Abstract
Autophagy is a fundamental cellular mechanism responsible for bulk turnover of cytoplasmic components. It is broadly related to many cellular activities, physiological processes, and pathological conditions. Autophagy entails a spatiotemporal interaction between cytosolic factors and membranes that are remodeled to encapsulate autophagic cargo within an autophagosome. Although majority of the factors [autophagy-related gene (Atg) proteins] involved in autophagy have been identified by genetic studies, the mechanism accounting for how these factors act upon the membrane to remodel it and efficiently recruit cargo for degradation is unclear. In vitro reconstitution of several different aspects of autophagy has provided important insights into the understanding of the mechanistic details underlying autophagic membrane remodeling and cargo recruitment. Here, we highlight these efforts toward studying autophagy through in vitro approaches.
Keywords: autophagy, autophagosome, in vitro reconstitution, membrane, vesicle
Introduction
Autophagy, activated by stress, is a fundamental cellular mechanism required for bulk turnover of cytoplasmic contents through lysosomal degradation. It is essential for maintaining cellular homeostasis and cell survival under stressed conditions. Dysfunctional autophagy has been implicated in aging and human diseases such as cancer, neurodegeneration, and metabolic syndromes (1–3).
Autophagy is initiated by stress signals that activate a cascade of cytosolic components, which then mobilize and remodel membranes from intra-cellular organelles, also known as the endomembrane system. The membrane precursors generated after autophagy initiation are delivered to specific sites on the endoplasmic reticulum (ER) called the phagophore assembly site (PAS). These precursors then fuse to form a cup-shaped phagophore that elongates and engulfs cytoplasmic cargo. Once properly elongated, the phagophore closes to form a double-membrane autophagosome that encapsulates autophagic cargos and delivers them to the lysosome for degradation (4–8) (Fig. 1). After the degradation is complete, the lysosomes are regenerated from autolysosomes through a process called lysosome reformation (9–12).
Fig. 1.
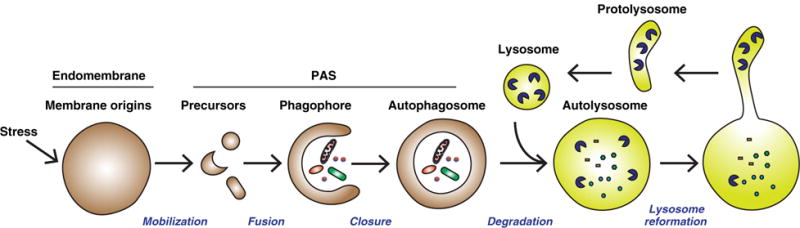
Major steps of membrane remodeling during autophagy. Stress signals, such as starvation, induce signaling cascades that are transmitted into a membrane remodeling signal that acts on the autophagosome membrane origin. Early precursors are then generated from the membrane origin (endomembrane) as a result of a membrane remodeling process (mobilization). These precursors are targeted to the phagophore assembly site (PAS) and fuse to form a cup-shaped phagophore that engulfs autophagic cargos (fusion). The phagophore further extends and encloses to form the double-membrane autophagosome that encapsulates cargos (closure). The autophagosome fuses with the lysosome to form the autolysosome where the autophagic cargos are degraded (degradation) (4–8). Lysosomes are regenerated through membrane tubulation and fission from the autolysosome after the completion of degradation (9–12).
Genetic studies have identified 41 autophagy-related gene (Atg) proteins in yeast, as well as corresponding mammalian homologs (4,13–17). The core regulatory modules include a serine/threonine kinase complex (mammals: ULK1/ULK2, FIP200/RB1CC1, ATG13, and ATG101; yeast: Atg1, Atg17, Atg13, plus Atg29 and Atg31 in Saccharomyces cerevisiae or Atg101 in Schizosaccharomyces pombe) (18–21), a class III phosphatidylinositol-3 kinase (PI3K) complex (mammals: ATG14/Barkor, Beclin-1, P150, and VPS34; yeast: Atg14, Atg6, Vps15, Vps34) (22,23), and two ubiquitin-like conjugation machineries {mammals: ATG7, ATG10, ATG3, ATG5, ATG12, ATG16L, and LC3 [LC3 here stands for multiple ATG8 family proteins] (24,25); yeast: Atg7, Atg10, Atg3, Atg5, Atg12, Atg16, and Atg8} (26–29). Additionally, the transmembrane protein(s) {mammals: ATG9a and ATG9b [Atg9b is only found expressed in the placenta and pituitary gland] (30); yeast: Atg9} (31,32), the PI3–phosphate-binding effector(s) (mammals: WIPIs; yeast: Atg18) (33,34), and the peripheral membrane protein (mammals: ATG2a and ATG2b; yeast: Atg2) (33–36) are also required for autophagy. In addition, a phosphatidylinositol-3 phosphate (PI3P)–binding protein DFCP1 decorates a special membrane structure (omegasome) associated with the autophagosomes in mammalian cells (37,38). These modules work in a hierarchical manner to activate autophagy and form the autophagosome (13,39–42). In brief, the serine/threonine kinase complex is activated by stress signals followed by the activation of PI3K complex to generate PI3P on the target membrane. PI3P effectors, such as WIPIs, and downstream factors, such as ATG2s, are then recruited to the membrane to organize phagophore formation together with the serine/threonine kinase complex and ATG9. The two ubiquitin-like conjugation machineries work in conjunction to covalently link the ubiquitin-like protein Atg8/LC3 to phosphatidylenthanolamine (PE) located on the autophagosomal membrane precursors in a process called lipidation. Once lipidated, Atg8/LC3 binds to autophagic cargo adaptors that recruit cargo to the autophagic membrane. Fusion and closure of the autophagic membrane complete the autophagosome formation and encapsulate cargo (26,28,43–45). Detailed molecular actions of these Atg proteins are described in other review articles in this same issue.
Three important aspects govern autophagy activity: (1) organizing spatiotemporal interactions of autophagic factors with the endomembrane system, (2) remodeling the endomembrane into the autophagic membranes, and (3) in the case of selective autophagy, targeting the autophagic membrane specifically to autophagic cargo. However, it is mechanistically unclear how these steps are conducted. There has been a burgeoning field of research focused on in vitro reconstitution of either specific stages of autophagy or certain autophagic complexes and provided more molecular details regarding the mechanism of autophagosome formation. This review briefly summarizes the efforts toward reconstituting autophagy and the important insights gained from these studies (Table 1).
Table 1.
A brief summary of the studies on in vitro reconstitution of autophagy
| Reconstitution | Main conclusion | Citation | |
|---|---|---|---|
| Protein–membrane interaction | Atg8/LC3 lipidation | (1) Reconstitution of Atg8/LC3 lipidation with purified proteins and liposomes demonstrated that Atg7, Atg3, and Atg12−Atg5 act as E1, E2, and E3-like enzymes for conjugating Atg8/LC3 to PE. | (46–49) |
| (2) Reconstitution of Atg8 lipidation with different kinds of liposomes indicated that Atg16 selectively promotes Atg8 lipidation on less curved membrane by facilitating the membrane targeting of Atg12−Atg5. | (50) | ||
| (3) Reconstitution of LC3 lipidation with different sized liposomes indicated that ATG3 prefers curved membrane for LC3 lipidation. | (51) | ||
| Membrane targeting of autophagic factors | (1) Sedimentation assays determined that Atg14 is recruited to membrane that is highly curved and enriched in PI3P and PI(4,5)P2, when mTORC1 is inhibited. | (52,53) | |
| (2) Cell-free reconstitution and membrane floatation indicate a starvation-induced recruitment of ATG14 to the ERGIC membrane. | (54) | ||
| Autophagic membrane remodeling | Autophagosome membrane origin | (1) Reconstitution of autophagic signal-regulated LC3 lipidation in a cell-free system and membrane fractionation identify the ERGIC as an essential membrane source of the autophagosome. | (54) |
| Autophagosome precursor generation | (1) Reconstitution of autophagic signal-regulated early autophagic precursor generation identifies a PI3K-induced switch of COPII location from the ER to the ERGIC as an essential mechanism for mobilizing the donor membrane for early autophagic precursor biogenesis. | (55) | |
| Membrane tethering and fusion | (1) Atg8 family proteins, either lipidated in vitro or chemically conjugated to PE, are capable of tethering vesicles and promote membrane fusion. | (56–59) | |
| (2) Structural and binding analyses indicated that Atg1/Atg13/Atg17/Atg29/Atg31 complex and TRAPPIII complex may be capable of tethering autophagic vesicles. | (60,61) | ||
| (3) Cell-free reconstitution indicated that the autophagosome can fuse with endosomes and lysosomes and that this is dependent on cytosolic factors. | (62) | ||
| (4) Liposome tethering and fusion assay indicates that ATG14 is capable of tethering membrane and mediating membrane hemifusion and fusion that is important for autophagosome and endolysosome fusion. | (63) | ||
| Phagophore shape and asymmetry | (1) Reconstitution of the Atg12−Atg5/Atg16 assembly on GUVs with lipidated Atg8 indicated that Atg16 anchors Atg12−Atg5 and lipidated Atg8 by forming a tetramer, which facilitates the formation of a two-dimensional meshwork likely to be a scaffold shaping the phagophore. | (64) | |
| (2) Cargo adaptor Atg32 competes with the Atg12−Atg5/Atg16 complex to associate with Atg8, which could be an explanation for the asymmetrical localization of cargo and autophagic factors on the concave and convex face of the phagophore. | (64) | ||
| Lysosome reformation | (1) Cell-free reconstitution pinpointed the role of clathrin and clathrin adaptors and PI(4,5)P2 in lysosome reformation after autophagy. | (10) | |
| Selective Autophagy | Localized signal activation | (1) Cell-free reconstitution with a genetically engineered Atg1 indicates that a regulatory chain from cargo, adaptor and scaffold activates Atg1 through direct protein interaction, which accounts for the local activation of autophagy around the cargo during selective autophagy. | (65) |
| Efficient cargo recognition | (2) The yeast cargo adaptor Atg19 has multiple AIMs. Reconstitution of membrane and cargo binding indicates that cargo binding enables the availability of AIMs to interact with lipidated Atg8, which enhances the recruitment of autophagic membranes. | (66) | |
| (3) The mammalian cargo adaptor P62 has one LIR. Reconstitution of membrane and cargo binding indicates that oligomerization enables multiple LIRs in one protein complex. This strengthens the binding of P62 to LC3 and ubiquitinated cargos as a mechanism to enhance the recruitment of autophagic membranes to the cargo. | (67) |
Reconstituting the Protein–Membrane Interactions in Autophagy
Autophagy requires extensive interactions between autophagic factors and the endomembrane system (4), the details of which are poorly understood. Two known types of interactions are involved. One is covalent, involving a linkage between Atg8/LC3 and PE in the membrane (lipidation). The other is noncovalent, which represents a majority of other autophagic factors, where they dynamically associate with the periphery of the membrane. To date, significant progress has been made toward reconstituting the lipidation process and decoding the mechanism. Efforts toward reconstructing the spatiotemporal association between autophagic factors and membrane as autophagy progresses are also underway.
Molecular mechanism of Atg8/LC3 lipidation
One of the key steps of autophagosome formation is Atg8/LC3 lipidation (26–28). In the early 2000s, genetic studies identified two ubiquitin-like conjugation processes that occur sequentially and lead to Atg8/LC3 lipidation. The first step generates the protein conjugate Atg12−Atg5 and requires the sequential activity of Atg7 (E1) and Atg10 (E2). The second ubiquitin-like process requires Atg7 (E1), Atg3 (E2), Atg12−Atg5 (E3), and Atg8. It covalently links Atg8 to the phospholipid PE located on autophagosome membrane precursors (27,29,68–72). Although genetic studies have indicated a sequential relationship between the first and second ubiquitin-like conjugation machineries in Atg8 lipidation, the mechanism of action of each component was unclear.
A pioneering study by the Ohsumi group biochemically reconstituted yeast Atg8 lipidation by combining purified Atg7, Atg3, Atg8, and PE-containing liposomes (Fig. 2A) (46). This study pinpointed that Atg7 and Atg3 act as the E1- and E2-like enzymes of the ubiquitin-like conjugation process. In addition, it also identified the minimal components required for Atg8–PE formation as Atg7, Atg3, Atg8, and PE. However, efficient lipidation required a higher than physiological concentration of PE on liposomes (50%–70%) (46). Moreover, this study left unanswered the question of why the product of the first ubiquitin-like conjugation process, Atg12−Atg5, is required for Atg8 lipidation in vivo. To study the role of the Atg12−Atg5 conjugate in Atg8−PE formation, they used the same Atg8 lipidation system in their subsequent study and added a purified Atg12−Atg5 conjugate (47). Interestingly, they found that the Atg12−Atg5 conjugate enhanced Atg8 lipidation on liposomes with physiological PE levels (20%) by acting as an E3-like enzyme to facilitate the transfer of Atg8 from Atg3 to PE (47). Therefore, these two biochemical reconstitution studies clarified a sequential action of E1 (Atg7), E2 (Atg3), and E3 (Atg12−Atg5) for efficient Atg8 lipidation. Similar systems were also employed using purified mammalian (48) and Arabidopsis (49) proteins with similar results.
Fig. 2.
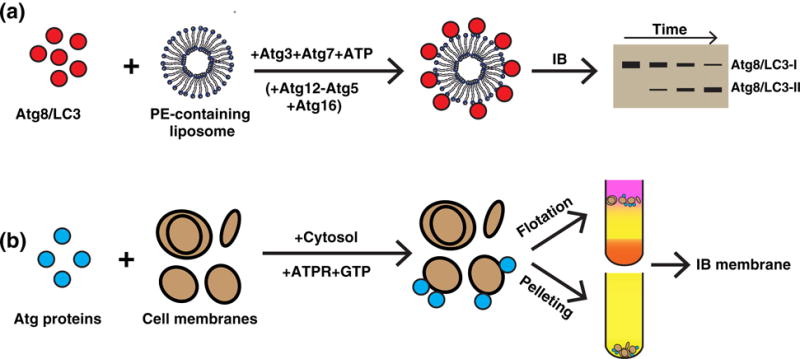
A summary of reconstituting the autophagic protein–membrane interaction. (a) In vitro lipidation. Purified lipidation components including Atg8/LC3 (C-terminal glycine exposed), Atg3, and Atg7 (plus Atg12−Atg5 and Atg16 at times) were incubated with PE-containing liposomes and ATP. The reaction led to the covalent conjugation of Atg8/LC3 to PE (and PS) on the liposome. The presence of lipidated Atg8/LC3 (Atg8/LC3 II, the higher mobility band) was determined by immunoblot (IB) (46–49,73). (b) Cell-free membrane targeting of ATG proteins. Purified ATG proteins (such as ATG14) were incubated with cytosol and cellular membrane (52,53). Alternatively, cytosol expressing ATG proteins (such as ATG14) was incubated with the cell membrane, GTP, and an ATP regeneration system (ATGPR) (54). After the reaction, the membrane fraction was collected by either centrifugation to obtain the membrane pellet or by density gradient to obtain the membrane layer. The membrane-associated ATG proteins were determined by IB.
Atg16 is a co-factor of the Atg12−Atg5 conjugate. The exact role of Atg16 in Atg8 lipidation has been unclear due to inconsistent results between in vivo and in vitro studies (47,74–76). Recent studies reconstituting Atg8 lipidation with different sized liposomes provided a possible explanation. One group found that Atg16 dramatically increases Atg8 lipidation efficiency on giant unilamellar vesicles (GUVs) possibly by promoting Atg12−Atg5 membrane association (50). However, Atg16 did not enhance Atg8 lipidation on small liposomes, even though it promotes Atg12−Atg5 membrane association under these conditions (50). These data suggest that the role of Atg16 in Atg8 lipidation is membrane curvature dependent. A later study on mammalian ATG3 further indicated that the lipidation machinery is dependent on membrane curvature because ATG3 harbors an amphipathic alpha helix that preferentially binds highly curved membrane primed for LC3 lipidation (51). It is likely that small liposomes with higher intrinsic curvature have enhanced Atg3 membrane association and activity, bypassing an in vitro requirement for Atg16. In contrast, larger liposomes have less intrinsic membrane curvature, which may therefore require Atg16 to activate Atg3 by recruiting it to the membrane.
Regulated membrane targeting of autophagic factors
Autophagy involves dynamic interactions between autophagic factors and the target membrane (4). Targeting specific autophagic machinery to the membrane is essential for regulating autophagy activity. Considering that the genetic hierarchy of each major regulatory module of autophagy has been uncovered in vivo (39,40), reconstituting membrane targeting of autophagic modules would provide important implications about how and what regulates autophagy during each step. One critical step in the autophagic signaling cascade is PI3K complex membrane localization and activation through ATG14 membrane association (77–81). The Zhong group used a co-sedimentation approach with purified ATG14 and autophagosome-enriched membrane fractions and found that ATG14 associates with autophagosomes in the absence of additional cytosolic factors (Fig. 2b) (52). Cytosolic factors do seem to regulate ATG14 membrane association, as it is blocked upon addition of cytosol from nutrient-rich cells in an mTORC1-dependent manner (52). Therefore, although Atg14 can bind autophagosomes, its ability to do so is regulated by stress signals such as mTORC1. This group also observed that the purified Barkor/ATG14 Autophagosome Targeting Sequence domain of ATG14 is specifically recruited to highly curved liposomes or those enriched in PI3P or PI(4,5)P2 (53). To look for the cellular membrane physiologically required for ATG14 recruitment, we established a cell-free ATG14 membrane recruitment assay based on membrane floatation where starvation enhanced cytosolic ATG14 membrane association (Fig. 2b). Moreover, we found that a stable ER–Golgi intermediate compartment (ERGIC) is required for ATG14 membrane association, as the membrane isolated from cells with a disrupted ERGIC cannot efficiently recruit ATG14 from the cytosol (54). The role of the ERGIC in autophagosome biogenesis is discussed in detail below. In the future, further studies using either purified or fractionated membrane in cell-free reconstitution assays will be advantageous to better understand the specific membrane microenvironment and hierarchy of membrane association responsible for organizing the autophagic machinery at a molecular level.
Reconstituting Autophagic Membrane Remodeling
During autophagy, the membrane morphology changes through several different steps such as (1) remodeling the endomembrane (membrane origin of the autophagosome) into autophagic precursors, (2) fusing autophagic precursors to form the phagophore, (3) closing the phagophore to complete the double-membrane autophagosome, (4) fusing autophagosomes with endosomes, and (5) regenerating lysosomes after content degradation (Fig. 1) (4–11). Efforts have been made thus far to reconstitute steps 1, 2, 4, and 5. However, the precise mechanism of how these steps occur is still unknown.
Autophagosome membrane origin
Autophagosome formation requires large amounts of membrane, but the specific organelles that are remodeled to produce the autophagosomal membrane have remained elusive. Although multiple membrane sources of the autophagosome have been proposed as the autophagosome membrane donor, such as the ER, Golgi, plasma membrane, and mitochondria, it has been difficult to functionally validate these sources (7,8,82). Pinpointing the membrane origin of the autophagosome will help us better understand the subsequent membrane morphology changes required for autophagosome biogenesis. It will also help us establish physiologically meaningful cell-free assays that utilize the appropriate physiological cell membranes instead of synthetic liposomes. We established a cell-free LC3 lipidation reaction that reconstitutes an early step of autophagosome biogenesis by combining cytosol from wild type cells, membrane from autophagy-deficient mutant (Atg5 knockout) cells, and nucleotides (Fig. 3a) (54). LC3 lipidation, in this reaction, recapitulates many regulatory landmarks of autophagy, such as the starvation–mTORC1–ULK1 regulatory axis and the autophagic PI3K pathway (13). We combined this cell-free assay with a membrane fractionation approach to look for the membrane fraction that triggers LC3 lipidation. Interestingly, lipidation activity is specifically enriched in the ERGIC, which has not been previously identified as an autophagic membrane origin (83). Reconstituting early autophagic factor membrane recruitment identified ATG14 as a key autophagic factor targeted to the ERGIC that initiates the signaling cascade resulting in LC3 lipidation. Pharmacological or genetic depletion of the ERGIC impairs ATG14 membrane recruitment and consequently inhibits autophagosome biogenesis (54). Accordingly, two other studies also found a close relationship between the ER-exit sites in yeast, a functional equivalent of mammalian ERGIC, and the PAS indicating the involvement of ER-exit sites to autophagosome biogenesis in yeast (60,84,85).
Fig. 3.
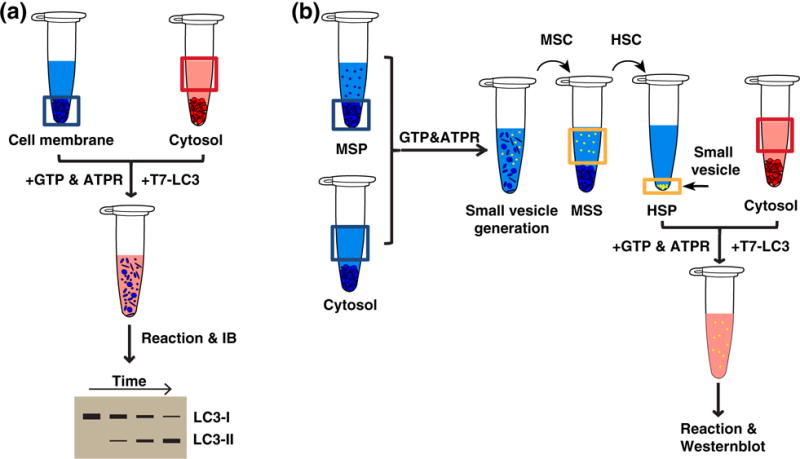
The procedure for reconstituting lipidation precursor generation. (a) Cell-free lipidation. Cytosol and membrane were incubated with purified T7-LC3 (C-terminal glycine exposed), GTP, and an ATP regeneration system. After the reaction, lipidated T7-LC3 was determined by IB (54). (b) Cell-free lipidation precursor generation. Cytosol and medium-speed membrane pellet (MSP donor membrane with small vesicles removed) were incubated with GTP and an ATP regeneration system. After the reaction, the in vitro generated small vesicles [high-speed pellet (HSP)] were enriched by a medium-speed centrifugation (MSC) followed by high-speed centrifugation (HSC) of the medium-speed supernatant (MSS). Cell-free lipidation as shown in (a) was performed on the HSP to determine the generation of small lipidation membrane precursors (55).
Autophagosome precursor generation
To form the double-membrane autophagosome, autophagic machinery should be capable of remodeling the endomembrane system to form phagophore precursors. However, this mechanism has not been fully understood. We employed our cell-free LC3 lipidation reaction to functionally trace the generation of these precursors. We found that the activation of the autophagic PI3K complex induces the production of small vesicles that are capable of LC3 lipidation (55). It is possible that these small vesicles are early phagophore precursors.
To generate these small membrane vesicles, the relatively flat target membrane undergoes a drastic physical change and is remodeled into a small, highly curved structure. Once formed, this vesicle is released from the donor membrane through membrane fission (86). It is also critical that the small vesicles form at the correct location (86). To understand how these small, lipidation-competent vesicles form, we established a cell-free assay that reconstitutes their production (Fig. 3b). Vesicle generation is enhanced by starvation and dependent on PI3K complex subunits, ATG14 and Beclin-1 and its product, PI3P. In addition, this process requires the upstream autophagic regulator FIP200. Membrane fractionation uncovered the ERGIC as the primary donor membrane responsible for generating small LC3 lipidation-competent vesicles. We then looked for the molecular machinery responsible for small vesicle budding from the ERGIC (55). Interestingly, we found that the COPII machinery, a protein complex that induces vesicle formation by generating membrane curvature and fission, is required for LC3 lipidation-competent vesicle budding (55,87). To determine how the COPII machinery is activated on the ERGIC under autophagic conditions, we isolated the ERGIC under different conditions and found that the autophagic PI3K induces COPII machinery ERGIC localization after starvation. (55). Therefore, our cell-free assay indicates that the autophagic PI3K specifies the ERGIC as the site of autophagic vesicle formation and the COPII machinery is responsible for creating the small, lipidation-competent membrane precursors.
Autophagic membrane tethering and fusion
After they are generated, autophagic precursors are then targeted to the PAS where they converge with other autophagic factors and undergo multiple membrane fusion steps to form the autophagosome (Fig. 1). Membrane fusion requires a concerted mechanism of fusogens that includes tethering factors that identify and link target and vesicle membrane, and several soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) proteins that bring these membranes close together to induce fusion (88). However, it is unknown which autophagic factors drive membrane tethering and fusion. In vitro reconstitution has provided some insight into the mechanisms and machinery required for membrane fusion and phagophore formation. The first possible autophagy-specific fusogen was proposed as Atg8−PE. Further utilization of the Atg8 lipidation reconstitution reaction with synthetic liposomes described above found that Atg8−PE is concentrated at membrane contact sites and promotes membrane tethering and hemifusion (Fig. 4a) (56). Mutants that affected Atg8 membrane tethering capabilities also failed to support autophagosome membrane expansion in vivo, confirming its physiological importance (56). The ability of lipidated Atg8 family proteins to promote vesicle tethering and fusion was further uncovered by another two studies that chemically conjugated mammalian LC3B and GATE-16 (57) or Caenorhabditis elegans LGG1 and LGG2 (homologs of LC3 in nematode) to PE in liposomes (58).
Fig. 4.
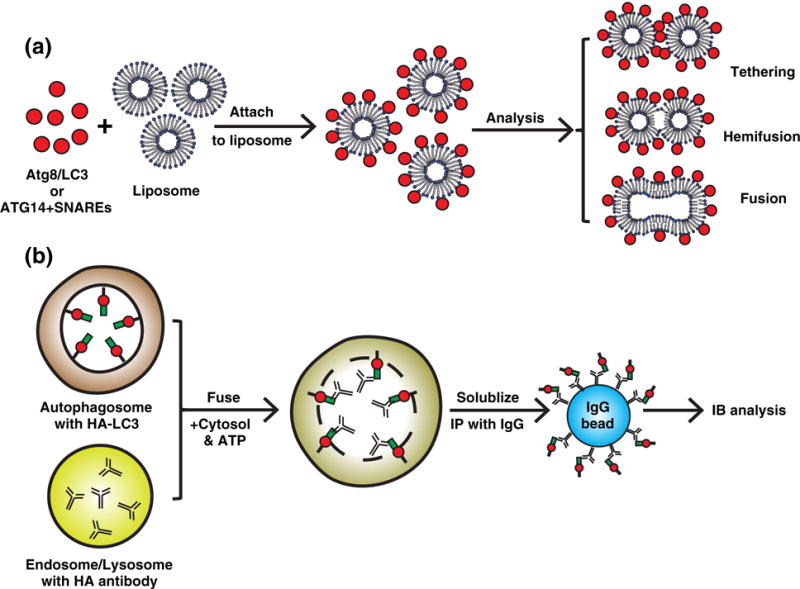
A summary of reconstituting autophagic membrane tethering and fusion. (a) The tethering and fusogenic effect of autophagic factors. Atg proteins, such as Atg8/LC3 or ATG14, were attached to liposomes via lipidation (C-terminal glycine exposed Atg8) to PE (56), chemical cross-linking (LC3 G/C mutant) to PE-maleimide (57,58) or direct association (ATG14) (63). Attachment of the ATG proteins to liposomes was reported to cause liposome tethering, hemifusion, and fusion. (b) Cell-free fusion between autophagosomes and endolysosomes. Autophagosomes from cells expressing HA-LC3 were isolated and contained HA-LC3 on the inner membrane of the double-membrane structure. Endosomes and lysosomes with internalized HA antibodies were also isolated. Then the autophagosomes and endolysomes were incubated with cytosol and ATP in a cell-free reaction. Autophagosome and endolysome fusion led to HA antibodies binding HA-LC3. These fusion events could be detected by immunoprecipitating HA antibody bound HA-LC3 with IgG beads (62). However, the mechanism by which the autophagosomal inner membrane is breached after outer membrane fusion to allow for HA antibody and HA-LC3 association is unknown.
However, it is still not entirely clear if Atg8 is a major autophagic fusogen because its fusogenic activity was most efficiently observed using non-physiological conditions by using either liposomes with a high PE concentration (55%) (56,59) or chemical cross-linking (57,58). Employment of more physiological conditions, for example, conjugating Atg8 to 30% PE liposomes using the lipidation machinery, almost entirely eliminated the vesicle tethering and fusing activity of Atg8−PE (59). This suggests that other factors may work synergistically with Atg8−PE to promote membrane tethering and fusion. Indeed, later studies identified several SNARE proteins that are also required for phagophore growth (59,89). In addition, another integral component of the lipidation machinery, Atg12−Atg5/Atg16, was also reported to tether GUVs (50). In this study, both the Atg12−Atg5/Atg16 complex and lipidated Atg8 localize at contact sites between GUVs similar to what was observed previously (50,56). Therefore, it is likely that under physiological conditions, synergistic effects mediated by Atg8, Atg12−Atg5/Atg16, SNAREs, and other factors contribute to autophagic membrane tethering and fusion. It will be necessary in the future to establish in vitro systems using more physiological conditions and a wider array of autophagy machinery to clarify the orchestration of factors that contribute to autophagosome membrane tethering and fusion.
Genetic hierarchical analyses rank the lipidation machinery farthest downstream in the autophagic cascade (39,40). However, as lipidation-deficient cells can still form phagophores, it is likely that early autophagic factors support the membrane tethering and fusion required to form this early structure. A structural study of the Atg17/Atg29/Atg31 complex, the first complex activated upon autophagy induction (39,90) determined that it could be an early membrane tether. This Atg17/Atg29/Atg31 complex forms an elongated dimer with Atg17, forming two crescent-shaped structures on both ends (61). Based on the 100 Å radius of curvature for the Atg17 crescent structure, this complex may be capable of binding and tethering small vesicles, such as the Atg9 vesicles required for the membrane nucleation step of autophagosome biogenesis (31,91). Indeed, a truncated version of the Atg1/Atg13/Atg17/Atg29/Atg31 complex was capable of binding and tethering small vesicles in vitro (61). Consequently, the Atg1–Atg13–Atg17/Atg29/Atg31 complex could be an early membrane-tethering factor required for autophagy initiation. Additionally, the transport protein particle (TRAPP) III complex was proposed to tether vesicles in early autophagy (60,92,93). It would be enticing to reconstitute the vesicle tethering activity of these complexes with other factors that promote membrane fusion, such as SNAREs to understand how they cooperate to facilitate phagophore formation.
Once autophagosome formation is complete, it fuses with endosomes and the vacuole/lysosome so that autophagic cargo can be degraded. To explore the molecular requirements of autophagosome and endosome fusion in more detail, the Tooze lab established a cell-free content mixing assay by first isolating autophagosomes containing HA-tagged LC3 and endosomes that contained internalized HA antibody. These two fractions were then combined and membrane fusion was determined by the presence of HA antibody bound to HA-LC3 (Fig. 4b) (62). This study determined that autophagosome/endosome fusion requires both cytosolic and endosome associated factors. Later, several different fusion machinery proteins were implicated in this process, including soluble SNAREs (Syntaxin 17), RabGTPases, and the homotypic fusion and vacuole protein sorting (HOPS) tethering complex (89,94–100).
Another study indicated that the membrane targeting subunit of the PI3K, ATG14/Barkor, has fusogenic activity and can promote autophagosome fusion with endolysosomes (63). Using a single-molecule vesicle/liposome-tethering assay in conjunction with lipid and content mixing assays, the Zhong and Brunger labs found that recombinant ATG14 alone promotes vesicle tethering facilitated by ATG14 homodimerization (Fig. 4A) (63). When ATG14 is incubated with liposomes incorporated with autophagy-specific SNARES (Syntaxin 17, SNAP29, and VAMP8 (99,101)), it enhances hemi-and full fusion of liposomes (Fig. 4a). An ATG14 homodimerization mutant (C43A/C46A/C55A/C58A) that cannot tether vesicles blocks autophagosome and endolysosome fusion, suggesting that that this tethering activity is required for later-stage autophagy (63). Because ATG14 usually formsaproteincomplex with Beclin-1 and VPS34 (77,78,80,81,102), in the future it will be important to validate this model by showing that ATG14 exists asa homodimerinthe cell. It would also be intriguing to employ a cell-free reconstitution to validate the direct role of the indicated factors, such as ATG14, HOPS, and SNAREs, in the autophagosome and endolysosome fusion.
Phagophore shape and asymmetry
The phagophore forms in an asymmetrical cup shape where the concave face surrounds autophagic cargo (103,104) and the convex face associates with other autophagic factors such as Atg12−Atg5 and Atg16 (69,105). Forming this asymmetrical structure is critical for autophagy activity, yet it is unclear how phagophore shape and asymmetry is established. A recent study has provided some preliminary implications. The Wollert lab reconstituted both Atg8 lipidation and Atg12−Atg5 and Atg16 membrane recruitment based on fluorescence imaging of GUVs (Fig. 5a). They found that the Atg12− Atg5 conjugate bridges Atg16 to the lipid-anchored Atg8 on the buffer soluble and convex side of a GUV through a noncanonical Atg8-interacting motif (AIM), similar to the LC3 interaction motif (LIR) (106). This study also found that Atg16 immobilizes Atg8 and Atg12−Atg5 through tetramer formation (Fig. 5b) (64). Atomic force microscopy indicated that Atg16 together with Atg12−Atg5 and Atg8−PE forms a two-dimensional meshwork on supported lipid bilayers indicating that scaffold formation possibly contributes to supporting convex phagophore shape (64). In addition to the potential scaffolding capabilities of the Atg16/Atg12−Atg5 complex, a recent study indicated that microfilaments are also required for correctly shaped autophagosomes (107).
Fig. 5.
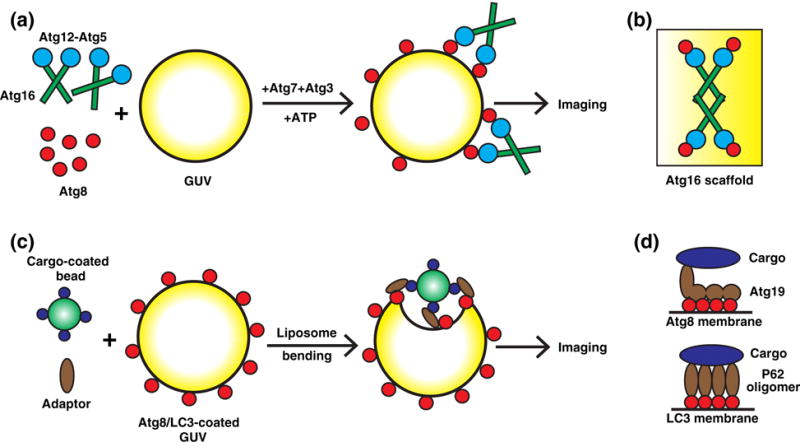
Reconstituting autophagic events on giant unilamellar vesicles (GUVs). (a) Autophagic scaffold assembly. Atg8 lipidation was performed on PE-containing GUVs with Atg3, Atg7, Atg12−Atg5, and Atg16. The quantitative fluorescence imaging of Atg recruitment to GUVs indicates that Atg12−Atg5 and Atg16 membrane association is enhanced by the interaction between Atg12 and lipid-conjugated Atg8 (64). (b) A tetramer of Atg16 with Atg12−Atg5 immobilizes Atg8 on the membrane and possibly acts as a unit for an autophagic membrane scaffold (64). (c) Autophagic membrane recruitment to cargo. Cargo-coated beads, prApe1 propeptide (for Atg19 binding) or diubiquitin (to mimic polyubiquitin chain for P62 binding), were incubated with cargo adaptors Atg19 or P62 and GUVs coated with Atg8 or LC3. GUV membrane bending by bead attachment was determined as the effect of autophagic membrane recruitment to cargo (66,67). (d) Atg19 mediates efficient autophagic membrane recruitment by harboring multiple Atg8-binding motifs that are allosterically exposed by cargo binding (upper panel). P62 oligomerizes to combine multiple LC3 and ubiquitin-binding motifs, therefore enhancing its interaction with both LC3-coated autophagic membrane and polyubiquitinated cargo (66,67).
Surprisingly, the cargo adaptor protein Atg32 competes with Atg12−Atg5 for Atg8 binding (64). Because Atg8 decorates the entire phagophore surface (108), this mutually exclusive Atg8-binding competition between Atg32 and Atg12−Atg5 may explain phagophore asymmetry. Hence, either Atg12−Atg5 is associated with Atg8 on the convex face of the phagophore or the cargo bound by adaptor proteins, such as Atg32, are bound to Atg8 on the concave face (64). Moreover, another two studies investigated the topological distribution of different autophagic factors on the growing phagophore and found that autophagic factors are distinctly localized on different regions of the cup-shaped membrane such as the tips or throughout membrane (84,85). Consequently, phagophore morphology is likely determined by a concerted mechanism involving all of these factors. It will be important in the future to establish a cell-free system that reconstitutes phagophore formation and closure to functionally evaluate the role of these factors.
Lysosome reformation
After prolonged starvation and autophagy activity, the autolysosome becomes enlarged and needs to be reformed into smaller lysosomes to reestablish lysosomal homeostasis. To accomplish this, mTORC1 is reactivated and stimulates the formation of tubules that extend from the large LC3-positive autolysosomes that, in time, become separate lysosomes (9–12). The Yu lab isolated lysosome reformation tubules by membrane fractionation and identified a regulatory module containing phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) and clathrin that is involved in forming new, mature lysosomes (9–11). They then established a cell-free reconstitution assay to demonstrate a direct requirement of PI(4,5)P2 and clathrin in autophagic lysosome reformation (10). Consequently, cell-free assays have been valuable in determining the molecular requirements for this late step of autophagy and could be used to further uncover other factors required for lysosome reformation after autophagy.
Reconstituting Selective Autophagy
Unlike starvation-induced autophagy (also known as macroautophagy), selective autophagy refers to the selective degradation of proteins and organelles under steady-state conditions (26,43,45). Because macroautophagy is inhibited under nutrient-rich conditions, selective autophagy activation requires the cell to overcome the autophagy inhibitory signals such as the fact that mTORC1 is active (43,109,110). In addition, because selective autophagic cargos are usually unwanted or toxic to the cells, autophagic membrane should be efficiently and specifically targeted to these cargos. Studies from two labs using in vitro systems have shed light on these two aspects of selective autophagy.
Localized signal activation
In the absence of nutrients, starvation-induced autophagy stimulates nonselective degradation of cytoplasmic components. In this process, a signaling cascade activates downstream autophagic factors to facilitate autophagosome formation throughout the cell to nonselectively degrade the cytoplasm (26,43). Under nutrient-rich conditions, the autophagic machinery can also be used to selectively eliminate damaged proteins and organelles (43,110). Unlike the cell-wide transduction of autophagic signals in starvation-induced autophagy during selective autophagy, autophagy is only locally active near specific cargo. How this localized autophagy is triggered remains unclear. A study using cell-free reconstitution brought some insight into this question. Atg1 is a protein kinase and master regulator of autophagy (18). The Denic lab genetically engineered Atg1 by replacing the bulky “gatekeeper residue” in the ATP-binding pocket with a glycine residue. Through this mutation, Atg1 could utilize an unnatural, bulky ATP derivative (N6-PhEt-ATPγS) to add thiophosphorylate to its substrates. Following alkylation with paranitrobenzyl mesylate, substrates that were labeled by Atg1 could be easily detected by immunoblotting with an anti-thiophosphate ester antibody. Based on this principle, the Denic lab established a cell-free assay to determine the activity of the engineered Atg1. Interestingly, they found that through direct interaction, a signaling cascade from the cargo protein to the adaptor Atg19 via the scaffold Atg11 triggers Atg1 activation (65). This bind-and-activate model provides an important rationale for the specific local activation of autophagic machinery during selective autophagy. Besides autophagy regulation through cargo adaptors, ATG13 and FIP200 complex formation was also shown to enhance the kinase activity of ULK1 in vitro (111). In the future, it will be necessary to understand if Atg1/ULK1 stimulation by different factors in selective autophagy and macroautophagy is mechanistically similar in yeast and mammalian systems.
Efficient cargo recognition
Unrestricted accumulation of aggregated proteins or damaged organelles is toxic to cells. To restrict this damage, the autophagic membrane must be efficiently recruited to these cargos to package them for removal and degradation. The mechanism by which the phagophore membrane is efficiently targeted to cargo has been elusive, yet two successive studies from the Martens group have provided insight. In yeast, Atg19 is a cargo adaptor that tethers autophagic cargo to lipidated Atg8 through a canonical AIM located at the C terminus of Atg19 (106,112). In the first study, they found that Atg19 contains Atg8-binding regions outside of this canonical AIM. Importantly, these additional binding regions are exposed after cargo association, enabling one Atg19 molecule to interact with multiple Atg8 proteins (Fig. 5d). This cooperative binding of Atg19 to many Atg8 molecules enhances the interaction between autophagic cargo and Atg8 vesicles. Because autophagic cargos are usually protein aggregates, a peptide corresponding to the Atg19-binding region on the cargo was attached to beads to mimic protein aggregates. The Martens group then established a system to reconstitute the Atg19-facilitated recruitment of cargo-coated beads to Atg8-conjugated GUVs (Fig. 5c). In this assay, full-length Atg19 efficiently recruited beads to the GUV; mutation of the Atg8-binding sites, either canonical or noncanonical, compromised the recruitment process (66). This study indicates a cargo-enabled association of Atg19 with multiple membrane-anchored Atg8s for efficient targeting of autophagic membranes to the cargo.
In the next study, the Martens group employed a similar in vitro system to investigate the mammalian autophagic cargo adaptor P62/SQSTM1 (67,113). They found that P62 contains only one LIR but oligomerizes to generate multiple LC3-binding sites in one protein complex, which enhance cargo binding to membrane-anchored LC3 (Fig. 5d). In addition, P62 oligomerization also enhanced its association with K63-linked polyubiquitin chain, a modification that flags cargo for autophagic degradation (67,114,115).
Together, these studies on Atg19 and P62 indicate that the binding affinity of cargo to the autophagic membrane is strengthened by the presence of multiple Atg8/LC3-binding sites, either on one protein or through oligomerization.
Concluding Remarks
Genetics and cellular imaging have dramatically contributed to our understanding of autophagy including identifying the major molecular players and steps of autophagosomal membrane maturation. However, the mechanism of autophagosome formation and regulation is still unclear. In vitro reconstitution offers an opportunity to deeply dissect the molecular mechanism of autophagy. As discussed in this review, significant progress has been made toward reconstituting different steps of autophagy with different in vitro systems. However, autophagy reconstitution work is still in its early stages. Several key steps of autophagosome biogenesis, including early autophagosomal membrane fusion, phagophore shape formation, and phagophore closure, have not been reconstituted yet. Additionally, the role that each key molecular component, such as the ULK1/Atg1 protein kinase complex and the PI3K phospholipid kinase complex, plays in remodeling target membranes is still unclear. It is necessary to establish new and robust in vitro systems to reconstitute these major steps of autophagy, as well as to functionally determine the mechanism of membrane modeling mediated by these autophagic factors. Considering that autophagy is a highly regulated process dependent on sophisticated spatiotemporal interactions between the autophagic factors and the target membrane, a big challenge in the future will be to establish an in vitro system that combines all these factors to recapitulate autophagosome biogenesis faithfully regulated by the major landmark signals of autophagy in vivo.
Acknowledgments
We are grateful for Dr. Randy Schekman’s mentorship. We thank Dr. James Hurley (UC Berkeley) for his helpful advice on the manuscript. L.W.B., M.Z., and L.G. are postdoctoral fellows in the Schekman lab at UC Berkeley. L.W.B. and M.Z. are HHMI associates. L.G. is a fellow of the NIH Pathway to Independence Award (Parent K99/R00) National Institute of General Medical Sciences (Grant No. 1K99GM114397-01).
Abbreviations used
- ER
endoplasmic reticulum
- Atg
autophagy-related gene
- PI3K
phosphatidylinositol-3 kinase
- PI3P
phosphatidylinositol-3 phosphate
- PE
phosphatidylenthanolamine
- GUVs
giant unilamellar vesicles
- ERGIC
ER–Golgi intermediate compartment
- SNARE
soluble N-ethylma-leimide-sensitive factor attachment protein receptors
- LIR
LC3 interaction motif
- AIM
Atg8-interacting motif
- PI(4,5)P2
phosphatidylinositol-4,5-bisphosphate
Footnotes
Edited by R.E. Hurley
References
- 1.Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge L, Baskaran S, Schekman R, Hurley JH. The protein-vesicle network of autophagy. Curr Opin Cell Biol. 2014;29:18–24. doi: 10.1016/j.ceb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Burman C, Ktistakis NT. Autophagosome formation in mammalian cells. Semin Immunopathol. 2010;32:397–413. doi: 10.1007/s00281-010-0222-z. [DOI] [PubMed] [Google Scholar]
- 6.Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22:R29–R34. doi: 10.1016/j.cub.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Hamasaki M, Shibutani ST, Yoshimori T. Up-to-date membrane biogenesis in the autophagosome formation. Curr Opin Cell Biol. 2013;1–6 doi: 10.1016/j.ceb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 9.Rong Y, McPhee CK, Deng S, Huang L, Chen L, Liu M, et al. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci U S A. 2011;108:7826–7831. doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong Y, Liu M, Ma L, Du W, Zhang H, Tian Y, et al. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat Cell Biol. 2012;14:924–934. doi: 10.1038/ncb2557. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang J, Lee S, Blackstone C. Spastic paraplegia proteins spastizin and spatacsin mediate autophagic lysosome reformation. J Clin Invest. 2014;124:5249–5262. doi: 10.1172/JCI77598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 14.Yao Z, Delorme-Axford E, Backues SK, Klionsky DJ. Atg41/Icy2 regulates autophagosome formation. Autophagy. 2015;13:2288–2299. doi: 10.1080/15548627.2015.1107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reggiori F, Klionsky DJ. Autophagic processes in yeast: mechanism, machinery and regulation. Genetics. 2013;194:341–361. doi: 10.1534/genetics.112.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 17.Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature. 2015;522:359–362. doi: 10.1038/nature14506. [DOI] [PubMed] [Google Scholar]
- 18.Noda NN, Fujioka Y. Atg1 family kinases in autophagy initiation. Cell Mol Life Sci. 2015;72:3083–3096. doi: 10.1007/s00018-015-1917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi S, do Kim J, Stjepanovic G, Hurley JH. Structure of the human Atg13–Atg101 HORMA heterodimer: an interaction hub within the ULK1 complex. Structure. 2015;23:1848–1857. doi: 10.1016/j.str.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Kaizuka T, Mizushima N, Noda NN. Structure of the Atg101−Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat Struct Mol Biol. 2015;22:572–580. doi: 10.1038/nsmb.3036. [DOI] [PubMed] [Google Scholar]
- 22.Obara K, Ohsumi Y. Atg14: a key player in orchestrating autophagy. Int J Cell Biol. 2011;2011:1–7. doi: 10.1155/2011/713435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12:226. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 27.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 28.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 30.Yamada T, Carson AR, Caniggia I, Umebayashi K, Yoshimori T, Nakabayashi K, et al. Endothelial nitric-oxide synthase antisense (NOS3AS) gene encodes an autophagy-related protein (APG9-like2) highly expressed in trophoblast. J Biol Chem. 2005;280:18,283–18,290. doi: 10.1074/jbc.M413957200. [DOI] [PubMed] [Google Scholar]
- 31.Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webber JL, Tooze SA. New insights into the function of Atg9. FEBS Lett. 2010;584:1319–1326. doi: 10.1016/j.febslet.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Proikas-Cezanne T, Takacs Z, Dönnes P, Kohlbacher O. WIPI proteins: essential PtdIns3P effectors at the nascent autophagosome. J Cell Sci. 2015;128:207–217. doi: 10.1242/jcs.146258. [DOI] [PubMed] [Google Scholar]
- 34.Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velikkakath AKG, Nishimura T, Oita E, Ishihara N, Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell. 2012;23:896–909. doi: 10.1091/mbc.E11-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obara K, Sekito T, Niimi K, Ohsumi Y. The Atg18−Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J Biol Chem. 2008;283:23,972–23,980. doi: 10.1074/jbc.M803180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 38.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 40.Koyama-Honda I, Itakura E, Fujiwara TK, Mizushima N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy. 2013;9:1491–1499. doi: 10.4161/auto.25529. [DOI] [PubMed] [Google Scholar]
- 41.Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Ohsumi Y. Current knowledge of the preautophagosomal structure (PAS) FEBS Lett. 2010;584:1280–1286. doi: 10.1016/j.febslet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 46.Ichimura Y, Imamura Y, Emoto K, Umeda M, Noda T, Ohsumi Y. In vivo and in vitro reconstitution of Atg8 conjugation essential for autophagy. J Biol Chem. 2004;279:40,584–40,592. doi: 10.1074/jbc.M405860200. [DOI] [PubMed] [Google Scholar]
- 47.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al. The Atg12−Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37,298–37,302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 48.Sou YS, Tanida I, Komatsu M, Ueno T, Kominami E. Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J Biol Chem. 2006;281:3017–3024. doi: 10.1074/jbc.M505888200. [DOI] [PubMed] [Google Scholar]
- 49.Fujioka Y, Noda NN, Fujii K, Yoshimoto K, Ohsumi Y, Inagaki F. In vitro reconstitution of plant Atg8 and Atg12 conjugation systems essential for autophagy. J Biol Chem. 2008;283:1921–1928. doi: 10.1074/jbc.M706214200. [DOI] [PubMed] [Google Scholar]
- 50.Romanov J, Walczak M, Ibiricu I, Schüchner S, Ogris E, Kraft C, et al. Mechanism and functions of membrane binding by the Atg5–Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31:4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nath S, Dancourt J, Shteyn V, Puente G, Fong WM, Nag S, et al. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol. 2014;16:415–424. doi: 10.1038/ncb2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan X, Sun Q, Ji J, Zhu Y, Liu Z, Zhong Q. Reconstitution of leucine-mediated autophagy via the mTORC1-Barkor pathway in vitro. Autophagy. 2012;8:213–221. doi: 10.4161/auto.8.2.18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan W, Nassiri A, Zhong Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L) Proc Natl Acad Sci U S A. 2011;108:7769–7774. doi: 10.1073/pnas.1016472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge L, Melville D, Zhang M, Schekman R. The ER–Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. elife. 2013;2:1–23. doi: 10.7554/eLife.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge L, Zhang M, Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER–Golgi intermediate compartment. elife. 2014;3:1–13. doi: 10.7554/eLife.04135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell. 2011;20:444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Wu F, Watanabe Y, Guo XY, Qi X, Wang P, Zhao HY, et al. Structural basis of the differential function of the two C. elegans Atg8 homologs, LGG-1 and LGG-2, in autophagy. Mol Cell. 2015;60:914–929. doi: 10.1016/j.molcel.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen WL, et al. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan D, Cai Y, Wang J, Zhang J, Menon S, Chou HT, et al. The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc Natl Acad Sci U S A. 2013;110:19,432–19,437. doi: 10.1073/pnas.1316356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ragusa MJ, Stanley RE, Hurley JH. Architecture of the Atg17 complex as a scaffold for autophagosome biogenesis. Cell. 2012;151:1501–1512. doi: 10.1016/j.cell.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morvan J, Kochl R, Watson R, Collinson LM, Jefferies HBJ, Tooze SA. In vitro reconstitution of fusion between immature autophagosomes and endosomes. Autophagy. 2009;5:676–689. doi: 10.4161/auto.5.5.8378. [DOI] [PubMed] [Google Scholar]
- 63.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaufmann A, Beier V, Franquelim HG, Wollert T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell. 2014;156:469–481. doi: 10.1016/j.cell.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 65.Kamber RA, Shoemaker CJ, Denic V. Receptor-bound targets of selective autophagy use a scaffold protein to activate the Atg1 kinase. Mol Cell. 2015;59:372–381. doi: 10.1016/j.molcel.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sawa-Makarska J, Abert C, Romanov J, Zens B, Ibiricu I, Martens S. Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane-cargo apposition during selective autophagy. Nat Cell Biol. 2014;16:425–433. doi: 10.1038/ncb2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wurzer B, Zaffagnini G, Fracchiolla D, Turco E, Abert C, Romanov J, et al. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. elife. 2015;4 doi: 10.7554/eLife.08941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanida I, Tanida-Miyake E, Nishitani T, Komatsu M, Yamazaki H, Ueno T, et al. Murine Apg12p has a substrate preference for murine Apg7p over three Apg8p homologs. Biochem Biophys Res Commun. 2002;292:256–262. doi: 10.1006/bbrc.2002.6645. [DOI] [PubMed] [Google Scholar]
- 71.Tanida I, Tanida-Miyake E, Ueno T, Kominami E. The human homolog of Saccharomyces cerevisiae Apg7p is a protein-activating enzyme for multiple substrates including human Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem. 2001;276:1701–1706. doi: 10.1074/jbc.C000752200. [DOI] [PubMed] [Google Scholar]
- 72.Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the approximately 350-kDa Apg12−Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277:18,619–18,625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- 73.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. (3rd) 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsushita M, Suzuki NN, Obara K, Fujioka Y, Ohsumi Y, Inagaki F. Structure of Atg5–Atg16, a complex essential for autophagy. J Biol Chem. 2007;282:6763–6772. doi: 10.1074/jbc.M609876200. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2008;105:19,211–19,216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, et al. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J Cell Biol. 2010;190:511–521. doi: 10.1083/jcb.200911141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itakura E, Mizushima N. Atg14 and UVRAG: mutually exclusive subunits of mammalian Beclin 1-PI3K complexes. Autophagy. 2009;5:534–536. doi: 10.4161/auto.5.4.8062. [DOI] [PubMed] [Google Scholar]
- 80.Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1–phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 82.Hurley JH, Schulman BA. Atomistic autophagy: the structures of cellular self-digestion. Cell. 2014;157:300–311. doi: 10.1016/j.cell.2014.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Appenzeller-Herzog C, Hauri HP. The ER–Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- 84.Graef M, Friedman JR, Graham C, Babu M, Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013;24:2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki K, Akioka M, Kondo-Kakuta C, Yamamoto H, Ohsumi Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J Cell Sci. 2013;126:2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- 86.Budnik A, Stephens DJ. ER exit sites—localization and control of COPII vesicle formation. FEBS Lett. 2009;583:3796–3803. doi: 10.1016/j.febslet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 87.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 88.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Publ Group. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 89.Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146:303–317. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawamata T, Kamada Y, Kabeya Y, Sekito T, Ohsumi Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol Biol Cell. 2008;19:2039–2050. doi: 10.1091/mbc.E07-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, et al. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol. 2012;198:219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, et al. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A. 2010;107:7811–7816. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J, Menon S, Yamasaki A, Chou HT, Walz T, Jiang Y, et al. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc Natl Acad Sci U S A. 2013;110:9800–9805. doi: 10.1073/pnas.1302337110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, et al. The HOPS complex mediates autophagosome-lysosome fusion through interaction with Syntaxin 17. Mol Biol Cell. 2014;8:1327–1337. doi: 10.1091/mbc.E13-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16:956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- 96.Reggiori F, Klionsky DJ. Atg9 sorting from mitochondria is impaired in early secretion and VFT-complex mutants in Saccharomyces cerevisiae. J Cell Sci. 2006;119:2903–2911. doi: 10.1242/jcs.03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tooze SA, Dooley HC, Jefferies HBJ, Joachim J, Judith D, Lamb CA, et al. Assessing mammalian autophagy. Methods Mol Biol. 2014;1270:155–165. doi: 10.1007/978-1-4939-2309-0_12. [DOI] [PubMed] [Google Scholar]
- 98.Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell. 2013;154:1285–1299. doi: 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takáts S, Pircs K, Nagy P, Varga Á, Kárpáti M, Hegedűs K, et al. Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell. 2014;25:1338–1354. doi: 10.1091/mbc.E13-08-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE Syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Baskaran S, Carlson LA, Stjepanovic G, Young LN, Kim DJ, Grob P, et al. Architecture and dynamics of the autophagic phosphatidylinositol 3-kinase complex. eLife. 2014;4:1–19. doi: 10.7554/eLife.05115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kondo-Okamoto N, Noda NN, Suzuki SW, Nakatogawa H, Takahashi I, Matsunami M, et al. Autophagy-related protein 32 acts as autophagic degron and directly initiates mitophagy. J Biol Chem. 2012;287:10,631–10,638. doi: 10.1074/jbc.M111.299917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mizushima N. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12−Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 106.Noda NN, Ohsumi Y, Inagaki F. Atg8-family interacting motif crucial for selective autophagy. FEBS Lett. 2010;584:1379–1385. doi: 10.1016/j.febslet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 107.Mi N, Chen Y, Wang S, Chen M, Zhao M, Yang G, et al. CapZ regulates autophagosomal membrane shaping by promoting actin assembly inside the isolation membrane. Nat Cell Biol. 2015;17:1112–1123. doi: 10.1038/ncb3215. [DOI] [PubMed] [Google Scholar]
- 108.Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, et al. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sudarsanam S, Johnson DE. Functional consequences of mTOR inhibition. Curr Opin Drug Discov Devel. 2010;13:31–40. [PubMed] [Google Scholar]
- 110.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol Mech Dis. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 111.Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1–ATG13–FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12,297–12,305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on Huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Long J, Gallagher TR, Cavey JR, Sheppard PW, Ralston SH, Layfield R, et al. Ubiquitin recognition by the ubiquitin-associated domain of p62 involves a novel conformational switch. J Biol Chem. 2008;283:5427–5440. doi: 10.1074/jbc.M704973200. [DOI] [PubMed] [Google Scholar]
- 115.Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004;24:8055–8068. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


