-tumor activity when elotuzumab was combined with PD-1 blockade.19 A phase I clinical trial for relapsed or refractory MM patients showed no objective responses to single-agent elotuzumab, however, it was generally well tolerated, with stable disease in a subset of patients.20 Improved responses and progression-free survival were observed when elotuzumab was used in combination with bortezomib plus dexamethasone or lenalidomide plus dexamethasone.21,22 These findings were confirmed when the antibody was administered in combination with dexamethasone and lenalidomide in a recent phase III clinical trial.23 The FDA has approved elotuzumab to be used in combination with lenalidomide and dexamethasone for treating MM patients with one to 3 prior lines of therapy.
We hypothesized that elotuzumab-mediated engagement with SLAMF7 may directly enhance NK cell activation and cytotoxicity, in addition to promoting ADCC through CD16. We demonstrate that elotuzumab strongly stimulates NK cell degranulation and activation in a CD16-dependent manner. Interestingly, we also show that soluble elotuzumab can effectively co-stimulate calcium signaling in NK cells when added in combination with antibodies targeting NKp46 and NKG2D.
Results
Elotuzumab promotes NK cell degranulation that correlates with SLAMF7 expression on myeloma target cell lines
We tested the in vitro impacts of elotuzumab (Elo) on the NK cells within PBMCs in the presence or absence of myeloma target cells to mimic conditions in treated patients. The impact of Elo on degranulation of primary NK cells was first investigated using in vitro CD107a-expression assays, in which healthy donor PBMCs were co-cultured with myeloma target cell lines. It has been shown that CD107a expression on NK cells correlates with target cell lysis.24,25 Adding 1µg/ml of Elo strongly increased the proportion of NK cells degranulating in response to MM.1R target cells from mean values of 0.65 ± 0.4% (targets alone) to 14.9 ± 7.6% for 7 healthy donors (Fig. 1A). To test whether SLAMF7 expression on target cells is important for inducing NK cell degranulation by Elo, we used a panel of myeloma target cell lines expressing a broad range of cell surface SLAMF7 levels. These cell lines were: RPMI8226 cells that express low levels of SLAMF7, RPMI8226 cells that were retrovirally transduced to generate intermediate expression of SLAMF7 (RPMI8226+SLAMF7), and MM.1R cells, which express high cell surface SLAMF7 (Fig. 1B). PBMCs from healthy donors were exposed to these myeloma lines in the presence or absence of Elo (1µg/ml), and NK cell degranulation was measured. Under these conditions, Elo alone or Elo plus RPMI8226 target cells induced similar low-level NK cell degranulation. In contrast, Elo induced more potent degranulation when added in combination with the RPMI8226+SLAMF7 and MM.1R target cells (Fig. 1C), and the level of degranulation directly correlated with the surface expression of SLAMF7 on the myeloma target cells (Fig. 1B). It should be noted that additional co-stimulatory ligands on MM1.R cells may have contributed to its enhanced capacity to stimulate Elo-mediated degranulation as compared with RPMI8226+SLAMF7 cells, but clearly exogenous expression of SLAMF7 on RPMI8226 cells significantly potentiated stimulatory capacity, as compared with the parent target cell line. Our results are consistent with previous reports of NK cell-mediated ADCC responses triggered by Elo,13,15-17 and our data demonstrate that the intensity of in vitro degranulation correlates with the SLAMF7 expression on myeloma target cells.
Figure 1.
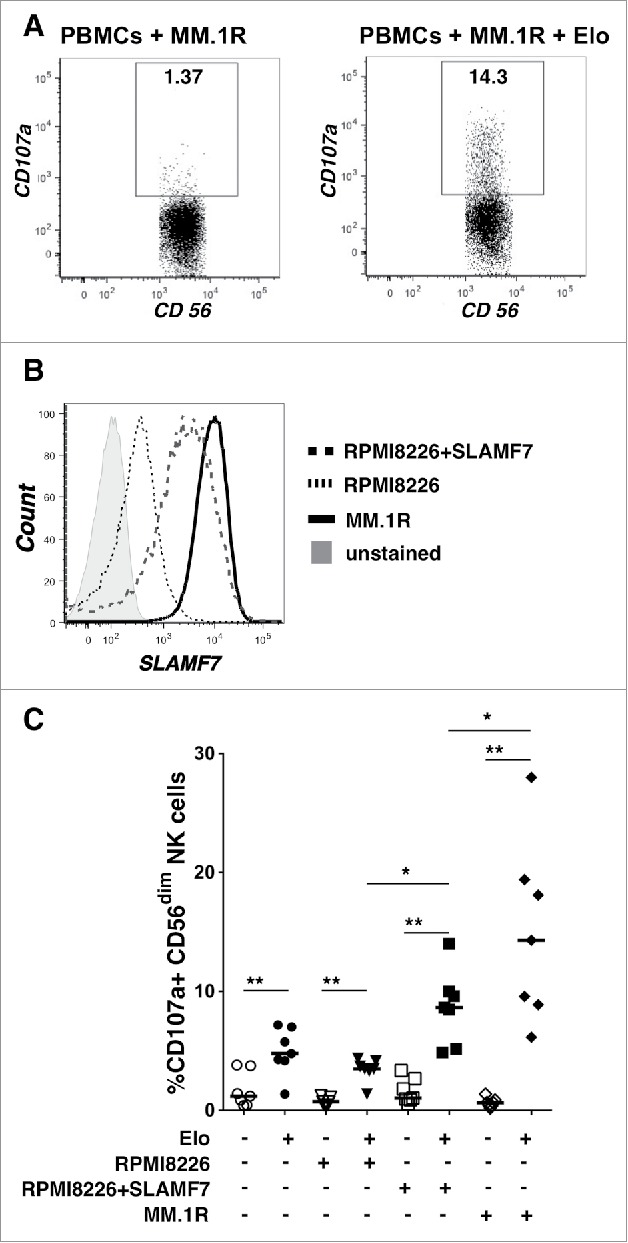
Elotuzumab promotes NK cell degranulation that correlates with SLAMF7 expression on myeloma target cell lines. A) NK cell degranulation (CD107a+) from a representative healthy donor after 2 hours incubation with MM.1R targets alone (left; PBMC to target ratio 1:1) or with 1µg/ml Elo. Percentage degranulating CD56dim NK cells is indicated in the box gates. B) SLAMF7 expression on myeloma target cell lines using biotinylated Elo and streptavidin-APC. Unstained cells = gray shaded, parental RPMI8226 = dotted (MFI 422), SLAMF7-transduced RPMI8226 = dashed (MFI 2254), and MM.1R = solid (MFI 10,973). C) Degranulation responses by NK cells in PBMCs from healthy donors (n = 7) alone (circles) or exposed to RPMI-8226 (inverted triangles), RPMI-8226+SLAMF7 (squares) or MM.1R target cells (diamonds) in the presence (+; filled) or absence (-; empty) of 1µg/ml Elo. Horizontal lines = medians; each datapoint = a healthy donor. Overhead bars mark statistical comparisons between indicated groups using one-sided or 2-sided Wilcoxon matched-pairs signed rank test, **- P < 0.01, *-P < 0.05.
NK cell activation and degranulation correlate with elotuzumab concentration
We next performed a dose response study to test the concentration of Elo (1ng/ml to 100µg/ml) promoting NK cell degranulation and activation. This dose response range reflects therapeutic levels, as clinical trials have observed serum concentrations of consistently greater than 70µg/ml in patients treated with therapeutic doses of Elo, and these patients achieved >90% occupancy of SLAMF7 on CD38+ bone marrow cells at serum concentrations between 30–100µg/ml.22,26 We measured NK cell degranulation in PBMCs from healthy donors in response to Elo alone or Elo plus MM1.R target cells (Fig. 2A). Elo alone promoted weak degranulation that was first evident at 10ng/ml and reached a maximum response at 10µg/ml. In contrast, when co-cultured with MM.1R targets, Elo stimulated robust degranulation over the same concentration range. Interestingly, the degranulation response was significantly reduced at 100µg/ml, as compared with 10µg/ml. This reduced response at higher antibody concentration is consistent with lower efficacy in patients treated with higher Elo dose in a phase 2 clinical trial, in which the objective response rate was 92% with 10mg/kg dose compared with 76% in patients treated with 20mg/kg in combination with lenalidomide and dexamethasone, although response was not compared with serum concentrations of Elo in that study.27 We observed only a modest decline in NK cell viability in response to Elo in these samples (Fig. 2B), suggesting minimal NK cell fratricide. The reduction in viability inversely correlated with degranulation response, consistent with ADCC-induced apoptosis of NK cells.28-30 Interestingly, Elo was far superior in promoting NK cell degranulation in the presence of MM.1R target cells, as compared with the human IgG1/mouse chimeric anti-SLAMF7 antibody, ChLuc90 (Fig. S1), which binds a different epitope on SLAMF7.15
Figure 2.
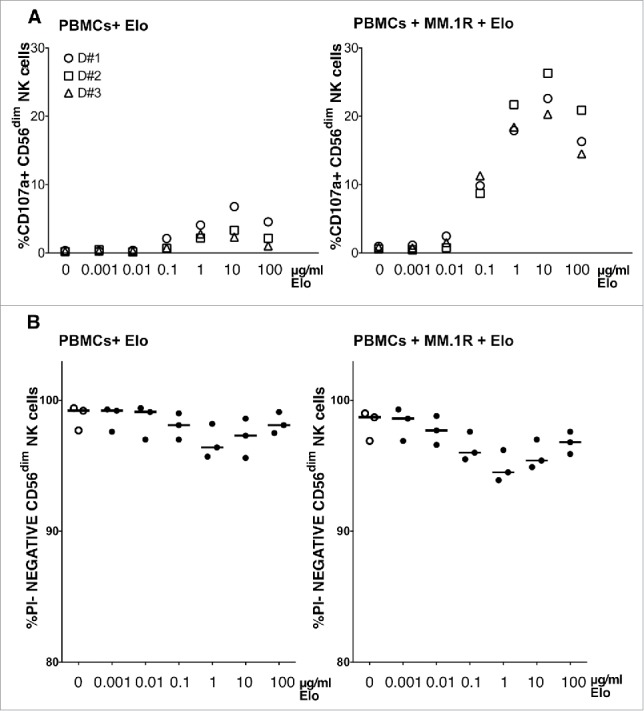
NK cell degranulation correlates with elotuzumab concentration. A) Percentage CD56dim NK cells in PBMC from 3 healthy donors degranulating after 2 hours with various concentrations (0.001–100µg/ml) of Elo alone (left) or with MM.1R targets (right; PBMC to target cell ratio 1:1). B) Viability of CD56dim NK cells as the percentage of propidium iodide (PI)-negative CD56dim NK cells for each condition.
As a further measure of NK cell activation, we quantified the surface expression of the activation marker CD69 on NK cells within PBMCs exposed to Elo for 24 hours. In this assay, Elo alone (1µg/ml) induced strong CD69 expression on NK cells (Fig. 3A). In a dose response experiment, increased CD69 expression was first detected at 100ng/ml Elo, peaked at 10µg/ml, and remained similarly elevated at 100µg/ml (Fig. 3B). Elo caused only a minimal decline in NK cell viability, even in these 24-hour assays (Fig. 3C). The addition of MM.1R target cells with Elo also stimulated significantly more robust expression of CD69 on NK cells in a 2-hour assay (Fig. S2).
Figure 3.
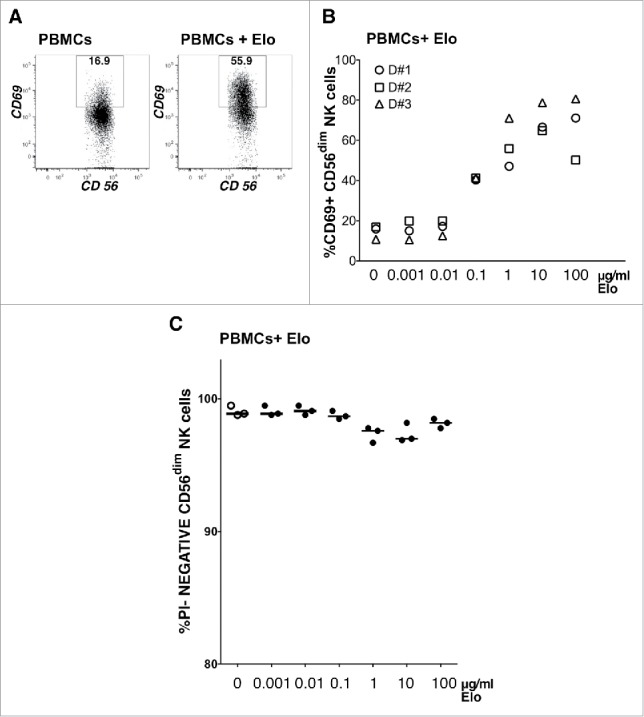
NK cell activation correlates with elotuzumab concentration. A) CD56dim NK cell activation from a representative healthy donor was measured as percentage of CD69+ cells after overnight incubation without (left) or with 1ug/ml Elo (right), as indicated in the box gate. B) Percentage of CD56dim NK cells in PBMCs from 3 healthy donors expressing CD69 after overnight exposure to indicated concentrations of Elo alone. C) Viability of CD56dim NK cells as the percentage of PI-negative CD56dim NK cells for each condition.
NK cell degranulation/activation requires the Fc portion of elotuzumab
Using Elo to stain healthy donor PBMCs, we observed high SLAMF7 expression on CD56dim NK cells and non-classical monocytes, whereas moderate expression was seen on CD56+ T cells and CD56bright NK cells (Fig. S3). The expression of SLAMF7 on multiple leukocyte populations in PBMCs suggests that the capacity of Elo alone to induce weak degranulation and CD69 expression of NK cells (Figs. 2 and 3) could be due to: 1) ADCC responses toward other NK cells or monocytes opsonized with Elo, or 2) direct engagement of SLAMF7 by the mAb. Therefore, we asked whether the Fc portion of Elo is required to promote NK cell degranulation and activation by first testing whether F(ab')2 Elo stimulates degranulation toward MM.1R targets. Elo alone promoted weak degranulation of NK cells from healthy donors, whereas F(ab')2 Elo was completely ineffective, even in the presence of MM.1R target cells (Fig. 4A). These results indicate that NK cell degranulation in presence of Elo is CD16-dependent.
Figure 4.
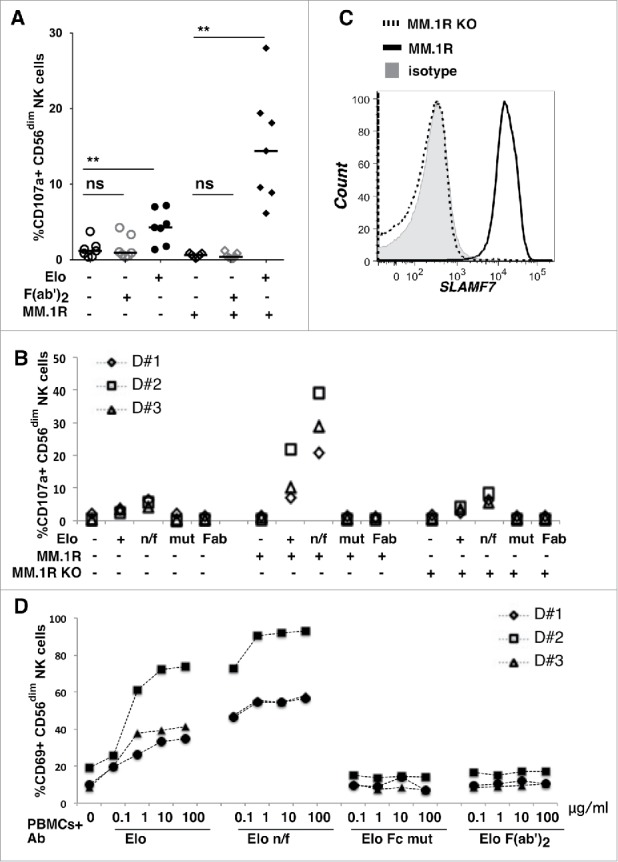
NK cell degranulation and activation requires the Fc portion of elotuzumab. A) Percentage of CD56dim NK cells that degranulated (CD107a+) in PBMCs from 7 healthy donors after exposure to whole Elo or F(ab')2 Elo at 1ug/ml with (+) or without (-) MM.1R targets. Horizontal lines are medians. Overhead bars mark statistical comparisons between indicated groups using one-sided Wilcoxon matched-pairs signed rank test, **- P < 0.01, ns – not significant. B) NK cell degranulation in PBMCs from 3 healthy donors after exposure to various forms of Elo alone (+ = native mAb, n/f = non-fucosylated Elo, mut = Elo Fc mutant, and Fab = F(ab')2 Elo; 1ug/ml each) with (+) or without (-) MM.1R or SLAMF7-deficient (KO) MM.1R targets (PBMC to target ratio 1:1). C) SLAMF7 expression on myeloma target cells using biotinylated Elo and streptavidin-APC. Isotype treated = gray shaded histogram, SLAMF7-deficient (KO) MM.1R = dashed line, and MM.1R = solid line. D) Activation of CD56dim NK cells in PBMC from 3 healthy donors upon exposure to indicated concentrations (µg/ml) of various forms of Elo overnight as a percentage CD56dim NK cells expressing CD69.
We next studied the effects of 2 Fc-engineered forms of Elo on NK cell degranulation: 1) a variant with a mutation in the Fc region that abrogates interaction with CD16 (Elo Fc mut) and 2) a non-fucosylated version (Elo n/f) that exhibits higher binding affinity to CD16 (Table 1). As expected, both Elo and Elo n/f increase the fraction of CD107a+ NK cells in the presence or absence of MM.1R target cells, and responses to Elo n/f stimulated more potent responses in all donors (Fig. 4B). Despite more potent degranulation response to Elo n/f, NK cell viability was only minimally reduced in these 2-hour assays (data not shown). In sharp contrast to the native or n/f antibody, Elo Fc mutant or Elo F(ab')2 did not induce degranulation in the presence or absence of MM.1R targets (Fig. 4B). We also knocked out SLAMF7 expression in MM.1R cells using zinc finger nucleases to generate MM.1R KO cells (Fig. 4C). The impacts of Elo and Elo n/f on degranulation in the presence of MM.1R KO targets were similar to these antibodies alone, demonstrating that the enhanced degranulation results from ADCC toward SLAMF7+ myeloma target cells.
Table 1.
KD values measured for the interactions between indicated form of Elo and human recombinant forms of the extracellular domain of CD16 (low affinity 158V or higher affinity 158F alleles), CD32a (low affinity 131R or higher affinity 131H alleles), CD32b, and CD64.
| KD, μM |
|||
|---|---|---|---|
| Elo | Elo n/f | Elo Fc mut | |
| CD16–158F | 2–5 | ≈0.1 | >>100 |
| CD16–158V | 0.36 ± 0.04 | ≈0.014 | >100 |
| CD32a-131H | 1.3 ± 0.1 | 1.6 ± 0.2 | >>200 |
| CD32a-131R | 1.4 ± 0.1 | 0.88 ± 0.08 | ≥10 |
| CD32b | ≥ 10 | ≥ 10 | >>10 |
| CD64 | 0.000073 ± 0.00001 | 0.000047 ± 0.00001 | >10 |
CD69 expression was also measured in response to treatment of PBMCs with 1–100µg/ml of Elo, Elo n/f, Elo Fc mutant and Elo F(ab')2. Consistent with degranulation experiments, Elo n/f induced greater increases in NK cell activation than Elo, while Elo Fc mutant and Elo F(ab')2 had no effect (Fig. 4D). These results indicate that CD107a and CD69 expression induced by Elo in the absence of myeloma target cells is due to Fc-mediated engagement with CD16 upon Elo binding to the surface of nearby SLAMF7+ cells within the PBMCs. Taken together, our data indicate that the activation of NK cells by Elo in vitro is primarily Fc-mediated and due to engagement with CD16, at least in these assays in which PBMCs are treated with soluble antibody.
Costimulatory effect of SLAMF7 engagement by elotuzumab
We next used the Fc-mutant form of Elo to test whether direct engagement with SLAMF7 can induce early signaling in NK cells, especially in soluble, unaggregated form. Previous work showed that antibody engagement of SLAMF7 induces activation of the human NK-like cell line, NK-92, through PLCγ1, PLCγ2 and PI3K signaling.31 Accordingly, antibody engagement of SLAMF7 increased intracellular calcium in wild type primary mouse NK cells.32 Therefore, we tested whether Elo Fc mutant could induce calcium mobilization alone, or in conjunction with simultaneous antibody crosslinking of other activating receptors in human NK cells. Biotinylated anti-CD16 mAb stimulated a robust calcium response when crosslinked with streptavidin (Fig. 5A). In sharp contrast, Elo alone or Elo Fc mutant alone did not induce calcium mobilization on human NK cells, even when these biotinylated mAbs were aggregated with streptavidin (Fig. 5A). Crosslinking of NKp46 with biotinylated mAb and streptavidin induced a modest calcium response in NK cells (Fig. 5B). Interestingly, streptavidin-mediated crosslinking of this activating receptor in combination with non-biotinylated Elo triggered even stronger calcium mobilization (Fig. 5B). Similar results were observed for combined streptavidin-mediated crosslinking of NKp46 and NKG2D in combination with non-biotinylated Elo (Fig. 5C). Importantly, co-crosslinking these activating receptors with non-biotinylated Elo Fc mutant (mimicking physiologic conditions) induced a similar potentiation of the calcium responses, demonstrating that this response is not due to Fc-mediated engagement with CD16 (Fig. 5B,C). Costimulation of NKp46-initiated calcium signals upon addition of non-biotinylated Elo or Elo Fc mutant was observed in both CD56dim and CD56bright NK cells from most of 9 healthy donors (Fig. 5D, E), despite significantly lower SLAMF7 expression on CD56bright NK cells (Fig. S4). On the other hand, co-stimulation of calcium signals initiated by the combined engagement of NKp46+NKG2D was only observed in CD56dim NK cells (Fig. 6E), which are the cytolytic cells that express consistently high levels of SLAMF7 (Fig. S3). These results demonstrate that Elo can costimulate calcium signaling in an Fc-independent manner, which does not require direct co-engagement with streptavidin-aggregated NKp46 and/or NKG2D.
Figure 5.
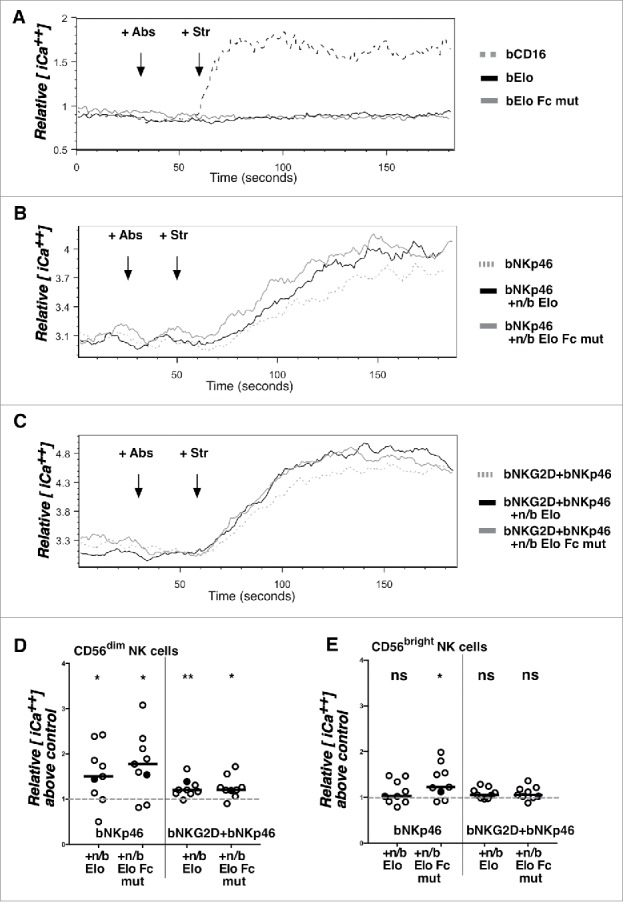
Co-activation of calcium signaling responses by SLAMF7 engagement with elotuzumab. A) PBMCs from healthy donors were loaded with indo-1-AM and stained for CD56 and CD3 before analysis by flow cytometry as in Materials and Methods. Cells were analyzed for 30 seconds, treated with antibodies (+Abs) [biotinylated anti-CD16 (bCD16; dashed gray line), bElo (solid black line), or bElo Fc mutant (solid gray line)], and analyzed for 30 seconds, exposed to streptavidin (+Str) to aggregate antibodies, and analyzed for 2 additional minutes. Relative mean intracellular calcium concentration [iCa2+] is indicated from the mean ratio of violet (405 nm) to blue (485 nm) emission over time in gated CD56dim NK cells. B) PBMCs were stimulated using biotinylated anti-NKp46 alone (+bNKp46; dashed gray line) or in combination with non-biotinylated (+n/b) Elo (black solid line) or +n/b Elo Fc mutant (gray solid line) at 30 seconds, then streptavidin 30 seconds later. C) PBMCs were stimulated using biotinylated anti-NKG2D + biotinylated anti-NKp46 mAbs (bNKG2D+bNKp46; dashed gray line) or these 2 biotinylated mAbs in combination with non-biotinylated (+n/b) Elo (black solid line) or +n/b Elo Fc mutant (gray solid line) at 30 seconds, then streptavidin 30 seconds later. D,E) Individual icons represent the increase of relative mean intracellular calcium concentration above control (+bNKp46 or bNKG2D+bNKp46 alone normalized to a value of 1) for CD56dim NK cells (D) or CD56bright NK cells (E) from 9 healthy donors. Relative intracellular calcium values for the gated NK cell populations from each donor were calculated by determining the area under the curve for mean 405nm/485nm emission ratios between 120–180 seconds in Elo-treated samples (+bNKp46 ± bNKG2D + Str as indicated) divided by area under the curve values for the same time window in samples treated with bNKp46 + Str alone (left) or bNKp46 + bNKG2D + Str alone (right). Conditions with and without n/b Elo or Elo Fc mutant were compared by 2-sided Wilcoxon matched-pairs signed rank test, *- P < 0.05, **- P < 0.01. Filled circles indicate ratio values for the representative donor shown in panels (B) and C.
Discussion
In this work, we studied the mechanism by which Elo promotes NK cell-mediated cytotoxicity of myeloma cells. Elo is a humanized IgG1 monoclonal antibody specific for SLAMF7, which is expressed on the surface of most myeloma tumor cells.13 Our studies also found high expression of SLAMF7, as assessed by Elo binding, on NK cells and CD16+ monocytes in the PBMCs of healthy donors, as well as expression on minor subsets of T and B cells, most notably CD56+ T cells (Fig. S3). In light of previous work demonstrating that SLAMF7 is a co-activating receptor on NK cells, we postulated that Elo may be directly stimulating and costimulating NK cells in patients, in addition to mediating ADCC responses through Fc-mediated engagement with CD16.13,15-17
Our results demonstrate robust degranulation when NK cells were exposed to SLAMF7+ myeloma target cells in the presence of Elo, and the degree of degranulation was directly dependent on the amount of SLAMF7 expressed on the myeloma cells. Interestingly, another SLAMF7 antibody, Chluc90, stimulated much weaker degranulation, indicating that Elo has unique activation properties, possibly due to higher affinity or targeting a key epitope on SLAMF7. We also showed that modest degranulation and CD69 induction in NK cells occurs when PBMCs are exposed to Elo alone, which is Fc-dependent and presumably due to CD16 engagement with Elo bound to adjacent SLAMF7+ cells within the PBMCs. It is important to note that only modest NK cell degranulation was observed when Elo was added to PBMC in the absence of myeloma target cells. This modest activation response to engagement with adjacent SLAMF7+ cells within the PBMCs may reflect the capacity of NK cell inhibitory receptors (e.g. killer cell Ig-like receptors or NKG2A/CD94) to impede detrimental degranulation responses toward normal MHC class I-bearing haematopoietic cells.
Importantly, we did not observe substantial loss of NK cell viability in Elo-treated cultures, even after overnight CD69 induction assays, demonstrating that fratricide between SLAMF7+ NK cells is minimal in response to Elo addition to PBMC cultures, even at high concentrations. Perhaps this lack of fratricide is also reflective of the inhibitory receptors engaging with MHC class I on adjacent NK cells. Furthermore, forms of Elo that are unable to engage with CD16 (Elo Fc mutant and Elo F(ab')2) could not stimulate degranulation or induce CD69 expression on NK cells in PBMCs. We conclude that the most potent stimulatory signal provided to NK cells by Elo is through CD16-mediated engagement with the Fc portion of the antibody, which is consistent with previous publications.13,15-17 Further studies are necessary to establish whether Elo can also impact immune responses by affecting the functions of SLAMF7-expressing non-classical monocytes (which also express CD16 to mediate ADCC) and CD56+ T cells. It is also possible that Elo could facilitate physical bridging between SLAMF7 on myeloma cells with SLAMF7 on NK cells to promote adhesion and co-stimulation of NK cell-mediated lysis.33,34 The lack of any enhanced degranulation or CD69 induction by adding Elo Fc mutant or F(ab')2 in the presence or absence of myeloma target cells, however, indicates that such a mechanism is not adequate to stimulate these specific responses.
In contradiction to our results, Collins et al. previously demonstrated direct stimulation of NK cells with soluble Elo F(ab')2 and a Fc mutant form of Elo (elo-G2M3).34 Their elo-G2M3 mutant consisted of an IgG2 backbone with Fc mutations designed to minimize CD16 binding.35 The basis for discrepancy with our results is unclear, but it is possible that 1) their elo-G2M3 mutant and F(ab')2 preparations retained some residual capacity to bind to CD16, which was not formally tested in that work, or 2) since their experiments used purified NK cell preparations, the antibodies could potentially have enhanced the “bridging” of SLAMF7 molecules expressed on adjacent NK cells. We consistently used freshly thawed aliquots of -80°C cryopreserved antibody preparations in our experiments and failed to observe any stimulation of degranulation or CD69 induction when using soluble Fc-compromised antibodies to treat PBMCs (in which NK cells are more distributed, making up only 5–20% of lymphocytes). Furthermore, our Elo Fc mutant preparation contained an IgG1 Fc region with 5 mutations, resulting in extremely low to undetectable affinities for CD16, CD32, or CD64 (see Table 1).
In addition, we provide evidence that direct engagement of SLAMF7 with soluble Elo can effectively mediate co-stimulatory signaling. The addition of soluble Elo potentiated intracellular calcium signaling responses triggered by antibody-mediated aggregation of NKp46 alone or in combination with NKG2D. The results illustrate that Elo binding to NK cells can effectively co-stimulate ITAM-mediated signaling through NKp46,36 even on top of costimulation by NKG2D in CD56dim NK cells. This co-stimulation was independent of CD16, as the same potentiation was observed upon addition of Elo Fc mutant. These data indicate that direct binding of soluble Elo to SLAMF7 on the NK cell surface provides a costimulatory priming signal that can effectively potentiate calcium mobilization responses triggered by multimeric aggregation of other activating receptors at target cell interfaces. Costimulation of calcium signaling by Elo is consistent with previous evidence that SLAMF7 associates with the cytosolic adaptor protein, EAT-2,31,32 which recruits PLCγ to augment calcium signaling.37
Importantly, Elo did not have to be co-aggregated by streptavidin with the activating receptors to mediate costimulation, as non-biotinylated forms of Elo or Elo Fc mutant effectively co-stimulated the calcium response. This observation suggests that SLAMF7 engagement by Elo can trigger co-stimulation from outside the site of activating receptor engagement with ligands on myeloma cells at an immune synapse. In this way, the Elo-mediated costimulation functions in “trans,” in contrast to costimulation in “cis,” which involves direct coengagement with the activating receptors. Similarly, trans-inhibition of FcεRI activation signaling was recently described when the FcγRIIB inhibitory receptor was independently engaged on the surface of mast cells and basophils.38 Trans-costimulation by Elo has the potential to enhance NK cell activation toward myeloma tumors that express appropriate ligands to engage NKp46, NKG2D, or potentially other ITAM-signaling receptors. DNAM-1, NKp46, and NKG2D play important roles in NK cell-mediated natural cytotoxicity of myeloma target cell lines,39,40 and while their known ligands are often expressed on myeloma cells,41,42 surface expression of these receptors can be reduced on NK cells in patients, especially in bone marrow.39,43,44 Our results suggest that trans-costimulation by Elo has the potential to enhance signaling by these receptors, even if their surface expression is reduced. Interestingly, lenalidomide can upregulate the expression of NKG2D ligands on myeloma cells,42,45 further increasing susceptibility to NK cells and potentially contributing to the improved therapeutic activity when combined with Elo.
In summary, our data demonstrate that Fc-mediated (CD16-dependent) NK cell activation is a dominant mechanism of action for Elo in vitro, while some co-stimulatory activation of NK cells can be induced through direct binding of Elo to SLAMF7 in a CD16-independent manner. Trans-costimulation by Elo has potential to boost activation signals mediated through other receptors engaging with ligands on the surface of myeloma cells, thereby providing an additive benefit to enhance myeloma-targeted cytolytic responses. In this way, Elo would promote NK cell cytolytic responses toward myeloma tumor cells through initiating ADCC and enhancing activation signals initiated through other receptors, without promoting promiscuous degranulation that may be detrimental to normal tissues. Clinical data has shown that Elo is more effective in combination with conventional treatments, such as lenalidomide, which also can potentiate NK cell cytotoxicity responses,18 further pointing to an important role for NK cells in therapeutic efficacy. Lenalidomide has recently been shown to lower the threshold of NK cell activation through CD16, which corresponds with enhanced cytolytic granule release toward target cells.46 Our data also demonstrate that non-fucosylated Elo, which has a higher affinity binding to CD16 (Table 1), can stimulate stronger NK cell degranulation and CD69 induction. This suggests that therapeutic use of non-fucosylated Elo could potentially result in improved clinical outcomes, although potential for toxicity must be considered. Furthermore, combination therapeutic strategies incorporating Elo with other drugs that can potentiate NK cell functions should be considered as promising strategies for MM treatment.
Methods
Blood sample preparation
Blood was collected into heparinized tubes from healthy volunteer donors that were consented using HIPAA-compliant procedures approved by the Institutional Review Board of Fox Chase Cancer Center (FCCC). Fresh blood was mixed with complete RPMI-1640 medium in 1:1 ratio, peripheral blood mononuclear cells (PBMCs) were isolated as a buffy coat on Lymphoprep (Axis-Shield POC AS, Oslo, Norway) after centrifuging at 900 RCF x30 minutes, and PBMC were washed x3 with HBSS. Cells were resuspended in complete RPMI-1640 medium (supplemented with penicillin/streptomycin, L-glutamine, sodium pyruvate, 10mM HEPES, 50µM 2-mercaptoethanol, and 10% heat-inactivated fetal bovine serum) and containing 10% donor plasma.
Cells and cell lines
Myeloma cell lines were cultured in complete RPMI-1640 medium. MM1.R and RPMI-8226 were obtained from ATCC as fresh stocks at the initiation of this project. Human SLAMF7 cDNA was subcloned into pBMN-IRES-EGFP retroviral vector. RPMI-8226 cells were retrovirally transduced to express higher level of cell surface SLAMF7 and sorted (RPMI-8226+SLAMF7) as described.47 Zinc finger nucleases were purchased to permanently disrupt the SLAMF7 gene (CompoZr Knockout Zinc Finger Nucleases, CKOZFND19392–1KT, Sigma-Aldrich). MM.1R cells were nucleofected with SLAMF7 zinc finger nuclease mRNA using a Lonza Nucleofector Device [program T-020 and Nucleofector Kit V (Lonza, Walkersville, MD)] and SLAMF7-deficient cells were sorted (MM1.R SLAMF7KO) by FACS. Cell lines were thawed fresh every 2 months and tested for mycoplasma at least yearly in the FCCC Cell Culture Facility.
Reagents
Elotuzumab (Elo), Elo with a mutation in the Fc region that prevents recognition by CD16 (Elo Fc mut), and non-fucosylated Elo with higher affinity to CD16 (Elo n/f) were provided by Bristol-Myers Squibb (BMS; Princeton, NJ). The anti-SLAMF7 antibody, ChLuc90, which consists of chimerized human IgG1 Fc fused to mouse CDR variable regions,15 was provided by AbbVie Inc. (North Chicago, IL). Elo n/f antibody was generated by expression of Elo variable regions cDNA cloned into pUCE (Millipore) in the FUT8−/− Ms 704-PF CHO cell line (BioWa). Elo Fc mut antibody was expressed by the CHO-S cell line cotransfected with vectors pICOFSCneoK (encoding Elo variable regions) and pODpurIgG1.1f (encoding IgG1 heavy chain constant region with L234A-L235E-G237A-A330S-P331S mutations). Antibodies were produced and purified using standard cultivation and purification techniques. F(ab')2 fragments were generated using immobilized pepsin (ThermoFisher Scientific, #20343). Complete digestion of the IgG1 HC Fc domain by pepsin was confirmed by SDS-PAGE. Isotype control human IgG1κ was obtained from SouthernBiotech (#0151K-01).
Antibody staining of cells
PBMCs were stained x20 minutes on ice and rinsed twice with staining buffer (0.1% Na azide and 1% FBS in HBSS) and 100 ng/ml of propidium iodide (Invitrogen) in the final wash. SLAMF7 expression was identified using biotinylated elotuzumab or biotinylated isotype control conjugated antibody [prepared using EZ-Link NHS-Biotin (Thermo Scientific #20217)] and streptavidin-APC (Biolegend, #405207). NK cells were gated in flow cytometry as CD45+CD3−CD56+ lymphocytes using anti-CD45-PercP-Cy5.5 (eBioscience, 2D1), anti-CD3-APC-H7 (BD, SK7) and anti-CD56-APC (Biolegend, NCAM 16.2) antibodies. Cell activation was measured with anti-CD69-Pacific Blue (Biolegend, FN50) antibody.
NK cell degranulation assay
Freshly isolated PBMCs (106 cells) were incubated either alone, with elotuzumab, with targets (106 cells), or with targets and elotuzumab for 2 hours at 37°C in 200 µl complete RPMI-1640. Samples were centrifuged at 150 RCF for 3 minutes before incubation. Anti-CD107a-PE (BD, H4A3), anti-CD45-PerCP-Cy5.5, anti-CD3-APC-H7, and anti-CD56-APC antibodies were added in the last 30 minutes of culture. Cells were centrifuged and rinsed twice, with propidium iodide in second wash. NK cell degranulation was measured as percent CD107a+ NK cells.
NK cell activation assay
PBMCs were added to a 96-well flat bottom plate (106 cells/well) and soluble antibodies were added to cultures (10μg/ml). After overnight incubation, cells were stained with anti-CD45-PerCP-Cy5.5, anti-CD56-APC, anti-CD3-APC-H7, and anti-CD69-Pacific Blue antibodies, washed x2 (second wash with 100ng/ml propidium iodide), and analyzed by flow cytometry.
Measuring intracellular calcium concentrations by flow cytometry
Intracellular calcium signaling was measured in human NK cells using previously described conditions.48 PBMCs were resuspended at 5 × 106 cells/2 ml in biotin- and phenol red-deficient RPMI-1640 (DRPMI), mixed thoroughly with 4.4µM Indo-1 AM (Invitrogen, Eugene OR), and incubated for 30 minutes at 37°C.49 Cells were washed with DRPMI and stained with anti-CD3-FITC (Biolegend, clone SK7) and anti-CD56-APC antibodies for 10 minutes at 37°C. PBMCs were rinsed twice with DRPMI and propidium iodide in last wash. Cells (3–5 × 106 cells) were resuspended in 500μl of DRPMI medium and maintained in the dark at room temperature before analysis on a BD Aria II flow cytometer with a UV laser at about 1000–1500 events/second. After 30 seconds, 1 μg of biotinylated Elo or Elo Fc mutant with or without biotinylated anti-NKG2D+anti-NKp46 antibodies (1µg each) were added to the PBMCs. Thirty seconds later, 2μg of streptavidin was added per 1μg of biotinylated antibody to aggregate biotinylated mAbs, and PBMCs were analyzed for 2 minutes. Single cells were gated by FSC-height vs. FSC-area and viable NK cells were gated as CD56+CD3−propidium iodide−. Relative intracellular calcium concentration was detected through ratio analysis of violet (405 nm) over blue (485 nm) emission.
Binding affinity of elotuzumab preparations to human Fc receptors
Antibodies were amine-coupled to a ProteOn GLC sensor chip (BioRad). Affinities were measured by Surface Plasmon Resonance in a ProteOn XPR36 Interaction Array System instrument (BioRad) at 25°C, using a running buffer of PBS (pH 7.4) supplemented with 0.05% Tween 20 (v/v). Recombinant versions of the extracellular domains of human Fc receptors were expressed with a C-terminal His-tag in CHO cells and purified by affinity (Ni2+) and size exclusion chromatograpy. Fc receptors were injected over all spots with a flow rate of 30µL/min in a 6-membered dilution series. The top concentration and dilution factor, as well as injection times, were receptor-specific. All data were double-referenced using interspots and buffer injections. Where possible, binding was fitted to a 1:1 binding model (steady-state fit for fast interactions and kinetic fit for slow interactions).
Flow cytometry and data analysis
Stained cells were analyzed on a BD ARIA II flow cytometer. Data was collected with BD FACS Diva software (v6) and analyzed with FlowJo (v9.7; Tree Star Inc., Ashland, OR), Microsoft Excel (v12), and Graphpad Prism (v.6.0; Graphpad Software, La Jolla, CA) software. Statistical analysis was performed using Wilcoxon matched-pairs signed rank with p < 0.05 considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs. Glenn Rall and Hossein Borghaei at FCCC for reviewing the manuscript, Dr. Audie Rice for initiating discussions to develop this project, and FCCC core facilities: Cell Culture, Genomics, Flow Cytometry, and Biostatis. We also thank BMS for valuable reagents and financial support.
Funding
This work was supported by grants from Bristol-Myers Squibb (BMS), which markets elotuzumab. NAB, KAH, CB, RFG, and MDR are employees of BMS. KSC and ADC have served on scientific advisory boards for BMS.
Financial support
Supported by Collaborative Science Center of Excellence grants CA204–013 and CA-204–156 to ADC and KSC from Bristol-Myers Squibb, FCCC Bucks County and Main Line Boards of Associates, NIH Training grant T32 CA9035 (AMJ) and NCI Comprehensive Cancer Center Support Grant CA06927 (FCCC).
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Eng J Med 2011; 364:1046-60; PMID:21410373; https://doi.org/ 10.1056/NEJMra1011442 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30 [DOI] [PubMed] [Google Scholar]
- 3.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol 2012; 9:135-43; PMID:22349016; https://doi.org/ 10.1038/nrclinonc.2012.15 [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317-27; PMID:20414205; https://doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palumbo A, Sonneveld P. Preclinical and clinical evaluation of elotuzumab, a SLAMF7-targeted humanized monoclonal antibody in development for multiple myeloma. Expert Rev Hematol 2015; 8(4):481-91:1-11 [DOI] [PubMed] [Google Scholar]
- 6.Laubach JP, Richardson PG. CD38-Targeted Immunochemotherapy in Refractory Multiple Myeloma: A New Horizon. Clin Cancer Res 2015; 21(12):2660-2; https://doi.org/ 10.1158/1078-0432.CCR-14-3190 [DOI] [PubMed] [Google Scholar]
- 7.Tassone P, Goldmacher VS, Neri P, Gozzini A, Shammas MA, Whiteman KR, Hylander-Gans LL, Carrasco DR, Hideshima T, Shringarpure R, et al.. Cytotoxic activity of the maytansinoid immunoconjugate B-B4-DM1 against CD138+ multiple myeloma cells. Blood 2004; 104:3688-96; PMID:15292058; https://doi.org/ 10.1182/blood-2004-03-0963 [DOI] [PubMed] [Google Scholar]
- 8.Ocio EM, Richardson PG, Rajkumar SV, Palumbo A, Mateos MV, Orlowski R, Kumar S, Usmani S, Roodman D, Niesvizky R, et al.. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia 2014; 28:525-42; PMID:24253022; https://doi.org/ 10.1038/leu.2013.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretz-Rommel A, Qin F, Dakappagari N, Cofiell R, Faas SJ, Bowdish KS. Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J Immunol 2008; 180:699-705; https://doi.org/ 10.4049/jimmunol.180.2.699 [DOI] [PubMed] [Google Scholar]
- 10.Bensinger W, Maziarz RT, Jagannath S, Spencer A, Durrant S, Becker PS, Ewald B, Bilic S, Rediske J, Baeck J, et al.. A phase 1 study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol 2012; 159:58-66; PMID:22861192; https://doi.org/ 10.1111/j.1365-2141.2012.09251.x [DOI] [PubMed] [Google Scholar]
- 11.Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, Craigen J, Yates J, Gliddon L, Fieles W, et al.. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014; 123:3128-38; PMID:24569262; https://doi.org/ 10.1182/blood-2013-10-535088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein R, Gupta P, Chen X, Cardillo TM, Furman RR, Chen S, Chang CH, Goldenberg DM. Therapy of B-cell malignancies by anti-HLA-DR humanized monoclonal antibody, IMMU-114, is mediated through hyperactivation of ERK and JNK MAP kinase signaling pathways. Blood 2010; 115:5180-90; PMID:20101022; https://doi.org/ 10.1182/blood-2009-06-228288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, et al.. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14:2775-84; PMID:18451245; https://doi.org/ 10.1158/1078-0432.CCR-07-4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annual Rev Immunol 2011; 29:665-705; PMID:21219180; https://doi.org/ 10.1146/annurev-immunol-030409-101302 [DOI] [PubMed] [Google Scholar]
- 15.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al.. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112:1329-37; PMID:17906076; https://doi.org/ 10.1182/blood-2007-08-107292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, Garg TK, Shi J, Moreno-Bost AM, Yun R, et al.. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Therapeutics 2009; 8:2616-24; PMID:19723891; https://doi.org/ 10.1158/1535-7163.MCT-09-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasa B, Yun R, Belmar NA, Fox M, Chao DT, Robbins MD, Starling GC, Rice AG.. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-alpha pathways. Cancer Immunol Immunother 2015; 64:61-73; PMID:25287778; https://doi.org/ 10.1007/s00262-014-1610-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentlik James A, Cohen AD, Campbell KS. Combination immune therapies to enhance anti-tumor responses by NK cells. Frontiers Immunol 2013; 4:481; PMID:24391651; https://doi.org/ 10.3389/fimmu.2013.00481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezman NA, Jhatakia A, Kearney AY, Brender T, Maurer M, Henning K, et al.. PD-1 blockade enhances elotuzumab efficacy in mouse tumor models. Blood Adv 2017; 1:753-65; https://doi.org/ 10.1182/bloodadvances.2017004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J, Afar DE, Singhal AK. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012; 120:552-9; PMID:22184404; https://doi.org/ 10.1182/blood-2011-06-360552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowiak A, Offidani M, Pegourie B, De La Rubia J, Garderet L, Laribi K, Bosi A, Marasca R, Laubach J, Mohrbacher A, et al.. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016; 127:2833-40; PMID:27091875; https://doi.org/ 10.1182/blood-2016-01-694604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL, Leleu X, Tsao LC, Westland C, et al.. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30:1953-9; PMID:22547589; https://doi.org/ 10.1200/JCO.2011.37.2649 [DOI] [PubMed] [Google Scholar]
- 23.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H, et al.. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Eng J Med 2015; 373(7):621-31; PMID:26035255; https://doi.org/ 10.1056/NEJMoa1505654 [DOI] [PubMed] [Google Scholar]
- 24.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294:15-22; PMID:15604012; https://doi.org/ 10.1016/j.jim.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 25.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med 2005; 202:1001-12; PMID:16203869; https://doi.org/ 10.1084/jem.20051143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibiansky L, Passey C, Roy A, Bello A, Gupta M. Model-based pharmacokinetic analysis of elotuzumab in patients with relapsed/refractory multiple myeloma. J Pharmacokinetics Pharmacodynamics 2016; 43(3):243-57; PMID:26993283; https://doi.org/ 10.1007/s10928-016-9469-x [DOI] [PubMed] [Google Scholar]
- 27.Richardson PG, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, Vij R, White D, Reece DE, Benboubker L, et al.. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol 2015; 2:e516-27; PMID:26686406; https://doi.org/ 10.1016/S2352-3026(15)00197-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eischen CM, Schilling JD, Lynch DH, Krammer PH, Leibson PJ. Fc receptor-induced expression of Fas ligand on activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J Immunol 1996; 156:2693-9; PMID:8609385 [PubMed] [Google Scholar]
- 29.Jewett A, Cavalcanti M, Giorgi J, Bonavida B. Concomitant killing in vitro of both gp120-coated CD4+ peripheral T lymphocytes and natural killer cells in the antibody-dependent cellular cytotoxicity (ADCC) system. J Immunol 1997; 158:5492-500; PMID:9164972 [PubMed] [Google Scholar]
- 30.Warren H. Target-induced natural killer cell loss as a measure of NK cell responses. Curr Protocols Immunol 2013; Chapter 14: Unit 14.29.1-21; https://doi.org/ 10.1002/0471142735.im1429s101 [DOI] [PubMed] [Google Scholar]
- 31.Tassi I, Colonna M. The cytotoxicity receptor CRACC (CS-1) recruits EAT-2 and activates the PI3K and phospholipase Cgamma signaling pathways in human NK cells. J Immunol 2005; 175:7996-8002; https://doi.org/ 10.4049/jimmunol.175.12.7996 [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol 2009; 10:297-305; PMID:19151721; https://doi.org/ 10.1038/ni.1693 [DOI] [PubMed] [Google Scholar]
- 33.Veillette A, Guo H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol 2013; 88:168-77; PMID:23731618; https://doi.org/ 10.1016/j.critrevonc.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 34.Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling GC, Ciarlariello D, Bhaskar S, Briercheck EL, et al.. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 2013; 62:1841-9; PMID:24162108; https://doi.org/ 10.1007/s00262-013-1493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole MS, Anasetti C, Tso JY. Human IgG2 variants of chimeric anti-CD3 are nonmitogenic to T cells. J Immunol 1997; 159:3613-21; PMID:9317161 [PubMed] [Google Scholar]
- 36.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annual Rev Immunol 2001; 19:197-223; PMID:11244035; https://doi.org/ 10.1146/annurev.immunol.19.1.197 [DOI] [PubMed] [Google Scholar]
- 37.Perez-Quintero LA, Roncagalli R, Guo H, Latour S, Davidson D, Veillette A. EAT-2, a SAP-like adaptor, controls NK cell activation through phospholipase Cgamma, Ca++, and Erk, leading to granule polarization. J Exp Med 2014; 211:727-42; PMID:24687958; https://doi.org/ 10.1084/jem.20132038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malbec O, Cassard L, Albanesi M, Jonsson F, Mancardi D, Chicanne G, Payrastre B, Dubreuil P, Vivier E, Daëron M. Trans-inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Sci Signal 2016; 9:ra126; PMID:27999175; https://doi.org/ 10.1126/scisignal.aag1401 [DOI] [PubMed] [Google Scholar]
- 39.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, et al.. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res 2007; 67:8444-9; PMID:17875681; https://doi.org/ 10.1158/0008-5472.CAN-06-4230 [DOI] [PubMed] [Google Scholar]
- 40.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, et al.. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005; 105:251-8; PMID:15328155; https://doi.org/ 10.1182/blood-2004-04-1422 [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Sanchez MV, Periago A, Legaz I, Gimeno L, Mrowiec A, Montes-Barqueros NR, Campillo JA, Bolarin JM, Bernardo MV, López-Álvarez MR, et al.. Overexpression of KIR inhibitory ligands (HLA-I) determines that immunosurveillance of myeloma depends on diverse and strong NK cell licensing. Oncoimmunology 2016; 5:e1093721; PMID:27141379; https://doi.org/ 10.1080/2162402X.2015.1093721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, et al.. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113:3503-11; PMID:19098271; https://doi.org/ 10.1182/blood-2008-08-173914 [DOI] [PubMed] [Google Scholar]
- 43.Costello RT, Boehrer A, Sanchez C, Mercier D, Baier C, Le Treut T, Sébahoun G. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology 2013; 139:338-41; PMID:23360454; https://doi.org/ 10.1111/imm.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Lilienfeld-Toal M, Frank S, Leyendecker C, Feyler S, Jarmin S, Morgan R, Glasmacher A, Märten A, Schmidt-Wolf IG, Brossart P, et al.. Reduced immune effector cell NKG2D expression and increased levels of soluble NKG2D ligands in multiple myeloma may not be causally linked. Cancer Immunol Immunother 2010; 59:829-39; PMID:20024547; https://doi.org/ 10.1007/s00262-009-0807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fionda C, Abruzzese MP, Zingoni A, Cecere F, Vulpis E, Peruzzi G, Soriani A, Molfetta R, Paolini R, Ricciardi MR, et al.. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget 2015; 6:23609-30; PMID:26269456; https://doi.org/ 10.18632/oncotarget.4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagrue K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood 2015; 126:50-60; PMID:26002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miah SM, Campbell KS. Expression of cDNAs in human Natural Killer cell lines by retroviral transduction. Methods Mol Biol 2010; 612:199-208; PMID:20033642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacFarlane AWT, Oesterling JF, Campbell KS. Measuring intracellular calcium signaling in murine NK cells by flow cytometry. Methods Mol Biol 2010; 612:149-57; PMID:20033639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.June CH, Abe R, Rabinovitch PS. Measurement of intracellular calcium ions by flow cytometry. Curr Protocols Cytom 2001; Chapter 9:Unit 9.8; https://doi.org/ 10.1002/0471142956.cy0908s02 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


