Abstract
To investigate brain–pituitary–gonadal inter-relationships, we have compared the effects of mutations that perturb the hypothalamic–pituitary–gonadal axis in male mice. Specifically, serum and pituitary gonadotrophin concentrations, gonadotrophin gene expression, and gonadotroph structure and number were measured. Follicle-stimulating hormone (FSH)β knockout (FSHβKO), FSH receptor knockout (FSHRKO), luteinising hormone (LH) receptor knockout (LuRKO), hypogonadal (hpg), testicular feminised (tfm) and gonadectomised mice were compared with control wild-type mice or heterozygotes. Serum levels of LH were similar in FSHβKO, FSHRKO and heterozygote males despite decreased androgen production in KO males. As expected, there was no detectable FSH in the serum or pituitary and an absence of expression of the FSHβ subunit gene in FSHβKO mice. However, there was a significant increase in expression of the common α and LHβ subunit genes in FSHRKO males. The morphology of FSHβKO and FSHRKO gonadotrophs was not significantly different from controls, except that the subpopulation of granules consisting of an electron-dense core and electron-lucent ‘halo’ was not observed in FSHβKO gonadotrophs and the granules were smaller in diameter. In the gonadotrophin-releasing hormone deficient hpg mouse, gonadotrophin mRNA and hormone levels were significantly lower compared to control mice and gonadotrophs were correspondingly smaller, with less abundant endoplasmic reticulum and reduced secretory granules. In LuRKO, tfm and gonadectomised mice, hyperstimulation of LHβ and FSHβ mRNA and serum protein concentrations was reflected by subcellular changes in gonadotroph morphology, including more dilated rough endoplasmic reticulum and more secretory granules distributed adjacent to the plasma membrane. In summary, major differences in pituitary content and serum concentrations of the gonadotrophins LH and FSH have been found between normal and mutant male mice. These changes are associated with changes in transcriptional activity of the gonadotrophin subunit genes and are reflected by changes in the cellular structure and secretory granule architecture within the gonadotroph cells.
Keywords: gonadotrophs, LH, FSH, anterior pituitary, ultrastructure
The gonadotrophins luteinising hormone (LH) and follicle-stimulating hormone (FSH) are synthesised within a single cell type, the gonadotroph, within the anterior pituitary gland. The gonadotrophins consist of a common α subunit and a specific β subunit responsible for conferring biological activity on the heterodimer (1). All three subunits are encoded by separate genes localised on different chromosomes (2). Different secretory granule populations have been identified in gonadotrophs of the rat, mouse and sheep; namely small LH and secretogranin II (SgII) positive granules, large FSH and chromogranin A (CgA) positive granules, and intermediate-sized granules containing an electron dense LH and SgII positive core and an electron lucent CgA-positive outer region (3). How this difference in storage relates to release remains unclear. Synthesis and secretion of the gonadotrophins is dependent on the decapeptide, gonadotrophin-releasing hormone (GnRH), produced within specific hypothalamic neurones. GnRH has long been regarded as the master hormone of the hypothalamic–pituitary–gonadal (HPG) axis because, in the absence of this hormone, synthesis of LH and FSH is reduced such that mice carrying a mutation in this gene do not reach sexual maturity but remain pre-pubertal throughout life (4). The discovery of Kiss1 stimulation of GnRH via Kiss1 receptors on the GnRH neurones (5) has introduced an additional level of control to the HPG axis. Gonadal steroids can act at both the hypothalamus and pituitary to moderate gonadotrophin synthesis. Androgens have been shown to decrease GnRH mRNA levels in the hypothalamus (6,7) and GnRH peptide (8,9) in rats, leading to decreased synthesis of LH and FSH. In addition, sex steroids differentially regulate expression of Kiss1 in neurones of the arcuate and anteroventral periventricular nucleus nuclei of the hypothalamus (10) providing a further mechanism whereby these steroids can modify GnRH output. The differential regulation of LH and FSH synthesis and secretion in different physiological situations when both are produced within a single cell has remained an enigma. The findings of a study by Knobil (11), showing that high GnRH pulse frequency favours LH and slow pulse frequencies FSH, suggested one mechanism, although the use of the immortalised mouse LβT2 gonadotroph cell line, which expresses GnRH, androgen and activin receptors and produces activin and follistatin, has provided important insights into the differential activation of the gonadotrophin subunit genes. Androgen repression of LHβ subunit gene expression in LβT2 cells occurs by indirect mechanisms that do not require androgen receptor binding to the LHβ gene (12,13), whereas androgen acts to stimulate FSHβ subunit gene expression through two conserved androgen response elements together with an activin response element within the FSHβ gene (14). In addition, androgen can stimulate FSHβ gene expression indirectly by increasing GnRH receptor expression on gonadotrophs (12,15). Current evidence supports a mainly pulsatile secretion of LH via pulsatile GnRH input, whereas pulsatile secretion of FSH is limited and independent of pulsatile GnRH (16). Most FSH appears to be constitutively released, whereby the amount of FSH released directly relates to the rate of synthesis (16,17). The differential packaging of LH and FSH in secretory granules is likely to form the point of control for distinct intracellular mechanisms enabling the discrete release of LH and/or FSH (3), although the precise mechanisms are not known.
In the present study, we investigated in vivo the effect of mutations, both naturally occurring and genetically engineered, which perturb the HPG axis. Specifically we have compared FSHβ knockout (FSHβKO), FSH receptor knockout (FSHRKO), LH receptor knockout (LuRKO), hypogonadal (hpg), testicular feminised (tfm) and gonadectomised mice. By contrast to studies in immortalised cell lines these mutant models allow us to characterise primary gonadotroph biology in situ under conditions of altered endogenous GnRH, LH, FSH and testosterone signalling.
The FSHKβO mouse has a genetically engineered deletion in the gene encoding the FSHβ subunit (18). As a result, there is no production of biologically active FSH within the pituitary. Despite the absence of FSH, FSHβKO male mice are fertile under laboratory conditions but show a decrease in testis size from 6 weeks of age (19). In adult mice, testis weight is approximately 50% that of control littermates. There is a reduction in Sertoli cell number and tubule diameter relative to control males. Leydig cell number is within the normal range despite an apparent increase as a result of the decrease in tubule size. All stages of spermatogenesis can be identified within the testis and mature sperm can be seen in the tubule lumen.
The FSHRKO mouse also carries a genetically engineered deletion in the gene encoding the FSH receptor (20). The resulting inability to respond to circulating FSH results in morphological changes within the testis similar to those seen in the FSHβKO mouse. FSHRKO males are also fertile under laboratory conditions but, in contrast to the FSHβKO mouse, cannot respond to exogenous FSH.
The LuRKO mouse has a genetically engineered deletion of exon 11 of the LH receptor (21). Males are born phenotypically normal but, postnatally, testicular growth, descent, external genital and accessory sex organ development do not occur and spermatogenesis is arrested at the round spermatid stage. The number and size of Leydig cells within the testis are markedly reduced and testosterone production is reduced. LuRKO male mice are infertile and are unable to respond to exogenous LH. However, testosterone replacement from day 21 for 60 days induces a male type phenotype, full spermatogenesis and testis descent. Spermatozoa can fertilise oocytes but mating remains subfertile (22).
The hpg mouse is a naturally occurring mutant with a deletion in the gene encoding the hypothalamic GnRH (4,23). Anatomically, there are no apparent differences between mutant and normal mice at birth. By 21 days, hpg males can be distinguished from normal littermates by an undeveloped scrotum, a small penis and reduced anogenital distance. Internally, the testes are small and remain intra-abdominal. All components of the male reproductive tract are present but remain pre-pubertile. Histological examination of the testes shows that spermatogenesis has arrested at the first meiotic division, the interstitial tissue is atrophic and there is no evidence of stimulation of secondary sexual tissues. Male mice are infertile and, although sexual development can be induced by exogenous hormone administration, mating behaviour remains impaired (4).
The tfm mouse is also a naturally occurring mutant with a single base deletion in the gene encoding the androgen receptor (24). This deletion alters the open reading frame such that a stop codon is introduced into the first exon. When transcribed, a truncated mRNA is produced whose protein product is nonfunctional as a result of the loss of the DNA and androgen binding domain (25). By contrast to LuRKO males, tfm animals fail to undergo masculinisation in utero and genetic males carrying the tfm mutation have female genitalia, lack Wolffian duct structures and do not respond to pharmacological doses of testosterone (26,27). Tfm males have small abnormal testes that remain within the abdomen and contain few germ cells. A small percentage of germ cells make it to the first meiotic division and the most advanced germ cells are arrested at the first meiotic division (28). FSH and LH are produced by the pituitary and testosterone production is stimulated in the testis, although there is no response to this hormone within the testis or at the level of the pituitary or hypothalamus.
The present study aimed to investigate pituitary synthesis and regulation of LH and FSH in the above mutant mice and to relate our findings to the ultrastructure of the gonadotroph relative to wild-type mice. Throughout the study, we refer to these mice in the order described above, namely FSH mutants, LH receptor mutant, hpg, tfm and gonadectomised mice, because this reflects the severity of the consequences of the mutations on gonadal axis function.
Materials and methods
Mutant mice
Breeding colonies of FSHβ deficient, FSHβKO mice (18), FSH receptor deficient FSHRKO mice (20) and LH receptor deficient, LuRKO mice (21) were established in our laboratory. Both FSHRKO and FSHβKO males are fertile. Breeding pairs of KO males and heterozygous females were used to generate heterozygous and KO offspring in a 1 : 1 ratio. Heterozygous males were used as controls for these two lines. FSHβ, FSHR and LHR mutations were identified as described by Hirst et al. (29). The hpg mice (4) and tfm mice (24) from the original colonies discovered at the MRC Laboratories (Harwell, Oxford, UK) were bred within our department. The hpg mutation was identified by polymerase chain reaction (PCR) analysis of tail DNA as described previously (30). Tfm mice were identified by an external female phenotype and coat colouration within a litter and confirmed on dissection by the presence of abdominal testes and the absence of any ductal system. The hpg and tfm mice were on a C3H/HeH-101/H genetic background and the KO mice on a mixed C57B16/129 background. All procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986 and with the approval of a local ethical review committee.
Serum and tissue collection
All procedures were carried out under anaesthesia [Rompun /Ketaset: 0.1 ml/kg of a 20% : 4% (v/v) solution; Veterinary Supplies, University of Oxford, Oxford, UK]. For the analysis of gonadotrophin hormones, blood was collected from the jugular sinus, serum separated and frozen at −20 °C for assays. For the analysis of gonadotrophin subunit mRNA or gonadotrophin hormone, pituitaries were dissected out, weighed, snap frozen in liquid nitrogen and stored at −70 °C until assayed. Testes were dissected out, weighed and prepared for histology as described previously (20). Seminal vesicles were removed and weighed.
Gonadectomy
Animals were anaesthetised as described above. A 1.0-cm vertical incision was made in skin of the lower abdomen. The fat pad overlying the testis on the right side was located and pulled forward to reveal the testis. The testicular artery was ligated and the testis removed. The procedure was repeated on the other side and the incision sutured.
Hormone assays
Serum and pituitary levels of FSH and LH were measured using in-house imunofluorimetric assays (Delfia, Wallac OY, Turku, Finland) as described previously (31, 32). A new pair of antibodies was used in the FSH assay: a monoclonal against recombinant human FSHβ (FSH56A) and a polyclonal against recombinant human FSHα (R93–2705); both donated by Organon (Oss, The Netherlands). The sensitivity of the LH assay was 0.75 pg/tube (0.03 μg/l in 25 μl), with an intra-assay coefficient of variation (CV) of 19% at 0.04 μg/l and < 5% above 1.0 μg/l, and an inter-assay CV of 12.5% at 0.24 μg/l and 7.8% at 0.78 μg/l. The sensitivity of the FSH assay was 0.1 μg/l, with an intra-assay CV of 4.3% and an inter-assay CV of 10.4% at 4.8 μg/l. Hormone assays were conducted on plasma collected between 09.00 h and 11.00 h from unstimulated mice. The hormone concentration values measured therefore represent random samples of a pulsatile pattern of gonadotrophin secretion.
RNA extraction
Total RNA was extracted from individual pituitaries with Trizol (Life Technologies, Paisley, UK) and residual genomic DNA was removed by DNAse treatment (DNA-free; Ambion Inc., supplied by AMS Biotechnology, Abingdon, UK). DNAse-treated RNA was quantified by spectrophotometric measurement at λ 260 nm. In total, 1 μg of RNA was reverse transcribed using Random hexamers (Ambion) and Moloney murine leukaemia virus reverse transcriptase (Life Technologies).
Quanitative real-time PCR
PCR experiments were carried out in a 25-μl volume using a 96-well plate format. Primers and probes were designed using Primer Express (Applied Biosystems, Warrington, UK) and probes were synthesised with FAM 5′ and TAMRA 3′ (Taqman®, Applied Biosystems). Primers were used at a final concentration of 300 nM and probes at a concentration of 150 nM in ABI universal master mix (Applied Biosystems). Primers and probes were selected from sequences generated using Primer Express (Applied Biosystems). Primer and probe sequences are listed in Table 1. To our knowledge, pituitary thyrotrophs are not responsive to gonadotrophin stimulation and thyroid-stimulating hormone (TSH)β subunit gene activity was measured for comparison with gonadotrope β subunit activity. Fluorescence was detected on an ABI 7700 system (Applied Biosystems). No reverse transcription controls for each sample were screened to check for the presence of residual genomic DNA.
Table 1.
Sequences of Mouse Primer and Probe Sets for Gonadotrophin Subunit Genes. Probes are Dual Labelled FAM 5′ and TAMRA 3′ (Taqman®).
| Subunit gene | Primer | Sequence 5′- to 3′ | ID Number |
|---|---|---|---|
| Common α | Forward | CTGTTGCTTCTCCAGGGCATA | NM009889 |
| Reverse | TTCTTTGGAACCAGCATTGTCTT | ||
| Probe | CCCACTCCCGCCAGGTCCAA | ||
| LHβ | Forward | TGGCCGCAGAGAATGAGTTC | MM25145 |
| Reverse | CTCGGACCATGCTAGGACAGTAG | ||
| Probe | CCCAGTCTGCATCACCTTCACCACC | ||
| FSHβ | Forward | GGAGAGCAATCTGCTGCCATA | MM12932 |
| Reverse | GCAGAAACGGCACTCTTCCT | ||
| Probe | CTGTGAATTGACCAACATCACCATCTCAGTAGA | ||
| TSHβ | Forward | ACTTCATCTACAGAACGGTGGAAAT | MMTSHB1 |
| Reverse | GCGACAGGGAAGGAGAAATAAG | ||
| Probe | CCAGGATGCCCGCACCATGTTACT |
To measure cDNA levels, a threshold cycle (Ct) was selected within the exponential phase of the amplification for all standards and samples. Arbitrary standards were generated by serial dilutions of a cDNA pool from normal adult male pituitaries. A standard curve was generated by plotting standards against Ct values, sample values were read from this standard curve and then mRNA levels were normalised relative to an endogenous control mGapdh mRNA (Applied Biosystems) to allow comparison of different mRNAs between samples. A comparison between mGapdh and wbscr (33) demonstrated that mGapdh showed a more stable pattern of expression across pituitary glands from normal and mutant mice and was therefore selected as the housekeeping gene of choice.
Tissue processing and electron microscopy
Pituitary glands for electron microscopy analysis were processed and analysed as described previously (34). Briefly, the tissue was contrasted with uranyl acetate [2% (w/v) in distilled water], dehydrated in ethanol and embedded in LR Gold resin (London Resin Company Ltd., Reading, UK). Ultrathin sections (50–80 nm) were prepared using a Reichart–Jung ultracut microtome and mounted on nickel grids (Agar Scientific, Stanstead, UK). For identification of bihormonal gonadotroph secretory granules, sections were labelled for LH on day 1 and for FSH on the following day. Pituitary sections from normal and transgenic mice were incubated for 2 h at room temperature with LH primary antibody [1 : 2000 dilution of guinea-pig anti-rat LH; National Hormone and Peptide Program (NHPP, Torrance, CA, USA)] followed by a 1-h incubation with a 15-nm protein A gold-conjugated donkey antiguinea pig secondary antibody (dilution 1 : 60; British Biocell, Cardiff, UK). On day 2, the sections were incubated with an FSH primary antibody (1 : 500 dilution of rabbit anti-rat FSH; ABCAM, Cambridge, UK) followed by a 5-nm protein A gold-conjugated goat anti-rabbit secondary antibody (dilution 1 : 60; British Biocell). All antibodies were diluted in 0.1 M phosphate-buffered saline (containing 0.1% egg albumin). Specificity of antibody labelling was confirmed in negative control sections in which the primary antibody was replaced with non-immune serum. Finally, sections were counterstained with lead citrate and uranyl acetate and examined on a JOEL 1010 transmission electron microscope (JOEL USA Inc., Peabody, MA, USA). For each pituitary (n = 4), four randomly orientated sections, each containing ten grid squares of intact tissue, were counted for individual gonadotrophs identified by immunogold labelling for LH and FSH. The total secretory cell population was determined by counting the total number of nucleated pituitary (granulated and folliculo-stellate) cells per grid. Gonadotroph number was expressed as a percentage of the total secretory cell population. Immunogold identification of somatotrophs using a rabbit antimouse GH primary antibody (dilution 1 : 4000; NHPP) was also carried to quantify the percentage of somatotrophs. This was intended as a control for changes in the size of the gonadotroph population because changes in the somatotroph population were not expected in the mutant mice investigated.
Electron microscopy morphological studies
For analysis of cell morphology, ten micrographs of gonadotrophs per animal (n = 4 mice per group) were taken at a magnification of × 4000 and scanned into ADOBE PHOTOSHOP, version 5.5 (Adobe Corp., San Jose, CA, USA) and analysed using AXIOVISON, version 4.5 (Zeiss,, Oberkochen, Germany). The analyst was blind to the sample code. The parameters calculated were: cytoplasmic, nuclear and total cell areas; granule area, granule density, granule diameter and percentage of secretory granules located in a 300-nm depth of the plasma membrane. For measurement of the cell and nuclear areas, margins were drawn around the cell or nucleus, respectively, and the area was calculated. Cytoplasmic area was determined by subtracting nuclear area from total cell area. Granule density was calculated by dividing total granule area by cytoplasmic area. Sections from four animals per group were examined. Expansion of the rough endoplasmic reticulum (rough ER) and Golgi apparatus was assessed visually and graded on a scale of 0–4 (0, no expansion; 4 the most expansion). These estimates do not provide absolute measurements but do provide a basis for comparison.
Statistical analysis
Normal and heterozygous mice from the hpg and LuR colonies were initially analysed separately. Where there was no significant difference, data from normal mice and heterozygous mice were combined and expressed as heterozygous/normal (H/N). Heterozygous males were used as normal controls in FSHR and FSHβ colonies. Each mutant line was compared with its own control; comparisons were not made across different lines. Means were compared by one-way ANOVA. Where a significant overall difference was detected between groups, differences between individual means were assessed by the Bonferroni test. P < 0.01 was considered statistically different between mice and controls in the same line.
Results
Pituitary and serum gonadotrophin concentrations and pituitary gene expression
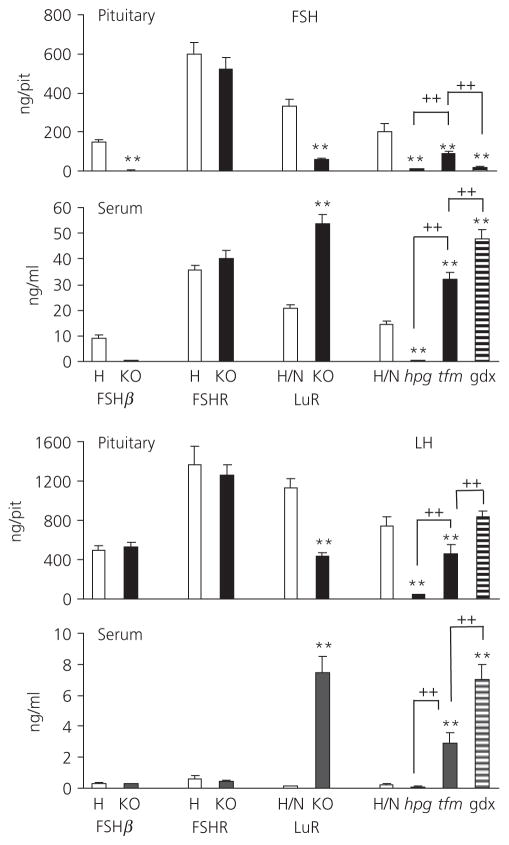
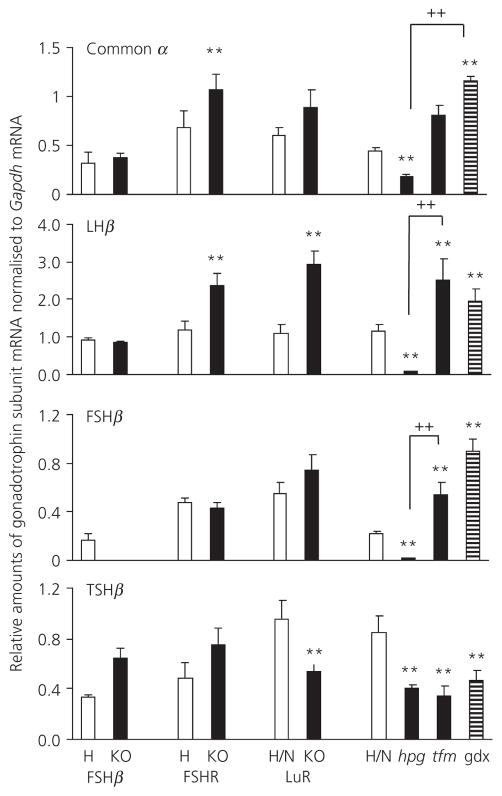
Figure 1 shows pituitary and serum gonadotrophin concentrations. Fig. 2 shows levels of gonadotrophin gene expression. Table 2 provides a summary of changes measured for each mutant and control. In FSHβKO adult males, pituitary and serum levels of FSH were at or below the limit of detection of the FSH assay throughout and no expression of the FSHβ gene was detected, confirming the absence of synthesis of FSH in this mutant.
Fig. 1.
Pituitary and serum luteinising hormone (LH) and follicle-stimulating hormone (FSH) levels in adult (8 week) FSHβH (5), FSHβKO (5), FSHRH (5), FSHRKO (4), LuRH/N (21), LuRKO (7), hpgH/N (15), hpg (9), tfm (5) and gonadectomised males (14) (normals from hpg colony, 4 weeks post gonadectomy). The number of mice in each group is shown in brackets. Results are expressed as the mean ± SEM. **P < 0.01 versus respective strain control; ++P < 0.01 versus comparison indicated by lines. Open columns, heterozygote/ normal control; filled columns, knockout/mutant; striped columns, gonadectomised (gdX) mice. KO, knockout; hpg, hypogonadal; tfm, testicular feminised.
Fig. 2.
Gonadotroph common α, luteinising hormone (LH)β, follicle-stimulating hormone (FSH)β and thyroid-stimulating hormone (TSH)β subunit mRNA levels, normalised to mGapdh mRNA in adult (8 week) FSHβH (3), FSHβKO (4), FSHRH (3), FSHRKO (4), LuRH/N (4), LuRKO (4), hpgH/N (4), hpg (4), tfm (4) and gonadectomised (gdX) males (3). Number of mice in each group is shown in brackets. Results are expressed as the mean ± SEM. **P < 0.01 versus respective strain control; ++P < 0.01 versus comparison indicated by lines. Open columns, heterozygote/normal control; filled columns, knockout/mutant; striped columns, gonadectomised (gdX) mice. KO, knockout; hpg, hypogonadal; tfm, testicular feminised.
Table 2.
Summary of the Changes in Pituitary and Serum Follicle-Stimulating Hormone (FSH) and Luteinising Hormone (LH) Concentration and mRNA Levels in Mouse Mutant Lines.
| FSHβKO | FSHRKO | LuRKO | hpg | tfm | gdX | |
|---|---|---|---|---|---|---|
| Pituitary FSH | Absent | ↔ | ↓ | ↓ | ↓ | ↓ |
| Serum FSH | Absent | ↔ | ↑ | ↓ | ↑ | ↑ |
| Pituitary mRNA FSHβ | Absent | ↔ | ↔ | ↓ | ↑ | ↑ |
| Pituitary mRNA Common α | ↔ | ↑ | ↔ | ↓ | ↑ | ↑ |
| Pituitary LH | ↔ | ↔ | ↓ | ↓ | ↓ | ↔ |
| Serum LH | ↔ | ↔ | ↑ | ↔ | ↑ | ↑ |
| Pituitary mRNA LHβ | ↔ | ↔ | ↑ | ↓ | ↑ | ↑ |
| Pituitary mRNA TSHβ | ↔ | ↔ | ↓ | ↓ | ↓ | ↓ |
Direction of arrows indicate direction of change, horizontal arrows indicate no overall change. LuR, LH receptor; KO, knockout; gdX, gonadectomised; hpg, hypogonadal; tfm, testicular feminised; TSH, thyroid-stimulating hormone.
In FSHβH males, serum FSH was 6.1% of pituitary content of FSH, similar to that seen in FSHRH, LuRH and hpgH/N adult males. Serum LH levels were 0.05% of pituitary content in FSHβH males and 0.06% in FSHβKO males. mRNA levels of both common α and LHβ subunit genes were similar in FSHβH and FSHβKO males.
In FSHRH and FSHRKO males, serum levels of FSH expressed as a % of pituitary content (5.9 and 7.7, respectively) and of LH (0.04 and 0.03, respectively) were similar despite significantly higher expression of common α and LHβ subunit genes in the FSHRKO males relative to heterozygote males. There was no significant difference in expression of FSHβ or TSHβ subunit genes between KO and heterozygous males in either FSHβ or FSHR males.
By contrast, in LuRKO males, serum levels of FSH were significantly higher compared to levels in LuRH/N males and this was associated with a significantly reduced pituitary content of FSH (P < 0.01). A similar pattern was seen for LH. The levels of common α, LHβ and FSHβ mRNA were higher in LuRKO males but only reached significance with LHβ. TSHβ mRNA levels were significantly lower relative to heterozygous males.
In hpg males, pituitary content of FSH and LH was a small fraction of that measured in normal and heterozygous males. Serum levels of FSH were also significantly lower than in control mice 2.7% (P < 0.01). Serum LH levels, although not significantly different, were only 35% of control levels. mRNA levels of all subunit genes were significantly lower compared to control levels.
Serum levels of both FSH and LH were significantly elevated in tfm males relative to control mice but did not reach the levels measured in normal male mice gonadectomised 1 month earlier. The high serum levels of FSH were associated with a significant reduction in pituitary content of FSH in both tfm and gonadectomised male mice. In tfm mice, pituitary content of LH was also significantly reduced, whereas, in gonadectomised males, pituitary content remained at a similar level to that of control males. In tfm mice, mRNA levels of LHβ and FSHβ were significantly higher than in control males and, in gonadectomised mice, mRNA levels of common α and FSHβ were significantly increased. By contrast, TSHβ mRNA levels were significantly lower in hpg, tfm and gonadectomised mice relative to control mice. There was no significant difference in TSHβ expression between hpg, tfm and gonadectomised mice.
Gonadotroph morphology
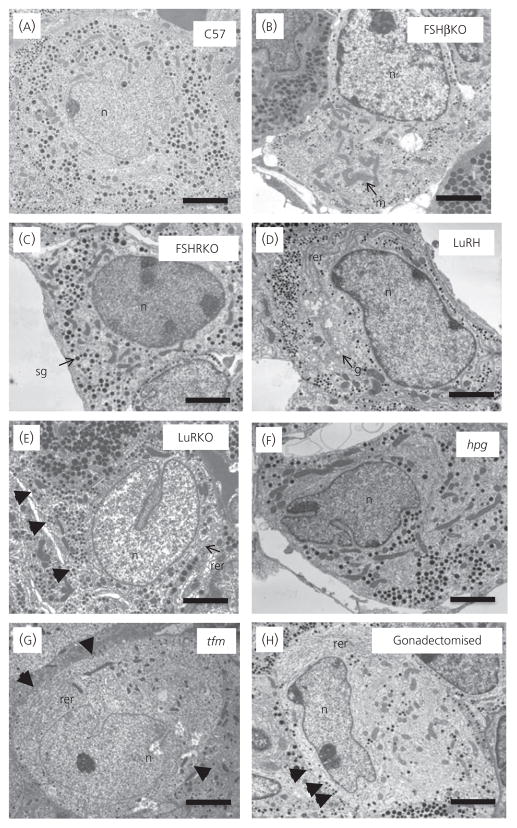
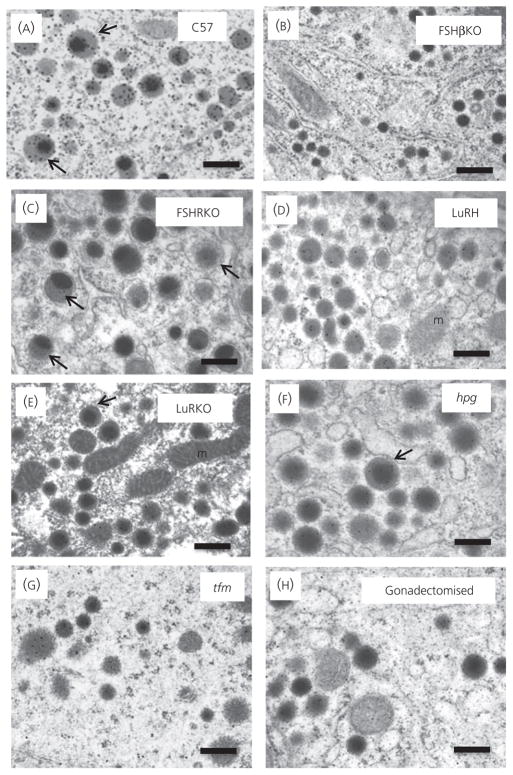
Figure 3 shows representative electron micrographs of gonadotrophs in control and mutant mice. Table 3 shows the quantitative analysis of gonadotrophs and their organelles. The gonadotrophs were round in shape and usually adjacent to a capillary wall. Gonadotrophs can be identified by their distinctive population of secretory granules, which have variable size and electron density. In the control mice (Figs 3A and 4A), secretory granules were mainly moderately electron-dense of variable size (100–250 nm) and a small subpopulation of granules displayed an electron-dense core and a relatively electron-lucent ‘halo’ (indicated by arrows). Cell and cytoplasmic areas showed no significant difference between controls and FSHβKO (Figs 3B and 4B) and FSHRKO (Figs 3C and 4C) mice. However, gonadotrophs in LuRKO (Figs 3E and 4E) males were significantly (P < 0.01) smaller compared to control mice (Figs 3A and 4A) and LuRH (Figs 3D and 4D). Gonadotrophs were also significantly smaller in hpg mice (Figs 3F and 4F). Gonadotrophs in tfm (Figs 3G and 4G) and gonadectomised (Figs 3H and 4H) mice were similar to control gonadotrophs in size. No significant difference was measured in nuclear area in any of the mutant mice relative to control mice. Secretory granule numerical density represents the balance between gonadotrophin synthesis, granule formation, storage and release (35). Granule density was significantly (P < 0.01) reduced compared to controls in LuRKO, hpg, tfm and gonadectomised mice (Table 3). Secretory granule diameter was significantly reduced in FSHβKO (P < 0.05; Fig. 4B), LuRKO (P < 0.01; Fig. 4E) and tfm (P < 0.01; Fig. 4G) gonadotrophs compared to controls (Fig. 4A) but not significantly different in other groups (Table 3). Interestingly, the subpopulation of granules consisting of an electron-dense core and electron-lucent ‘halo’ was observed in all except the FSHβKO gonadotrophs (Fig. 4B). Figure 5 shows the distribution of immunogold labelling of LH and FSH on secretory granules in control C57 mice. FSH (5 nm) immunolabel distributed more strongly to larger electron-lucent granules and the ‘halo’ of granules with an electron-dense core, whereas LH immunogold was distributed to smaller electron-dense granules. Secretory granule diameter was significantly (P < 0.05) smaller in FSHβKO gonadotrophs compared to FSHRKO but granule distribution and density were not significantly different. Rough ER expansion reflects increased secretory protein synthesis. Rough ER was significantly (P < 0.01) reduced in the hpg mice but significantly (P < 0.01) increased in the LuRKO (Fig. 3E), tfm (Fig. 3G) and gonadectomised (Fig. 3H) mice (Table 3). The percentage of granules at the perimeter of the cell (which reflects the readily releasable pool of granules) was similar in control (Fig. 3A) FSHβKO (Fig. 3B) and FSHRKO (Fig. 3C) gonadotrophs, whereas this parameter was significantly (P < 0.01) increased in LuRKO, tfm and gonadectomised gonadotrophs (Fig. 3E, G, H). No obvious changes were measured in the thyrotroph population for any of the morphology parameters quantified (data not shown).
Fig. 3.
Representative electron micrographs of gonadotrophs in 8-week-old normal and reproductive mutant mice, namely (A) C57 Normal; (B) FSHβKO; (C) FSHRKO; (D) LuRKO; (E) LuRKO Het; (F) hpg; (G) tfm; (H) gonadectomised. Scale bar = 2 μm. Representative organelles are labelled: m, mitochondria; rer, rough endoplasmic reticulum; sg, secretory granule; n, nucleus; g, Golgi apparatus. Arrowheads in (E), (G) and (H) indicate granules at the periphery of the gonadotroph. KO, knockout; hpg, hypogonadal; tfm, testicular feminised.
Table 3.
Subcellular Morphology of Gonadotrophs in Male 8-Week-Old Normal and Mutant Mice.
| Control | FSHβKO | FSHRKO | LuRKO | hpg | tfm | Gonadectomised | |
|---|---|---|---|---|---|---|---|
| Cell area (μm2) | 101 ± 4 | 94 ± 6 | 115 ± 9 | 68 ± 3b | 55 ± 3a | 106 ± 7 | 108 ± 3 |
| Cytoplasm area (μm2) | 78 ± 4 | 73 ± 5 | 90 ± 9 | 48 ± 2b | 33 ± 2a | 80 ± 5 | 87 ± 4 |
| Nuclear area (μm2) | 21 ± 3 | 19 ± 3 | 26 ± 4 | 21 ± 4 | 20 ± 1 | 25 ± 2 | 20 ± 2 |
| Granule diameter (nm) | 181 ± 5 | 162 ± 8c | 185 ± 15 | 107 ± 5b | 194 ± 8 | 106 ± 4b | 188 ± 9 |
| Granule density (/μm2) | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.02 | 0.06 ± 0.01b | 0.07 ± 0.005a | 0.06 ± 0.01b | 0.05 ± 0.01b |
| Rough ER (units) | 2.6 ± 0.2 | 2.1 ± 0.2 | 2.3 ± 0.2 | 3.2 ± 0.2c | 1.3 ± 0.3a | 3.9 ± 0.1b | 3.8 ± 0.3b |
| % Granules at perimeter | 39 ± 4 | 32 ± 2 | 29 ± 5 | 55 ± 4b | 39 ± 3 | 57 ± 4b | 60 ± 2b, |
P < 0.01 versus normal C3H;
P<0.01,
P < 0.05 versus control.
Values expressed as the mean ± SEM (n = 4 animals). Three groups of controls were independently assessed C57BL6/129 (transgenic mouse background), C3H (hpg background) and LuRH (transgenic heterozygote). No significant differences were found for each of the parameters measured and, for clarity, the control data shown are from the C57BL6/129 mice.
Fig. 4.
Representative electron micrographs to show the morphological appearance of gonadotroph secretory granules in 8-week-old male, normal and reproductive mutant mice: (A) C57 Normal; (B) FSHβKO; (C) FSHRKO; (D) LuRKO; (E) LuRKO Het; (F) hpg; (G) tfm; (H) gonadectomised. Scale bar = 200 nm. Characteristic secretory granules comprising a dense core and electron lucent ‘halo’ are indicated by arrows. M, mitochondria. KO, knockout; hpg, hypogonadal; tfm, testicular feminised.
Fig. 5.
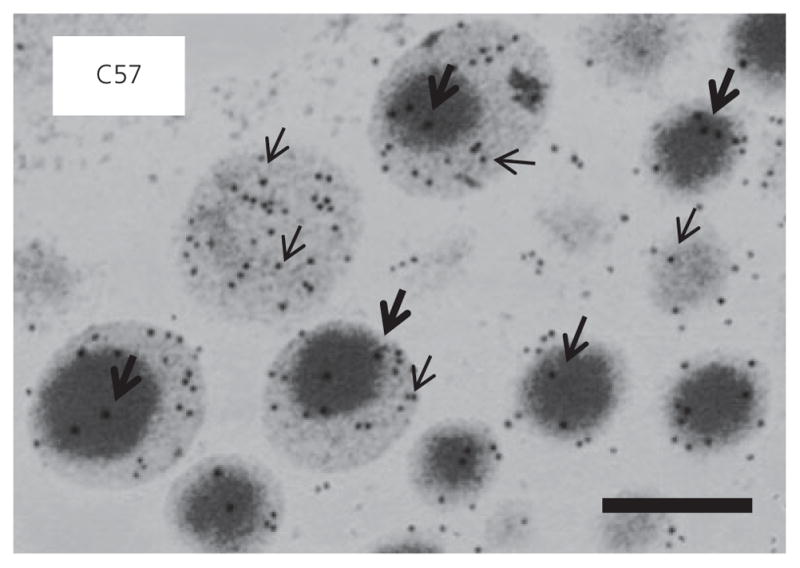
High power electron micrograph illustrating the distribution of luteinising hormone (LH) (15 nm; indicated by thickset arrows) immunogold and follicle-stimulating hormone (FSH) immunogold (5 nm; thinset arrows) in gonadotroph secretory granules in C57BL6/129 mouse pituitary. Scale bar = 200 nm.
Gonadotroph number
No significant difference in the percentage of gonadotrophs or somatotrophs (as a percentage of total secretory cell number) was measured between control and FSHβKO, FSHRKO, LuRKO, hpg, tfm and gonadectomised mice (Table 4).
Table 4.
Percentage of Luteinising Hormone (LH) and follicle stimulating hormone (FSH) Positive Cells (Gonadotrophs) and Growth Hormone (GH)-Positive Cells (Somatotrophs) in Wild-Type and Mutant Mice as a Percentage of Total Anterior Pituitary Secretory Cell Number.
| Control | FSHβKO | FSHRKO | LuRKO | hpg | tfm | Gonadectomised | |
|---|---|---|---|---|---|---|---|
| % LH & FSH positive cells | 12 ± 2 | 15 ± 2 | 16 ± 1 | 17 ± 1 | 19 ± 1 | 19 ± 2 | 20 ± 3 |
| % GH positive cells | 52 ± 2 | 54 ± 3 | 60 ± 3 | 53 ± 3 | 53 ± 3 | 53 ± 3 | 54 ± 4 |
Values expressed as the mean ± SEM (n = 4 animals). hpg, hypogonadal; tfm, testicular feminised; FSH, follicle-stimulating hormone; LuR, LH receptor; KO, knockout.
Testis weight and histology
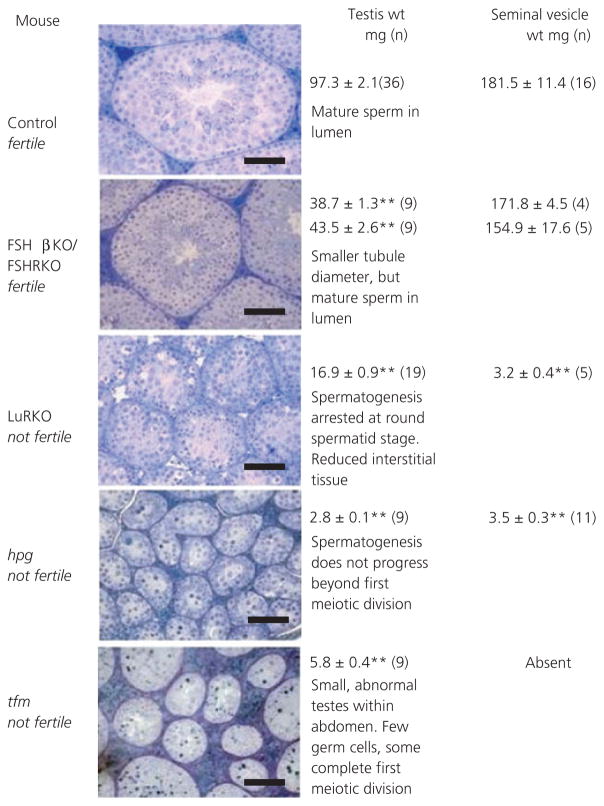
Testis weights in adult FSHβKO and FSHRKO mice were 46% and 43% of the corresponding heterozygote control males. In adult LuRKO males, testes weights were 20% of control weights. In adult mice, hpg testes were 3% and tfm were 6% of control weights (Fig. 6). Testis weights in all mutant males were significantly (P < 0.01) lower than weights in corresponding control males. Representative histology has been included in Fig. 6 to illustrate the major features of testicular morphology relevant to the present study (19–22).
Fig. 6.
Testis histology and weights in adult 8 week old control, FSHβKO, FSHRH, FSHRKO, LuRKO, hpg and tfm male mice. Also shown are seminal vesicle weights in control, FSHβKO, FSHRKO, LuRKO and hpg mice. Results are expressed as the mean ± SEM, **P < 0.01 versus control. The number of mice in each group is shown in brackets. Scale bars = 100 μm. KO, knockout; hpg, hypogonadal; tfm, testicular feminised.
Seminal vesicle weights
Seminal vesicle weights in adult FSHβKO and FSHRKO males did not differ from weights measured in control males (Fig. 6). Seminal vesicles remained pre-pubertal in adult LuRKO and hpg males and were significantly reduced in weight (2%; P < 0.01) relative to control males. Seminal vesicles did not develop in tfm males as a result of androgen insensitivity.
Discussion
The present study has directly compared the effects of mutations that perturb the HPG axis in male mice (specifically, effects on plasma gonadotrophin concentrations, gonadotrophin gene expression and pituitary content) and relates these findings to gonadotroph ultrastructure. These findings advance knowledge of the structure and function of primary gonadotrophs in situ in conditions of altered GnRH, LH, FSH and androgen signalling.
Previous studies have shown that adult FSHβKO and FSHRKO male mice present with a similar phenotype despite the difference in genetic modifications. The absence of biologically active FSH in FSHβKO males and the absence of a functional FSH receptor in FSHRKO males leads to a reduction in Sertoli cell number as a result of a lack of stimulation of Sertoli cell mitosis in the early postnatal period and a consequent reduction in testis size in the adult animal (18–20). Despite the similar phenotypes, differences have been found between these adult males, with FSHRKO males having a reduced number of Leydig cells and lower levels of expression of genes in the steroidogenic pathway, leading to a decreased intra-testicular testosterone levels (19).
We have previously shown that the FSH receptor shows constitutive activity in the absence of ligand stimulation in vitro and differences between these two KO lines may reflect residual activity of the FSH receptor in vivo in the FSHβKO mouse (19). In the present study, serum levels of LH were similar in FSHβKO, FSHRKO and heterozygote males, suggesting that GnRH and or Kiss 1 activity within the hypothalamus were not increased by decreased androgen production in KO males. However, the significant increase in expression of the common α and LHβ subunit genes in FSHRKO males may reflect the lower androgen levels because androgen has been reported to decrease LHβ mRNA levels in pituitary cells in vitro (12,13). Although androgen has been reported to increase activity of FSHβ subunit genes in vitro (14), it is likely that in vivo additional factors such as inhibin regulate expression of this gene and account for the similarity in expression in FSHRKO and FSHβKO males. The absence of detectable FSH in both serum and pituitary and the lack of expression of FSHβ subunit gene confirmed the expected lack of synthesis of FSH in the FSHβKO males. The secretory granules in FSHKβO gonadotrophs were significantly smaller than controls and there was a lack of the subpopulation of granules characterised by an electron-dense core and electron-lucent halo. Immunogold labelling localised FSH to the electron-lucent halo and this compartment appears to be lost in the absence of FSH synthesis and storage. Therefore, it would appear that high FSH content determines the presence of the ‘halo’. The LH and FSH immunogold distribution that we have observed in mouse gonadotrophs confirms studies in the rat (3,36), which have previously shown differential storage of LH and FSH. In the rat, large-sized granules (500 nm in diameter) of moderate electron density were found to be immunoreactive for FSH and LH, whereas small-sized electron-dense granules (200 nm diameter) were immunoreactive exclusively for LH (3,36). Furthermore, differential association between granins (secretogranin II and chromogranins A and B) and LH and differing granule morphology has been reported to facilitate differential storage in rats and mice, although it is not known how the granins might contribute to the the mechanism of constitutive FSH release (36,37). Serum levels of LH were similar in FSHβKO and heterozygote males, confirming the original observations of Kumar et al. (18), and reflected the absence of any differences in pituitary content of this hormone found in the present study. However, Kumar et al. (18) suggested that disruption of the FSHβ gene could have implications for the synthesis and assembly of the common α and LHβ subunits within the pituitary. Although no differences in pituitary content of LH were observed between FSHβKO and H males, it was interesting to find that both serum level and pituitary content of LH (and FSH in H mice) were considerably lower in the FSHβKO line compared to both FSHRKO and FSHRH mice.
By contrast to the loss of FSH stimulation of the Sertoli cell, the absence of LH activation of Leydig cells induced profound effects on serum levels of both FSH and LH and LHβ mRNA in the LuRKO mouse. In these mice, the Sertoli cell complement is normal but Leydig cells are reduced in number and size and testosterone production is severely compromised in the absence of LH stimulation (21). Gonadectomy is well known to increase concentrations of both gonadotrophins in mice (38), with the removal of androgen negative-feedback increasing hypothalamic GnRH levels that stimulate the increased synthesis of LH and FSH in gonadotrophs. In the present study, in both LuRKO males and gonadectomised males, pituitary content of FSH was significantly reduced relative to normal/heterozygote controls, suggesting that all newly-synthesised FSH is released from the gonadotrophs in the absence of androgen feedback. Therefore, androgens may contribute to the regulation of the tonic release of FSH in the intact male. A similar profile of results was measured in tfm males where the absence of functional androgen receptors prevents the action of testosterone at either the hypothalamus or pituitary. Hyperstimulation of gonadotrophin secretion, consistent with a lack of feedback in LuRKO, tfm and gonadectomised mice, is reflected by changes in their subcellular gonadotroph morphology. Rough ER and Golgi apparatus appear dilated, reflecting an elevated protein synthesis and glycosylation. The area occupied by granules per cytoplasmic profile (granule density) and the number of granules is reduced, reflecting an increased granule release. Also, granules are relatively marginated to the plasma membrane, representing a larger readily releasable pool. In the hpg, mouse there were more secretory granules than would be expected for the amount of hormone measured. As observed in previous studies (39), despite very low stores of LH and FSH (< 5% than control), secretory granule density was comparatively high (50% of control). However, LH and FSH immunogold labelling in granules appeared to be reduced in hpg gonadotrophs, indicating that the intensity of immunogold labelling was correlated better with amounts of hormone measured by an enzyme-linked immunoabsorbent assay than the number of granules. In the hpg mouse, the reduced size of gonadotrophs correlated with a lack of synthesis and secretion of FSH and LH. However, an increase in synthesis and secretion did not necessarily correlate with an increase in cell area. Gonadotrophs in LuRKO mice, although significantly larger than hpg gonadotrophs, remain smaller than control gonadotrophs despite the fact that all functional parameters of gonadotrophin synthesis and secretion were increased.
By contrast, pituitary content of LH in 1-month gonadectomised males did not differ from that in control mice, despite the significantly higher serum levels of LH, suggesting that synthesis was able to keep pace with secretion over this time period. The finding that granule diameter in gonadectomised was not significantly different from control was consistent with this. In LuRKO and tfm mice, pituitary content of LH was significantly depleted relative to control males and this may reflect the failure to establish negative feedback regulation in these mice where testosterone is not produced or recognised from birth. Increased levels of LHβ mRNA were seen in LuRKO and tfm males corresponding to the high output of LH in these mice. Gonadectomy results not only in steroid withdrawal, but also in the loss of any peptide feedback to the pituitary. In gonadectomised mice, mRNA levels of common α and FSHβ subunit genes were significantly increased, suggesting that the loss of factors in addition to androgen was allowing increased activity of these gonadotrophin genes. FSHβ mRNA levels were also significantly increased in tfm mice but not in LuRKO males. The Sertoli cell complement is normal in LuRKO males and the increased testis mass in these mice, which is predominantly Sertoli cells (21), relative to tfm may account for the lower activity of the FSHβ subunit gene. Although tfm males have a full complement of Sertoli cells, spermatogenesis is compromised not only by the absence of functional androgen receptors, but also by the retention of the testes within the abdominal cavity. Sertoli cells have been seen within the lumen of the seminiferous tubules in these mice, suggesting a loss of cell viability and physiological function (40).
In the GnRH deficient hpg mouse, pituitary content and serum levels of both LH and FSH were a fraction of that seen in the normal mouse, as reported in earlier studies (4), emphasising the primary role of GnRH in stimulating synthesis of LH and FSH in gonadotrophs. Transcription of the three gonadotrophin genes in the pituitary was minimal, with mRNA levels significantly below that seen in controls and all mutant mice, reflecting the lack of GnRH activation above basal levels. However TSHβ mRNA levels in hpg males were similar to levels measured in other mutant mice, confirming that the effects of the GnRH mutation are confined to the gonadotrophin axis. Interestingly, levels of common α subunit mRNA were higher than the sum of the β subunit mRNA levels, a pattern not seen in any other mouse, suggesting that the activity of the common α subunit gene may be less dependent on GnRH stimulation compared to the β subunit genes. This may reflect the presence of a GnRH independent region, within the promoter region of the common α gene, regulating basal secretion, in addition to a GnRH responsive element (41). Transcriptional activity of the common α subunit gene within thyrotroph cells will have contributed to the mRNA levels recorded in the present study. Although disruption of the pituitary α subunit has been shown to result in thyrotroph hyperplasia (42), in the present study, no obvious changes were observed in thyrotroph morphology in our mutant mice relative to control males.
In summary, major differences in pituitary content and serum concentrations of the gonadotrophins LH and FSH have been found between normal and mutant male mice in the present study. These differences are associated with changes in mRNA levels of the gonadotrophin subunit genes and are reflected by changes in the cellular structure and granular architecture within the gonadotroph cells. Consistent between the mutants are the finding that increased rough ER density corresponds with increased gonadotrophin synthesis, and also that increased circulating LH and FSH associate with a decrease in granule size, numerical density and increase in proximity to the plasma membrane. These ultrastructural changes in gonadotrophs may therefore be useful for predicting reproductive endocrine status. A loss of androgen feedback allows the gonadotroph to free run with depletion of hormone stores, initially more severe for FSH but, in the long term, also depleting LH stores within the pituitary. Androgens are the major factor regulating GnRH control of both LH and FSH synthesis, although, in mice with functional Sertoli cells, additional factors may act to regulate FSH production. The major role of GnRH in controlling gonadotrophin synthesis is through regulation of subunit gene mRNA levels in the gonadotroph. The significantly lower levels of TSHβ mRNA within the pituitaries of LuRKO, hpg, tfm and gonadectomised males relative to control males suggests that androgens may also have a role in the expression of the TSHβ subunit gene and/or effects on thyrotroph differentiation, and this requires further investigation. The present study has shown differences between mutant mice at the single time point of 8 weeks of age. Follow-up studies are investigating changes with advancing age. Knowledge of reproductive mutant models such as those reported here has corroborated information from human mutations (43) and provides useful tools for exploring pathogenesis and treatments for human infertility.
Acknowledgments
We thank Vivienne Wilkins, Lynne Scott and Mohan Masih for their excellent technical assistance and the Staff of the Biological Services Unit, Oxford, for care of the animals.
References
- 1.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 2.Naylor SL, Chin WW, Goodman HM, Lalley PA, Grzeschik KH, Sakaguchi AY. Chromosome assignment of genes encoding the alpha and beta subunits of glycoprotein hormones in man and mouse. Somatic Cell Genet. 1983;9:757–770. doi: 10.1007/BF01539478. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Uchiyama Y, Grube D. Topology of chromogranin A and secretogranin II in the rat anterior pituitary: potential marker proteins for distinct secretory pathways in gonadotrophs. Histochemistry. 1991;96:285–293. doi: 10.1007/BF00271348. [DOI] [PubMed] [Google Scholar]
- 4.Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- 5.Goodman RL, Lehman MN. Kisspeptin from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toranzo D, Dupont E, Simard J, Labrie C, Couet J, Labrie F, Pelletier G. Regulation of pro-gonadotropin-releasing hormone gene expression by sex steroids in the brain of male and female rats. Mol Endocrinol. 1989;3:1748–1756. doi: 10.1210/mend-3-11-1748. [DOI] [PubMed] [Google Scholar]
- 7.Selmanoff M, Shu C, Petersen SL, Barraclough CA, Zoeller RT. Single cell levels of hypothalamic messenger ribonucleic acid encoding luteinizing hormone-releasing hormone in intact, castrated, and hyperprolactinemic male rats. Endocrinology. 1991;128:459–466. doi: 10.1210/endo-128-1-459. [DOI] [PubMed] [Google Scholar]
- 8.Gross DS. Effect of castration and steroid replacement on immunoreactive gonadotropin-releasing hormone in hypothalamus and preoptic area. Endocrinology. 1980;106:1442–1450. doi: 10.1210/endo-106-5-1442. [DOI] [PubMed] [Google Scholar]
- 9.Kalra PS, Kalra SP. Modulation of hypothalamic luteinizing hormone-releasing hormone levels by intracranial and subcutaneous implants of gonadal steroids in castrated rats: effects of androgen and estrogen antagonists. Endocrinology. 1980;106:390–397. doi: 10.1210/endo-106-1-390. [DOI] [PubMed] [Google Scholar]
- 10.Colledge WH. GPR54 and kisspeptins. Results Probl Cell Differ. 2008;46:117–143. doi: 10.1007/400_2007_050. [DOI] [PubMed] [Google Scholar]
- 11.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- 12.Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA. Androgen suppression of GnRH-stimulated rat LHβ gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol. 2001;15:1906–1917. doi: 10.1210/mend.15.11.0723. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen JS, Nilson JH. AR suppresses transcription of the LHβ subunit by interacting with steroidogenic factor-1. Mol Endocrinol. 2001;15:1505–1516. doi: 10.1210/mend.15.9.0691. [DOI] [PubMed] [Google Scholar]
- 14.Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL. Androgen regulates follicle-stimulating hormone beta gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol. 2004;18:925–940. doi: 10.1210/me.2003-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik SI, Young LS, Charlton HM, Clayton RN. Pituitary gonadotropin-releasing hormone receptor regulation in mice. I. Males. Endocrinology. 1984;115:106–113. doi: 10.1210/endo-115-1-106. [DOI] [PubMed] [Google Scholar]
- 16.McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl. 2003;61:463–476. [PubMed] [Google Scholar]
- 17.Nicol L, McNeilly JR, Stridsberg M, McNeilly AS. Differential secretion of gonadotrophs: investigation of the role of secretogranin A in the release of LH and FSH in LbT2 cells. J Mol Endocrinol. 2004;32:467–480. doi: 10.1677/jme.0.0320467. [DOI] [PubMed] [Google Scholar]
- 18.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 19.Baker PJ, Pakarinen P, Huhtaniemi IT, Abel MH, Charlton HM, Kumar TR, O’Shaughnessy PJ. Failure of normal Leydig cell development in follicle-stimulating hormone (FSH) receptor-deficient mice, but not FSHbeta-deficient mice: role for constitutive FSH receptor activity. Endocrinology. 2003;144:138–145. doi: 10.1210/en.2002-220637. [DOI] [PubMed] [Google Scholar]
- 20.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 21.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 22.Pakarainen T, Zhang FP, Makela S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146:596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- 23.Mason AJ, Pitts SL, Nikolics K, Szonyi E, Wilcox JN, Seeburg PH, Stewart TA. The hypogonadal mouse: reproductive functions restored by gene therapy. Science. 1986;234:1372–1378. doi: 10.1126/science.3097822. [DOI] [PubMed] [Google Scholar]
- 24.Lyon MF. Hawkes SG X-linked gene for testicular feminisation in the mouse. Nature. 1970;277:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 25.Gasper ML, Meo T, Bougarel P, Guenet JL, Tose M. A single base deletion in the tfm androgen receptor gene creates a short lived messenger RNA that directs internal translation initation. Proc Natl Acad Sci USA. 1991;88:8606–8610. doi: 10.1073/pnas.88.19.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dofuku R, Tettenborn U, Ohno S. Testosterone – ‘regulon’ in the mouse kidney. Nature. 1971;232:5–7. doi: 10.1038/newbio232005a0. [DOI] [PubMed] [Google Scholar]
- 27.Lyon MF, Hendry I, Short RV. The submaxillary salivary glands as test organs for response to androgen in mice with testicular feminization. J Endocrinol. 1973;58:357–362. doi: 10.1677/joe.0.0580357. [DOI] [PubMed] [Google Scholar]
- 28.Lyon MF, Glenister PH, Lynn Lamoreux M. Normal spermatozoa from androgen-resistant germ cells of chimeric mice and the role of androgen in spermatogenesis. Nature. 1975;258:620–622. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- 29.Hirst RC, Abel MH, Wilkins V, Simpson C, Knight PG, Zhang FP, Huhtaniemi I, Kumar TR, Charlton HM. Influence of mutations affecting gonadotropin production or responsiveness on expression of inhibin subunit mRNA and protein in the mouse ovary. Reproduction. 2004;128:43–52. doi: 10.1530/rep.1.00176. [DOI] [PubMed] [Google Scholar]
- 30.Lang J. Assay for deletion in GnRH (hpg) locus using PCR. Mouse Genome. 1991;89:857. [Google Scholar]
- 31.Haavisto A-M, Pettersson K, Bergendahl M, Perheentupa A, Roser J, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- 32.van Casteren JI, Schoonen WG, Kloosterboer HJ. Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol Reprod. 2000;62:886–894. doi: 10.1095/biolreprod62.4.886. [DOI] [PubMed] [Google Scholar]
- 33.O’Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod. 2002;66:966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- 34.Christian HC, Chapman LP, Morris JF. Thyrotrophin releasing hormone, vasoactive intestinal peptide, prolactin-releasing peptide and dopamine regulation of prolactin secretion by different lactotroph morphological subtypes in the rat. J Neuroendocrinol. 2007;19:605–613. doi: 10.1111/j.1365-2826.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 35.Lewis CE, Morris JF, Fink G, Johnson M. Changes in the granule population of gonadotrophs of hypogonadal (hpg) and normal female mice associated with the priming effect of LH-releasing hormone in vitro. J Endocrinol. 1986;109:35–44. doi: 10.1677/joe.0.1090035. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Banno T, Jeziorowski T, Ohsawa Y, Waguri S, Grube D, Uchiyama Y. Effects of sex steroids on secretory granule formation in gonadotropes of castrated male rats with respect to granin expression. Endocrinology. 1998;139:2765–2773. doi: 10.1210/endo.139.6.6059. [DOI] [PubMed] [Google Scholar]
- 37.Crawford J, McNeilly JR, Nicol L, McNeilly AS. Promotion of intragranular co-aggregation with LH by enhancement of secretogranin II storage resulted in increased intracellular storage in gonadotrophs of GnRHdeprived male mice. Reproduction. 2002;124:267–277. doi: 10.1530/rep.0.1240267. [DOI] [PubMed] [Google Scholar]
- 38.Kovacic N, Parlow AF. Alterations in serum FSH-LH ratios in relation to the estrous cycle, pseudopregnancy, and gonadectomy in the mouse. Endocrinology. 1972;91:910–915. doi: 10.1210/endo-91-4-910. [DOI] [PubMed] [Google Scholar]
- 39.McDowell IFW, Morris JF, Charlton HM. Characterization of the pituitary gonadotroph cells of hypogonadal (hpg) male mice: comparison with normal mice. J Endocrinol. 1982;95:321–330. doi: 10.1677/joe.0.0950321. [DOI] [PubMed] [Google Scholar]
- 40.Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O’Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 41.Maurer RA, Kim KE, Schoderbek WE, Robertson MS, Glenn DJ. Regulation of glycoprotein hormone alpha-subunit gene expression. Rec Prog Horm Res. 1999;54:455–485. [PubMed] [Google Scholar]
- 42.Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 43.Kumar TR. What have we learned about gonadotropin function from gonadotropin and receptor knockout mice? Reproduction. 2005;130:293–302. doi: 10.1530/rep.1.00660. [DOI] [PubMed] [Google Scholar]