ABSTRACT
We recently described the CD56lowCD16low subset of Natural Killer (NK) cells that both mediate cytotoxic activity and produce IFNγ, being more abundant in bone marrow (BM) than in peripheral blood (PB) of pediatric normal subjects. Given the multifunctional properties of this subset, we examined its development and functional recovery in a cohort of children undergoing α/β T-cell depleted HLA-haploidentical haematopoietic stem cell transplantation (HSCT). The results obtained indicate that CD56lowCD16low NK cells are present in both PB and BM already at one month post-HSCT, with an increased frequency in BM of graft recipients as compared with normal subjects. During the first 6 months after HSCT, no difference in CD56lowCD16low NK cells distribution between PB and BM was observed. In comparison to normal subjects, CD56lowCD16low NK cells from transplanted patients show lower expression levels of CD25 and CD127 and higher levels of CD122, and accordingly, produce higher amounts of IFNγ after stimulation with IL-12 plus IL-15. The recovery of NK-cell cytotoxicity after HSCT was strictly restricted to CD56lowCD16low NK cells, and their ability to degranulate against K562 target cells or autologous leukemic blasts was completely restored only one year after HSCT.
Based on the phenotypic and functional ability of reconstituted CD56lowCD16low NK cells, we suggest that they play an important role in host defense against leukemia relapse and infections after HSCT, and represent an ideal candidate for approaches of adoptive immunotherapy.
KEYWORDS: Natural Killer cell subsets, Haematopoietic stem cell transplantation, haematological malignancies, Phenotype, Cytokine production, Cytotoxicity
Introduction
Natural Killer (NK) cells, classified as type 1 innate lymphoid cells (ILC1), are important effectors in the early phase of innate immune response due to their ability to kill both transformed and infected cells; moreover, they play a crucial role as immune regulators of adaptive immunity through the production of cytokines and chemokines.1,2,3
NK cells are endowed with a complex system of both activating and inhibitory receptors that either positively or negatively modulate their functions.4,5,6 The inhibitory NK-cell receptor families include the killer-immunoglobulin-like receptors (KIRs), which recognize the classical HLA-A, HLA-B and HLA-C molecules, and CD94/NKG2A, which recognize the non-classical HLA-E molecule on target cells.7,8 Activating signals are mediated by receptor families, such as natural cytotoxicity receptors (NKp46, NKp30 and NKp44), C-type lectin receptors NKG2C/CD94, NKG2D, signaling lymphocytic activation molecule family molecule (2B4/CD244), the DANAX accessory molecule (DNAM-1/CD226), activating KIRs, and the low-affinity Fc-γ receptor IIIA (CD16).9,10,11,12 The expression and activation of MHC class I-recognizing receptors is fundamental for the acquisition of functional competence by NK cells, through a process, defined as education or licensing, that makes NK cells capable to distinguish self from non-self.13,14 According to NK-cell receptor expression profile, distinct NK-cell populations have been identified in different tissues, likely representing specialized subsets capable of mediating different functions and endowed with distinct migratory ability.15 In humans, 2 major NK-cell subsets have been characterized on the basis of the cell surface density of both CD56 (NCAM) and CD16: CD56lowCD16high are mainly cytotoxic and represents 90% of circulating NK cells, while, CD56highCD16−/+ NK cells constitute 10% of peripheral blood (PB) NK cells and are mainly cytokine producers.16,17
We recently described a subset of NK cells endowed with low expression levels of both CD56 and CD16 more abundant in bone marrow (BM) than in PB of healthy donors (HD). CD56lowCD16low NK cells are able to release IFNγ upon cytokine stimulation and represent the major cytotoxic NK-cell population against human HLA class-I-deficient K562 target cells or acute leukemia blast cells. Moreover, we showed that in BM and PB of pediatric ALL patients the frequency of CD56lowCD16low NK cells was higher than in healthy donors, even if they were not fully functional. Interestingly, we demonstrated that the multifunctional CD56lowCD16low NK cell subset isolated from haematopoietic stem cell (HSC) donors is able to degranulate in response to leukemia cells of the corresponding recipients, suggesting a role of this cell population in mediating a graft-versus-leukemia (GvL) effect.18
Haematopoietic stem cell transplantation (HSCT) from an HLA-identical donor represents the treatment of choice for many malignant and non-malignant hematological diseases, being able to cure a high percentage of patients. HLA-haploidentical HSCT (haplo-HSCT), where donor and recipient share one HLA haplotype while the other is fully mismatched, represents a valid alternative, in the absence of an HLA-identical sibling or suitable HLA-compatible unrelated volunteer.19
NK cells play a key role in HSCT, representing the first donor-derived lymphocyte subset to reconstitute after transplantation; pioneering studies in adult acute myeloid leukemia (AML) demonstrated that in T cell-depleted haplo-HSCT, the GvL is mainly mediated by NK cells differentiating from donor HSC. This NK cell-mediated GvL effect is due to the release of inflammatory cytokines and direct target cell lysis.20,21,22 More specifically, in haplo-HSCT, a strong GvL response occurred in those patients transplanted from an alloreactive donor according to the KIR/KIR ligand model.20 In addition to their anticancer activity, NK cells have been shown to become activated and to proliferate in response to viral infections, like that caused by cytomegalovirus (CMV), which represents one of the main problems during the early months post-HSCT.5,23,24
Since NK-cell phenotypic and functional recovery after HSCT still remains a major field of investigation, in this study, we analyzed at different time points after the allograft NK-cell reconstitution in 46 acute leukemia patients given α/β T cell-depleted haplo-HSCT. Through this type of graft manipulation, patients receive not only donor HSC, but also high numbers of mature NK cells. In particular, we evaluated the frequency, phenotype and effector functions of CD56lowCD16low NK cell subset at different time points after transplantation in both BM and PB.
Methods
Cell source
PB and BM cells were obtained from 46 leukemia patients at different time-points after α/β T cell-depleted haplo-HSCT performed at Bambino Gesù Children's Hospital, Rome, Italy, (see also Supplementary Table 1 for further details on patient, donor and transplant characteristics). The study (ClinicalTrial.gov identifier: NCT01810120) was approved by the institutional ethics committees and informed assent/consent was obtained from donors, patients and/or their legal guardians.
Multicolor immunofluorescence and cytofluorimetric analysis
Freshly isolated PB and BM mononuclear cells were stained using the appropriate antibody (Ab) combination and subjected to cytofluorimetric analysis. Intracellular staining with appropriate mAb was performed after fixation with 1% paraformaldehyde and permeabilization (0.5% saponin, 1% FCS). Sample acquisition was performed on FACSCantoII (BD Biosciences, San Jose, CA) flow cytometer and cytofluorimetric analysis was performed with FlowJo 9.2.3 (TreeStar, Ashland, OR).
Degranulation assay and IFNγ production
NK cells from PB or BM were co-cultured with either the MHC class I negative human erythroleukemia cell line K562 or leukemia blast cells at 1:1 effector/target (E/T) ratio for 3 h, in the presence of 50 µM monensin (BD Biosciences) for the last 2 h, and degranulation was assessed by evaluating CD107a expression on NK-cell subsets.
In some experiments, NK cells were co-cultured with the FcγR+ murine mastocytoma cell line, P815, in the presence of mAbs directed against the relevant activating NK-cell receptors. Degranulation was assessed upon 2 h co-culture at 37°C.
To assess intracellular IFNγ production, mononuclear cells were incubated with IL-12 (25 ng/mL) plus IL-15 (50 ng/mL) (PeproTech, London, UK) at 37°C. After 1 h, 10 µg/ml brefeldin A were added, and cells were incubated for additional 12 h. Cells were subsequently fixed, permeabilized, stained with anti-IFNγ-APC, and analyzed by flow cytometry.
Statistical analysis
t-test or Mann-Whitney U test were used to compare independent groups; paired t-test or Wilcoxon matched test were used to compare matched groups. Statistical analyses were performed using PRISM 6.0 (GraphPad, La Jolla, CA)
Results
CD56lowCD16low NK cell recovery early after HSCT
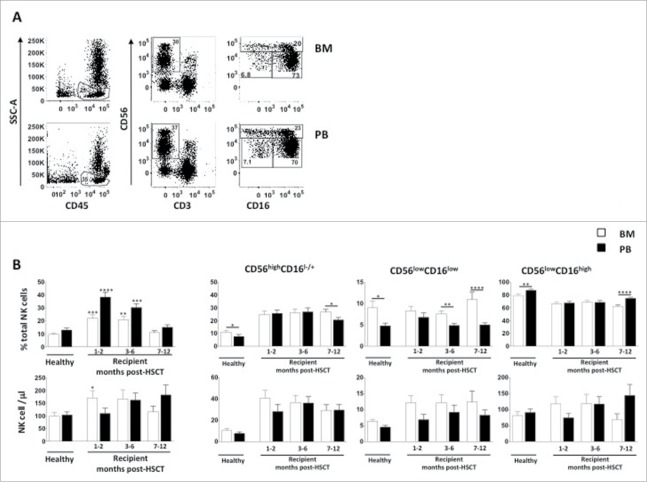
NK-cell differentiation has been proposed to occur through a linear process: accordingly CD56highCD16−/+ NK cells are believed to be precursors of CD56lowCD16high NK cells, and in our previous work, we proposed that the CD56lowCD16low NK cell subset represents an intermediate stage of differentiation between CD56high and CD56low.18,25 Herein, our goal was to follow the kinetics of recovery of CD56lowCD16low NK-cell number and functionality to predict their protective effect post HSCT. To this purpose, we analyzed BM and PB of 46 leukemia patients at different time-points after α/β T cell-depleted haplo-HSCT and compared them with BM and PB of age-matched HD (Supplementary Table 1). In line with previous observations, we found that NK cells engrafted early after HSCT and their frequency, but not their absolute number, was significantly higher with respect to HD both in BM and PB, reaching comparable levels only 6 months after transplantation (Fig. 1 panel A/B). Further dissection of NK cells into CD56highCD16+/−, CD56lowCD16low and CD56lowCD16high subsets by multiparametric flow-cytometry, revealed that the NK-cell population consisted of all 3 subsets already at one month post-HSCT, and that the absolute number of cells belonging to the most immature populations, namely CD56highCD16+/− and CD56lowCD16low, was increased in BM with respect to age-matched controls. Furthermore, unlike what was found in HD, there were no difference in CD56highCD16+/− and CD56lowCD16low NK cells frequency between BM and PB during the first 6 months after HSCT (Fig. 1 panel B). Neither the type of leukemia (ALL vs. AML) nor the occurrence of viral infections after the allograft significantly affected the CD56lowCD16low NK-cell recovery (data not shown).
Figure 1.
BM and PB CD56lowCD16low NK-cell recovery after α/β T-cell depleted HSCT. Cells freshly isolated by density gradient centrifugation from BM and PB of pediatric healthy donors and α/β T-cell depleted haplo-HSCT recipients were analyzed through flow cytometry to dissect CD56+CD3− NK cells into 3 subsets based on CD56 and CD16 expression levels, namely CD56highCD16+/−, CD56lowCD16low and CD56lowCD16high NK cell subsets (panel A). Histograms represent the percentage and absolute cell number/μl of total NK cells among lymphocytes and of CD56highCD16+/−, CD56lowCD16low and CD56lowCD16high NK cell subsets in BM (white) and PB (black) (panel B). Error bars represent SEM. BM n = 65; PB n = 62. Unpaired t-test, *p < 0,05; **p < 0,005; ***p < 0,0002; ****p < 0,0001.
Phenotype of reconstituting CD56lowCD16low NK cells
We previously demonstrated that CD56lowCD16low NK cells isolated from BM and PB of pediatric normal subjects exhibit a peculiar less-mature NK-cell phenotype. Since expression of NK-cell surface receptors often parallels progression toward a functional maturation state, we further characterized, at different time points both in BM and PB, the phenotypic profile of CD56lowCD16low NK cells that arise after HSCT.
By multi-parametric flow cytometry, we examined the expression patterns and the kinetics of acquisition of some activating (NKG2D, NKG2C, NKp46 and DNAM-1) and inhibitory (NKG2A, KIR2DL1/CD158a, KIR2DL37CD158b and KIR3DL1/CD158e1) receptors (expressed as a percentage of positive cells, Fig. 2 panel A/B), and as mean fluorescence intensity (MFI) (data not shown). Starting from the first month after HSCT, the phenotypic profile of CD56lowCD16low NK cells was similar to that of HD for all the activating receptors analyzed, while donor and recipient differed for the expression of CD158a/KIR2DL1 and CD158b/KIR2DL3 receptors on CD56lowCD16low NK cells, being expressed by 20% of recipients while being undetectable in cells from HD.
Figure 2.
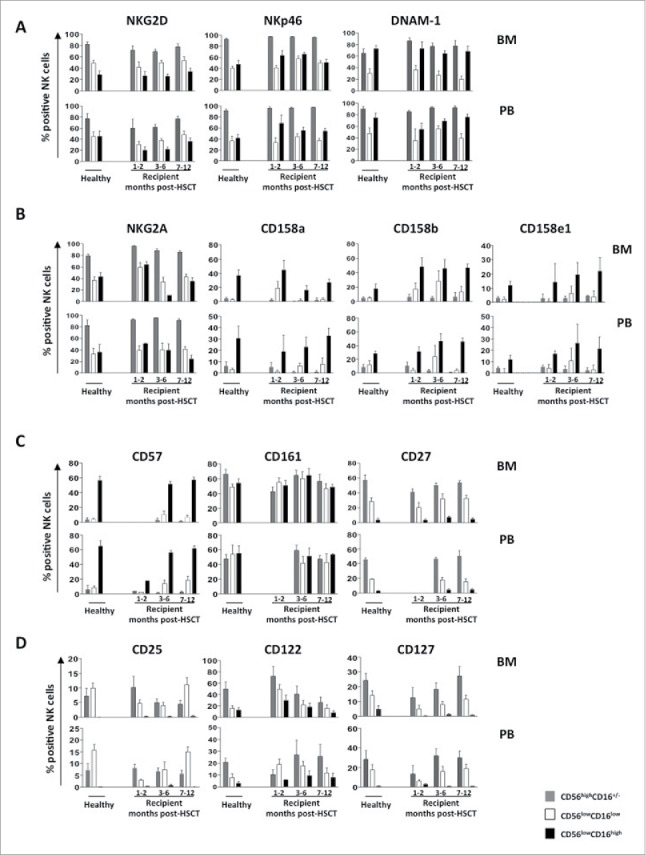
BM and PB CD56lowCD16low NK-cell phenotype at different time points after transplant. Cells freshly isolated by density gradient centrifugation from BM and PB of pediatric healthy donors and haplo-HSCT recipients were analyzed by flow cytometry for the expression of activating receptors (panel A), MHC class I-specific inhibitory receptors (panel B), differentiation markers (panel C) and cytokines receptors (panel D). The CD56highCD16+/− (gray), CD56lowCD16low (white) and CD56lowCD16high (black) NK cell subsets were gated on CD56+CD3− NK cells. Histograms represent the mean value ± SD of the percentage of positive cells analyzed at different time-points after HSCT starting from the first month until 12th month.
We next evaluated whether and when NK cells reconstituting after haplo-HSCT recover a mature phenotype. To this purpose, we analyzed the expression levels of CD161, a marker of NK-cell lineage commitment, CD27, a TNF receptor superfamily member that has been associated with more immature stages of differentiation, and CD57, a marker of terminally differentiated NK cells (Fig. 2 panel C). Both BM and PB CD56lowCD16low NK cells expressed CD27 and CD161 receptors, while were mostly negative for CD57. These data suggest that CD56lowCD16low NK cells that arise after HSCT exhibit a less mature stage of differentiation than CD56lowCD16high NK cells.
The phenotypic features of CD56lowCD16low NK cells at one month after HSCT showed several differences from those of HD as regards to the expression of CD25 (IL-2Rα), CD122 (IL-2/IL-15Rβ) and CD127 (IL-7Rα), all these molecules being receptors of cytokines (namely IL-2, IL-15 and IL-7, respectively) sustaining NK-cell growth and differentiation.26,27,28 CD56lowCD16low NK cells from haplo-HSCT recipients showed a peculiar cytokine receptor profile, displaying lower expression levels of CD25 and CD127 and higher levels of CD122, both in terms of frequency and MFI (data not shown), in comparison to healthy age matched controls counterpart (Fig 2 panel D). The recovery of an expression profile comparable to that of healthy individuals was gradually taking place over the course of 6 to 9 months.
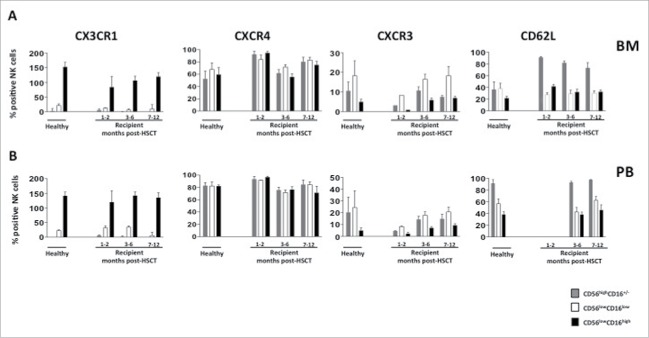
Since CD56lowCD16low NK cells are more abundant in BM than in PB both in healthy subjects and transplanted patients, we investigated the expression levels of some chemokine receptors and adhesion molecules known to regulate NK-cell tissue distribution. The chemokine receptor profile of reconstituted CD56lowCD16low NK cells resembled that of healthy individuals, since they were endowed with low surface density of CXCR3 (CD183) and CX3CR1, the receptors of CXCL10/IP-10 and CX3CL1/fractalkine, respectively, while expressing high levels of CXCR4 (CD184), the receptor of CXCL12 (SDF-1), this finding supporting the preferential BM enrichment of this subset (Fig. 3 panel A/B).
Figure 3.
CD56lowCD16low NK-cell chemokine receptor and adhesion molecules profile after α/β T-cell depleted haplo-HSCT. Cells freshly isolated by density gradient centrifugation from BM (panel A) and PB (panel B) of pediatric healthy donors and haplo-HSCT recipients were analyzed by flow cytometry at different time-points after HSCT. Chemokine receptors and adhesion molecule expression of CD56highCD16+/− (gray), CD56lowCD16low (white) and CD56lowCD16high (black) NK cell subsets, gated on CD56+CD3− NK cells are shown. The mean values ± SD of the percentage of positive cells are shown.
We also investigated the expression levels of the adhesion molecule CD62L (L-selectin) involved in lymphocyte migration, which is downregulated, like CD16, after NK cell activation. In both BM and PB, the CD56lowCD16low NK-cell subset expressed levels of CD62L comparable to that of the CD56lowCD16high NK-cell subset. Given the role that ADAM-17 protease has in the shedding of CD16 and CD62L from NK cell surface, we also investigated the presence of soluble ADAM-17 in both BM and PB plasma of transplanted patients by ELISA assay (supplementary Fig.1).29 Plasma of most HSCT patients, like HD, resulted negative for ADAM-17. Collectively these data support the notion that CD56lowCD16low NK cells of transplanted patients, as we have described previously for healthy individuals, represent a distinct NK cell subset.30
Timing differences in recovery of cytokine production and cytotoxic function by CD56lowCD16low NK cells
Given the multifunctional properties of CD56lowCD16low NK cells isolated from healthy donors, we analyzed the functions of this distinct NK-cell subset isolated from BM and PB of patients at different time-points after α/β T cell-depleted HSCT.
We first evaluated the ability of CD56lowCD16low NK cells to degranulate upon binding to HLA class-I deficient K562 target cells, by measuring the percentage of CD107a positive cells.
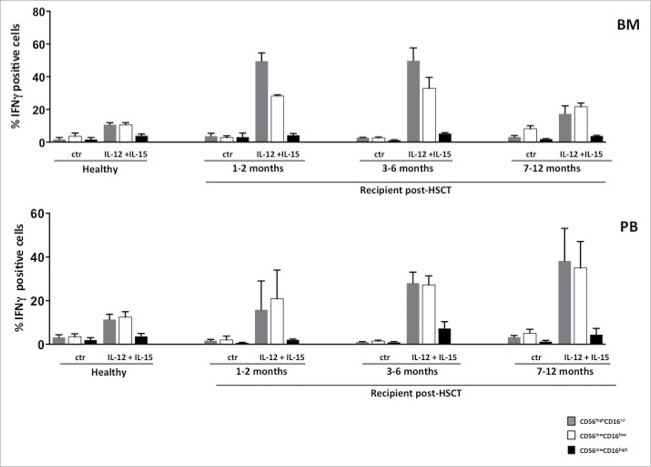
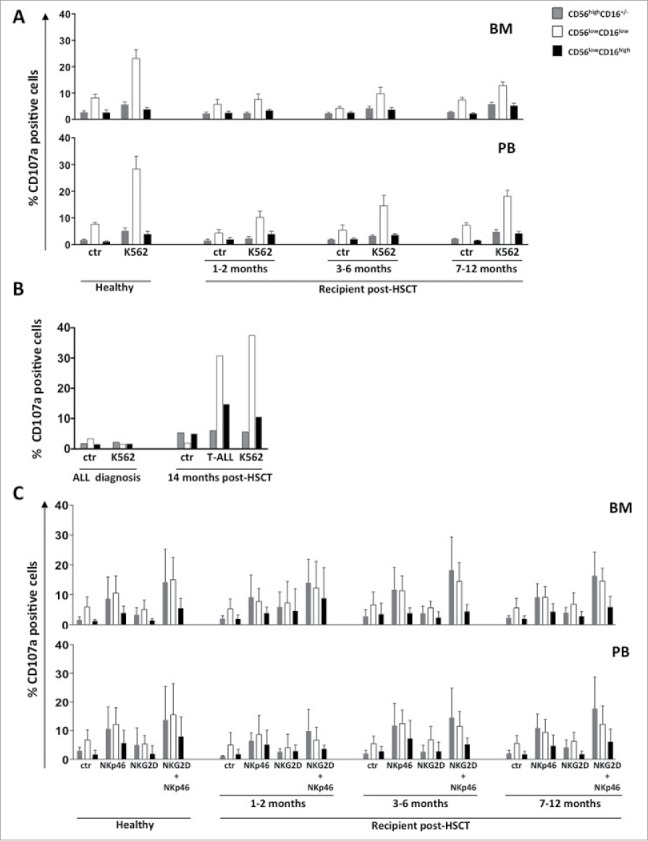
As previously shown for total NK cells, also CD56lowCD16low NK cell response to K562 target cells was significantly impaired during the first 6 months after transplantation.31 However, at one year after HSCT, in BM and PB reconstituting CD56lowCD16low NK cells represent the only subset capable of degranulation, although less efficiently than age-matched controls (Fig. 4 panel A). Interestingly, by comparing the degranulation ability of CD56lowCD16low NK cells from PB of an ALL patient at diagnosis and 14 months after HSCT, we observed complete recovery of its degranulation ability toward both autologous blasts and K562 target cells (Fig. 4 panel B). To investigate whether the impairment of target cell killing represents a global defect in degranulation process, we also performed a reverse ADCC assay on NK-cell subsets isolated from transplanted patients at different times. We found that CD56lowCD16low NK cells both from BM and PB degranulated in response to stimulation with anti-NKp46 mAb, used in combination with anti-NKG2D mAb, already at the early months post HSCT, and this response was similar to that of CD56highCD16−/+ NK cells and comparable to that of HDs (Fig. 4 panel C). Thus, the degranulation defect of CD56lowCD16low NK cells cannot be due to deficiencies in activating receptor signaling or degranulation machinery. In sharp contrast to killer ability, the IL-12 plus IL-15 cytokine-induced IFNγ production by CD56lowCD16low and CD56highCD16−/+ NK cells was significantly enhanced in the first 6 months after HSCT with respect to healthy individuals, both in BM and PB (Fig. 5). This increased ability correlated with the high expression levels of CD122 receptor observed on CD56lowCD16low NK cells in the first months after HSCT.
Figure 4.
CD56lowCD16low NK cells IFNγ producing ability during the first year after HSCT. Freshly isolated cells from BM or PB of pediatric transplanted patients and healthy donors were stimulated overnight with IL-12 (25 ng/ml) plus IL-15 (50 ng/ml). The percentage of IFNγ positive cells into CD56highCD16+/− (gray), CD56lowCD16low (white) and CD56lowCD16high (black) NK-cell subsets gated on CD56+CD3− NK cells was assessed by cytofluorimetric analysis. The histograms represent the mean value ± SEM of the percentage of positive cells analyzed at different time-points after haplo-HSCT starting from the first month until 12th month after transplantation.
Figure 5.
Degranulation ability of BM and PB CD56lowCD16low NK cells at different time points after HSCT. Cells freshly isolated from BM or PB of pediatric transplanted patients and healthy donors were co-cultured for 3 hours with K562 target cells at 1:1 effector/target cells ratio and degranulation ability of CD56highCD16+/−(gray), CD56lowCD16low (white) and CD56lowCD16high (black) NK-cell subsets gated on CD56+CD3− NK cells was assessed by measuring the percentage of CD107a positive cells by flow cytometry. The histograms represent the mean value ± SD of the percentage of positive cells in BM and PB analyzed at different time-points after haplo-HSCT starting from the first month until 12th month (panel A). NK cells from PB of ALL patient at diagnosis or 14 months after HSCT were co-cultured with K562 target cells or autologous blast cells (1:1 effector/target cells ratio) and the degranulation ability of CD56highCD16+/−(gray), CD56lowCD16low (white) and CD56lowCD16high (black) NK cell subsets gated on CD56+CD3− NK cells was assessed as above described (panel B). NK cells from BM and PB were stimulated for 2 hours with P815 target cells pre-coated with anti-NKG2D, anti-NKp46, anti-DNAM-1 antibodies used alone or in combination (1:1 effector/target cells ratio) and degranulation ability of CD56highCD16+/−(gray), CD56lowCD16low (white) and CD56lowCD16high (black) NK cell subsets was assessed as above described (panel C). The histograms represent the mean value ± SD of the percentage of positive cells analyzed at different time-points after HSCT starting from the first month until 12th month after transplantation.
Collectively these data indicate the existence of a different timing between recovery of cytotoxic function and the ability to produce cytokine in post-HSCT CD56lowCD16low NK cells.
Discussion
In this study, we investigated NK-cell reconstitution in patients undergoing α/β T cell-depleted haplo-HSCT for high-risk hematological malignancies. Since recipients of T-cell depleted allograft cannot benefit from the anti-infectious and GVL effect mediated by donor T cells adoptively transferred with the graft, they could be theoretically exposed to an increased risk of viral infections and leukemia recurrence until recovery of adaptive immunity is completed. NK cells are the first lymphocytes that reconstitute after HSCT and may compensate the lack of donor T cells, playing a role in protection against both infection and tumors.32,33,34,35
We focused our attention on a subset of NK cells characterized by low expression levels of CD56 and CD16, defined as CD56lowCD16low NK cells. We had previously demonstrated that CD56lowCD16low NK cells isolated from haploidentical HSCT donors represent the only NK cell subset capable of degranulating against leukemia blasts of the corresponding recipients.18 Therefore, we hypothesized that CD56lowCD16low NK cells could play a fundamental protective role in patients undergoing haplo-HSCT.
We analyzed the reconstituting NK cells isolated from BM and PB of children with leukemia at different time-points after haploidentical α/β T cell-depleted haplo-HSCT, until one year after transplantation. In line with previously published results, we found an increase of total NK-cell frequency both in BM and in PB of transplanted patients within the first 6 months after HSCT.36,37,38 Among reconstituting NK cells, the percentage of the more immature CD56highCD16+/− NK cell subset was higher at the expense of the more mature CD56lowCD16high NK cell subset, while with regard to CD56lowCD16low NK cells they are present since the first month after HSCT with a frequency comparable to that of HD.
Miller and colleagues have reported that CD56lowCD16high NK cells could go toward CD16 loss by the action of ADAM-17, a protease known to shed also CD62L. Unlike our previous results in ALL patients showing higher plasma concentrations of ADAM-17 in BM with respect to HD, here we found that the distribution of CD56lowCD16low NK cells in transplanted patients does not correlate with the presence of soluble ADAM-17, in that no differences in BM and PB plasma levels of this enzyme between HD and HSCT recipients were observed.29,30 These findings suggest that CD56lowCD16low NK cells arising after HSCT represent a distinct NK-cell subset rather than a post-activation state of CD56lowCD16high NK cells.
The NK-cell receptor profile of reconstituting CD56lowCD16low NK cells was investigated at various time points after HSCT and no major differences were found in the expression of activating/inhibitory receptors, chemokine receptors and differentiation markers at any time-point with respect to HD. Interestingly, the analysis of the cytokine receptor profile revealed that CD56lowCD16low NK cells from haplo-HSCT patients display a lower expression of CD25 and CD127, while they express higher levels of CD122/IL-12Rβ with respect to healthy age-matched controls. In humans, most if not all innate lymphocyte populations (ILCs) are characterized by CD127 expression and may be further dissected into different subsets using phenotypic markers and cytokine expression pattern.39,40,41 Human NK cells, the main cytotoxic members of ILC1 group, can also express the marker CD127, but can be distinguished from other ILCs for the co-expression of key markers like CD56 and CD94/NKG2A and for the absence of NKp44 and CD103 expression. Distinct pattern of receptors have been shown to define different stages of NK-cell development: however, developmental pathways of other lymphoid subsets can overlap/intersect that described previously for NK cells.42,43,44 Although, this is controversially discussed, a linear developmental relationship between CD56high and CD56low NK cell subsets is supported by accumulating evidence.45,25,46 According to this model, we have previously proposed that, based on NK-cell receptor profile, CD56lowCD16low NK cells represent an intermediate stage of differentiation between the more immature CD56highCD16+/− NK cells and the terminally differentiated CD56lowCD16high NK cells.18 Moreover, the finding that this subset represents a phenotypic and functional intermediary in NK-cell development was corroborated by our observation that although the CD56lowCD16low NK cell subset comprises CD127 positive cells, it includes CD94 positive cells and unlike other ILC1 subsets, lacks NKp44 and CD103 markers (Stabile et al. data not shown).
In regard to the higher CD122/IL-12Rβ expression levels shown by CD56lowCD16low NK cells during the first 6 months after HSCT, previous evidences described that the more immature NK-cell subsets after HSCT are characterized by higher IL-12Rβ expression, which correlates with a strong responsiveness to IL-12, a cytokine that is critical for IFNγ production by NK cells.47 Accordingly, we observed that CD56lowCD16low NK cells from haplo-HSCT patients are able to produce even more IFN-γ than cells from HD upon IL-12 plus IL-15 stimulation, suggesting that this subset may exert a crucial role in tumor suppression and in the control of viral infections in the early post-transplant period.48 Concerning the recovery of cytotoxic function, our results show that K562-induced CD107a expression in reconstituting NK cells was strictly restricted to CD56lowCD16low NK cell subset, and reached that of HD only one year after HSCT, when these cells were also able to kill autologous blasts. However, direct and simultaneous triggering of 2 activating receptors (NKp46 and NKG2D) on recipient NK cells isolated at one month after HSCT is able to induce a degranulation response similar to that of HD, implying the absence of a global defect in degranulation ability.
The existence of a dissociation between the recovery of cytokine-producing and cytotoxic functions following allo-HSCT was described previously in adult patients transplanted with a differ graft composition.31 In particular, Foley et al. demonstrated that the NK-cell ability to kill reconstituted before the capacity to produce IFN-γ upon target stimulation in recipients of T-cell depleted graft even if to a lower extent when compared with that of HD. Conversely, IFNγ production upon cytokine stimulation was recovered early after HSCT, suggesting that cell requirements for IFNγ production are different according to the stimuli and are acquired with a different kinetics after HSCT. Our observations are in line with the data reported by Foley et al., since we found that the production of IFNγ upon cytokine stimulation precedes the acquisition of degranulation ability after interaction with target cells. However, we cannot undisputedly support a dissociation between the recovery of the 2 major functions of NK cells, since we could not obtain sufficient purified NK cells to investigate the ability to produce IFNγ after challenge with target cells.
Overall, our results show that, although the effector functions of CD56lowCD16low NK cells are partially impaired after HSCT, they still represent the most functional NK cell subset. We propose that CD56lowCD16low NK cells could play an important role in the defense of infections and tumor growth post-HSCT, due to their higher ability to produce IFNγ, and represent ideal candidates for approaches of adoptive immunotherapy, provided that they can be successfully isolated and ex vivo expanded. Because of the crucial role played by NK cells in terms of anti-leukemic and anti-infectious effects, methods to enhance NK-cell function early after HSCT are warranted to improve transplants outcome. In particular, these strategies could include adoptive transfer of CD56lowCD16low NK cell subset after in vitro expansion and activation.
Supplementary Material
Disclosure of potential conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
A special thanks to all patients and healthy donors who contributed to this study.
Funding
This work was supported by grants from the Italian Association for Cancer Research (AIRC: project #16014 and AIRC 5xmille: project #9962), Istituto Pasteur-Fondazione Cenci Bolognetti and Ministero dell'Istruzione, dell'Università e della Ricerca (Ricerche Universitarie) and from the Italian Institute of Technology (A2 project).
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol 2005; 23:225-74; PMID:15771571; https://doi.org/ 10.1146/annurev.immunol.23.021704.115526 [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Biology of natural killer cells. Adv Immunol 1989; Volume 47:187-376; PMID:2683611; https://doi.org/10.1016/s0065-2776(08)60664-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier E. Functions of natural killer cells. Nat Immunol 2008; 9(5):503-10; PMID:18425107; https://doi.org/ 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 4.Vivier E. Natural killer cell signaling pathways. Science (80-) 2004; 306(5701):1517-9; PMID:15567854; https://doi.org/ 10.1126/science.1103478 [DOI] [PubMed] [Google Scholar]
- 5.Cichocki F, Schlums H, Theorell J, Tesi B, Miller JS, Ljunggren HG, Bryceson YT. Diversification and functional specialization of human NK cell subsets. Curr Top Microbiol Immunol 2015; 358(January):63-93; PMID:26472216; https://doi.org/ 10.1007/82_2015_487 [DOI] [PubMed] [Google Scholar]
- 6.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006; 107(1):159-66; PMID:16150947; https://doi.org/ 10.1182/blood-2005-04-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A Moretta, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol 1996; 14:619-48; PMID:8717527; https://doi.org/ 10.1146/annurev.immunol.14.1.619 [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. NK cell receptors. Annu Rev Immunol 1998; 16:359-93; PMID:9597134; https://doi.org/ 10.1146/annurev.immunol.16.1.359 [DOI] [PubMed] [Google Scholar]
- 9.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31:227-58; PMID:23516982; https://doi.org/ 10.1146/annurev-immunol-020711-075005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003; 3(10):781-90; PMID:14523385; https://doi.org/ 10.1038/nri1199 [DOI] [PubMed] [Google Scholar]
- 11.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol 2006; 16(5):348-58; PMID:16893656; https://doi.org/ 10.1016/j.semcancer.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 2006; 214:73-91; PMID:17100877; https://doi.org/ 10.1111/j.1600-065X.2006.00457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlazzo G, Münz C. NK cell compartments and their activation by dendritic cells. J Immunol 2004; 172(3):1333-9; PMID:14734707; https://doi.org/ 10.4049/jimmunol.172.3.1333 [DOI] [PubMed] [Google Scholar]
- 14.Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björklund AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME, et al.. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56 dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010; 116(19):3853-64; PMID:20696944; https://doi.org/ 10.1182/blood-2010-04-281675 [DOI] [PubMed] [Google Scholar]
- 15.Carrega P, Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Front Immunol 2012; 3(NOV):347; PMID:23230434; https://doi.org/ 10.3389/fimmu.2012.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punt J, Owen J, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22(11):633-40; PMID:11698225; https://doi.org/ 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- 17.Fauriat C, Ivarsson MA, Ljunggren H-G, Malmberg K-J, Michaëlsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood 2010; 115(6):1166-74; PMID:19903900; https://doi.org/ 10.1182/blood-2009-09-245746 [DOI] [PubMed] [Google Scholar]
- 18.Stabile H, Nisti P, Morrone S, Pagliara D, Bertaina A, Locatelli F, Santoni A, Gismondi A. Multifunctional human CD56lowCD16lownatural killer cells are the prominent subset in bone marrow of both healthy pediatric donors and leukemic patients. Haematologica 2015; 100(4):489-98; PMID:25596273; https://doi.org/ 10.3324/haematol.2014.116053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354(17):1813-26; PMID:16641398; https://doi.org/ 10.1056/NEJMra052638 [DOI] [PubMed] [Google Scholar]
- 20.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al.. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (80-.) 2002; 295(5562):2097-100; PMID:11896281; https://doi.org/ 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk acute leukaemia. Semin Immunol 2014; 26(2):173-9; PMID:24613727; https://doi.org/ 10.1016/j.smim.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 22.Moretta L, Pietra G, Montaldo E, Vacca P, Pende D, Falco M, Del Zotto G, Locatelli F, Moretta A, Mingari MC. Human NK cells: From surface receptors to the therapy of leukemias and solid tumors. Front Immunol 2014; 5:87; PMID:24639677; https://doi.org/ 10.3389/fimmu.2014.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Della Chiesa M, Falco M, Podestà M, Locatelli F, Moretta L, Frassoni F, Moretta A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus? Blood 2012; 119(2):399-410; PMID:22096237; https://doi.org/ 10.1182/blood-2011-08-372003 [DOI] [PubMed] [Google Scholar]
- 24.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 2012; 189(10):5082-8; PMID:23077239; https://doi.org/ 10.4049/jimmunol.1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagnani C, Juelke K, Falco M, Morandi B, D'Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, et al.. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol 2007; 178(8):4947-55; PMID:17404276; https://doi.org/ 10.4049/jimmunol.178.8.4947 [DOI] [PubMed] [Google Scholar]
- 26.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 2005; 86:209-39; PMID:15705423; https://doi.org/ 10.1016/S0065-2776(04)86006-1 [DOI] [PubMed] [Google Scholar]
- 27.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med 2008; 205(5):1213-25; PMID:18458113; https://doi.org/ 10.1084/jem.20071913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol 2005; 174(3):1213-21; PMID:15661875; https://doi.org/ 10.4049/jimmunol.174.3.1213 [DOI] [PubMed] [Google Scholar]
- 29.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, Luo X, Cooley S, Verneris M, Walcheck B, et al.. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013; 121(18):3599-608; PMID:23487023; https://doi.org/ 10.1182/blood-2012-04-425397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stabile H, Nisti P, Pagliara D, Locatelli F, Santoni A, Gismondi A. Response to comment on multifunctional human CD56lowCD16low NK cells are the prominent subset in bone marrow of both pediatric healthy donors and leukemic patients. Haematologica 2015; 100(8):e332-3; PMID:26314084; https://doi.org/ 10.3324/haematol.2015.130831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: Dissociation between recovery of cytokine-producing and cytotoxic functions. Blood 2011; 118(10):2784-92; PMID:21757615; https://doi.org/ 10.1182/blood-2011-04-347070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, et al.. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 1998; 339(17):1186-93; PMID:9780338; https://doi.org/ 10.1056/NEJM199810223391702 [DOI] [PubMed] [Google Scholar]
- 33.Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, Nagler A, Di Bartolomeo P, Lacerda JF, Lupo Stanghellini MT, et al.. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: A risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112(9):3574-81; PMID:18606875; https://doi.org/ 10.1182/blood-2008-02-140095 [DOI] [PubMed] [Google Scholar]
- 34.Palma J, Salas L, Carrión F, Sotomayor C, Catalán P, Paris C, Turner V, Jorquera H, Handgretinger R, Rivera GK. Haploidentical stem cell transplantation for children with high-risk leukemia. Pediatr Blood Cancer 2012; 59(5):895-901; PMID:22238059; https://doi.org/ 10.1002/pbc.24022 [DOI] [PubMed] [Google Scholar]
- 35.André-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G, Vilmer E, Fischer A, Cavazzana-Calvo M. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: A phase 1/2 study. Lancet 2002; 360(9327):130-7; PMID:12126823; https://doi.org/ 10.1016/S0140-6736(02)09413-8 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N, Carosella ED, Boudifa A, Debré P, Vieillard V. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: Immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood 2005; 105(10):4135-42; PMID:15687235; https://doi.org/ 10.1182/blood-2004-10-4113 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen S, Kuentz M, Vernant J-P, Dhedin N, Bories D, Debré P, Vieillard V. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia 2008; 22(2):344-52; PMID:18033316; https://doi.org/ 10.1038/sj.leu.2405041 [DOI] [PubMed] [Google Scholar]
- 38.Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M, Carmagnat M, Loiseau P, Tamouza R, Scieux C, et al.. An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol 2008; 181(3):2227-37; PMID:18641363; https://doi.org/ 10.4049/jimmunol.181.3.2227 [DOI] [PubMed] [Google Scholar]
- 39.Juelke K, Romagnani C. Differentiation of human innate lymphoid cells (ILCs). Curr Opin Immunol 2016; 38:75-85; PMID:26707651; https://doi.org/ 10.1016/j.coi.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 40.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of il-12- and il-15-responsive ifn-??-producing cells. Immunity 2013; 38(4):769-81; PMID:23453631; https://doi.org/ 10.1016/j.immuni.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al.. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013; 14(3):221-9; PMID:23334791; https://doi.org/ 10.1038/ni.2534 [DOI] [PubMed] [Google Scholar]
- 42.Freud AG, Yu J, Caligiuri MA. Human natural killer cell development in secondary lymphoid tissues. Semin Immunol 2014; 26(2):132-7; PMID:24661538; https://doi.org/ 10.1016/j.smim.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol 2013; 34(12):573-82; PMID:24055329; https://doi.org/ 10.1016/j.it.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, Serafini N, Puel A, Bustamante J, Surace L, et al.. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 2017; 168(6):1086-100.e10; PMID:28283063; https://doi.org/ 10.1016/j.cell.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 45.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56 dim NK cells. Blood 2010; 116(8):1299-307; PMID:20505160; https://doi.org/ 10.1182/blood-2009-11-253286 [DOI] [PubMed] [Google Scholar]
- 46.Chan A, Hong D-L, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: Role of contact with peripheral fibroblasts. J Immunol 2007; 179:89-94; PMID:17579025; https://doi.org/ 10.4049/jimmunol.179.1.89 [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010; 116(19):3865-74; PMID:20733159; https://doi.org/ 10.1182/blood-2010-04-282301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.a Biron C, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu Rev Immunol 1999; 17:189-220; PMID:10358757; https://doi.org/ 10.1146/annurev.immunol.17.1.189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.