Abstract
The spinal cord is the initial stage that integrates temperature information from peripheral inputs. Here we used molecular genetics and in vivo calcium imaging to investigate the coding of cutaneous temperature in the spinal cord in mice. We found that heating or cooling the skin evoked robust calcium responses in spinal neurons, and their activation threshold temperatures distributed smoothly over the entire range of stimulation temperatures. Once activated, heat-responding neurons encode the absolute skin temperature without adaptation and receive major inputs from TRPV1+ dorsal root ganglion (DRG) neurons. By contrast, cold-responding neurons rapidly adapt to ambient temperature and selectively encoded temperature changes. These neurons receive TRPM8+ DRG inputs as well as novel TRPV1+ DRG inputs that were selectively activated by intense cooling. Our results provide a comprehensive examination of the temperature representations in the spinal cord and reveal fundamental differences in the coding of heat and cold.
Thermosensation enables mammals to maintain the homeostasis of body temperature and to avoid noxious temperature that may cause tissue damage. Ambient temperature is detected by the free nerve endings of primary sensory neurons in the skin. These neurons, whose cell bodies are located in the DRG, synapse onto the dorsal horn neurons in the spinal cord, where information is further processed before being transmitted to the brain. In the past decades, significant progress has been made in identifying molecular temperature sensors that transduce temperature stimulus into action potentials in the primary sensory neurons1–12. These molecular sensors mostly belong to the family of transient receptor potential (TRP) ion channels. When characterized in heterologous expression systems, these thermoTRPs are activated at specific threshold temperatures and function as dedicated transducers of distinct thermal modalities, including cold, cool, warmth and heat3,6–9,13. Among all thermoTRPs, TRPM8 and TRPV1 have been most intensively studied. These two thermoTRPs are expressed in largely separated populations of DRG neurons and are activated below ~25 °C or above ~43 °C, respectively6–9,14–16. Therefore, TRPM8- and TRPV1-expressing DRG neurons were considered to be dedicated pathways for cold and noxious heat, respectively.
In contrast to the well-characterized molecular anatomy of the primary sensory neurons, our understanding of the representation of temperature information in its next relay center, the spinal cord, is still limited17,18. Early elegant in vivo electrophysiological studies have investigated this question and have revealed considerably heterogeneous stimulus-response relationships and adaptation kinetics, reflecting the complexity of the spinal cord circuitry19–22. However, the low throughput nature of in vivo electrophysiological recording method constrains its sampling from a small number of neurons per animal22. Moreover, the contributions of each molecularly defined DRG input to temperature response in the spinal cord are still far from clear. Specifically, since only a small proportion of spinal neurons respond to innocuous cold and warmth stimuli19,22, the contributions of TRPM8- or TRPV1- expressing DRG inputs to spinal responses evoked by these temperatures stimuli have been difficult to examined with in vivo electrophysiological recording. Therefore, understanding how the entire spinal circuitry orchestrates and represents temperature information requires recording from a large number of neurons and delivering thermal stimuli in a precisely controlled manner.
We therefore developed an in vivo two-photon spinal cord imaging preparation that permits simultaneous optical recording of activities of a large number of neurons with single cell resolution. We found that the activation threshold temperatures of spinal neurons are distributed smoothly across the entire range of temperature stimuli. After reaching individual neurons’ thresholds, spinal neurons signal temperature change in the cold range, but absolute temperature in the heat range. We also found a substantial number of spinal neurons that respond to both cooling and heating (broadly tuned neurons), and the percentage of these broadly tuned neurons increase with thermal stimulus intensity. Furthermore, combining in vivo imaging with ablation of genetically defined peripheral sensory inputs, we found that spinal neurons that are activated by mild cold stimuli received input from TRPM8-expressing DRG neurons, whereas TRPV1-expressing neurons mediate spinal responses to heat and strong cold. Together, our study provides the first comprehensive examination of the representations of temperature in the spinal cord.
RESULTS
In vivo two-photon calcium imaging of mouse spinal cord
We performed two-photon imaging to record thermal stimuli-evoked calcium signals in a large population of spinal dorsal horn neurons in anesthetized mice (Fig. 1a). To simultaneously activate a large number of neurons in the spinal cord, we depilated the hind limb, which is innervated by the L4 nerve, and placed it in a stimulation chamber that was constantly superfused with 32 °C water to maintain the skin temperature close to its physiological setting. Superfusion of water at temperatures ranging from 5 °C to 50 °C led to rapid and even changes of cutaneous temperature on the surface of the hind limb (Fig. 1b,c, see Methods). Temperature measured subcutaneously did not differ from the temperature measured on the surface by more than 1 °C, demonstrating the rapid change of temperature across the thickness of the skin (Supplementary Fig. 1). We then performed a dorsal laminectomy at spinal level L4 and bulk-loaded dorsal horn neurons with the calcium-sensitive dye Oregon Green 488 BAPTA-1 AM (OGB) for functional imaging (Fig. 1d–f)23–25. OGB was chosen over genetically encoded calcium indicators, such as GCaMPs26,27, due to the lack of proper transgenic mouse line that pan-neuronally expresses GCaMP in the superficial laminae of the spinal cord and the inflammation after intra-spinal injection of adeno-associated virus expressing GCaMP weeks prior to imaging. We focused on neurons 25–85 µm below the spinal cord surface, since temperature-sensing DRG neurons largely innervate the superficial laminae of the dorsal horn3. In this region, we usually imaged ~400 cells in a field of view (438 µm × 438 µm), consistent with the high neuronal density in the superficial laminae of the spinal cord (Fig. 1g,h).
Figure 1. In vivo two-photon calcium imaging in the spinal cord.
(a) Schematic illustrating the imaging preparation. The colored dots show the four different locations where stimulation temperature was recorded. (b) Recorded stimulation temperature at the four locations in a during the thermal stimulation. (c) Overlay of temperature curves in b. (d) An example field of view (438 µm × 438 µm) of 413 OGB labeled neurons. Neurons that showed positive responses to the cooling stimulus (see Methods) are marked in green. Similar observations were made in more than 55 experiments. Scale bar, 50 µm. (e) Representative ΔF/F traces from neurons responded to the cooling stimulus. Colored traces are averaged ΔF/F from two individual trials (grey traces). Scale bars are 10 s and 10% ΔF/F, respectively. (f) ΔF/F heat maps for the 193 cold-responding neurons in the FOV in d during a cooling stimulus to 16 °C. Scale bar, 10 s. (g) Example images showing NeuN stained sections of the spinal superficial laminae (left) and layer 2/3 of the motor cortex (right). Scale bars, 50 µm. (h) Quantification of g (spinal cord, n = 5 FOVs from 3 mice; cortex, n = 6 FOVs from 3 mice, ** P = 0.0043, U = 0.0000, Mann Whitney test, Error bars represent s.e.m.). (i) Top: recorded stimulation temperature during ten cooling trials. Bottom: heat maps showing the activity of all 102 neurons that were activated in any one of the ten trials in one mouse, sorted by their maximum response amplitudes. Each row in the heat map represents the response from the same neuron in different trials. Scale bar, 10 s. (j) A rank-ordered plot of peak ΔF/F of all 102 neurons in i. Gray, maximum response amplitudes of individual trials; red, mean.
Cooling-evoked responses in the spinal cord
We first examined the response to skin cooling and observed robust, reliable calcium transients in subsets of spinal dorsal horn neurons. Most cold-responding neurons were found in superficial laminae within 60 µm from the surface (Supplementary Fig. 2a). On average, the peak amplitudes of cooling-evoked responses varied within 6% of the mean across 10 trials (Fig. 1i,j), and these responses were blocked by direct application of glutamate receptor antagonists APV and NBQX onto the spinal cord, indicating that the calcium signals were mediated by synaptic transmission (Supplementary Fig. 2b). Although astrocyte can be labeled with OGB, no response to cold stimuli was detected in astrocytes (Supplementary Fig. 2c).
In general, cooling to lower temperatures activated more neurons and evoked larger calcium signals when averaging the peak amplitudes across all responders (Fig. 2a–c). Seventy present of all cold-responding neurons responded to temperature drops less than 6 °C, revealing their exquisite sensitivity to mild cooling (Fig. 2c). The onsets of calcium transients in cold-responding neurons tiled the entire period of thermal stimulation, suggesting that each individual cold-responding neuron is activated at a specific threshold temperature, and that unique ensemble of these neurons represent specific cooling stimuli applied to the skin (Fig. 2d).
Figure 2. Representation of cold intensity in the spinal cord.
(a) Neuronal responses evoked by cooling the skin from 32 °C to different target temperatures. Top: recorded temperature trace of each cooling stimulus. Middle: an example field of view (FOV) showing neurons activated by the corresponding cooling stimulus. Similar observations were made in 12 mice. Neurons that showed positive responses to cold are marked in green. Bottom: ΔF/F heat maps for all 138 cold-responding neurons in the same FOV during cooling to different temperatures. Neurons are rank-ordered by their response onset times during the cooling stimulus to 5 °C. Each row in the heat maps represents responses from the same neuron to different cooling stimuli. Scale bar, 10 s. (b) Top: stimulation temperature traces. Bottom: calcium traces of 5 representative neurons with different activation temperature thresholds. Cooling period is marked with cyan. Scale bars, 10 s and 10% ΔF/F. (c) Left Y axis, quantification of the percentage of total OGB labeled neurons that responded to the given cooling stimuli in an FOV (squares). Right Y axis, the averaged peak ΔF/F (circles) at a given temperature indicated on the Y axis from all cold responders to any cooling stimuli (n = 12 mice). Error bars represent s.e.m. (d) An overlay of the temperature trace of a cooling stimuli (white, from 32 °C to 16 °C) with neuronal responses during this stimulus (heat map). Scale bar, 10 s.
Besides the absolute temperature value, our perception of temperature is also influenced by the rate of temperature change28–30. To evaluate the impact of cooling rate on spinal sensory responses, we used a two-stage stimulus: a "change" stage in which temperature was changed at a constant rate, followed by a "stable" stage in which a specific target temperature was maintained for an extended period before returning to the holding temperature (32 °C; Fig. 3a). We did not observe noticeable effects of cooling rate on the percentage of cold-responding neurons or their peak response amplitudes (Fig. 3a–c). Notably, the vast majority of cold-responding neurons peaked during the "change" stage, and their responses drastically decreased when entering the "stable" stage (Fig. 3a,b). Therefore, since a longer "change" stage was required to reach the same target temperature at a lower cooling rate, the calcium transients were wider when cooling was slower (Fig. 3b,d). Together, these results indicate that cold-responding neurons robustly respond to cooling but rapidly adapt to a steady cold temperature, suggesting that cold-responding neurons detect temperature changes (ΔT).
Figure 3. Rapid adaptive responses to cooling.
(a) Heat maps of normalized ΔF/F traces illustrating the response kinetics to cooling. Top: recorded temperature trace of each cooling stimulus with different temperature change rates. Green: slow, 0.31 °C·s−1, teal: intermediate, 0.56 °C·s−1, blue: fast, 0.99 °C·s−1. Bottom: heat maps of the response kinetics of all 113 cold-responding neurons in one FOV. The peak ΔF/F of each responding neuron is normalized to 100%. Neurons are rank-ordered by their response onset times to the slowest cooling stimulus. Each row in the heat maps represents the response of the same neuron to different cooling rates. Scale bar, 10 s. (b) Top: stimulation temperature traces. Bottom: calcium traces of 5 representative neurons to cooling from 32 °C to 22 °C at different cooling rates. Scale bars, 10 s and 10% ΔF/F. (c) Quantification of the number of cold-responding neurons (squares) and the averaged peak ΔF/F (circles) in response to three different cooling stimuli. (d) Quantification of the full width at half maximum (FWHM, illustrated in the insert) of ΔF/F traces evoked by cooling at different rates (n = 6 mice). ** P = 0.0017, Friedman statistic = 10.33, Friedman Test. Error bars represent s.e.m.
If cold-responding neurons selectively encode temperature change, their response amplitude should correlate with ΔT regardless of the absolute temperature. To test this hypothesis, we performed experiments in which the absolute temperature and ΔT were changed separately. First, we examined the response to the same target temperature (19 °C) with different ΔTs by adjusting the adaptation temperature (AT, see method). Consistent with our hypothesis, cooling from higher ATs to the same target temperature activated more neurons and evoked larger responses, as a result of the larger ΔTs (Fig. 4a,b). Second, we found that when the AT was lower than the normal skin temperature (32 °C), cooling the skin with the same ΔT (8 °C) activated the same group of neurons with similar response amplitudes regardless of the AT, revealing the exquisite accuracy of encoding ΔT in cold-responding neurons (Fig. 4c,d). Third, we combined these two stimulation conditions, cooled the skin from two different ATs (32 °C and 27 °C) with different ΔTs. We found a larger ΔT evoked larger responses but different ATs had minimal effects when ΔT was the same (Supplementary Fig. 3). Notably, when the ATs were higher than 32 °C, the same ΔT from higher ATs evoked smaller spinal responses (Fig. 4c,d). Nevertheless, a large number of cold-responding neurons were activated even when cooling from an AT of 42 °C, suggesting that these neurons could potentially mediate the cool sensation of a breeze in hot environments. Collectively, these results indicate that when cooling the temperature below their activation thresholds, cold-responding neurons encode temperature change. This rapid adaption to steady temperature may enable the system to signal temperature change over a wide range of ambient temperatures.
Figure 4. Neurons encode the change of temperature for cold.
(a) Neuronal responses to cooling to 19 °C from different adaptation temperatures (ATs). Top: recorded temperature traces of cooling by different ΔTs (3 °C, 8 °C, 13 °C and 18 °C). Bottom: ΔF/F heat maps demonstrating fluorescence changes of all 163 cold-responding neurons in the same FOV to the corresponding cooling stimulus. Neurons are rank-ordered by their response onset times to the cooling by ΔT = 18 °C. Each row in the heat map represents the responses from the same neuron to a different cooling stimulus. Scale bar, 10 s. (b) Quantification of the number of cold-responding neurons (squares, left Y axis) and the peak ΔF/F (circles, right Y axis) averaged across all cold-responders to any of the four cooling stimuli (ΔT = 18 °C, n = 3 mice; other data points, n = 4 mice). (c) Neuronal responses to cooling by 8 °C from different ATs. Top: recorded temperature traces of cooling stimuli with different ATs (42 °C, 37 °C, 32 °C, 27 °C and 22 °C). Bottom: ΔF/F heat maps demonstrating fluorescence changes of all 141 cold-responding neurons the corresponding cooling stimulus. Neurons are rank-ordered by their response onset times to cooling from 22 °C to 14 °C. Scale bar, 10 s. (d) Quantification of c (AT = 22–37 °C, n = 12 mice; AT = 42 °C, n = 4 mice). Error bars represent s.e.m.
Heating-evoked responses in the spinal cord
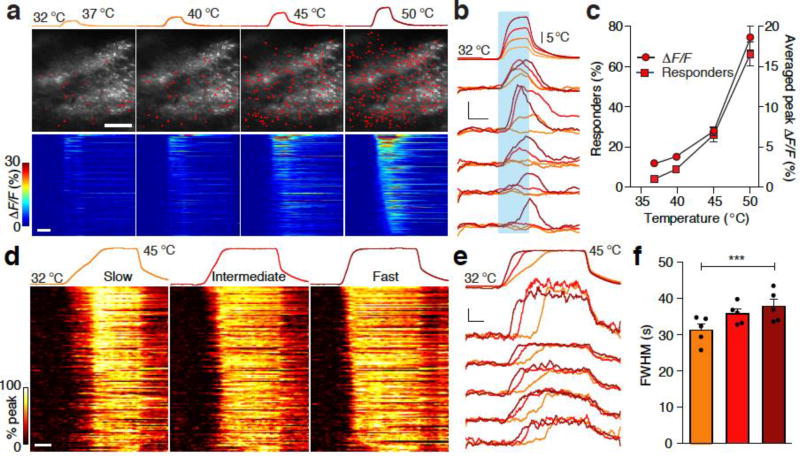
The representation of heat in the dorsal horn was drastically different from that of cold. Mild heating from the normal skin temperature (32 °C, ΔT = 5–8 °C) only activated less than 15% of heat-responding neurons, whereas stronger heating (ΔT > 8 °C) was required to activate the rest (Fig. 5a–c). The percentage of neurons that responded to strong heating gradually increased with depth (Supplementary Fig. 4a). Different heating rates had no noticeable effects on the percentage of activated heat-responding neurons or their peak response amplitudes (Supplementary Fig. 4b). In contrast to cooling-evoked responses, responses to heat showed no adaptation and remained high during the "stable" stage of the stimulus. Therefore, calcium transients were the widest when the heating was the fastest (Fig. 5d–f).
Figure 5. The representation of heating in the spinal cord.
(a) Neuronal responses to heating the skin from 32 °C to different target temperatures. Top: recorded temperature trace of each heating stimulus. Middle: an example FOV image showing neurons activated by the corresponding heating-stimulus. Heat-responding neurons are marked in red. Bottom: ΔF/F heat maps demonstrating fluorescence changes of all 276 heat-responding neurons in the same FOV to different temperatures. Neurons are rank-ordered by their response onset times to the 50 °C stimulus. Each row in the heat maps represents responses from the same neuron to different temperatures. Similar observations were made in ten mice. Scale bar, 100 µm. (b) Top: recorded stimulation temperature traces. Bottom: calcium traces of 5 representative neurons with different activation temperature thresholds. Heating period is marked with cyan. Scale bars, 10 s and 10% ΔF/F. (c) Left Y axis, quantification of the percentage of total OGB loaded neurons that respond to the given heating stimuli in an FOV (squares). Right Y axis, the averaged ΔF/F (circles) at a given temperature indicated on the Y axis from all heat responders to any heating stimuli (n = 10 mice). (d) Heat maps of normalized ΔF/F traces illustrating response kinetics to heating. Top: temperature traces of each heating stimuli with different temperature change rates. Orange: slow 0.31 °C·s−1, red: intermediate 0.56 °C·s−1, cardinal: fast 0.99 °C·s−1. Bottom: heat maps of the response kinetics of all 137 heat-responding neurons in one FOV. Peak ΔF/F of each responding neuron is normalized to 100%. Neurons are rank-ordered by their response onset times for the slowest heat stimulus. Each row in the heat map represents responses from the same neuron to different heating rates. Scale bar, 10 s. (e) Top: stimulation temperature traces. Bottom: calcium traces from 5 representative neurons to heating from 32 °C to 45 °C, at different heating rates. ΔF/F signals closely followed the temperature curve of the heat stimuli. Scale bars, 10 s and 10% ΔF/F. (f) Quantification of FWHM of ΔF/F traces evoked by heating at different rates (n = 5 mice). *** P = 0.0008, Friedman statistic = 10.00, Friedman Test. Error bars represent s.e.m.
We also examined the effect of changing ATs on heat-evoked responses in the spinal cord and observed an opposite stimulation-response relationship compared to that in the cold range. When heating to the same target temperature from different ATs, the same group of neurons was activated with similar response amplitudes (Fig. 6a,b). In contrast, when heating with the same ΔT from different ATs, higher absolute target temperature evoked stronger responses (Fig. 6c,d). Together, these results suggest that spinal neurons encode the absolute heat temperature and faithfully reflect stimulus kinetics after reaching their activation thresholds. The lack of adaptation to heat stimuli may provide persistent warning signals against potential tissue damage.
Figure 6. Neurons encode absolute temperature for heat.
(a) Neuronal responses to heating to 43 °C from different ATs. Top: recorded temperature traces of heating by different ΔTs (6 °C, 11 °C, 16 °C and 21 °C). Bottom: ΔF/F heat maps demonstrating fluorescence changes of all 69 heat-responding neurons in the same FOV to the corresponding heating stimulus. Neurons are rank-ordered by their response onset times to the cooling stimulus with ΔT = 21 °C. Each row in the heat maps represents the responses from the same neuron to a different heating stimulus. Scale bar, 10 s. (b) Quantification of the number of heat-responding neurons (squares, left Y axis) and the peak ΔF/F (circles, right Y axis) averaged across all heat-responders to any of the four cooling stimuli (n = 7 mice). (c) Neuronal responses to heating by 8 °C from different ATs. Top: recorded temperature traces of heating stimuli with different ATs (22 °C, 27 °C, 32 °C, 37 °C and 42 °C). Bottom: ΔF/F heat maps demonstrating fluorescence changes of all 296 heat-responding neurons in the same FOV to the corresponding stimulus. Neurons are rank-ordered by their response onset times to cooling from 42 °C to 50 °C. Scale bar, 10 s. (d) Quantification of c (AT = 42 °C, n = 3 mice; other data points, n = 4 mice). Error bars represent s.e.m.
Contribution of specific DRG inputs to spinal responses
We next examined how thermal information was transmitted through different peripheral inputs to the spinal cord. Using cell ablation strategies, previous studies demonstrated that TRPV1- and TRPM8-expressing DRG inputs are dedicated pathways for hot and cold stimuli, respectively31,32. Administration of diphtheria toxin (DT) to transgenic mice co-expressing the human diphtheria toxin receptor (DTR) and enhanced green fluorescent protein (eGFP) under the control of endogenous Trpv1 or Trpm8 regulatory sequences (referred as TRPV1-DTR and TRPM8-DTR, respectively) selectively ablated TRPV1- and TRPM8-expressing DRG neurons31. This ablation was validated by the lack of in situ hybridization and immunohistochemistry signals against TRP channels and eGFP (Supplementary Fig. 5)31. Previous behavior analysis suggests a role of the MrgprD-expressing DRG neurons in response to extreme temperature stimuli, such as rapid cooling the plantar surface to −10 to −20 °C using dry ice or heating to 55 °C31. Because of the confounding possibility of tissue injury at extreme temperature conditions, we restricted temperature stimulation from 5 °C to 50 °C in our imaging experiments. Thus, we focused on examining the contributions of TRPV1- and TRPM8-expressing DRG inputs to spinal responses in these DT-treated DTR mice.
Ablation of TRPV1-expressing DRG neurons led to a significant reduction in heating-evoked neuronal activities in the spinal cord (Fig. 7a–c), supporting the prominent role of TRPV1+ DRG inputs in heat sensation5,33,34. Notably, the extent of response reduction (~80%) was similar for all stimuli from 37 °C to 50 °C, revealing an unexpected role for TRPV1-expressing DRG neurons in detecting innocuous warmth (Fig. 7b,c). The residual heat responses in these mice indicate the existence of TRPV1− DRG neurons that also detect heat ranging from 37 °C to 50 °C. Interestingly, ablation of TRPM8-expressing DRG neurons increased spinal response to warmth, indicating a tonic inhibition from the TRPM8+ inputs onto warmth-responding neurons in the dorsal horn (Fig. 7a–c). This suggests that the sense of warmth is synthesized by the presence of activities in both the cold and heat pathways.
Figure 7. Spinal responses to temperature are mediated by specific DRG inputs.
(a) Heat maps of the activities of all heat-responding neurons in representative FOVs in wild type (WT, 396 neurons), TRPV1-DTR (188 neurons) and TRPM8-DTR (467 neurons) mice after diphtheria toxin treatment. Scale bar, 10 s. (b) A histogram showing the distribution of activation thresholds of heat-responding neurons in WT, TRPV1-DTR and TRPM8-DTR mice. (Black: WT, n = 10 mice, orange: TRPV1-DTR, n = 9 mice, blue: TRPM8-DTR, n = 4 mice. Dunn's multiple comparisons test following Kruskal-Wallis test. TRPV1-DTR vs. WT, P = 0.0049, P = 0.0446, P = 0.0149, P = 0.0004 for the four temperature ranges, respectively. TRPM8-DTR vs. WT, P = 0.6377, P = 0.4073, P > 0.9999, P > 0.9999.) (c) The percentage of reduction (calculate from b) of heat-responding neurons in DTR mice compare to WT. (d) Heat maps of the activities of all cold-responding neurons in representative FOVs in WT (299 neurons), TRPV1-DTR (146 neurons) and TRPM8-DTR (138 neurons) mice after diphtheria toxin treatment. Scale bar, 10 s. (e) A histogram showing the distribution of activation thresholds of cold-responding neurons in WT, TRPV1-DTR and TRPM8-DTR mice. (Black: WT, n = 12 mice, orange: TRPV1-DTR, n = 7 mice, blue: TRPM8-DTR, n = 8 mice. Dunn's multiple comparisons test following Kruskal-Wallis test. TRPV1-DTR vs. WT, P > 0.9999, P = 0.2075, P = 0.0296, P = 0.0281, P = 0.0033 for the five temperature ranges, respectively. TRPM8-DTR vs. WT, P = 0.0004, P = 0.0030, P = 0.3078, P = 0.6914, P = 0.2411.) (f) The percentage of reduction (calculate from e) of cold-responding neurons in DTR mice compare to WT. Dashed bars indicate the sum of reduction of cold-responding neurons of TRPV1-DTR and TRPM8-DTR mice. Error bars represent s.e.m.
When examining the responses to cooling in DT-treated TRPV1-DTR mice, we observed a reduction in the number of neurons that were activated specifically by strong cooling (ΔT > 6 °C) (Fig. 7d–f). Ablation of TRPM8-expressing DRG neurons caused a selective reduction in the number of neurons activated by mild cooling stimuli (29 °C and 26 °C), but did not change the percentage of responders whose activation thresholds were in the lower temperature range (below 26 °C, Fig. 7e). The sum of the reduction of cold responders after ablation of TRPM8 and TRPV1 inputs was close to 100%, suggesting that TRPM8+- and TRPV1+-cold DRG neurons account for the majority of peripheral cold sensors (Fig. 7f). Together, these observations not only substantiate the important role of TRPM8-expressing DRG neurons in sensing cold stimuli, but also identify a population of TRPV1-expressing DRG neurons that selectively detect strong cold.
Interactions of DRG inputs in the spinal cord
We identified four different DRG inputs (TRPM8+-cold and TRPV1+-cold; TRPV1+-heat and TRPV1−-heat) that contribute to thermal sensory responses in the spinal cord. How do these different DRG inputs interact in the spinal cord? Besides the large number of singly tuned neurons, we also found spinal neurons that responded to both heating and cooling (Fig. 8a–c)19. These broadly tuned neurons were spatially intermingled with the singly tuned neurons (Fig. 8a), and the percentage of broadly tuned neurons in the total number of thermoresponsive spinal neurons increased with stimulus intensity (Fig. 8d). For example, about 7% of thermosensitive spinal neurons responded to both 29 °C and 37 °C, whereas 44% of thermosensitive spinal neurons responded to both 5 °C and 50 °C stimuli. Compare to the singly tuned neurons, the broadly tuned spinal neurons had longer response latencies to cooling, suggesting that these neurons might receive more TRPV1+-cold inputs, which were activated at lower temperatures and required longer cooling to reach their thresholds (Fig. 8e). Consistent with this prediction, ablation of TRPV1+-DRG inputs reduced the percentage of broadly tuned neurons (Fig. 8f,g). Meanwhile, at 29 °C, only TRPM8+ cold-inputs were activated at this temperature, about 40% of cold-responding neurons also responded to heating to 45 °C in WT mice. Fewer but a substantial number of these broadly tuned neurons remained after ablation of TRPV1-expressing DRG neurons, indicating a convergence of TRPV1+ heat-, TRPV1− heat- and TRPM8+ cold-DRG inputs in the dorsal horn (Fig. 8f,g). TRPV1+-cold DRG inputs could also contribute to the broad tuning of these neurons, as some of them should respond to both heat and cold. Future works are needed to identify more specific molecular markers defining each group of DRG neurons, which will allow selective manipulation of their activities and examining the interactions between these pathways in the spinal cord.
Figure 8. Broadly tuned thermal responding neurons in the spinal cord.
(a) An example FOV image showing neurons activated by cooling to 16 °C (green), heating to 45 °C (red) or both (yellow). Similar observations were made in 55 mice. Scale bar, 100 µm. (b) Top: stimulation temperature traces. Bottom: representative ΔF/F traces from neurons responded only to cooling (Neurons #1 and #2), only to heating (Neurons #3 and #4) and to both cooling and heating (Neurons #5 and #6). Colored traces are averaged ΔF/F from three individual trials (grey traces) per thermal stimulus. Scale bars, 10 s and 10% ΔF/F. (c) Heat maps showing the activities of all thermal-responding neurons (86 cold only, 89 heat only and 61 broadly tuned) in a. Each row in the heat maps represents responses from the same neuron to different stimuli. Neurons are sorted into 3 different groups based on their tuning properties (indicated by the color bar on the right). In each group, neurons are rank-ordered based on their response onset times. (d) A heat map showing the percentage of broadly tuned neurons in total number of thermosensitive neurons at different stimulation temperatures (n = 6 mice). (e) Averaged response latencies of singly- and broadly-tuned neurons in response to a cooling stimulus to 16 °C and a heating stimulus to 45 °C (n = 55 mice, Cold, P = 0.0008, W = 786.0; heat, P = 0.1053, W = −388.0, Wilcoxon matched-pairs signed rank test). (f) An example FOV image showing neurons that selectively respond to cooling to 29 °C (green), or respond to both heating to 45 °C and cooling to 29 °C (yellow) in wild type and DT treated TRPV1-DTR mice. Scale bar, 100 µm. (g) Quantification of g. WT, n = 11 mice, TRPV1-DTR, n = 8 mice. P = 0.0287, U = 17.00, Mann Whitney test.) * P < 0.05, ** P < 0.01. Error bars represent s.e.m.
DISCUSSION
Compared to the well-characterized molecular and cellular mechanisms of temperature detection at periphery, how temperature information is processed in the spinal cord circuitry is still largely unknown. To study this question, we developed a precisely controlled temperature stimulation system that allows activation of a large number of spinal neurons through evenly changing the cutaneous temperature over the surface of the hindlimb. Combining this stimulation apparatus with a novel in vivo two-photon calcium imaging platform, we systematically examined the representation of cutaneous temperature in the spinal cord and the contributions of genetically defined DRG inputs to temperature evoked responses in the spinal neurons.
Cutaneous temperature stimuli evoked robust calcium responses in the spinal neurons, and their activation temperature thresholds were smoothly distributed across the entire range of stimulation temperatures. Thus, specific ensembles of spinal neurons with continuously distributed activation temperature thresholds could precisely encode temperature stimuli on the skin. Classical single-fiber recording and recent molecular profiling studies have classified peripheral thermosensing DRG inputs into four main types, which correlate with human perception of the four distinct temperature modalities: noxious cold, innocuous cool, innocuous warm, noxious heat2–4,35,36. However, how the ‘modality-specific’ coding at the periphery is transformed into the continuously distributed activation temperature thresholds in the spinal cord remains an outstanding question. Spinal neurons receive convergent innervation from direct or indirect DRG inputs. Strengths of different DRG inputs or local inhibition onto the spinal neurons may play important roles17,37. Future works are required to delineate the contribution of each factor to the distributed activation temperature thresholds.
Using temperature stimulation protocols with prolonged duration of temperature change, we found that the responses of cold-responding neurons peaked during the cooling stage and rapidly adapted to steady cold stimuli, whereas heat-responding neurons persistently responded to steady heat stimulus. Previous in vivo electrophysiological studies also observed different adaptation kinetics of heat and cold responding neurons in the spinal cord19–22,38. However, temperature-changing period in those studies was short. Therefore, it is not clear whether the rapid adaptation reflects the neurons' lack of ability to respond persistently (onset responses), or a precise code for temperature change. We also found that adapting the skin to different cold temperatures (ATs) prior to the stimuli had no effects on their sensitivity to cooling, which enables the cold-responding neurons to signal cooling over a wide temperature range. These features of cold responses were reminiscent of the visual system, in which rapid visual adaptation has been suggested as the key mechanism to maintain high sensitivity in environments with different luminance39. It has been shown that TRP channels adapt to persistent stimuli1,7,40–45. Indeed, using a similar ‘two-stage’ temperature stimulation protocol, Kenshalo and Duclaux reported rapid adaptation of both cold- and warm-fibers to steady temperature stimuli46,47. Thus, the rapid adaptation in the spinal cold-responding neurons may simply reflect the same feature of its DRG inputs, whereas circuitry mechanisms underlying the transformation from adaptive DRG heat inputs to the non-adaptive heat spinal response is a fascinating question for future study.
We also examined the contributions of molecular defined DRG inputs to the thermosensory responses in the spinal cord. Chemicals, such as menthol and capsaicin, directly bind to their receptors TRPM8 and TRPV1 and evoke robust responses in heterologous expression systems or cultured DRG neurons. However, when applied topically, these chemicals slowly penetrate the skin, their concentration at the nerve endings in the skin may change over the course of stimulation, and topically applied chemicals can stay in skin for a long time, which prohibits performing multiple stimulation trails in the same animal. These technical challenges make topical chemical application less ideal for in vivo imaging experiments. Therefore, we approached this question by recording calcium response to temperature stimuli in mice that lack TRPM8+ or TRPV1+ DRG inputs. The high throughput nature of the imaging method enabled us to quantify the effects of loss-of-function mutants on spinal response with unprecedented precision. This is particularly important for innocuous temperature stimuli as they activate extremely small proportions of neurons and do not evoke reliable behavioral reflex, so that loss-of-function mutants cannot be readily characterized by electrophysiological recordings or behavioral assays measuring thermal reflex. Our data reveal that spinal responses to mild cooling were mediated by TRPM8-expressing DRG neurons, whereas TRPV1-expressing neurons drove spinal responses to heat and strong cold. The contribution of TRPV1+ DRG inputs to strong cold spinal responses were not seen in our previous cold plantar test31. We note that the temperature drops rapidly from room temperature to ~ −10 °C within 5 seconds in cold plantar test. Paw withdrawal latency may not be a measure that is sensitive enough to evaluate and differentiate the contributions of TRPV1- and TRPM8-expressing DRG neurons in such a fast cooling assay. We believe that calcium imaging at single cell resolution and precisely controlled temperature stimulation protocol provided better sensitivity allowing us to unravel the contribution of TRPV1-expressing DRG neurons in detecting the strong cold. Because Trpa1 channel is expressed in a subset of TRPV1+ DRG neurons and has been suggested as a molecular sensor for strong cold13,48,49, it could be a good candidate for mediating the strong cold response in TRPV1+ DRG inputs. To test this hypothesis, we performed in vivo imaging in Trpa1 knockout mice and found no difference when compared to the responses to mild or strong cooling in wild type mice (Supplemental Fig. 6), suggesting that additional cold receptors are required for detecting strong cold in the TRPV1-expressing DRG neurons.
Our study provides a comprehensive examination of spinal neurons’ response to cutaneous temperature changes and therefore lays groundwork for future investigations. The dorsal horn is a highly heterogeneous structure containing genetically and anatomically diverse cell types, which have not been considered in current study17,37,50. Imaging sensory responses from genetically or anatomically defined neuronal types, and manipulating these neurons to examine its impact on the rest of spinal cord circuitry will help delineate the contributions of specific cell types to the processing of thermal information in the spinal cord. Combining in vivo imaging with animal models of inflammatory or neuropathic pain will help unravel maladaptive changes in the spinal circuitry that leads to chronic pain and provide therapeutic insights into these devastating disorders3,50.
Experimental Procedures
Animals
Young adult (4–8 weeks) WT (C57BL/6J), Mgfap-cre;Ai14 (a gift from Dr. Ben Barres), Trpa1 knockout (a gift from Dr. David Julius), TRPM8-DTR, TRPV1-DTR BAC transgenic mice and their littermates were used for all experiments. Male and female mice were used for in situ hybridization and immunohistochemistry, only female mice were used for calcium imaging. Mice were group-housed on a 12 h light cycle and were randomly assigned for experiments. All procedures were in accordance with the US National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and were approved by Stanford University’s Administrative Panel on Laboratory Animal Care.
Diphtheria Toxin (DT) Treatment
DT treatment was performed as described with minor modifications. Briefly, DT treatment started in young adult mice (~4 weeks old). For TRPM8-DTR mice and their littermate controls, 0.1 mL DT (1 mg·L−1 in PBS, Sigma) solution was injected intraperitoneal (i.p.) daily for 7 days. For TRPV1-DTR mice, 0.2 mL DT solution was i.p. injected daily for 5 days, followed by 2 days off, for 3 weeks. Imaging experiments were performed between 6–20 days after DT administration. No significant difference was observed between DT-treated littermate controls and untreated wild type mice. Thus, data from both groups were combined.
In Situ Hybridization and Immunohistochemistry
In situ hybridization was performed at high stringency (washed 30 min, 0.2 × SSC, 70 °C) as described previously. Immuohistochemistry was performed with primary antibodies mouse anti-NeuN (1:250, Millipore, MAB377), guinea pig anti-TRPV1 (1:1000, Millipore, AB5566), rabbit anti-GFP (1:1000, ThermoFisher, A-21311, Alexa Fluor 488-conjugated), secondary antibodies Cy3-conjugated donkey anti-guinea pig and Alexa Fluor 488 conjugated donkey anti-rabbit or donkey anti-mouse antibodies (Jackson ImmunoResearch). Neurons were stained with NeuroTrace Blue (1:500, Invitrogen). Images were obtained using a Zeiss 780 confocal microscope or a Zeiss epifluorescence microscope. For Supplementary Fig. 5 the number of cells that were positively stained for TRPV1/GFP were counted and normalized to NeuroTrace Blue positive cells in a blind fashion.
Surgery
Animals were anesthetized with urethane (2 mg·g−1), delivered by two i.p. injections separated by 30 minutes. Surgery started 30 minutes after the second urethane injection. Corneal reflex was examined throughout the experiment, and up to 0.6 mg·g−1 additional urethane might be given to animals with corneal reflex response. A tracheotomy was then performed, and 0.7 mg·g−1 atropine was administrated if needed. The right hind limb was gently depilated with hair removal cream. Paravertebral muscles at vertebrae level T10-L1 were retracted, and spinal clamps (STS-A, Narishige) were used to clamp the exposed vertebral column and stabilize the preparation. A dorsal laminectomy was performed at vertebra level T12 to expose the spinal cord. A custom-designed plastic chamber was placed around the vertebrae and was sealed with agarose to create a watertight compartment for the use of water immersion objective. The exposed spinal cord was keep at stable temperature with normal Ringer solution (in mM: 135 NaCl, 5.4 KCl, 5 Hepes, 1.8 CaCl2, pH 7.2, 30–32 °C). The dura mater was carefully removed, and the animal was rotated around the longitudinal axis by ~30 degrees for imaging. Blood flow through the central vessel was closely monitored as an indicator of tissue health throughout the experiment.
Dye Injections
Neurons in the superficial lamina of the dorsal horn were bulk-loaded with the Oregon Green 488 BAPTA-1 AM (OGB-AM, Invitrogen) under two-photon microscope as described previously. We used glass pipettes with 2–3 µm tips to inject a solution containing 1 mM OGB-AM, 50 µM Alexa Fluor 594, 10% dimethyl sulfoxide and 2% (w/v) Pluronic F-127 in Ca2+/Mg2+ free pipette solution (in mM: 150 NaCl, 2.5 KCl, 10 Hepes, pH 7.4). The dye solution was maintained at ~0 °C before filtered with 0.22 µm centrifuge filter (Millipore) and loaded into a glass pipette. The pipette tip was targeted 70–130 µm below the surface of the spinal cord, about 200 µm lateral to the central vessel. Spinal neurons were bulk-load for about 3 minutes by applying 900–1100 ms pulses of 15–25 psi to the pipette to pressure eject the dye at several sites approximately 200 µm apart from each other. After dye injection, the imaging site was covered with a No. 0 glass coverslip pre-cut to fit inside the custom chamber, sealed with 2% agarose in Ringer solution, except the experiments in Supplementary Fig. 2b, in which the imaging site was covered with a plastic coverslip with an access pore for drug application.
Two-photon Imaging
Calcium imaging experiments were performed with a two-photon microscope (Prairie Technologies) using a Nikon 16× water-immersion objective (IR, N.A. = 0.8) with 2× optical zoom. This provided a 438 × 438 µm field of view that was scanned at 1–2 Hz and recorded as a series of either 256 × 256 (when imaged at 2 Hz) or 512 × 512 (1 Hz) pixel images. No differences in results were seen between the two imaging settings. A Ti:Sapphire laser (Chameleon, Coherent) was tuned to 810 nm and fluorescence emission was filtered with a 580 dcxr dichroic and hq525/70 m-2p bandpass filter. In all experiments except Supplementary Fig. 3 and Supplementary Fig. 4b,c, imaging FOV were 25–45 µm below the spinal cord surface.
The right hind limb of the mouse was depilated and was placed in a custom-designed stimulation container (Fig. 1a), with the fifth toe glued onto the bottom of the container to maintain the limb slightly stretched during stimulation. Water at the adaptation temperature (AT) was infused into the stimulation container at a flow-rate of 5 mL·s−1. Stimulation temperature was monitored and recorded using a microprobe thermometer (BAT-12, Physitemp) with a Type-K thermocouple at 20 Hz. No difference was found when the tip of the thermocouple was placed at different positions in the stimulation container (Fig. 1b,c). For each trial, the spinal cord was imaged for 20 seconds at the AT to obtain baseline fluorescence and noise. Then, the flow was switched to water that was pre-incubated at various stimulation temperatures with the same flow rate. This switch led to rapid changes of temperature in stimulation container for 15 seconds before the flow was switched back to AT for at least another 70 seconds before the next trial. The electric valves that control the switch of water flow was triggered by TriggerSync plugin (Prairie Technologies) and synchronized with the image acquisition system. For experiments in Fig. 3a–d and Fig. 5d–f, temperature stimulation consisted of two stages: a "change" stage in which temperature was changed at a constant rate and a "stable" stage in which temperature was maintained at the target temperature. The duration of the two stage combined was 58 seconds. During the "change" stage, the electronic valves for both water at the AT and target temperature were opened simultaneously. The duty cycles of the two valves was gradually and constantly adjusted to mix the water from these two valves to achieve a constant temperature change rate while maintaining a constant flow rate.
Unlike studies in the olfactory, visual or auditory system where the stimuli can be delivered and removed within milliseconds, thus dozens of trials can be conducted. The need to deliver thermal stimuli to a relatively large and curved cutaneous surface, and to transfer heat to/from thermoreceptors in the skin necessitate long trial time, thus limiting the total number of trials that can be tested in any one experiment. Given the high consistency of responses (Fig. 1i,j) and the relatively large sample size (~400 neurons/FOV), 2–3 trials of each stimulus were imaged in each spinal FOV.
Imaging Analysis
The imaging data were analyzed as previously using custom software written in Matlab. We first corrected lateral motion artifacts using the TurboReg plugin in ImageJ, and averaged the corrected images data set across the entire t-series to generate a template that was used to delineate the outline of the neurons in the imaging FOV. Cell bodies were semi-automatically detected using a fast-normalized cross-correlation routine. Briefly, the averaged images were cross-correlated against a kernel with a size approximating that of an average cell; this image map was threshold to generate a binary mask that demarcated the cell bodies. The mask was then visually examined and errors were corrected manually. About 400 neurons were found in a typical FOV. Stimulation temperature, which was monitored at 20 Hz, was decimated to 1–2 Hz, generating a t-series of recorded temperature. The onset and offset of the stimulation were determined when the difference in temperature between two consecutive recorded time points exceeded 10%–15% of the maximum/minimum difference of the t-series, respectively. Cellular fluorescence intensity (Ft) was calculated for individual neurons at each time-point by averaging the intensity of pixels falling within the cell boundaries. Baseline fluorescence (F0) was assigned to each cell by averaging fluorescence intensity over the 9 seconds period prior to stimulation onset. ΔF/F was calculated as ΔF/F = (Ft − F0) / F0, and the standard deviation of the pre-stimulus baseline was determined (σ0). Neurons were considered responders when the maximum ΔF/F of each individual trial exceeded 5% and 2.5 times of σ0 above F0 of each individual trial, and the maximum ΔF/F of the averaged and smoothed response exceeded 5% and 3 times σ0 above F0 of the response averaged from all the trials of the same stimulus. Raw images and individual neurons' responses were visually examined, and experiments or neurons with failed image registration and irregular motion artifacts (typically thermal stimulus-induced reflex paw movement due to insufficient anesthesia) were excluded.
Analysis, Statistics, Data and Code availability
Mice are randomly assigned for experiments. For calcium imaging experiments, data collection and analysis were not performed blind to the conditions of the experiments. No data points were excluded from analyses. No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those generally employed in the filed. No assumptions concerning normality of equal variances were made, thus all statistical tests used in the manuscript were non-parametric. Dunn's multiple comparison test was used for nonparametric multiple comparisons. All tests in this study are two-sided.
The data that support the findings of this study and the custom Matlab code are available upon request.
A Supplementary Methods Checklist is available.
Supplementary Material
Acknowledgments
We thank L. Luo for his generous support during the entire project and Z.M. Shen for initial experiments and G. Kamalani for assistance; B.A. Barres (Stanford University) and D. Julius (University of California, San Francisco) for Mgfap-cre and Trpa1 knockout mice. We are grateful to X.J. Gao, C. Guenthner, B. Weissbourd and members of the Chen laboratory for helpful comments on the manuscript. This work was supported by grants from the intramural research program of NIDCR (M.A.H.), and the Whitehall Foundation, Terman Fellowship and start-up funding from Stanford University (X.K.C.).
Footnotes
Author Contributions:
C.R. and X.K.C. designed the study. C.R. conducted imaging experiments. C.R. and X.K.C. analyzed data. M.A.H. provided TRPM8- and TRPV1-DTR mice, and performed in situ hybridization experiments. C.R. and X.K.C. wrote the paper with help from M.A.H. X.K.C. supervised the research.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 2.Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palkar R, Lippoldt EK, McKemy DD. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34C:14–19. doi: 10.1016/j.conb.2015.01.010. doi:S0959-4388(15)00019-7 [pii] 10.1016/j.conb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. doi:8443 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 7.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 8.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. doi:S0092867402006529. [DOI] [PubMed] [Google Scholar]
- 9.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 10.Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Colburn RW, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 13.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. doi:S0092867403001582. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 15.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. doi:28/3/566 [pii] 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takashima Y, et al. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. The Journal of clinical investigation. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. Journal of neurophysiology. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- 20.Burton H. Responses of spinal cord neurons to systematic changes in hindlimb skin temperatures in cats and primates. Journal of neurophysiology. 1975;38:1060–1079. doi: 10.1152/jn.1975.38.5.1060. [DOI] [PubMed] [Google Scholar]
- 21.Bester H, Chapman V, Besson JM, Bernard JF. Physiological properties of the lamina I spinoparabrachial neurons in the rat. Journal of neurophysiology. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- 22.Andrew D, Craig AD. Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. The Journal of physiology. 2001;537:489–495. doi: 10.1111/j.1469-7793.2001.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johannssen HC, Helmchen F. In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. The Journal of physiology. 2010;588:3397–3402. doi: 10.1113/jphysiol.2010.191833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature biotechnology. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 28.Harrison JL, Davis KD. Cold-evoked pain varies with skin type and cooling rate: a psychophysical study in humans. Pain. 1999;83:123–135. doi: 10.1016/s0304-3959(99)00099-8. doi:S0304395999000998. [DOI] [PubMed] [Google Scholar]
- 29.Kenshalo DR, Holmes CE, Wood PB. Warm and cool thresholds as a function of rate of stimulus temperature change. Perception and Psychophysics. 1968;3:81–84. [Google Scholar]
- 30.Hensel H. Temperaturempfindung Und Intracutane Warmebewegung. Pflugers Archiv Fur Die Gesamte Physiologie Des Menschen Und Der Tiere. 1950;252:165–215. doi: 10.1007/Bf00361676. [DOI] [PubMed] [Google Scholar]
- 31.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knowlton WM, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanaugh DJ, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. The Journal of physiology. 2013;591:1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science (4th ed.) New York: McGraw-Hill, Health Professions Division; 2000. [Google Scholar]
- 36.Hensel H, Iggo A. Analysis of cutaneous warm and cold fibres in primates. Pflugers Arch. 1971;329:1–8. doi: 10.1007/BF00586896. [DOI] [PubMed] [Google Scholar]
- 37.Zeilhofer HU, Wildner H, Yevenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiological reviews. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urch CE, Dickenson AH. In vivo single unit extracellular recordings from spinal cord neurones of rats. Brain research. Brain research protocols. 2003;12:26–34. doi: 10.1016/s1385-299x(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 39.Rieke F, Rudd ME. The challenges natural images pose for visual adaptation. Neuron. 2009;64:605–616. doi: 10.1016/j.neuron.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Sarria I, Gu J. Menthol response and adaptation in nociceptive-like and nonnociceptive-like neurons: role of protein kinases. Molecular pain. 2010;6:47. doi: 10.1186/1744-8069-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nature neuroscience. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao J, Qin F. Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor. PLoS biology. 2009;7:e46. doi: 10.1371/journal.pbio.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tominaga M, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 45.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Archiv : European journal of physiology. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 46.Duclaux R, Kenshalo DR., Sr Response characteristics of cutaneous warm receptors in the monkey. Journal of neurophysiology. 1980;43:1–15. doi: 10.1152/jn.1980.43.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Kenshalo DR, Duclaux R. Response characteristics of cutaneous cold receptors in the monkey. Journal of neurophysiology. 1977;40:319–332. doi: 10.1152/jn.1977.40.2.319. [DOI] [PubMed] [Google Scholar]
- 48.Karashima Y, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 50.Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mice are randomly assigned for experiments. For calcium imaging experiments, data collection and analysis were not performed blind to the conditions of the experiments. No data points were excluded from analyses. No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those generally employed in the filed. No assumptions concerning normality of equal variances were made, thus all statistical tests used in the manuscript were non-parametric. Dunn's multiple comparison test was used for nonparametric multiple comparisons. All tests in this study are two-sided.
The data that support the findings of this study and the custom Matlab code are available upon request.
A Supplementary Methods Checklist is available.