Abstract
Skin cancer is the most commonly diagnosed type of cancer and is strongly associated with ultraviolet (UV) exposure and skin pigmentation. Recent advances in pharmacologic non-UV tanning methods open the possibility of preventing melanoma and non-melanoma skin cancer, especially in people who do not tan in the sun.
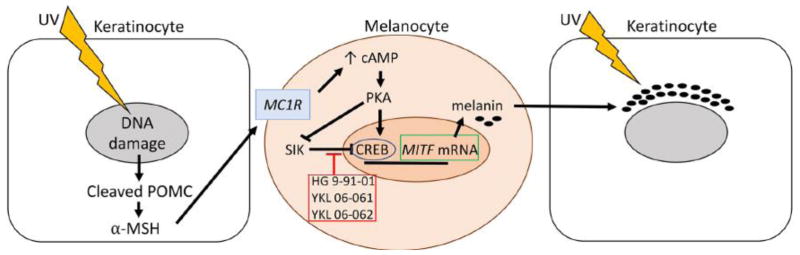
Each day in the United States there are an estimated 9,500 new skin cancer diagnoses and 1 in 5 people will develop skin cancer over their lifetime [1]. The incidence of both melanoma and non-melanoma skin cancer has been rising over the last few decades. Ultraviolet (UV) light exposure is strongly associated with the development of all skin cancers. Melanin in the skin provides valuable protection for keratinocytes and basal cells in the skin, and people with very pale and non-tanning skin are at the highest risk of all types of skin cancer [1]. UV induces tanning when DNA damage in keratinocytes engenders the production and secretion of alpha-melanocyte stimulating hormone (α-MSH), which binds to the melanocortin 1 receptor (MC1R) in melanocytes. The subsequent increase in cAMP and activation of protein kinase A phosphorylates the cAMP-responsive-element-binding protein (CREB), resulting in increased microphthalmia-associated transcription factor (MITF) transcription and increased melanin production (Figure 1). In the absence of UV activation, salt inducible kinase (SIK) inhibits CREB-dependent MITF transcription. Melanin is packaged into melanosomes and transferred to keratinocytes, which array the melanin over their nucleus, like a parasol used to protect against further UV damage.
Figure 1. Mechanisms of UV-Dependent and -Independent Tanning.
Ultraviolet (UV)-associated DNA damage in keratinocytes leads to cleavage of pre-opiomelanocortin (POMC) and production of alpha-melanocyte stimulating hormone (α-MSH), which binds to the melanocortin 1 receptor (MC1R) in melanocytes. MC1R binding results in increased cAMP, activation of protein kinase A (PKA), and phosphorylation of the c-AMP responsive element binding protein (CREB). CREB phosphorylation leads to increased transcription of MITF, which initiates the melanin production cascade resulting in increased melanin. Melanin is packaged in melanosomes and then transferred to keratinocytes to protect their nuclei against further UV damage. SIK inhibits MITF transcription in the absence of UV-damage, preventing melanin production. Pharmacological inhibition of SIK with HG 9-91-01, YKL 06-061, or YKL-060062 leads to UV-independent tanning [2].
Work by Mujahid et al. recently published in Cell Reports [2] describes how topical administration of first and second generation SIK inhibitors, HG 9-91-01 (HG), YKL 06-061 (YKL1) and YKL 06-062 (YKL2), can increase melanin production in vitro and in vivo. In vitro treatment with HG of normal human melanocytes and the human melanoma lines UACC62 and UACC257 resulted in increased expression of MITF mRNA and downstream MITF target TRPM1 mRNA, indicating that HG activated the melanin-producing pathway [2]. In vivo, topical HG treatment induced local black eumelanin skin pigmentation in “red-headed” Mc1r-deficient K14-Scf mice, but not in albino K14-Scf mice [2]. Since HG is not easily absorbed through human skin, the Fisher laboratory used a kinase screen, identifying second generation SIK inhibitors YKL1 and YKL2, which exhibited similar kinase activity and predicted skin penetration better than other agents [2]. YKL1 and YKL2 were used to treat human breast skin explants in vitro and induced melanin production and skin darkening. Fontana-Masson staining of human and mouse skin sections demonstrated appropriate arraying of melanin over keratinocyte nuclei, indicating that melanin was not only produced in response to SIK inhibitors, but properly packaged and transferred to keratinocytes [2]. This suggested that passive spray applications may be sufficient for clinical administration of second generation SIK inhibitors.
The potential benefits of SIK inhibitors are particularly important for individuals with red hair and pale, non-tanning skin, who have the highest risk of skin cancer [1]. Redheads often have loss-of-function mutations in MC1R, which prevents the optimal UV-induced tanning response, and higher relative production of reddish pheomelanin. Pheomelanin may actually enhance UV-associated DNA damage, as opposed to the production of protective black eumelanin in individuals with tanning skin [3]. The Fisher laboratory has previously demonstrated that cAMP regulators, which also act downstream of MC1R, can lead to increased eumelanin production and visible tanning in Mc1r-deficient mice; however, those findings could not be translated to human skin [4, 5]. Presumably, human MC1R-deficient melanocytes would exhibit a similar response to those of Mc1r-deficient mice. From a medical perspective, since redheads and others with non- or poorly-tanning skin stand to gain the most from this advance, testing to confirm protective eumelanin production in human skin samples without functional MC1R is warranted.
Many people still view tanned skin as “healthier” and more attractive, which helps fuel the 2-billion-dollar tanning industry in the United States. Some people believe that indoor tanning is safer than sun tanning or will protect skin from sunburn, but indoor UV tanning is associated with a higher risk of all types of skin cancer, especially when usage begins in adolescence or early adulthood [6]. Indoor tanning is particularly associated with an increased risk of melanoma in young women [6]. In 2010, approximately 30% of Caucasian women 18–25 and nearly 6% of all adults reported using indoor UV-tanning devices in the previous year [7]. Although tanning salon usage has been decreasing over the last few years, in 2015, 7% of high school students reported indoor tanning [7]. A topical SIK inhibitor might also potentially provide a localized, non-UV tanning option for individuals who tan for esthetic reasons and thereby reduce their tanning-associated UV exposure and skin cancer risks. However, people with “tanning addictions” [8] would not necessarily be aided by SIK inhibitors.
Additionally, potential risks and delivery methods for SIK inhibitors would need to be thoroughly investigated before treatments could reach the clinic. There may be risks associated with increasing MITF protein expression in melanocytes, or with inhibiting SIK in the skin. Indeed, MITF plays a paramount role in melanocyte development and differentiation. Although the link between MITF and melanoma is still far from clear, amplifications of MITF and increased expression of MITF mRNA and protein are seen in some melanomas. Additionally, melanin, especially pheomelanin, can enhance UVA-associated oxidative DNA damage in melanocytes [3, 9], raising the possibility that increased tanning in redheads could potentially lead to increased risk of melanoma. The health consequences associated with SIK inhibitor treatment, particularly with chronic usage, remain unknown. The role of SIK molecules in tumorigenesis is incompletely understood and both pro-tumorigenic and anti-metastatic functions have been reported [10]. SIK inhibitor treatments would likely need to be initiated in childhood to provide optimal protection against melanoma, associated with childhood sun exposure and sunburn [1]. The use of tanning agents in children and long-term or lifelong use of SIK inhibitors may confer additional or unique risks. Furthermore, melanin in the skin reduces the production of vitamin D in the skin[7], which has been a source of resistance to sunscreen use. The effect of non-UV tanning on vitamin D production would therefore need to be rigorously studied.
Unfortunately, pharmacological skin tanning would likely not be sufficient on its own to prevent UV-associated skin damage. Melanin, even in the darkest-skinned individuals, provides only the equivalent of ultraviolet protection factor (UPF) 4 [9], which is weaker than the lowest UPF sunscreens on the market. Sunscreen and/or protective clothing would remain the most important line of defense against UV-associated skin damage, cancer and aging. Compliance with sunscreen recommendations, including recommendations to reapply at least every 80–90 minutes, is thought to be relatively low; therefore, combining non-UV tanning and sunscreen could provide better baseline protection against UV damage for everyone.
SIK inhibitors are a novel avenue for skin cancer prevention and could help reverse the trend of ever-increasing skin cancer diagnoses. Although SIK inhibitors would not replace the need for sunscreen, they could provide valuable baseline UV protection for people with pale, non-tanning skin and reduce the risk of skin cancer. A topical tanning agent could also supplant dangerous UV tanning methods that currently contribute to skin cancer risk. Although a number of hurdles need to be overcome before topical SIK inhibitors reach the clinic, the work by Fisher’s laboratory provides a remarkable breakthrough that could have long-term effects in preventing skin cancer diagnoses and deaths.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AAD. [accessed July 10, 2017];Skin cancer Incidence rates. 2017 https://www.aad.org/media/stats/conditions/skin-cancer.
- 2.Mujahid N, et al. A UV-Independent Topical Small-Molecule Approach for Melanin Production in Human Skin. Cell Rep. 2017;19(11):2177–2184. doi: 10.1016/j.celrep.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valverde P, et al. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 4.D’Orazio JA, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443(7109):340–4. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 5.Khaled M, et al. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 2010;24(20):2276–81. doi: 10.1101/gad.1937710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulman JM, Fisher DE. Indoor ultraviolet tanning and skin cancer: health risks and opportunities. Curr Opin Oncol. 2009;21(2):144–9. doi: 10.1097/CCO.0b013e3283252fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. [accessed July 11, 2017];Skin Cancer. 2017 https://www.cdc.gov/cancer/skin/index.htm.
- 8.Fell GL, et al. Skin beta-endorphin mediates addiction to UV light. Cell. 2014;157(7):1527–34. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539–49. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du WQ, et al. The diverse oncogenic and tumor suppressor roles of salt-inducible kinase (SIK) in cancer. Expert Opin Ther Targets. 2016;20(4):477–85. doi: 10.1517/14728222.2016.1101452. [DOI] [PubMed] [Google Scholar]