Synopsis
The pancreas is a complex organ that may give rise to large number of neoplasms and non-neoplastic lesions. This article will focus on benign neoplasms such as serous neoplasms as well as tumor-like (pseudotumoral) lesions that may be mistaken for neoplasm not only by clinicians and radiologists, but also by pathologists. The family of pancreatic pseudotumors, by a loosely defined conception of that term, includes a variety of lesions including heterotopia, hamartoma, and lipomatous pseudohypertrophy. Autoimmue pancreatitis (covered in chronic pancreatitis chapter) and paraduodenal (“groove”) pancreatitis may also lead to pseudotumor formation. Knowledge of these entities will help in making an accurate diagnosis.
Keywords: Benign, serous, lymphoepithelial, squamoid, epidermoid, hamartoma, inflammatory, pancreatitis
SEROUS NEOPLASMS
Serous neoplasms of the pancreas are rare benign tumors accounting for approximately 1% of all pancreatic lesions. These tumors reveal a unique cytomorphology characterized by distinctive cuboidal epithelial cells with uniform round nuclei, dense, homogenous chromatin, and a prominent epithelium-associated microvascular meshwork 1, 2. They are generally regarded under the category of ductal-type tumors; however, they do not produce mucin despite their presumed ductal lineage, instead, they produce abundant glycogen.
Several morphologic variants of serous neoplasms have been described. These include microcystic and macrocystic (a.k.a. oligocystic) serous cystadenomas, solid serous adenoma, and von Hippel-Lindau (vHL)-associated serous cystic neoplasm. The microcystic serous cystic neoplasm consists of innumerable small, irregularly contoured tubular structures of variable shapes, the vast majority of which measure in submillimeters. The macrocystic (oligocystic) serous cystic neoplasms are predominantly or completely composed of fewer (typically less than 10) but much larger cysts, each measuring in centimeters. Although “serous cystadenoma (SCA)” and “serous cystic neoplasm (SCN)” terms technically refer to the microcystic variant; they are often used interchangeably for both microcystic and macrocystic variants. Solid serous adenoma is characterized by uniform, small, evenly shaped and sized nests or tubules with minimal or no lumen formation 1. vHL-associated serous cystic neoplasms are often more a patchy transformation in the pancreas, although some may form a well-defined localized mass 3–8.
Microcystic and Macrocystic (Oligocystic) Serous Cystadenomas
Serous cystadenomas can occur at any age but it is more common in elderly female patients 1, 7–21. They are often asymptomatic 11, 22, 23, discovered incidentally, either sporadically or as part of vHL disease 7, 8, 24–27. If the mass is located in the pancreatic head, it can obstruct the biliary tract 25, 28. Rarely, the lesions are multiple, specifically when associated with vHL.
A “honeycomb” appearance on CT or MRI, associated with a central scar that may or may not be calcified, is characteristic for microcystic variant 16, 17, 22, 29–31. However, the diagnosis is often not accomplished preoperatively by imaging studies. Similarly, macrocystic (oligocystic) variant, especially if only a single cyst is evident 32, radiographically simulates intraductal papillary mucinous neoplasms, mucinous cystic neoplasms, and pseudocysts 16, 19, 30, 33–35.
The FNA diagnosis of serous neoplasms has also proven to be unexpectedly challenging because of the very low aspirate cellularity, probably due to the cohesiveness and adhesion of the cells to the tissue 1, 36–38. The tumor cells are bland, cuboidal, and arranged in loose clusters or monolayers. The cytoplasm is usually cleared or vacuolated. However, the cells are frequently stripped of cytoplasm, showing only small, round nuclei with fine but dense, homogenous nuclear chromatin 31, 36, 39.
The presurgical diagnosis of pancreatic cysts has traditionally relied on measuring cyst fluid amylase as well as the tumor markers CA19-9 and CEA to identify and distinguish the mucinous neoplasms from non-mucinous lesions such as serous neoplasms 40. However, the sensitivity and specificity of these markers are relatively low 41. Recently, in an ELISA analysis of cyst fluid and tumoral tissue, Yip-Schneider et al. found that vascular endothelial growth factor-A (VEGF-A) was markedly elevated in serous cystic neoplasms when compared to pseudocysts, intraductal papillary mucinous neoplasms, mucinous cystic neoplasms, and pancreatic ductal adenocarcinoma 42. With a cut off of 8500pg/mL, VEGF-A had 100% sensitivity and 97% specificity as a marker of serous cystic neoplasms, making it a very promising biomarker for the diagnosis and distinction of serous cystic neoplasms from other pancreatic cysts, particularly when used in conjunction with CEA 42.
Similarly, the identification of cyst-specific somatic mutations (involving KRAS, GNAS, RNF43, CTNNB1, and VHL genes) offers great promise in the presurgical diagnosis of pancreatic cysts. Recently, KRAS and GNAS mutations have been shown to have 96% sensitivity and 100% specificity for the differentiation of intraductal papillary mucinous neoplasm from serous cystic neoplasm 41. Molecular assays containing a 5-gene panel may be especially useful in the pretreatment diagnosis of serous cystic neoplasms since isolated VHL mutations have not been identified in intraductal papillary mucinous neoplasms and mucinous cystic neoplasms. However, it should be kept in mind that pancreatic neuroendocrine tumors may also be cystic and may harbor VHL deletions in up to 25% of sporadic cases.
These new developments seem to be very promising for the preoperative diagnosis (and thus possible conservative management) of serous cystic neoplasms; however, they need to be verified in larger-scale studies before they can be put into daily clinical management.
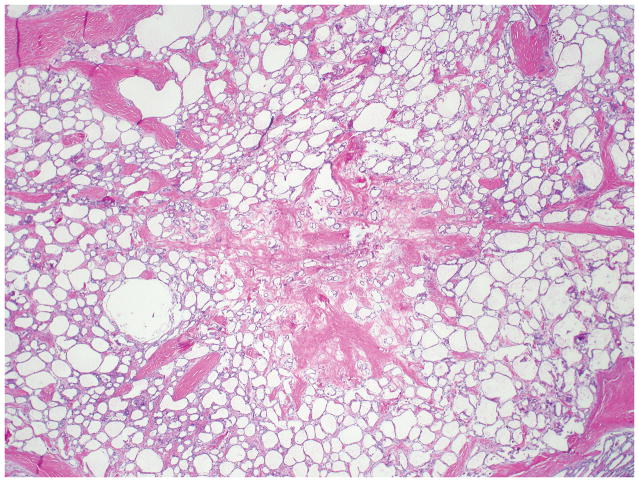
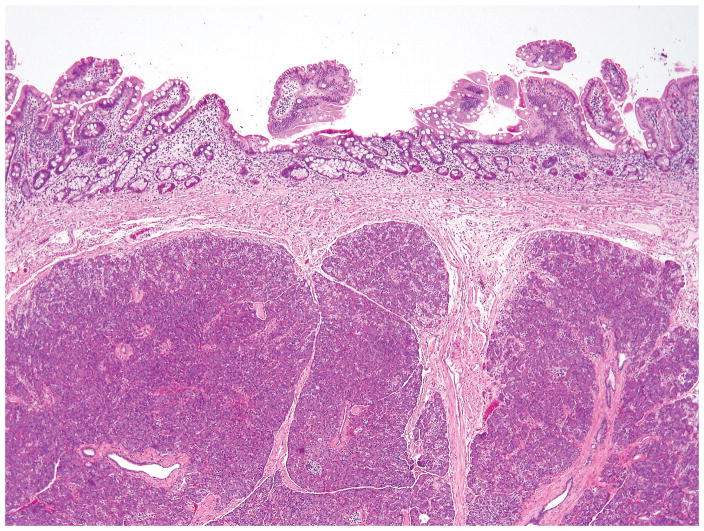
The mean diameter of serous cystic neoplasms is approximately 4 cm but now smaller lesions are found using improved imaging techniques 1, 9, 10. They occur anywhere in the organ and appear as circumscribed and well-demarcated from the surrounding pancreas. Microcystic serous cystic neoplasms form partly encapsulated, lobulated masses composed of innumerable tiny cysts, which impart the highly distinctive and entity-defining sponge-like appearance on sectioning (Figure 1). Irregular central scars, frequently calcified, may be seen in the larger tumors. The fluid in the cysts is clear and watery, appearing colorless, yellow, or blood stained. Foci of hemorrhage can occur 43. Macrocystic (oligocystic) serous cystic neoplasms, by definition, are composed of much larger cysts with fewer loculi and devoid of central fibrosis or calcification (Figure 2).
Figure 1.
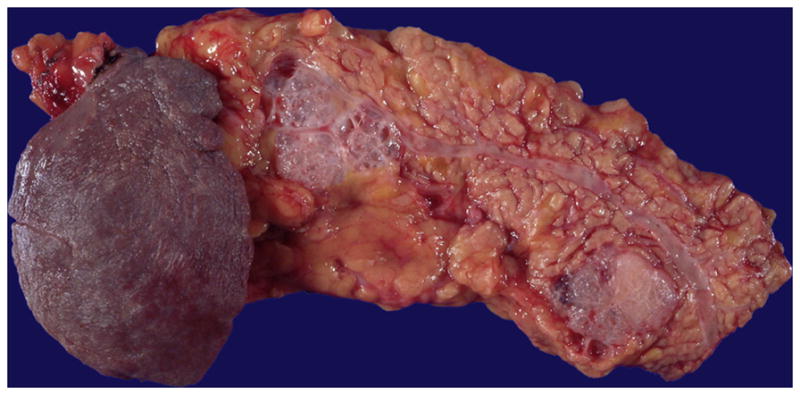
Two microcystic serous cystadenomas are present in this distal pancreas. The neoplasms are well-demarcated and composed of numerous small cysts, the majority of which measure in submillimeters.
Figure 2.
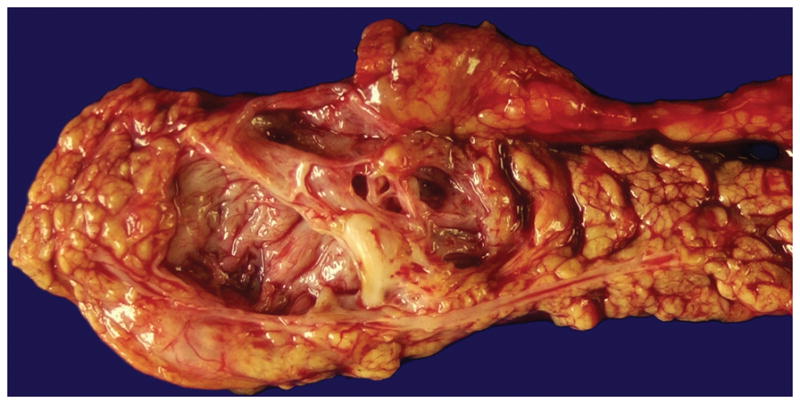
Macrocystic (oligocystic) serous cystadenoma has either a singular locule or a few locules (as illustrated here) with a flattened lining.
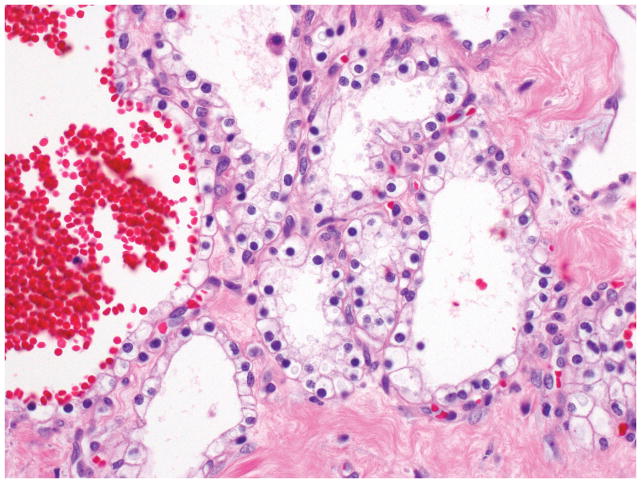
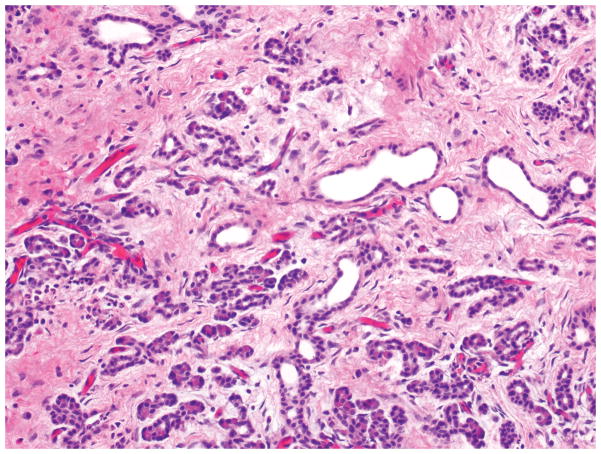
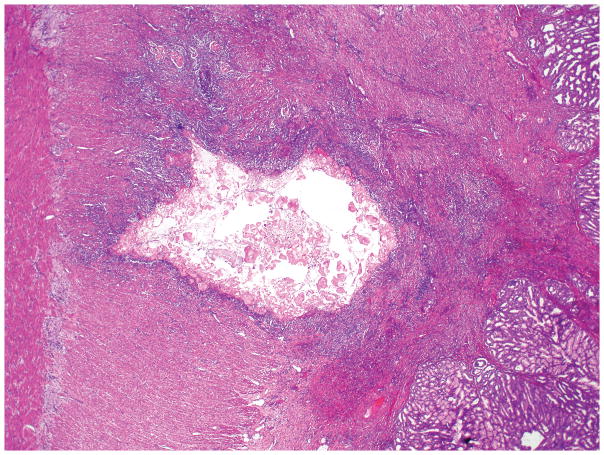
Microscopic examination of microcystic serous cystic neoplasms reveal back to-back tubules of variable size and shape (Figure 3), creating a characteristic microcystic pattern formed by cuboidal epithelium. Scattered larger (up to a centimeter in diameter), more irregular cysts lined by low cuboidal to flat cells may also be seen 7, 15, 17, 19–21, 44. Dense stroma can occur in the lesions with these larger cysts. Some of the stroma may be hyalinized or myxoid. The tumor cells usually have abundant clear cytoplasm due to abundant glycogen (Figure 4). Some cases reveal more oncocytoid cells with granular cytoplasm. Nuclei are small, round, compact, and uniform, with inconspicuous nucleoli. The presence of a capillary meshwork immediately adjacent to (almost within) the epithelium is highly characteristic 45, 46, and has been noted as a striking analogy with other clear cell tumors arising in association with vHL, including renal cell carcinomas and hemangioblastomas 45. Macrocystic (oligocystic) serous cystic neoplasms may be missing much of its lining, thus requiring multiple sections to find the epithelium. The lining of the cysts display the characteristic serous cytology (Figure 5).
Figure 3.
Microcystic serous cystadenoma is characterized by numerous small, irregular tubular structures of variable shapes. Note the hyalinized stroma at the centre.
Figure 4.
The cysts of serous cystadenomas are lined by cuboidal cells, with round, central to slightly eccentric nuclei and clear cytoplasm.
Figure 5.
Although the cysts are fewer and larger than in the microcystic serous cystadenoma, macrocystic (oligocystic) serous cystadenoma is also lined by the same cuboidal cells with clear cytoplasm (inset).
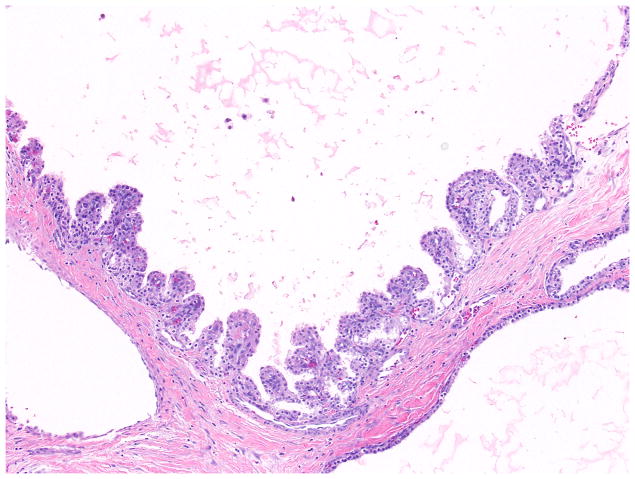
Serous cystic neoplasms often show degenerative changes (hemorrhage, inflammation, and cholesterol clefts). Calcifications, tufting/micropapillae (Figure 6), the presence of satellite nodules, and, frequent intermixing of neuroendocrine cell proliferation in a pseudoinvasive pattern are also common 1.
Figure 6.
Although the lining epithelium is usually flat, prominent but stubby papillary projections might be seen in some serous cystadenomas.
Both histologically and immunophenotypically, these neoplasms appear to recapitulate centroacinar cells 18, 46–48. The glycogen rich cytoplasm is typically PAS positive, diastase sensitive, and reacts with broad-spectrum and low-molecular-weight keratins, EMA, and inhibin 15, 49. Ductal mucin markers (B72.3, CA19-9, CEA and MUC1) are either negative or only focally positive, although MUC6 is usually positive 49. Molecules implicated in clear cell tumorigenesis [glucose uptake and transporter-1 (GLUT-1), hypoxia-inducible factor-1α (HIF-1α), and carbonic anhydrase IX (CA-IX)] are also consistently expressed 45.
At a molecular level, VHL gene allelic deletions (chromosome 3p) are detected in serous cystic neoplasms from patients with vHL, providing molecular evidence of their neoplastic nature and integral association with vHL disease 6–8. However, VHL gene alterations may also be detected in sporadic cases 50. To date, the genetic alterations seen in pancreatic ductal adenocarcinoma (TP53, KRAS, SMAD4, and p16/CDKN2A), neuroendocrine tumors (MEN1, DAXX, and ATRX), and intraductal papillary mucinous neoplasms (GNAS, RNF43, PIK3CA, and STK11/LKB1) have not been reported in serous neoplasms 41, 50.
Serous cystic neoplasms are very slow-growing tumors 9, 51, with an estimated doubling time of 12 years 52. Therefore, if a definitive diagnosis can be accomplished, watchful waiting is a distinct option for smaller tumors 9, 11, 14, 25, 44, 52–54. For symptomatic cases and/or larger ones, surgical removal is still the treatment of choice 44, 55. More recently, radiofrequency ablation, ethanol, and chemotherapeutic agent injection have been proposed as options in the non-surgical management of patients with limited disease 56, 57.
The 2010 WHO classification requires the presence of distant metastasis as the criterion for “malignancy” in serous cystic neoplasm. Thus, many of the cases that had been reported as “malignant” based on “vascular invasion” would no longer qualify by the WHO classification 58. Similarly, larger serous neoplasms (>11.0 cm) with inflammation and hemorrhage may show localized adhesion and/or penetration of neighboring organs, including lymph nodes, spleen, stomach, and colon, which does not seem to be an indicator of malignant behavior. Even for serous neoplasms occurring in the liver, the possibility of synchronous independent tumors may have to be considered before concluding metastasis, especially considering there was no fatality related to this and no reported metastases to other remote sites 1, 9. Of note, there are isolated cases reported in which a cytologically obvious carcinoma arose within a microcystic serous cystadenoma (“carcinoma ex microcystic adenoma”) 59. The biological behavior of these rare cases is yet to be defined.
Solid Serous Adenoma
Solid serous tumors with uniform, small, evenly shaped and sized nests or tubules with minimal or no lumen formation has also been described. These typically lack central fibrosis and often misinterpreted radiologically as neuroendocrine tumors. Tumor cells reveal typical glycogen-laden clear cytoplasm and bland, round to oval hyperchromatic nuclei 60–64. Distinguishing solid serous adenomas from other solid neoplasms, such as neuroendocrine tumors, or other clear-cell neoplasms, such as metastatic renal cell carcinoma, may be difficult; even more so in the setting of vHL, where both lesions may be concurrently present. Special studies and immunohistochemical analysis are helpful 65.
Serous Cystic Neoplasm Key Features Box.
Clinical
Present with nonspecific symptoms or detected incidentally
Have well-established association with von Hippel-Lindau syndrome
Molecular assays containing a 5-gene panel (KRAS, GNAS, RNF43, CTNNB1, and VHL genes) may be useful in the pretreatment diagnosis
Macroscopic
Medium size lesion (mean size = 4 cm)
Well-demarcated and sponge-like appearance with innumerable small cysts, each measuring a few millimeters is diagnostic
Central stellate scar common in microcytic variant
Microscopic
Conglomerate of cysts lined by simple cuboidal epithelium
Clear or eosinophilic cells with distinct cytoplasmic borders
Small, round nuclei with dense, homogenous chromatin
Similar to vHL syndrome-associated other clear cell tumors, a prominent epithelium-associated microvascular meshwork is present
INFLAMMATORY MYOFIBROBLASTIC TUMOR
Inflammatory myofibroblastic tumor is a rare, especially in the pancreas, and distinctive entity 66–69. The pancreatic head is affected most frequently by an ill defined and firm mass causing obstructive jaundice. Therefore, patients are often suspected to suffer from pancreatic ductal adenocarcinoma 70, 71.
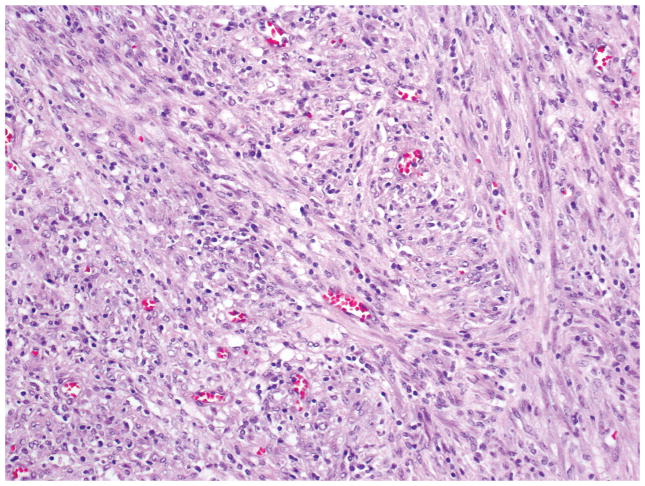
At low-power-magnification, inflammatory myofibroblastic tumor has a relatively pushing border (Figure 7). Fibroblasts and myofibroblasts, usually arranged in a storiform pattern, with moderate to marked inflammation composed of lymphocytes and plasma cells are characteristic (Figure 8). Cytologic atypia and mitotic figures are rarely observed.
Figure 7.
Inflammatory myofibroblastic tumor with well-defined margins.
Figure 8.
Proliferation of generally bland spindle mesenchymal cells arranged in irregular fascicles into a stroma with lymphocytes, plasma cells, and histiocytes is characteristic in inflammatory myofibroblastic tumor.
In approximately 50% of inflammatory myofibroblastic tumors, various gene aberrations including the anaplastic lymphoma kinase (ALK) gene at chromosome 2p23, leading to aberrant ALK expression in the myofibroblasts, have been identified 72–75. These observations have led to the development of the concept of inflammatory myofibroblastic tumor as a clonal neoplastic lesion rather than a reactive process 69. Of note, ALK gene abnormality is more often seen in children or young adults than in elderly people 67.
Inflammatory myofibroblastic tumors share, at least focally, the morphologic appearance of the IgG4-related sclerosing disease (discussed separately in this issue, see autoimmune pancreatitis section), particularly at areas with prominent fibroblastic/myofibroblastic proliferation mingled with lymphocytes and plasma cells 69, 76. However, the majority of inflammatory myofibroblastic tumors are different from IgG4-related lesions in terms of the ALK expression, low-level of IgG4+ cell infiltration and lack of obstructive phlebitis. Thus, inflammatory myofibroblastic tumor should be recognized to be a distinctive neoplastic entity 69.
Inflammatory Myofibroblastic Tumor Key Features Box.
Composed of fibroblasts and myofibroblasts, usually arranged in a storiform pattern, with moderate to marked inflammation
In half of inflammatory myofibroblastic tumors, various ALK gene abnormalities, leading to aberrant ALK expression in the myofibroblasts, have been identified
ALK expression and low-level of IgG4+ cell infiltration help distinguishing inflammatory myofibroblastic tumors from IgG4-related sclerosing disease
LYMPHOEPITHELIAL CYST
Lymphoepithelial cyst is usually asymptomatic, with the lesion found incidentally on imaging studies performed for unrelated reasons or at autopsy 77, 78. In contrast with its salivary gland analogues, no autoimmune disorder is identified and there is no syndrome association. Association with HIV also appears to be coincidental and exceedingly uncommon 79.
It typically present as a unilocular or multilocular cyst within, or protruding from, the pancreas. Imaging studies cannot consistently separate lymphoepithelial cyst from neoplastic mucinous cysts, such as intraductal pancreatic mucinous neoplasm or mucinous cystic neoplasm 80. Fine needle aspiration can support the diagnosis of a lymphoepithelial cyst when squamous cells, amorphous keratinaceous debris, cholesterol clefts and/or lymphocytes are present 81. However, fine needle aspiration may be inconclusive. Using cyst fluid CEA as a discriminating test has its limitations as several case reports have noted elevated levels of CA19-9 and/or CEA in lymphoepithelial cysts 82–84.
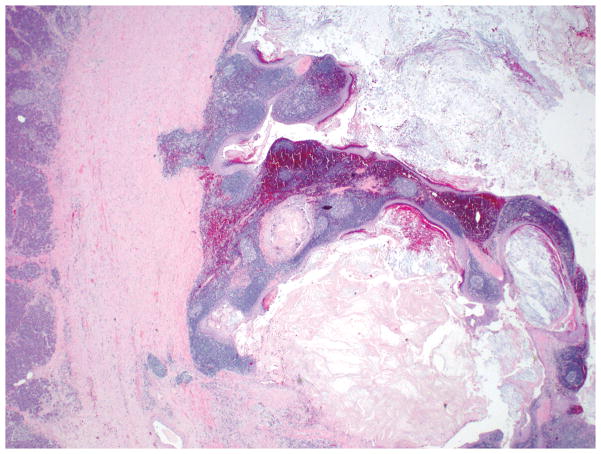
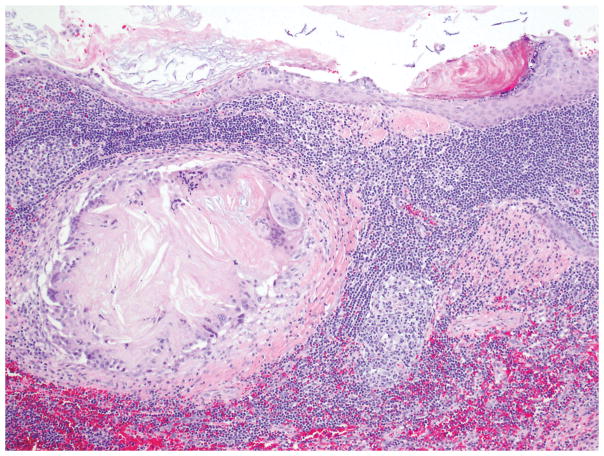
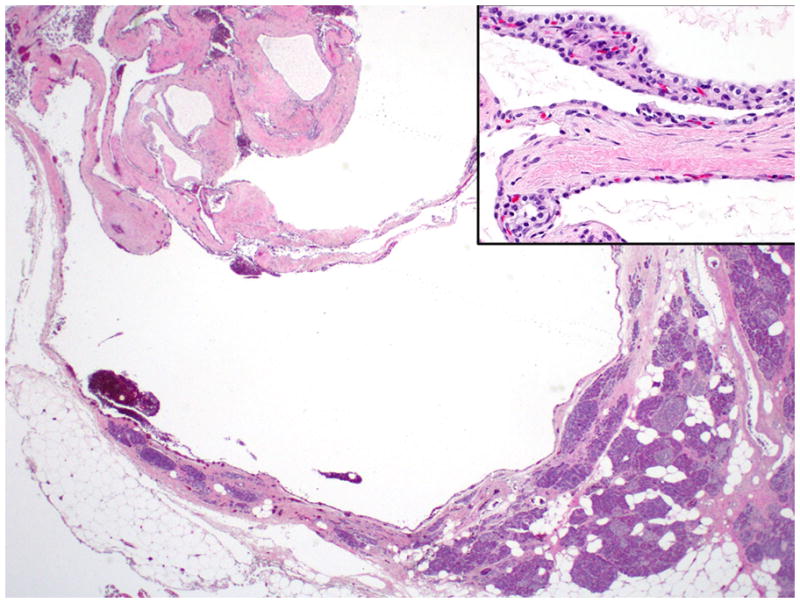
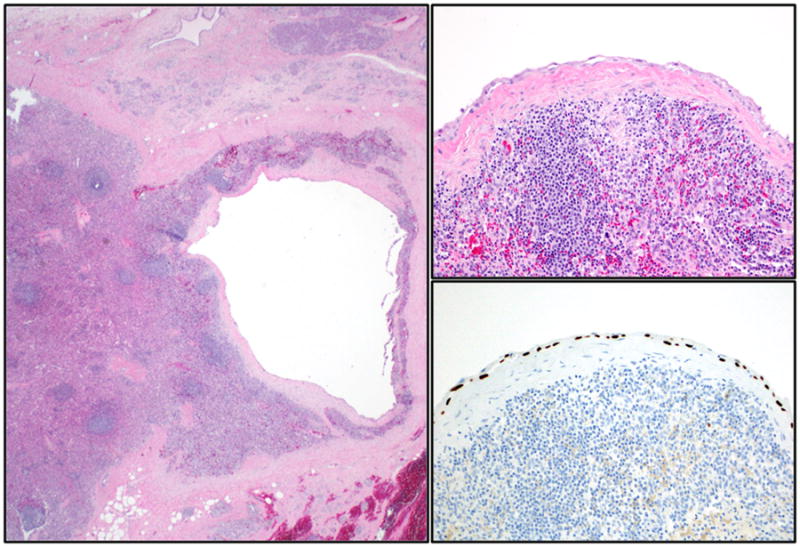
Gross examination shows a medium size (mean size = 5 cm), encapsulated cystic lesion. Depending on the degree of keratin formation, cyst content may vary from serous to cheesy/casseous-appearing. Microscopically, there is a dense band of mature lymphoid tissue with prominent, well-formed germinal centers subtending a cyst lining of mature stratified squamous epithelium occasionally containing keratinaceous debris (Figure 9). The adjacent pancreas may have granulomas, collections of foamy histiocytes and fat necrosis 77, 78 (Figure 10).
Figure 9.
A lymphoepithelial cyst lined by mature, stratified squamous epithelium with keratinization. Note the lymphoid tissue with germinal center formation immediately beneath the epithelium.
Figure 10.
Epithelioid granulomas and cholesterol clefts may also be present beneath the lining epithelium of lymphoepithelial cysts.
Although lymphoepithelial cysts might contain sebaceous glands, they are distinct from dermoid cysts (cystic monodermal teratomas) or teratomas because of the large amount of organized lymphoid tissue present and the lack of hair, cartilage, and occasionally neural tissue 77, 85.
Pancreatic lymphoepithelial cyst can be cured by conservative resection but if it is asymptomatic and diagnosed before surgery, no treatment is necessary. Neither malignant transformation nor recurrence after resection has been reported 86.
Lymphoepithelial Cyst Key Features Box.
Clinical
Mostly in male patients
Medium size, peripancreatic cyst
Microscopic
Has variable lining ranging from attenuated to transitional to stratified squamous epithelium with keratinization
Goblet cells or scanty sebaceous elements may be seen
Distinct band of lymphoid tissue, sometimes with prominent, well-formed germinal centers, composed of mature T lymphocytes surrounds the epithelium
EPIDERMOID CYST IN INTRAPANCREATIC ACCESSORY SPLEEN
These are very rare lesions seen in younger adults (2nd to 3rd decades). They occur almost exclusively in the tail of the pancreas where accessory spleens are not uncommon. Of note, accessory spleens are most frequently found at the perihilar region of the spleen (80% of cases) followed by the pancreatic tail 87.
Similar with lymphoepithelial cyst, epidermoid cyst in intrapancreatic accessory spleen can also be misdiagnosed preoperatively as a cystic pancreatic neoplasm, such as intraductal papillary mucinous neoplasm or mucinous cystic neoplasm, especially in the setting of elevated serum CA19-9 levels 88, 89.
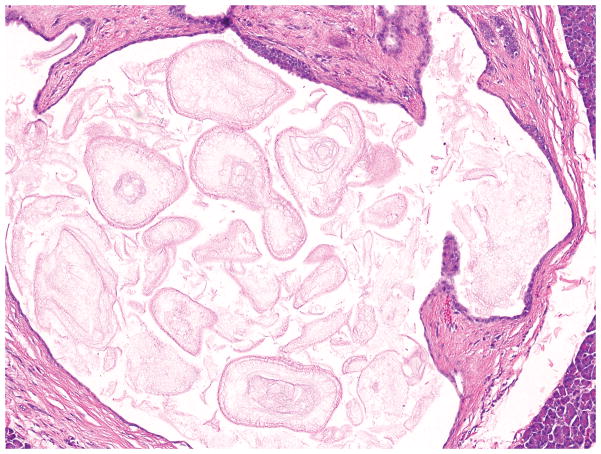
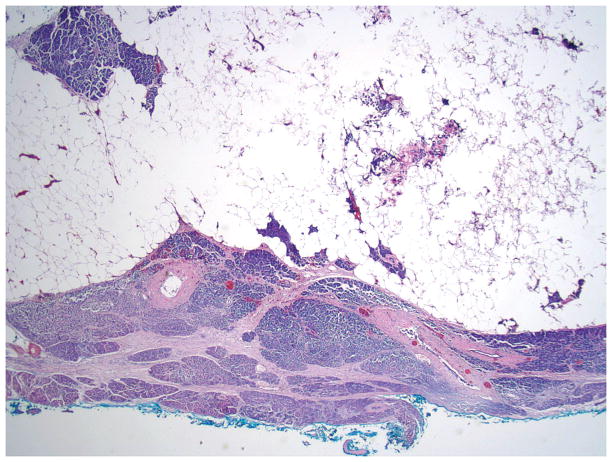
Grossly, a well-circumscribed dark red mass with a unilocular or multilocular cyst in the pancreatic tail is characteristic 88. The cyst contains serous fluid or keratinaceous debris and is lined by benign multilayered epithelium, which is reminiscent of squamous epithelium or urothelium, surrounded by unremarkable splenic tissue 78, 88162. The lining epithelium shows stratified cuboidal or columnar cell morphology in some areas, whereas it is flattened and attenuated in other areas (Figure 11) 159.
Figure 11.
Epidermoid cyst in intrapancreatic heterotopic spleen has a thin lining surrounded by splenic red and white pulp (left). Recognition of the splenic elements allows a correct diagnosis even if the squamous nature of the attenuated lining epithelium (top right) may not be appreciated without immunohistochemical staining (bottom right, p63 immunohistochemical stain).
SQUAMOID CYST OF PANCREATIC DUCTS
Squamoid cysts of pancreatic ducts are relatively small cysts, with a median size of 1.5 cm, and the vast majority of the cases are detected during work-up for other conditions 90, 91. These cysts typically result from unilocular cystic dilatation of the ducts and have variable lining ranging from attenuated, flat, non-stratified squamous, to transitional, to mucosal-type stratified squamous epithelium (Figure 12) as well as mucoproteinaceous acidophilic secretions within the lumen. The wall of the cyst is composed of a thin band of fibrous tissue, focally showing tributary ducts. Neither acute nor chronic inflammation is a feature of this lesion.
Figure 12.
Squamoid cyst of pancreatic duct showing unilocular and well-circumscribed cyst filled with dense mucoproteinaceous material, characteristic of enzymatic concretions.
Immunohistochemically, nuclear p63 expression is present in all cases, a finding that is not seen in any normal component of pancreas or in non-squamous cystic lesions of this organ 92.
It is important to distinguish squamoid cysts of pancreatic ducts from other cystic lesions, in particular, from mucinous tumors, since the latter often have malignant potential, whereas squamoid cysts of pancreatic ducts are innocuous lesions 93. Preoperative differential diagnosis of these may be difficult, especially in the setting of elevated fluid CEA levels and acellular cytology 94, 95. However, their distinction at the microscopic level is fairly straight forward 90.
Squamoid Cyst of Pancreatic Ducts Key Features Box.
Clinical
Usually small, unilocular cyst
Some might undergo resection with the clinical impression of being an intraductal papillary mucinous neoplasm
Microscopic
Has variable lining ranging from attenuated to transitional to stratified squamous epithelium
Contains distinctive mucoproteinaceous acidophilic secretions
The wall is composed of thin fibrous tissue devoid of any lymphoid tissue
Nuclear p63 expression confirms the nature of the lining
HETEROTOPIC PANCREAS
Heterotopic (ectopic) pancreatic tissue, independent from the vascular supply or anatomic connection to the pancreas, may occur from displacement of small amounts of pancreas during embryologic development 96–98. It is located most frequently in the stomach and proximal small intestine (Figure 13), but can be identified in other organs such as esophagus, gallbladder, hepatic or common bile ducts, spleen as well as in Meckel diverticulum 99–101.
Figure 13.
Heterotopic pancreas, containing all of the normal pancreatic elements, in the duodenal submucosa.
Depending on the size, location and the pathological changes similar to those observed in case of the normal pancreas, patients may present with symptoms such as epigastric pain, nausea, vomiting, ulcer, obstruction, and weight loss. However, it is often an incidental finding. Radiographically or endoscopically, it may be a challenge to differentiate it from a neoplasm 102, 103.
If it is large enough to be seen on gross inspection, it appears as a firm, pale, nodular mass. Microscopically, it has been classified into three types according to the histologic components (Heinrich classification). Type I is composed of complete structures consisting of ducts, acini, and islets of Langerhans cells (Figure 13). Type II is composed of ducts and acini. Type III is composed of ducts alone 104. Histologic type is not related to the clinical symptoms 105. Of note, small collections of acinar cells in other organs such as gastroesophageal region are regarded as metaplasia rather than heterotopia 106.
In symptomatic cases, surgical excision relieves symptoms. However, rarely, a more extensive treatment may be necessary due to secondary pancreatic neoplasms including adenocarcinomas arising within ectopic pancreatic tissue 101, 107–112.
Heterotopic Pancreas Key Features Box.
Clinical
Occurs in a variety of organs; most common in gastrointestinal tract
Usually an incidental finding
May rarely cause symptoms due to local complications or secondary pancreatic pathology
Microscopic
Components of the pancreas (acini, ducts or islets) in different combinations
PANCREATIC HAMARTOMA
The term hamartoma refers to a focal overgrowth of cells and tissues native to the organ in which it occurs. Thus, hamartoma is regarded as a malformation rather than a true neoplasm 96, 113.
Pancreatic hamartoma is rare, accounting for less than 1% of occurrences of tumor-like lesions 114. Usually presents as a well-demarcated, solid or solid and cystic mass. It is often located in the head of the pancreas 115–119. Cases with multiple lesions have been reported 115, 116.
Microscopically, it is characterized by small to medium-sized ductal structures, lined by columnar epithelium without atypia, surrounded by disarranged acini and reveals various amounts of fibrous stroma (Figure 14). Well-formed islets of Langerhans are not common. In fact, Pauser et al. 118, 120 and Yamaguchi et al. 121 defined the criteria for the diagnosis of pancreatic hamartoma as: (1) forming a well-demarcated mass, (2) being comprised of mature acini and ducts with distorted architecture, and (3) lacking discrete islets of Langerhans. Adjacent pancreatic parenchyma is usually unremarkable.
Figure 14.
Pancreatic hamartoma with cystic ductal structures and disorganized acini embedded in a fibroblastic stroma. Note that islets are not present.
Immunohistochemically, both acinar cells and ductal cells are positive for epithelial markers (AE1/AE3, CAM5.2, and EMA), and the acinar cells are positive for exocrine markers (trypsin, chymotrypsin, etc), similar to what is observed in a normal pancreas. The stromal spindle cells reportedly express CD34 and CD117 but are usually negative for S100, SMA, desmin, and bcl-2 116–121.
Although pancreatic hamartoma is usually not aggressive, it typically requires surgical resection because of the difficulty in prospective clinical imaging diagnosis 122–124.
NESIDIOBLASTOSIS
Nesidioblastosis is a descriptor of the morphologic changes seen in functional disorders of the endocrine pancreas, characterized by persistent hyperinsulinemic hypoglycemia, due to defective nonneoplastic β-cells 2. It is usually seen in newborns 130, 131 (a.k.a congenital hyperinsulinism 125, 126); however, rare cases of adult nesidioblastosis do occur 96, 127–131.
Nesidioblastosis can be diagnosed biochemically, usually within the first few weeks of life, through a series of highly specialized tests of glucose, insulin, C-peptide levels, ketones, and glucagon response coupled with arterial calcium stimulation or percutaneous transhepatic pancreatic venous sampling 132. However, heterogeneous clinical manifestation causes risk of late diagnosis or even misdiagnosis, which can lead to serious and permanent damage to the central nervous system and eventually, mental retardation 133.
Currently, there are three histological forms of nesidioblastosis: Focal, diffuse and atypical. Focal nesidioblastosis occurs when the abnormal islets are localized to a specific location in the pancreas. Diffuse form occurs when all the islets in the pancreas are abnormal. If a case is difficult to histologically categorize, it is regarded as atypical form 126, 134.
Alterations vary from patient to patient and may include
relatively large collections of islet cells displacing acinar tissue,
neoproliferation of islet cells from ducts (ductuloinsular complexes),
islet cell “dysplasia” or nesidiodysplasia (loss of the usual centrilobular concentration of larger islets, increased numbers of small aggregates of islet cells distributed irregularly in the lobules, irregularity of the contour of the islets) or
scattered islet cells (mostly β-cells cells) with enlarged and hypertrophic nuclei 130, 135–138.
However, none of these changes are specific for nesidioblastosis and would be confirmatory only in the right clinical context. Also, it should be kept in mind that in adults, hyperinsulinemic hypoglycemia is usually the result of a neuroendocrine neoplasm releasing insulin. Therefore, before nesidioblastosis diagnosis can be rendered, a diligent search for a neuroendocrine tumor is essential. In fact, the patient ought to be considered to have a neuroendocrine tumor unless definitively proven otherwise by systemic examination of the resected pancreas.
At a molecular level, genetic abnormalities in nine different genes [ATP binding cassette subfamily C member 8 (ABCC8), potassium channel, inwardly rectifying subfamily J, member 11 (KCNJ11), glutamate dehydrogenase 1 (GLUD1), glucokinase (GCK), hepatocyte nuclear factor 4 homeobox A (HNF4A), HNF1A, SLC16A1 (also known as a monocarboxylate transporter, MCT1), uncoupling protein 2 (UCP2) and hydroxyacyl-CoA dehydrogenase (HADH)] have been identified in approximately half of the cases, indicating that there are as yet unidentified mechanisms involved in the regulation of insulin secretion 133.
Genetic abnormalities are classified into two categories namely: ‘channelopathies’ and ‘metabolopathies’ 139. The former is attributed to the ATP-sensitive potassium channels (KATP) channel genes (ABCC8 and KCNJ11) and latter to genes regulating different metabolic pathways (GLUD1, GCK, HNF4A, HNF1A, SLC16A1, UCP2 and HADH). The most common disorders are those affecting the KATP channel genes and these are predominantly autosomal recessive. The other seven are less common but are autosomal dominant 133, 140, 141. In most cases, diffuse form is inherited in an autosomal recessive manner, whereas focal form is sporadic 142, 143. Patients with a homozygous recessive or a compound heterozygote mutation in their ABCC8 or KCNJ11 genes are usually medically unresponsive 140.
Partial to near-total pancreatectomy is the treatment of choice in cases refractory to aggressive medical management, although enucleation may be of value in controlling the symptoms in cases with focal nesidioblastosis. Diabetes mellitus and pancreatic insufficiency (malabsorption) may develop after pancreatectomy 127, 144–146.
Nesidioblastosis Key Features Box.
Clinical
Functional disorder of the β-cells
Associated with persistent hyperinsulinemic hypoglycemia
Microscopic
Can be focal or diffuse
Pathologic findings vary greatly patient to patient
Enlarged islets, abnormally shaped islets, ductuloinsular complexes and enlarged (a three-fold increase relative to the nuclei of adjacent islet cells) and hyperchromatic β-cell nuclei are common
However, none of these changes are specific and would be confirmatory only in the right clinical context
An insulinoma must be excluded by pathological examination before nesidioblastosis diagnosis can be rendered
Molecular
Genetic abnormalities in nine different genes (ABCC8, KCNJ11, GLUD1, GCK, HNF4A, HNF1A, SLC16A1, UCP2 and HADH) have been identified in approximately half of the cases
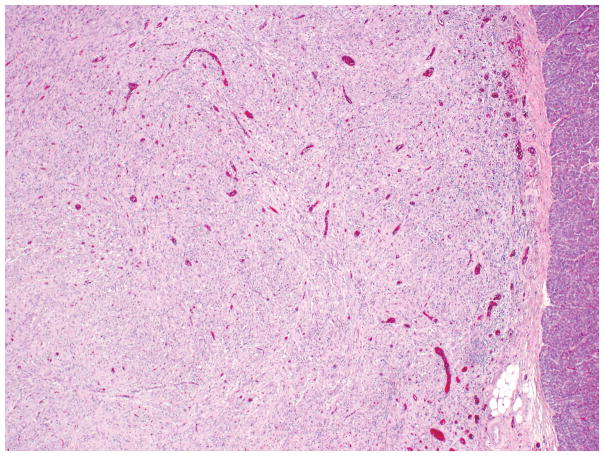
LIPOMATOUS PSEUDOHYPERTROPHY
Focal replacement of the exocrine pancreas with mature adipose tissue is common in the pancreas and usually correlates directly with body mass index 147–149. In contrast, lipomatous pseudohypertrophy is a distinct entity characterized by pseudotumor formation by adipose tissue, replacing almost an entire segment of exocrine parenchyma 150.
Most common symptom at presentation is abdominal pain 150. In some cases, lipomatous pseudohypertrophy seems to be associated with specific syndromes such as Shwachman-Diamond or Johanson-Blizzard 151, 152 syndromes. Because it forms a mass, it is often mistaken for a malignancy 153. On the other hand, if proper radiological evaluation is performed, it can be recognized that the lesion is actually composed of fat. Fine needle aspiration biopsy showing mature fat cells, without any atypia, might be helpful in such cases 154.
Approximately in 70% of the cases, there is a diffuse involvement of the pancreas; in 30%, the tumor is located in the head, body or tail only 150. Macroscopically, the appearance and consistency are those of adipose tissue. Histological examination shows massive replacement of pancreatic parenchyma by mature adipose tissue. Although it does not appear to have a well-formed capsule, it has well-defined borders (Figure 15). The islets of Langerhans are relatively preserved, and typically there are scattered, small but well-preserved acinar elements. There is no significant inflammation. Adjacent pancreatic or soft tissue may show signs of compression.
Figure 15.
Well-circumscribed lipomatous pseudohypertrophy, composed of mature adipose tissue, pushing into the adjacent pancreas is illustrated. Scattered acini within the adipose tissue are also visible.
At the microscopic level, the main differential diagnosis is with a well-differentiated liposarcoma, which is reported in the literature as individual case reports 66, 155. The findings that speak against this possibility are the perfect maturation of lipocytes, sharp demarcation of the lesion, lack of lipoblasts, and admixture of normal pancreatic parenchyma within the lesion 150.
Lipomatous Pseudohypertrophy Key Features Box.
Clinical
May be mistaken for a malignancy
Microscopic
Can be focal or diffuse
Characterized by mature adipose tissue replacing the pancreatic parenchyma, leaving only scattered clusters of pancreatic elements
Lipocytes are entirely normal, no lipoblast present
PARADUODENAL (GROOVE) PANCREATITIS
Paraduodenal pancreatitis (a.k.a. groove pancreatitis or cystic dystrophy of heterotopic pancreas) is a distinctive form of pancreatitis that occurs in the tissue between the duodenal wall and the pancreatic head. It often surrounds the minor ampulla and accessory duct 156–158.
The vast majority of patients are young males with a history of alcohol abuse. The most common symptom is severe waxing and waning upper abdominal pain. Frequent clinical findings include stenosis of duodenum, disordered gastric emptying, and postparandial vomiting. Weight loss is also a common finding and can be severe in some cases, complicating the differential diagnosis with pancreas cancer 159–161. ocal thickening and abnormal enhancement of the second portion of the duodenum and “tubulocystic” change in the vicinity of the accessory duct are specific features of this entity 162–164. Those that have predominantly solid lesions, often related to the sclerotic changes in the periampullary region, are commonly diagnosed as “pancreas cancer” or “ampullary/periampullary neoplasm” 158, 162.
The reasons for this process to develop are not known. However, the macroscopic and microscopic findings are quite distinctive: The process leads to narrowing of the duodenal lumen and the duodenal mucosa often acquires a nodular or cobblestone appearance 165. Upon sectioning, the duodenal wall shows a trabeculated appearance, often accompanied by cystic change, especially in the vicinity of the minor ampulla. In some cases, cyst formation may be prominent, measuring up to several centimeters in size, mimicking intestinal duplication.
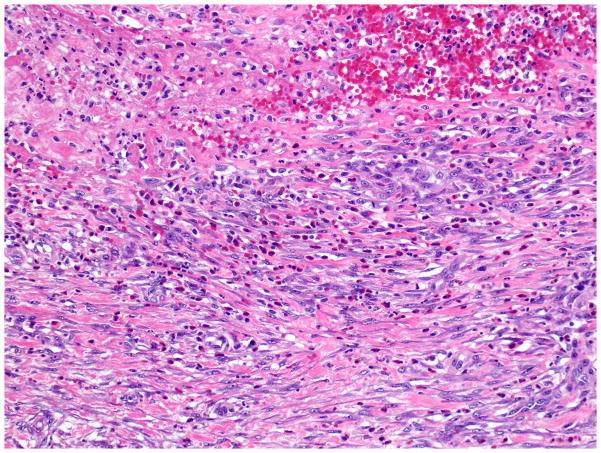
Microscopically, the duodenal mucosa often reveals Brunner’s gland hyperplasia and there is an exuberant myofibroblastic proliferation, often arranged in fascicles (Figure 16) accompanied by small, well-circumscribed lobules of pancreatic tissue (“myoadenomatosis” pattern) or variably sized ducts (“cystic dystrophy of heterotopic pancreas”). These ducts may contain inspissated acinar enzymes. The cyst contents may extravasate and lead to the development of a foreign-body giant cell reaction and stromal eosinophilia. Some cysts are devoid of epithelium (Figure 17). Instead, they are lined by more cellular fibroblastic tissue 158, 164. Prominence of nerve bundles mimicking traumatic neuroma is also common.
Figure 16.
In paraduodenal (groove) pancreatitis, there is a striking reactive spindle cell proliferation, including, smooth muscle cells and myofibroblasts, associated with inflammatory cells.
Figure 17.
Characteristically, the cysts of paraduodenal (groove) pancreatitis contain eosinophilic, amorphous inspissated enzymatic secretions and are surrounded by markedly inflamed fibrous tissue. Note the abundant Brunner’s glands on the right.
It should be kept in mind that, as in any case of pancreatic pathology, before the diagnosis of paraduodenal pancreatitis is rendered, the possibility of adenocarcinoma ought to be carefully excluded, since it can mimic any form of pancreatitis, and be associated with any of the changes characteristic of this lesion.
Paraduodenal Pancreatitis Key Features Box.
Clinical
Predominantly in 40 to 50 years old males
History of alcohol abuse is common
Patients present with waxing and waning severe upper abdominal pain and postparandial vomiting
Predominantly solid ones radiographically mimic pancreatic or ampullary/periampullary tumors
Macroscopic
Often centered in the region of minor papilla
Trabeculation of duodenal musculature with occasional cysts is common
Paraduodenal wall cyst (measuring up to 10 cm) mimicking intestinal duplication may occur
Microscopic
Dense myoid stromal proliferation with intervening rounded lobules of pancreatic tissue (“myoadenomatosis’)
Brunner’s gland hyperplasia
Extravasated (stromal) mucoprotein plugs surrounded by eosinophiles or multinucleated giant cells
CONCLUSIONS
Benign neoplasms and tumor-like (pseudotumoral) lesions of the pancreas can be challenging, mostly due to lack of familiarity because of the lower number of cases, compared to malignant neoplasms, pathologists encounter on a daily basis.
Well-demarcated and sponge-like appearance with innumerable small cysts of microcystic serous cystadenomas is so characteristic and usually does not create any diagnostic problems. In contrasts, macrocytic serous cystadenomas can be difficult to diagnose as the lining epithelium might be extremely attenuated. Inflammatory myofibroblastic tumors may have overlapping morphologic features with IgG4-related autoimmune pancreatitis (discussed separately in this issue). However, it is different from autoimmune pancreatitis in terms of the ALK expression, low-level of IgG4+ cell infiltration and lack of obstructive phlebitis.
A variety of non-neoplastic conditions may also form a cystic or solid mass mimicking a malignant neoplasm in the pancreas. Up to 5% of pancreatectomies performed with the preoperative clinical diagnosis of malignant neoplasm will prove to be non-neoplastic by pathologic examination, although this figure is decreasing with improved diagnostic modalities. Lymphoepithelial cyst, epidermoid cyst in intrapancreatic accessory spleen and squamoid cyst of pancreatic ducts are all cystic lesions lined by usually squamous epithelium and recognition of the accompanying elements (lymphoid tissue with or without germinal centers, splenic tissue, etc) is necessary for a correct diagnosis. Heterotopic pancreas may form a well-defined nodule within the duodenum and is typically mistaken for neuroendocrine neoplasm. Hamartomas are very rare if the entity is defined strictly. They are characterized by irregularly arranged mature pancreatic elements admixed with stromal tissue. None of the pathologic findings of nesidioblastosis such as enlarged islets, abnormally shaped islets, ductuloinsular complexes and enlarged/hyperchromatic β-cell nuclei are specific and would be confirmatory only in the right clinical context. Lipomatous hypertrophy is the replacement of pancreatic tissue with mature adipose tissue that occasionally leads to moderate to marked enlargement of the pancreas. Chronic inflammatory lesions are the leading cause of “pseudotumoral pancreatitis”, and among these, autoimmune and paraduodenal pancreatitides are most important.
In conclusion, it is important to recognize all these benign neoplasms and types of conditions that form pseudotumors in the pancreas so that they can be distinguished from malignant neoplasms, especially ductal adenocarcinomas.
Footnotes
Disclosure
The authors have no conflict of interest to declare.
References
- 1.Reid MD, Choi HJ, Memis B, et al. Serous Neoplasms of the Pancreas: A Clinicopathologic Analysis of 193 Cases and Literature Review With New Insights on Macrocystic and Solid Variants and Critical Reappraisal of So-called “Serous Cystadenocarcinoma”. Am J Surg Pathol. 2015;39:1597–610. doi: 10.1097/PAS.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 2.Hruban R, Pitman MB, Klimstra DS. AFIP Atlas of Tumor Pathology. Washington, DC: American Registry of Pathology; 2007. Tumors of the Pancreas. [Google Scholar]
- 3.Kobayashi N, Sato T, Kato S, et al. Imaging findings of pancreatic cystic lesions in von Hippel-Lindau disease. Intern Med. 2012;51:1301–7. doi: 10.2169/internalmedicine.51.7194. [DOI] [PubMed] [Google Scholar]
- 4.Kanno A, Satoh K, Hamada S, et al. Serous cystic neoplasms of the whole pancreas in a patient with von Hippel-Lindau disease. Intern Med. 2011;50:1293–8. doi: 10.2169/internalmedicine.50.4946. [DOI] [PubMed] [Google Scholar]
- 5.Matsubayashi H, Uesaka K, Kanemoto H, et al. Multiple endocrine neoplasms and serous cysts of the pancreas in a patient with von Hippel-Lindau disease. J Gastrointest Cancer. 2010;41:197–202. doi: 10.1007/s12029-010-9134-3. [DOI] [PubMed] [Google Scholar]
- 6.Moore PS, Zamboni G, Brighenti A, et al. Molecular characterization of pancreatic serous microcystic adenomas: evidence for a tumor suppressor gene on chromosome 10q. Am J Pathol. 2001;158:317–321. doi: 10.1016/S0002-9440(10)63971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohr VH, Vortmeyer AO, Zhuang Z, et al. Histopathology and molecular genetics of multiple cysts and microcystic (serous) adenomas of the pancreas in von Hippel-Lindau patients. Am J Pathol. 2000;157:1615–1621. doi: 10.1016/S0002-9440(10)64799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vortmeyer AO, Lubensky IA, Fogt F, et al. Allelic deletion and mutation of the von Hippel-Lindau (VHL) tumor suppressor gene in pancreatic microcystic adenomas. Am J Pathol. 1997;151:951–956. [PMC free article] [PubMed] [Google Scholar]
- 9.Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas) Gut. 2016;65:305–12. doi: 10.1136/gutjnl-2015-309638. [DOI] [PubMed] [Google Scholar]
- 10.Reid MD, Choi H, Balci S, et al. Serous cystic neoplasms of the pancreas: clinicopathologic and molecular characteristics. Semin Diagn Pathol. 2014;31:475–83. doi: 10.1053/j.semdp.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Fukasawa M, Maguchi H, Takahashi K, et al. Clinical features and natural history of serous cystic neoplasm of the pancreas. Pancreatology. 2010;10:695–701. doi: 10.1159/000320694. [DOI] [PubMed] [Google Scholar]
- 12.Colonna J, Plaza JA, Frankel WL, et al. Serous cystadenoma of the pancreas: clinical and pathological features in 33 patients. Pancreatology. 2008;8:135–41. doi: 10.1159/000123606. [DOI] [PubMed] [Google Scholar]
- 13.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Salvia R, Molinari E, et al. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg. 2003;27:319–323. doi: 10.1007/s00268-002-6570-7. [DOI] [PubMed] [Google Scholar]
- 15.Yasuhara Y, Sakaida N, Uemura Y, et al. Serous microcystic adenoma (glycogen-rich cystadenoma) of the pancreas: Study of 11 cases showing clinicopathological and immunohistochemical correlations. Pathol Int. 2002;52:307–312. doi: 10.1046/j.1440-1827.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- 16.Procacci C, Carbognin G, Biasiutti C, et al. Serous cystadenoma of the pancreas: imaging findings. Radiol Med. 2001;102:23–31. [PubMed] [Google Scholar]
- 17.Capella C, Solcia E, Kloppel G, et al. Serous cystic neoplasms of the pancreas. Tumors of the Exocrine Pancreas. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System. IARCPress; Lyon: 2000. pp. 231–233. [Google Scholar]
- 18.Compton CC. Serous cystic tumors of the pancreas. Semin Diagn Pathol. 2000;17:43–55. [PubMed] [Google Scholar]
- 19.Sperti C, Pasquali C, Perasole A, et al. Macrocystic serous cystadenoma of the pancreas: clinicopathologic features in seven cases. Int J Pancreatol. 2000;28:1–7. doi: 10.1385/IJGC:28:1:01. [DOI] [PubMed] [Google Scholar]
- 20.Alpert LC, Truong LD, Bossart MI, et al. Microcystic adenoma (serous cystadenoma) of the pancreas. A study of 14 cases with immunohistochemical and electron-microscopic correlation. Am J Surg Pathol. 1988;12:251–263. doi: 10.1097/00000478-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69:289–298. doi: 10.1093/ajcp/69.1.289. [DOI] [PubMed] [Google Scholar]
- 22.Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas. 2012;41:380–7. doi: 10.1097/MPA.0b013e31822a27db. [DOI] [PubMed] [Google Scholar]
- 23.Galanis C, Zamani A, Cameron JL, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–6. doi: 10.1007/s11605-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 24.Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg. 2012;16:1422–8. doi: 10.1007/s11605-012-1847-0. [DOI] [PubMed] [Google Scholar]
- 25.Khashab MA, Shin EJ, Amateau S, et al. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am J Gastroenterol. 2011;106:1521–6. doi: 10.1038/ajg.2011.117. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay B, Sahdev A, Monson JP, et al. Pancreatic lesions in von Hippel-Lindau disease. Clin Endocrinol (Oxf) 2002;57:603–8. doi: 10.1046/j.1365-2265.2002.01637.x. [DOI] [PubMed] [Google Scholar]
- 27.Girelli R, Bassi C, Falconi M, et al. Pancreatic cystic manifestations in von Hippel-Lindau disease. Int J Pancreatol. 1997;22:101–9. doi: 10.1007/BF02787467. [DOI] [PubMed] [Google Scholar]
- 28.Berman L, Mitchell KA, Israel G, et al. Serous cystadenoma in communication with the pancreatic duct: an unusual radiologic and pathologic entity. J Clin Gastroenterol. 2010;44:e133–5. doi: 10.1097/MCG.0b013e3181d3458d. [DOI] [PubMed] [Google Scholar]
- 29.Garcea G, Ong SL, Rajesh A, et al. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology. 2008;8:236–51. doi: 10.1159/000134279. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Lee JM, Kim SH, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192–1198. doi: 10.2214/AJR.05.0337. [DOI] [PubMed] [Google Scholar]
- 31.Lal A, Bourtsos EP, DeFrias DV, et al. Microcystic adenoma of the pancreas: clinical, radiologic, and cytologic features. Cancer. 2004;102:288–294. doi: 10.1002/cncr.20487. [DOI] [PubMed] [Google Scholar]
- 32.Chatelain D, Hammel P, O’Toole D, et al. Macrocystic form of serous pancreatic cystadenoma. Am J Gastroenterol. 2002;97:2566–71. doi: 10.1111/j.1572-0241.2002.06024.x. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Shimura T, Araki K, et al. Macrocystic serous cystadenoma mimicking branch duct intraductal papillary mucinous neoplasm. Int Surg. 2009;94:176–81. [PubMed] [Google Scholar]
- 34.Lee SE, Kwon Y, Jang JY, et al. The morphological classification of a serous cystic tumor (SCT) of the pancreas and evaluation of the preoperative diagnostic accuracy of computed tomography. Ann Surg Oncol. 2008;15:2089–95. doi: 10.1245/s10434-008-9959-1. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi H, Ishigami K, Inoue T, et al. Three cases of serous oligocystic adenomas of the pancreas; evaluation of cyst wall thickness for preoperative differentiation from mucinous cystic neoplasms. J Gastrointest Cancer. 2007;38:52–8. doi: 10.1007/s12029-008-9017-z. [DOI] [PubMed] [Google Scholar]
- 36.Collins BT. Serous cystadenoma of the pancreas with endoscopic ultrasound fine needle aspiration biopsy and surgical correlation. Acta Cytol. 2013;57:241–51. doi: 10.1159/000346911. [DOI] [PubMed] [Google Scholar]
- 37.Salomao M, Remotti H, Allendorf JD, et al. Fine-needle aspirations of pancreatic serous cystadenomas: Improving diagnostic yield with cell blocks and alpha-inhibin immunohistochemistry. Cancer Cytopathol. 2013 doi: 10.1002/cncy.21347. [DOI] [PubMed] [Google Scholar]
- 38.Belsley NA, Pitman MB, Lauwers GY, et al. Serous cystadenoma of the pancreas: limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2008;114:102–10. doi: 10.1002/cncr.23346. [DOI] [PubMed] [Google Scholar]
- 39.Huang P, Staerkel G, Sneige N, et al. Fine-needle aspiration of pancreatic serous cystadenoma: cytologic features and diagnostic pitfalls. Cancer. 2006;108:239–249. doi: 10.1002/cncr.21911. [DOI] [PubMed] [Google Scholar]
- 40.Lewandrowski K, Lee J, Southern J, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: a new approach to the preoperative assessment of pancreatic cystic lesions. AJR Am J Roentgenol. 1995;164:815–9. doi: 10.2214/ajr.164.4.7537015. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yip-Schneider MT, Wu H, Dumas RP, et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg. 2014;218:608–17. doi: 10.1016/j.jamcollsurg.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Panarelli NC, Park KJ, Hruban RH, et al. Microcystic serous cystadenoma of the pancreas with subtotal cystic degeneration: another neoplastic mimic of pancreatic pseudocyst. Am J Surg Pathol. 2012;36:726–31. doi: 10.1097/PAS.0b013e31824cf879. [DOI] [PubMed] [Google Scholar]
- 44.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–419. doi: 10.1097/01.sla.0000179651.21193.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thirabanjasak D, Basturk O, Altinel D, et al. Is serous cystadenoma of the pancreas a model of clear-cell-associated angiogenesis and tumorigenesis? Pancreatology. 2009;9:182–8. doi: 10.1159/000178890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki K, Eyden B. An immunohistochemical and ultrastructural study of pancreatic microcystic serous cyst adenoma with special reference to tumor-associated microvasculature and vascular endothelial growth factor in tumor cells. Ultrastruct Pathol. 2006;30:119–128. doi: 10.1080/01913120500407960. [DOI] [PubMed] [Google Scholar]
- 47.Basturk O, Singh R, Kaygusuz E, et al. GLUT-1 expression in pancreatic neoplasia: implications in pathogenesis, diagnosis, and prognosis. Pancreas. 2011;40:187–92. doi: 10.1097/MPA.0b013e318201c935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Y, Wang XN, Lou WH, et al. Serous cystic neoplasms of the pancreas: a clinicopathologic and immunohistochemical analysis. Chin J Dig Dis. 2006;7:39–44. doi: 10.1111/j.1443-9573.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 49.Kosmahl M, Wagner J, Peters K, et al. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004;28:339–346. doi: 10.1097/00000478-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–93. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malleo G, Bassi C, Rossini R, et al. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut. 2012;61:746–51. doi: 10.1136/gutjnl-2011-300297. [DOI] [PubMed] [Google Scholar]
- 52.El-Hayek KM, Brown N, O’Rourke C, et al. Rate of growth of pancreatic serous cystadenoma as an indication for resection. Surgery. 2013;154:794–800. doi: 10.1016/j.surg.2013.07.005. discussion 800–2. [DOI] [PubMed] [Google Scholar]
- 53.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanini N, Fantini L, Casadei R, et al. Serous cystic tumors of the pancreas: when to observe and when to operate: a single-center experience. Dig Surg. 2008;25:233–9. doi: 10.1159/000142947. discussion 240. [DOI] [PubMed] [Google Scholar]
- 55.Wargo JA, Fernandez-del-Castillo C, Warshaw AL. Management of pancreatic serous cystadenomas. Adv Surg. 2009;43:23–34. doi: 10.1016/j.yasu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Oh HC, Seo DW. Endoscopic ultrasonography-guided pancreatic cyst ablation (with video) J Hepatobiliary Pancreat Sci. 2015;22:16–9. doi: 10.1002/jhbp.179. [DOI] [PubMed] [Google Scholar]
- 57.DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664–8. doi: 10.1097/MPA.0b013e3182128d06. [DOI] [PubMed] [Google Scholar]
- 58.Terris B, Fukushima N, Hruban RH. Serous neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. IARC Press; Lyon, France: 2010. pp. 296–299. [Google Scholar]
- 59.Zhu H, Qin L, Zhong M, et al. Carcinoma ex microcystic adenoma of the pancreas: a report of a novel form of malignancy in serous neoplasms. Am J Surg Pathol. 2012;36:305–10. doi: 10.1097/PAS.0b013e3182393dc3. [DOI] [PubMed] [Google Scholar]
- 60.Lee SD, Han SS, Hong EK. Solid serous cystic neoplasm of the pancreas with invasive growth. J Hepatobiliary Pancreat Sci. 2013;20:454–6. doi: 10.1007/s00534-012-0575-x. [DOI] [PubMed] [Google Scholar]
- 61.Yasuda A, Sawai H, Ochi N, et al. Solid variant of serous cystadenoma of the pancreas. Arch Med Sci. 2011;7:353–5. doi: 10.5114/aoms.2011.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casadei R, D’Ambra M, Pezzilli R, et al. Solid serous microcystic tumor of the pancreas. JOP. 2008;9:538–40. [PubMed] [Google Scholar]
- 63.Stern JR, Frankel WL, Ellison EC, et al. Solid serous microcystic adenoma of the pancreas. World J Surg Oncol. 2007;5:26. doi: 10.1186/1477-7819-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reese SA, Traverso LW, Jacobs TW, et al. Solid serous adenoma of the pancreas: a rare variant within the family of pancreatic serous cystic neoplasms. Pancreas. 2006;33:96–9. doi: 10.1097/01.mpa.0000226890.63451.c4. [DOI] [PubMed] [Google Scholar]
- 65.Hoang MP, Hruban RH, Albores-Saavedra J. Clear cell endocrine pancreatic tumor mimicking renal cell carcinoma: a distinctive neoplasm of von Hippel-Lindau disease. Am J Surg Pathol. 2001;25:602–609. doi: 10.1097/00000478-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Kim JY, Song JS, Park H, et al. Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas. 2014;43:959–68. doi: 10.1097/MPA.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 67.Mizukami H, Yajima N, Wada R, et al. Pancreatic malignant fibrous histiocytoma, inflammatory myofibroblastic tumor, and inflammatory pseudotumor related to autoimmune pancreatitis: characterization and differential diagnosis. Virchows Arch. 2006;448:552–560. doi: 10.1007/s00428-006-0157-x. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto H, Watanabe K, Nagata M, et al. Inflammatory myofibroblastic tumor (IMT) of the pancreas. J Hepatobiliary Pancreat Surg. 2002;9:116–119. doi: 10.1007/s005340200013. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto H, Yamaguchi H, Aishima S, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009;33:1330–40. doi: 10.1097/pas.0b013e3181a5a207. [DOI] [PubMed] [Google Scholar]
- 70.Casadei R, Piccoli L, Valeri B, et al. Inflammatory pseudotumor of the pancreas resembling pancreatic cancer: clinical, diagnostic and therapeutic considerations. Chir Ital. 2004;56:849–858. [PubMed] [Google Scholar]
- 71.Wreesmann V, van Eijck CH, Naus DC, et al. Inflammatory pseudotumour (inflammatory myofibroblastic tumour) of the pancreas: a report of six cases associated with obliterative phlebitis. Histopathology. 2001;38:105–110. doi: 10.1046/j.1365-2559.2001.01056.x. [DOI] [PubMed] [Google Scholar]
- 72.Tothova Z, Wagner AJ. Anaplastic lymphoma kinase-directed therapy in inflammatory myofibroblastic tumors. Curr Opin Oncol. 2012;24:409–13. doi: 10.1097/CCO.0b013e328354c155. [DOI] [PubMed] [Google Scholar]
- 73.Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–80. [PubMed] [Google Scholar]
- 74.Bridge JA, Kanamori M, Ma Z, et al. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. 2001;159:411–5. doi: 10.1016/S0002-9440(10)61711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma Z, Hill DA, Collins MH, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 76.Ohara H, Nakazawa T, Sano H, et al. Histopathologic similarities of inflammatory pseudotumor to autoimmune pancreatitis: a morphologic and immunohistochemical study of 4 cases. Pancreas. 2006;32:115–7. doi: 10.1097/01.mpa.0000191646.01926.54. [DOI] [PubMed] [Google Scholar]
- 77.Adsay NV, Hasteh F, Cheng JD, et al. Lymphoepithelial cysts of the pancreas: a report of 12 cases and a review of the literature. Mod Pathol. 2002;15:492–501. doi: 10.1038/modpathol.3880553. [DOI] [PubMed] [Google Scholar]
- 78.Adsay NV, Hasteh F, Cheng JD, et al. Squamous-lined cysts of the pancreas: lymphoepithelial cysts, dermoid cysts (teratomas), and accessory-splenic epidermoid cysts. Semin Diagn Pathol. 2000;17:56–65. [PubMed] [Google Scholar]
- 79.Bedat B, Genevay M, Dumonceau JM, et al. Association between lymphoepithelial cysts of the pancreas and HIV infection. Pancreatology. 2012;12:61–4. doi: 10.1016/j.pan.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Kim WH, Lee JY, Park HS, et al. Lymphoepithelial cyst of the pancreas: comparison of CT findings with other pancreatic cystic lesions. Abdom Imaging. 2013;38:324–30. doi: 10.1007/s00261-012-9910-6. [DOI] [PubMed] [Google Scholar]
- 81.Vandenbussche CJ, Maleki Z. Fine-needle aspiration of squamous-lined cysts of the pancreas. Diagn Cytopathol. 2013 doi: 10.1002/dc.23080. [DOI] [PubMed] [Google Scholar]
- 82.Tewari N, Rollins K, Wu J, et al. Lymphoepithelial cyst of the pancreas and elevated cyst fluid carcinoembryonic antigen: a diagnostic challenge. JOP. 2014;15:504–7. doi: 10.6092/1590-8577/2799. [DOI] [PubMed] [Google Scholar]
- 83.Raval JS, Zeh HJ, Moser AJ, et al. Pancreatic lymphoepithelial cysts express CEA and can contain mucous cells: potential pitfalls in the preoperative diagnosis. Mod Pathol. 2010;23:1467–76. doi: 10.1038/modpathol.2010.144. [DOI] [PubMed] [Google Scholar]
- 84.Yamaguchi T, Takahashi H, Kagawa R, et al. Lymphoepithelial cyst of the pancreas associated with elevated CA 19–9 levels. J Hepatobiliary Pancreat Surg. 2008;15:652–4. doi: 10.1007/s00534-007-1314-6. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura T, Osaka Y, Ishikawa S, et al. Uncommon lymphoepithelial cyst with sebaceous glands of the pancreas. JOP. 2013;14:632–5. doi: 10.6092/1590-8577/1670. [DOI] [PubMed] [Google Scholar]
- 86.Mege D, Gregoire E, Barbier L, et al. Lymphoepithelial cyst of the pancreas: an analysis of 117 patients. Pancreas. 2014;43:987–95. doi: 10.1097/MPA.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 87.Halpert B, Gyorkey F. Accessory spleen in the tail of the pancreas. AMA Arch Pathol. 1957;64:266–9. [PubMed] [Google Scholar]
- 88.Hwang HS, Lee SS, Kim SC, et al. Intrapancreatic accessory spleen: clinicopathologic analysis of 12 cases. Pancreas. 2011;40:956–65. doi: 10.1097/MPA.0b013e318216815b. [DOI] [PubMed] [Google Scholar]
- 89.Motosugi U, Yamaguchi H, Ichikawa T, et al. Epidermoid cyst in intrapancreatic accessory spleen: radiological findings including superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2010;34:217–22. doi: 10.1097/RCT.0b013e3181c1b2bd. [DOI] [PubMed] [Google Scholar]
- 90.Othman M, Basturk O, Groisman G, et al. Squamoid cyst of pancreatic ducts: A distinct type of cystic lesion in the pancreas. Am J Surg Pathol. 2007;31:291–7. doi: 10.1097/01.pas.0000213349.42143.ec. [DOI] [PubMed] [Google Scholar]
- 91.Adsay NV. Cystic lesions of the pancreas. Mod Pathol. 2007;20(Suppl 1):S71–S93. doi: 10.1038/modpathol.3800706. [DOI] [PubMed] [Google Scholar]
- 92.Basturk O, Khanani F, Sarkar F, et al. DeltaNp63 expression in pancreas and pancreatic neoplasia. Mod Pathol. 2005;18:1193–1198. doi: 10.1038/modpathol.3800401. [DOI] [PubMed] [Google Scholar]
- 93.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification differential diagnosis and clinical implications. Arch Pathol Lab Med. 2009;133:423–38. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 94.Hanson JA, Salem RR, Mitchell KA. Squamoid cyst of pancreatic ducts: a case series describing novel immunohistochemistry cytology and quantitative cyst fluid chemistry. Arch Pathol Lab Med. 2014;138:270–3. doi: 10.5858/arpa.2012-0667-CR. [DOI] [PubMed] [Google Scholar]
- 95.Milanetto AC, Iaria L, Alaggio R, et al. Squamoid cyst of pancreatic ducts: a challenging differential diagnosis among benign pancreatic cysts. JOP. 2013;14:657–60. doi: 10.6092/1590-8577/1905. [DOI] [PubMed] [Google Scholar]
- 96.Thompson LDR, Basturk O, Adsay V. Pancreas. In: Mills S, editor. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer Health; China: 2015. [Google Scholar]
- 97.Ormarsson OT, Gudmundsdottir I, Marvik R. Diagnosis and treatment of gastric heterotopic pancreas. World J Surg. 2006;30:1682–1689. doi: 10.1007/s00268-005-0669-6. [DOI] [PubMed] [Google Scholar]
- 98.Eisenberger CF, Gocht A, Knoefel WT, et al. Heterotopic pancreas--clinical presentation and pathology with review of the literature. Hepatogastroenterology. 2004;51:854–858. [PubMed] [Google Scholar]
- 99.Lawrence AJ, Thiessen A, Morse A, et al. Heterotopic Pancreas within the Proximal Hepatic Duct, Containing Intraductal Papillary Mucinous Neoplasm. Case Rep Surg. 2015;2015:816960. doi: 10.1155/2015/816960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sumiyoshi T, Shima Y, Okabayashi T, et al. Heterotopic pancreas in the common bile duct, with a review of the literature. Intern Med. 2014;53:2679–82. doi: 10.2169/internalmedicine.53.3007. [DOI] [PubMed] [Google Scholar]
- 101.Cates JM, Williams TL, Suriawinata AA. Intraductal papillary mucinous adenoma that arises from pancreatic heterotopia within a meckel diverticulum. Arch Pathol Lab Med. 2005;129:e67–e69. doi: 10.5858/2005-129-e67-IPMATA. [DOI] [PubMed] [Google Scholar]
- 102.Lin M, Fu Y, Yu H, et al. Gastric heterotopic pancreas masquerading as a stromal tumor: A case report. Oncol Lett. 2015;10:2355–2358. doi: 10.3892/ol.2015.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zinczuk J, Bandurski R, Pryczynicz A, et al. Ectopic Pancreas Imitating Gastrointestinal Stromal Tumor (GIST) In The Stomach. Pol Przegl Chir. 2015;87:268–71. doi: 10.1515/pjs-2015-0052. [DOI] [PubMed] [Google Scholar]
- 104.von Heinrich H. Ein Beitrag zur Histologie des sogen akzessorischen Pankreas. Virchows Archiv Pathol. 1909;198:392–401. [Google Scholar]
- 105.Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg. 1986;151:697–700. doi: 10.1016/0002-9610(86)90045-0. [DOI] [PubMed] [Google Scholar]
- 106.Schneider NI, Plieschnegger W, Geppert M, et al. Pancreatic acinar cells-a normal finding at the gastroesophageal junction? Data from a prospective Central European multicenter study. Virchows Arch. 2013;463:643–50. doi: 10.1007/s00428-013-1471-8. [DOI] [PubMed] [Google Scholar]
- 107.Fukino N, Oida T, Mimatsu K, et al. Adenocarcinoma arising from heterotopic pancreas at the third portion of the duodenum. World J Gastroenterol. 2015;21:4082–8. doi: 10.3748/wjg.v21.i13.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee SH, Kim WY, Hwang DY, et al. Intraductal papillary mucinous neoplasm of the ileal heterotopic pancreas in a patient with hereditary non-polyposis colorectal cancer: A case report. World J Gastroenterol. 2015;21:7916–20. doi: 10.3748/wjg.v21.i25.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abraham J, Agrawal V, Behari A. Mucinous cystic neoplasm in heterotopic pancreas presenting as colonic polyp. JOP. 2013;14:671–3. doi: 10.6092/1590-8577/1943. [DOI] [PubMed] [Google Scholar]
- 110.Ginori A, Vassallo L, Butorano MA, et al. Pancreatic adenocarcinoma in duodenal ectopic pancreas: a case report and review of the literature. Pathologica. 2013;105:56–8. [PubMed] [Google Scholar]
- 111.Yan ML, Wang YD, Tian YF, et al. Adenocarcinoma arising from intrahepatic heterotopic pancreas: a case report and literature review. World J Gastroenterol. 2012;18:2881–4. doi: 10.3748/wjg.v18.i22.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chetty R, Weinreb I. Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. J Clin Pathol. 2004;57:314–317. doi: 10.1136/jcp.2003.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basturk O, Adsay NV. Pancreas. In: Cheng L, Bostwick DG, editors. Essentials of Anatomic Pathology. Springer; in press. [Google Scholar]
- 114.Kim HH, Cho CK, Hur YH, et al. Pancreatic hamartoma diagnosed after surgical resection. J Korean Surg Soc. 2012;83:330–4. doi: 10.4174/jkss.2012.83.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsushita D, Kurahara H, Mataki Y, et al. Pancreatic hamartoma: a case report and literature review. BMC Gastroenterol. 2016;16:3. doi: 10.1186/s12876-016-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawakami F, Shimizu M, Yamaguchi H, et al. Multiple solid pancreatic hamartomas: A case report and review of the literature. World J Gastrointest Oncol. 2012;4:202–6. doi: 10.4251/wjgo.v4.i9.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nagata S, Yamaguchi K, Inoue T, et al. Solid pancreatic hamartoma. Pathol Int. 2007;57:276–80. doi: 10.1111/j.1440-1827.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- 118.Pauser U, Kosmahl M, Kruslin B, et al. Pancreatic solid and cystic hamartoma in adults: characterization of a new tumorous lesion. Am J Surg Pathol. 2005;29:797–800. doi: 10.1097/01.pas.0000157748.18591.d7. [DOI] [PubMed] [Google Scholar]
- 119.Flaherty MJ, Benjamin DR. Multicystic pancreatic hamartoma: a distinctive lesion with immunohistochemical and ultrastructural study. Hum Pathol. 1992;23:1309–12. doi: 10.1016/0046-8177(92)90301-i. [DOI] [PubMed] [Google Scholar]
- 120.Pauser U, da Silva MT, Placke J, et al. Cellular hamartoma resembling gastrointestinal stromal tumor: a solid tumor of the pancreas expressing c-kit (CD117) Mod Pathol. 2005;18:1211–1216. doi: 10.1038/modpathol.3800406. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi H, Aishima S, Oda Y, et al. Distinctive histopathologic findings of pancreatic hamartomas suggesting their “hamartomatous” nature: a study of 9 cases. Am J Surg Pathol. 2013;37:1006–13. doi: 10.1097/PAS.0b013e318283ce4c. [DOI] [PubMed] [Google Scholar]
- 122.Al-Hawary MM, Kaza RK, Azar SF, et al. Mimics of pancreatic ductal adenocarcinoma. Cancer Imaging. 2013;13:342–9. doi: 10.1102/1470-7330.2013.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kersting S, Janot MS, Munding J, et al. Rare solid tumors of the pancreas as differential diagnosis of pancreatic adenocarcinoma. JOP. 2012;13:268–77. [PubMed] [Google Scholar]
- 124.Raman SP, Hruban RH, Cameron JL, et al. Pancreatic imaging mimics: part 2, pancreatic neuroendocrine tumors and their mimics. AJR Am J Roentgenol. 2012;199:309–18. doi: 10.2214/AJR.12.8627. [DOI] [PubMed] [Google Scholar]
- 125.Fournet JC, Junien C. Genetics of congenital hyperinsulinism. Endocr Pathol. 2004;15:233–40. doi: 10.1385/ep:15:3:233. [DOI] [PubMed] [Google Scholar]
- 126.Sempoux C, Guiot Y, Jaubert F, et al. Focal and diffuse forms of congenital hyperinsulinism: the keys for differential diagnosis. Endocr Pathol. 2004;15:241–246. doi: 10.1385/ep:15:3:241. [DOI] [PubMed] [Google Scholar]
- 127.Kloppel G, Anlauf M, Raffel A, et al. Adult diffuse nesidioblastosis: genetically or environmentally induced? Hum Pathol. 2008;39:3–8. doi: 10.1016/j.humpath.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Rumilla KM, Erickson LA, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol. 2009;22:239–45. doi: 10.1038/modpathol.2008.169. [DOI] [PubMed] [Google Scholar]
- 129.Raffel A, Krausch MM, Anlauf M, et al. Diffuse nesidioblastosis as a cause of hyperinsulinemic hypoglycemia in adults: a diagnostic and therapeutic challenge. Surgery. 2007;141:179–184. doi: 10.1016/j.surg.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 130.Anlauf M, Wieben D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol. 2005;29:524–533. doi: 10.1097/01.pas.0000151617.14598.ae. [DOI] [PubMed] [Google Scholar]
- 131.Service FJ, Natt N, Thompson GB, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemia hypoglycemia in adults independent of mutations in Kir6. 2 and SUR1 genes. J Clin Endocrinol Metab. 1998;84:1582–1589. doi: 10.1210/jcem.84.5.5645. [DOI] [PubMed] [Google Scholar]
- 132.Thompson SM, Vella A, Thompson GB, et al. Selective Arterial Calcium Stimulation With Hepatic Venous Sampling Differentiates Insulinoma From Nesidioblastosis. J Clin Endocrinol Metab. 2015;100:4189–97. doi: 10.1210/jc.2015-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilis-Januszewska A, Piatkowski J, Skalniak A, et al. Noninsulinoma pancreatogenous hypoglycaemia in adults--a spotlight on its genetics. Endokrynol Pol. 2015;66:344–54. doi: 10.5603/EP.2015.0044. [DOI] [PubMed] [Google Scholar]
- 134.Goossens A, Gepts W, Saudubray JM, et al. Diffuse and focal nesidioblastosis. A clinicopathological study of 24 patients with persistent neonatal hyperinsulinemic hypoglycemia. Am J Surg Pathol. 1989;13:766–75. [PubMed] [Google Scholar]
- 135.Suchi M, MacMullen C, Thornton PS, et al. Histopathology of congenital hyperinsulinism: retrospective study with genotype correlations. Pediatr Dev Pathol. 2003;6:322–333. doi: 10.1007/s10024-002-0026-9. [DOI] [PubMed] [Google Scholar]
- 136.Delonlay P, Simon A, Galmiche-Rolland L, et al. Neonatal hyperinsulinism: clinicopathologic correlation. Hum Pathol. 2007;38:387–399. doi: 10.1016/j.humpath.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 137.Ismail D, Werther G. Persistent hyperinsulinaemic hypoglycaemia of infancy: 15 years’ experience at the Royal Children’s Hospital (RCH), Melbourne. J Pediatr Endocrinol Metab. 2005;18:1103–1109. doi: 10.1515/jpem.2005.18.11.1103. [DOI] [PubMed] [Google Scholar]
- 138.Fournet JC, Mayaud C, de LP, et al. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: association with a reduction to homozygosity of a mutation in ABCC8 or KCNJ11. Am J Pathol. 2001;158:2177–2184. doi: 10.1016/S0002-9440(10)64689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dunne MJ, Cosgrove KE, Shepherd RM, et al. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84:239–75. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- 140.Rahman SA, Nessa A, Hussain K. Molecular mechanisms of congenital hyperinsulinism. J Mol Endocrinol. 2015;54:R119–29. doi: 10.1530/JME-15-0016. [DOI] [PubMed] [Google Scholar]
- 141.Senniappan S, Arya VB, Hussain K. The molecular mechanisms diagnosis and management of congenital hyperinsulinism. Indian J Endocrinol Metab. 2013;17:19–30. doi: 10.4103/2230-8210.107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Henquin JC, Nenquin M, Sempoux C, et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J Clin Invest. 2011;121:3932–42. doi: 10.1172/JCI58400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huopio H, Reimann F, Ashfield R, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest. 2000;106:897–906. doi: 10.1172/JCI9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suchi M, MacMullen CM, Thornton PS, et al. Molecular and immunohistochemical analyses of the focal form of congenital hyperinsulinism. Mod Pathol. 2006;19:122–129. doi: 10.1038/modpathol.3800497. [DOI] [PubMed] [Google Scholar]
- 145.Meissner T, Wendel U, Burgard P, et al. Long-term follow-up of 114 patients with congenital hyperinsulinism. Eur J Endocrinol. 2003;149:43–51. doi: 10.1530/eje.0.1490043. [DOI] [PubMed] [Google Scholar]
- 146.Adzick NS, Thornton PS, Stanley CA, et al. A multidisciplinary approach to the focal form of congenital hyperinsulinism leads to successful treatment by partial pancreatectomy. J Pediatr Surg. 2004;39:270–275. doi: 10.1016/j.jpedsurg.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 147.Pezzilli R, Calculli L. Pancreatic steatosis: Is it related to either obesity or diabetes mellitus? World J Diabetes. 2014;5:415–9. doi: 10.4239/wjd.v5.i4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol. 1984;15:677–683. doi: 10.1016/s0046-8177(84)80294-4. [DOI] [PubMed] [Google Scholar]
- 149.Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand A. 1978;86A:367–73. doi: 10.1111/j.1699-0463.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 150.Altinel D, Basturk O, Sarmiento JM, et al. Lipomatous pseudohypertrophy of the pancreas: a clinicopathologically distinct entity. Pancreas. 2010;39:392–7. doi: 10.1097/MPA.0b013e3181bd2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maunoury V, Nieuwarts S, Ferri J, et al. Pancreatic lipomatosis revealing Johanson-Blizzard syndrome. Gastroenterol Clin Biol. 1999;23:1099–101. [PubMed] [Google Scholar]
- 152.MacMaster SA, Cummings TM. Computed tomography and ultrasonography findings for an adult with Shwachman syndrome and pancreatic lipomatosis. Can Assoc Radiol J. 1993;44:301–3. [PubMed] [Google Scholar]
- 153.Okun SD, Lewin DN. Non-neoplastic pancreatic lesions that may mimic malignancy. Semin Diagn Pathol. 2016;33:31–42. doi: 10.1053/j.semdp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 154.Masuda A, Tanaka H, Ikegawa T, et al. A case of lipomatous pseudohypertrophy of the pancreas diagnosed by EUS-FNA. Clin J Gastroenterol. 2012;5:282–6. doi: 10.1007/s12328-012-0318-1. [DOI] [PubMed] [Google Scholar]
- 155.Dodo IM, Adamthwaite JA, Jain P, et al. Successful outcome following resection of a pancreatic liposarcoma with solitary metastasis. World J Gastroenterol. 2005;11:7684–5. doi: 10.3748/wjg.v11.i48.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zamboni G, Capelli P, Scarpa A, et al. Nonneoplastic mimickers of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:439–53. doi: 10.5858/133.3.439. [DOI] [PubMed] [Google Scholar]
- 157.Chatelain D, Vibert E, Yzet T, et al. Groove pancreatitis and pancreatic heterotopia in the minor duodenal papilla. Pancreas. 2005;30:e92–5. doi: 10.1097/01.mpa.0000161885.79373.1d. [DOI] [PubMed] [Google Scholar]
- 158.Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas” “para-duodenal wall cyst” and “groove pancreatitis”. Semin Diagn Pathol. 2004;21:247–54. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 159.Kloppel G, Adsay NV. Chronic pancreatitis and the differential diagnosis versus pancreatic cancer. Arch Pathol Lab Med. 2009;133:382–7. doi: 10.5858/133.3.382. [DOI] [PubMed] [Google Scholar]
- 160.Klöppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20(Suppl 1):S113–S131. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 161.Adsay NV, Bandyopadhyay S, Basturk O, et al. Chronic pancreatitis or pancreatic ductal adenocarcinoma? Semin. Diagn Pathol. 2004;21:268–276. doi: 10.1053/j.semdp.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 162.Kalb B, Martin DR, Sarmiento JM, et al. Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269:475–81. doi: 10.1148/radiology.13112056. [DOI] [PubMed] [Google Scholar]
- 163.Manzelli A, Petrou A, Lazzaro A, et al. Groove pancreatitis. A mini-series report and review of the literature. JOP. 2011;12:230–3. [PubMed] [Google Scholar]
- 164.Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging. 2008;33:342–8. doi: 10.1007/s00261-007-9245-x. [DOI] [PubMed] [Google Scholar]
- 165.Casetti L, Bassi C, Salvia R, et al. “Paraduodenal” pancreatitis: results of surgery on 58 consecutives patients from a single institution. World J Surg. 2009;33:2664–9. doi: 10.1007/s00268-009-0238-5. [DOI] [PubMed] [Google Scholar]