Abstract
Methamphetamine (METH) is a powerful and highly addictive stimulant that affects the central nervous system of users in the United States and worldwide, and its consumption is associated to the acquisition of HIV and AIDS-related infections. METH enhances cryptococcosis in mice, an opportunistic infection caused by the encapsulated fungus Cryptococcus neoformans. Due to its ability to survive within macrophages, C. neoformans is an ideal model to study pathogen-macrophage interactions. METH abrogates normal macrophage function, which might contribute to the higher rate and more rapid progression of infections in drug abusers. Hence, we investigated the role of complement and specific IgM to C. neoformans capsular polysaccharide on the function of J774.16 macrophage-like cells after exposure to METH. We found that complement and IgM significantly promotes complement-mediated phagocytosis of C. neoformans by J774.16 cells in comparison to co-incubation with complement alone. IgM enhances the expression of complement receptor 3 on the surface macrophages treated with the drug. Also, IgM-increased macrophage phagocytosis of C. neoformans may be associated with upregulation of GTPase-RhoA, a key regulator of the actin polymerization signaling cascade. Fungal cells incubated with complement and IgM in the presence of METH demonstrated higher number of cells per aggregate, a possible explanation for their enhanced ingestion by phagocytes. IgM increased killing of yeast cells by macrophages by inhibiting the alkalization of the phagosome and stimulating the intracellular production of nitric oxide. Together, our findings suggest that IgM stimulates the effector functions of macrophages against opportunistic pathogens in the setting of drug abuse.
Keywords: complement, IgM, infection, methamphetamine, nitric oxide, phagocytosis
1. INTRODUCTION
Methamphetamine (METH) is a major public health and safety problem in the United States and globally. METH is a strong addictive central nervous system (CNS) stimulant that alters judgment and reduces inhibitions, leading people to engage in unsafe activities [1]. The transmission of HIV [1, 2] hepatitis B and C [3], and other communicable diseases are possible consequences of METH use. These infectious diseases can be spread via contaminated paraphernalia used by multiple people who inject the drug [4, 5]. METH is also associated with high-risk sexual behavior and high rates of HIV acquisition among homosexual and bisexual men [1, 2]. METH consumption may facilitate brain and systemic HIV replication [6–8] and undermine the effectiveness of antiviral therapies even after optimal adherence [9]. In addition, METH addicts infected with HIV have more pronounced neuronal injury and cognitive impairment compared to HIV infected non-users [10, 11].
The encapsulated fungus Cryptococcus neoformans is acquired by inhalation and responsible for approximately 1 million cases annually of meningoencephalitis and 600,000 deaths worldwide, primarily in HIV-infected individuals [12]. The major capsular polysaccharide of C. neoformans, glucuronoxylomannan (GXM), is abundantly released during infection in tissue resulting in a variety of immunosuppressive effects such as impaired antibody responsiveness and cell-mediated immunity [13]. Interestingly, structural analysis of the capsular polysaccharide of METH-exposed cryptococci revealed that the drug alters the carbohydrate composition of this virulence factor, an event of adaptation to external stimuli that can be advantageous to the fungus during pathogenesis [14]. Similarly, METH enhances C. neoformans pulmonary infection and dissemination into the CNS of mice by stimulating fungal adhesion, GXM release, and biofilm formation in the lungs [14, 15]. Furthermore, recent cases of disseminated cryptococcosis have been associated with intravenous drug users without HIV infection suggesting that drug abuse may exacerbate the infection [16].
The interaction of C. neoformans with alveolar macrophages is critical for containing the infection in the lungs [17]. Fungal phagocytosis by macrophages in HIV patients with cryptococcosis is mediated by complement receptors [18]. However, the efficiency of complement-mediated opsonization varies, depending on the localization of the complement component 3 (C3) on the surface of C. neoformans [19]. Complement receptor 3 (CR3, CD11b/CD18) is an opsonic receptor that recognizes complement fragment C3bi deposited on the fungal surface resulting in the uptake of the fungus [20]. Passive administration of capsule binding IgM has shown to be important in host response against C. neoformans [21], including phagocytosis [22]. Although METH impairs fungal phagocytosis [23], the effect of METH on complement-mediated phagocytosis or the additive role of IgM in this process has not been yet investigated. C. neoformans is an excellent model organism for the study of host-pathogen interactions in the setting of METH due to the availability of tools such as specific antibodies, cell lines, and animal systems [14, 15].
METH is an immunosuppressive agent to phagocytic cells as it alkalizes normally acidic organelles within immune cells and inhibits antigen presentation [24]. After phagocytosis, C. neoformans can also replicate intracellularly and escape macrophages through lytic and non-lytic exocytosis [25]. The fungus has a unique and dynamic intracellular pathogenic strategy that involves cytoplasmic accumulation of polysaccharide-containing vesicles and intracellular replication leading to the formation of spacious phagosomes in which multiple cryptococcal cells are present [26]. Here, we evaluated the role of complement and specific IgM to C. neoformans capsular polysaccharide on J774.16 macrophage-like cell function after co-incubation with METH.
2. MATERIALS AND METHODS
2.1 C. neoformans
C. neoformans strain H99 (serotype A; isolated and kindly provided by John Perfect at Duke University) was inoculated in Sabouraud dextrose broth (Becton Dickinson) and incubated at 30 °C for 24 h in a rotary shaker set at 150 rpm (Thermo Fisher).
2.2 Rationale for METH doses used in studies
Controlled studies indicated that a single 260 mg dose peaks at a level of 7.5 μM [27]. Thus, a single dose of 260 mg/g would be expected to produce 7.5 to 28.8 μM blood METH levels. Intravenous drug users tend to self-administer METH in binges, and as the drug exhibits a half-life of 11.4 to 12 h, this can lead to higher drug levels.24, 25 Binge patterns of use in individuals have shown that the fourth administration of 260 mg during a single day produced blood levels of 17 μM and could reach 20 μM on the second day of such a binge [27]. Thus, binge doses of 260 to 1,000 mg produce 17 to 80 μM blood METH levels and levels in the micromolar range of hundreds in organs, including the brain and the spleen [28]. Therefore, we selected ~25 to 50 μM METH to perform our experiments.
2.3 Phagocytosis assay using J774.16 macrophage-like cells
J774.16 is a well-characterized murine macrophage-like cell line that originated from a murine reticulum cell sarcoma and has been extensively used to study C. neoformans-macrophage interactions [26, 29]. J774.16 cells were incubated in 96-well microtiter plates in the absence or presence of METH (25 or 50 μM; Sigma) for 2 h. Cytochalasin D (Cyt D; 25 μM; Sigma) is a potent inhibitor of actin polymerization and was used as a positive control. Monolayers of macrophage-like cells were washed thrice with phosphate-buffered saline (PBS) or feeding medium [Dulbecco’s Modified Eagle’s Medium (Mediatech) supplemented with 10% heat-inactivated fetal calf serum (Atlanta Biologicals), 10% NCTC-109 (Gibco), and 1% nonessential amino acids (Mediatech)], respectively, followed by the addition of pre-incubated cryptococci with complement alone (25% murine serum, Sigma), monoclonal antibody (mAb) 12A1 (anti-cryptococcal GXM (IgM) generated and generously provided by Arturo Casadevall at Johns Hopkins Bloomberg School of Public Health; 2 μg/mL), and combination of complement and mAb 12A1 in a macrophage:yeast ratio of 1:10. The plates were incubated for 2 h at 37 °C. The monolayer coculture was washed 3× with PBS to remove nonadherent cells, fixed with cold methanol, and viewed with light and fluorescence microscopy with use of an Axiovert 200 M inverted microscope (Carl Zeiss) at a magnification of 400×; images were collected using an AxioCam MrC digital camera using the Zen 2011 digital imaging software (Carl Zeiss). The phagocytic index was determined to be the number of internalized yeast cells per number of 100 macrophages per well. Internalized cells were differentiated from attached cells by the presence in a well-defined phagocytic vacuole.
2.4 Fluorescence Microscopy
For immunofluorescence studies after treatment with METH and incubation with complement, IgM, or combination, slides were coated with poly-l-lysine (0.1 mg/mL; Sigma), and 106 yeast cells were allowed to air dry on slides so that organisms adhered. MAb 18B7 (anti-GXM (IgG1) generated and provided by Arturo Casadevall; 2 μg/mL) was added in buffer (PBS with 1% bovine serum albumin; Sigma). FITC-labeled goat anti-mouse-IgG1 (Southern Biotech) was added at 2 μg/mL after application of unconjugated mAb. All incubations were done at 37 °C for 30 min, and slides were washed 3× with PBS between each application of reagents. Slides were washed again with PBS, 30 μL of mounting medium (0.1 M n-propyl gallate-50% glycerol in PBS; Sigma) was added, and coverslips were placed. The slides were then viewed as described above to determine the number of cell aggregates per field and the number of yeast cells per aggregate. Finally, fluorescent images were recorded.
2.5 Killing assay
J774.16 macrophage-like cells were first allowed to phagocytize C. neoformans H99 cells for 2 h. Each well containing interacting cells was gently washed with feeding medium 3× to get rid of fungal cells that were not phagocytize and incubated with feeding medium and either PBS (untreated), METH (25 or 50 μM), or Chloroquine, a weak base and a well-known inhibitor of endosomal acidification (Chlq; 25 μM) for 24 h [30]. Macrophage-like cells were lysed by forcibly pulling the culture through a 27-gauge needle 5–7×. A volume of 100 μL of suspension containing cryptococci was aspirated from the wells, transferred to a microcentrifuge tube with 900 μL of PBS. For each well, serial dilutions were performed and plated in triplicate onto Sabouraud dextrose agar plates, which were incubated at 30 °C for 48 h. Quantification of viable cryptococcal cells was determined by colony forming units (CFU).
2.6 Measurement of phagosomal pH
J774.16 macrophage-like cells were incubated with C. neoformans as described in the killing assay. Prior to fluorometry the monolayers were rinsed with ice-cold 10 mM phosphate buffer at pHs ranging from 4.0 to 7.0. The fluorescence was determined on a Synergy HT spectrofluorimeter (Bio-Tek) at both 450 nm and 490 nm excitation wavelengths and an emission wavelength of 515 nm. After the fluorescence of the intact phagosomes had been determined, the phagosomes were permeabilized with 0.1% Triton X-100 (Sigma). The fluorescence of phagosomes permeabilized in different pH buffers was used to generate a pH curve from which the pH of intact phagosomes was determined.
2.7 Nitric oxide (NO) determinations
NO produced in the supernatants of macrophages treated with PBS (untreated), Chlq, or METH was quantified 24 h after incubation with C. neoformans using a Griess method kit (Cayman Chemical), according to the manufacturer’s protocol. Additionally, macrophages were treated with aminoguanidine (AG; Sigma) as a control because it inhibits both constitutive and inducible NO synthase (iNOS). NO production was monitored by measuring the optical density at 540 nm using a microtiter plate reader (OD540; Bio-Tek).
2.8 Western blot analysis
To further explore the impact of METH on J774.16 phagocytosis and nitric oxide production after interaction with C. neoformans, we assessed macrophage CD11b, CD18, RhoA, and iNOS expression. Western blot analysis was conducted using cytoplasmic extracts made by NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Fisher). The mixture was centrifuged at 10,000 g for 10 min at 4 °C and the resulting protein content of the supernatant was determined using the Bradford method employing a protein assay kit (BioRad). Lysates were preserved in protease inhibitor cocktail (Roche) and stored at −20 °C until use. The supernatant was added with a sample buffer containing 1.6% sodium dodecylsulphate, 5% glycerol, 0.1 M dithiothreitol, 0.002% bromphenol blue and 62.5 mM TRIS-HCl (pH 6.8). The mixture was then heated to 100 °C for 5 min. Fifty-five micrograms of protein was applied to each lane of a gradient gel (10%, BioRad). After electrophoresis at a constant 130 V/gel, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (BioRad, 0.2 mm), and briefly stained with Ponceau S (Sigma) to verify efficient transfer of the protein. The PVDF membrane was incubated for 1 h at 37 °C in a blocking solution containing 5% non-fatty dry milk, 0.04 M TRIS-HCl, pH 7.6, 0.8% NaCl and 0.5% Tween 20, followed by an overnight incubation at 4 °C with a mouse monoclonal anti-CD11b (dilution 1:1000; Southern Biotech), CD18 (dilution 1:1000; BD), RhoA (dilution 1:1000; BD), or iNOS (dilution 1:3000; Abcam) antibodies and with subsequent addition of peroxidase linked anti-mouse secondary IgG antibody diluted (1:5000; Southern Biotech) in the blocking solution. Protein bands were detected and quantified with a lumino image analyzer (GE Typhoon 8600) after staining with chemiluminescence detection reagents (Thermo Fisher). Quantitative measurements of individual band intensity in western blot analyses for CD11b, CD18, RhoA, and iNOS were performed using Image J software (US National Institutes of Health). GAPDH, a cytoplasmic housekeeping protein, was used as a loading control to determine the relative intensity ratio.
2.9 Statistical analysis
All data were subjected to statistical analysis using Prism 7.0 (GraphPad Software). P values for multiple comparisons were calculated by analysis of variance (ANOVA) and were adjusted by use of the Bonferroni correction. P values for individual comparisons were calculated using student’s t-test analysis. P values of <0.05 were considered significant.
3. RESULTS
3.1 Specific IgM to C. neoformans capsular polysaccharide and complement stimulates J774.16 macrophage-like cells phagocytosis of the fungus after co-incubation with METH
We investigated the role of complement and IgM in the phagocytosis of C. neoformans strain H99 by J774.16 macrophage-like cells in the presence of METH. Light microscopy images of J774.16 cells co-incubated with 50 μM of METH, complement or complement + IgM, and C. neoformans demonstrated considerable difference in the uptake of the yeast cells (Fig. 1). Macrophages incubated in the presence of complement and METH were surrounded by many cryptococci indicating impaired phagocytosis (Fig. 1A; left panel). In contrast, METH-treated J774.16 cells supplemented with complement + IgM displayed substantial phagocytized C. neoformans cells and small number of fungi in their surroundings (Fig. 1A; right panel). We quantitatively assessed the phagocytic indices of J776.14 cells treated with PBS (untreated), 25 μM CytD (phagocytosis inhibitor; positive control), or 25 or 50 μM METH and co-incubated with complement or complement + IgM and C. neoformans (Fig. 1B). We did not observe any difference in fungal phagocytosis between untreated J774.16 cells incubated with complement or complement + IgM. We found that complement + IgM incubated with 25 and 50 μM METH significantly increased phagocytosis of yeast cells by J774.16 cells compared to cells incubated with complement alone (P<0.0001) (Fig. 1B). CytD inhibited cryptococcal phagocytosis by macrophages co-incubated with complement or complement + IgM (Fig. 1B). Untreated macrophages co-incubated with complement opsonized fungal cells showcased higher phagocytosis than leukocytes treated with 25 or 50 μM METH (P<0.0001) (Fig. 1B). Untreated macrophages co-incubated with complement + IgM opsonized cryptococci demonstrated significantly higher fungal uptake than macrophages treated with CytD (P<0.0001) but significantly lower phagocytosis than macrophages treated with METH (P<0.0001) (Fig. 1B). J774.16 macrophage-like cells treated with 50 μM METH and supplemented with complement + IgM showcased the highest engulfment and phagocytosis of C. neoformans cells (Fig. 1B). Macrophages co-incubated with irrelevant or non-specific IgM showed similar phagocytic index to those observed in PBS-treated macrophages (data not shown).
Figure 1. Anti-polysaccharide capsule specific IgM and complement stimulates phagocytosis of C. neoformans by J774.16 macrophage-like cells after co-incubation with methamphetamine (METH). (A).
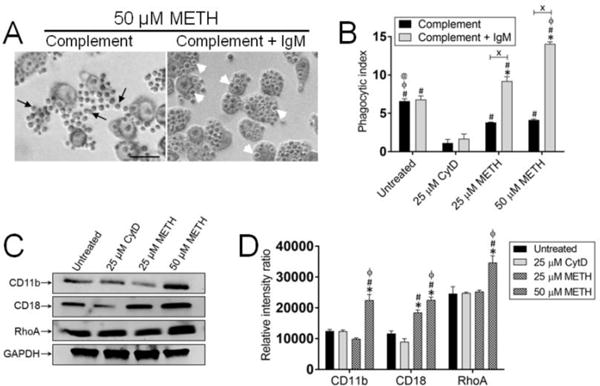
Light microscopy images of METH treated J774.16 cells incubated in presence of complement or complement + IgM (mAb 12A1, a capsular specific mAb) interacting with AIDS-associated yeast-like fungus C. neoformans. Images show activated macrophage-like cells with phagocytized yeast cells. Black arrows indicate C. neoformans cells outside of macrophages. White arrow heads denote yeast cells inside of macrophages. Scale bar: 10 μm. (B) The phagocytic indices (ratio of number of intracellular yeast cells to the number of macrophages counted) were determined after 2 h. Bars represent the means of four wells (100 cells per well) and error bars denote standard deviations (SDs). Symbols (*, #, ϕ, @) denote P-value significance (P<0.0001) calculated using analysis of variance analysis (ANOVA) and adjusted by use of the Bonferroni correction. *, #, ϕ, and @ indicate significantly higher fungal phagocytosis than in macrophages from untreated, 25 μM cytochalasin D (CytD, an inhibitor of actin polymerization and phagocytosis), 25 and 50 μM METH groups, respectively. Crosses (X) indicate P-value significance (P<0.0001) calculated using student’s t-test analysis. (C) Expression of CR3 (CD11b/CD18) and RhoA were determined by a western blot analysis. GAPDH was used as a control. (D) The levels of expression of CD11b, CD18, and RhoA were measured by determining the relative intensity ratios. Individual band intensities from the western blot in C were quantified using Image J software (US NIH). GAPDH was used to determine the relative intensity ratios shown in C. For panels B and D, symbols (*, #, ϕ) denote P-value significance (P<0.0001) calculated using analysis of ANOVA and adjusted by use of the Bonferroni correction. *, #, and ϕ indicate significantly higher fungal phagocytosis than in macrophages from untreated, 25 μM cytochalasin D (CytD, an inhibitor of actin polymerization and phagocytosis), and 25 μM METH groups. The experiments were performed twice with similar results obtained.
Macrophages recognize C. neoformans via CR3 for phagocytosis and destruction of fungal cell, particularly in presence of IgM which enhances the localization of C3 to outer edge of the polysaccharide capsule [20]. We evaluated the involvement of specific IgM to C. neoformans capsular polysaccharide and complement in stimulating the phagocytic activity of J774.16 cells against C. neoformans in the presence of METH by assessing the changes in the expression of CR3 (CD11b/CD18) (Fig. 1C–D). Western blot analysis revealed up-regulated expression of CD11b (25 μM METH) and CD18 (25 and 50 μM METH) relative to the other conditions (Fig. 1C). Moreover, to test whether METH affected CR3-mediated phagocytosis protein expression cascade, we measured the expression of GTPase RhoA, a regulatory protein involved in the cascade for actin polymerization and formation of the phagocytosis pocket. We found that 50 μM METH significantly increases the expression of RhoA in J774.16 cells co-incubated with complement and IgM, therefore, stimulating fungal internalization compared with the other conditions (Fig. 1C). Relative intensity ratios indicate a significant increase in the expression of CD11b, CD18 and RhoA in the cells exposed to 50 μM METH (P<0.0001). Interestingly, 25 μM METH treated cells only showed a marked increase in the expression of CD18 domain of CR3 (P<0.0001) whereas CytD has no impact in the expression of CR3 molecules or RhoA (Fig. 1D). These findings suggest that during METH use and infection, IgM may stimulate complement-mediated fungal elimination by phagocytic cells.
3.2 Specific IgM to C. neoformans capsular polysaccharide and complement promotes fungal cell aggregation during incubation with METH
Since we found that anti-capsular IgM enhances complement-mediated phagocytosis of C. neoformans by J774.16 cells in the setting of METH, we examined the role of this isotype in microbial aggregation under these conditions (Fig. 2). We incubated yeast cells with 25 μM METH alone (control), METH + specific IgM (IgM), METH + complement (complement), and METH + complement + specific IgM (complement + IgM) for 2 h and fluorescent microscopy was performed to document the number of cell aggregates per field and cells per aggregates. Only clusters of ≥ 3 cryptococci were considered and counted as aggregates. C. neoformans cells incubated with METH showed mainly budding cells or small number (~3) of cells per aggregate (~4) dispersed throughout the field (Fig. 2A–C). Fungi incubated with irrelevant IgM showed similar cell distribution and arrangement to those observed in the control group (data not shown). Yeast cells incubated with complement or IgM exhibited the highest number of cell aggregates per field and showed statistical significance relative to control (P<0.0001) and complement + IgM (P<0.0001) groups (Fig. 2A–B). However, the number of cells per aggregate in fungal cells incubated with IgM was higher than in complement-treated cryptococci (P<0.0001) (Fig. 2C). Although the number of cell aggregates per field in the complement + IgM groups was similar to those observed in the control group (Fig. 2A–B), C. neoformans cells cultured with complement + IgM showcased the highest number of cells per cluster (Fig. 2C). Our results indicate that combination of complement and IgM promotes aggregation of C. neoformans and phagocytosis of the fungus.
Figure 2. Specific IgM to C. neoformans polysaccharide capsule and complement promote fungal cell aggregation after incubation with METH. (A).

Immunofluorescent images of C. neoformans after co-incubation with METH and either PBS, complement, mAb 12A1 (IgM), or combination of complement and IgM, the cells were washed and incubated with mAb 18B7-FITC-conjugated goat anti-mouse IgG1 stained to label the capsular polysaccharide. Scale bar, 10 μm. (B) Number of cell aggregates per field. Only clusters of ≥ 3 cryptococci were considered aggregates. (C) Cells per aggregate. Each aggregate was closely examined and the number of cells clustered was counted and recorded. For panels B and C, the number of cell aggregates per field and cells per aggregate was assessed after co-incubation of fungi with METH alone or METH with complement, IgM, or complement + IgM. Bars represent the means of multiple measurements and error bars denote SDs. Symbols (*, #, ϕ, @) denote P-value significance (P<0.0001) calculated using ANOVA and adjusted by use of the Bonferroni correction. *, #, ϕ, and @ indicate significantly higher than control, complement, IgM, and complement + IgM groups, respectively. The experiments were performed twice with similar results obtained.
3.3 Specific IgM to C. neoformans capsular polysaccharide and complement prevent METH-induced alkalization of phagosomes in J774.16 cells resulting in killing of the fungus
METH causes alkalization of the phagosome in primary macrophages impairing the killing of yeast cells [23]. We explored whether capsule-specific IgM to C. neoformans and complement prevented METH-induced alkalization of phagosomes in macrophage-like cells resulting in killing of the fungi (Fig. 3). Chlq (pH 7.09) and METH (25 μM, pH 7.25; 50 μM, pH 7.34)-treated macrophages incubated with complement demonstrated higher phagosomal alkalization than untreated (pH 4.7) cells (P<0.0001) (Fig. 3A). This suggests that complement alone is unable to stimulate macrophage intraphagosomal microbial killing in the setting of METH use. In contrast, untreated (pH 4.89) and METH (25 μM, pH 4.89; 50 μM, pH 5.42)-treated macrophages co-incubated with complement + IgM evinced reduced intraphagosomal pH relative to the Chlq (pH 7.44) control group (P<0.0001) (Fig. 3A). Notably, METH-induced alkalization of phagosomes was prevented in J774.16 cells by complement + IgM compared to cells incubated with complement alone (P<0.01) (Fig. 3A).
Figure 3. Specific IgM to C. neoformans capsular polysaccharide and complement prevent METH-induced alkalization of phagosomes in J774.16 cells resulting in killing of the fungus.
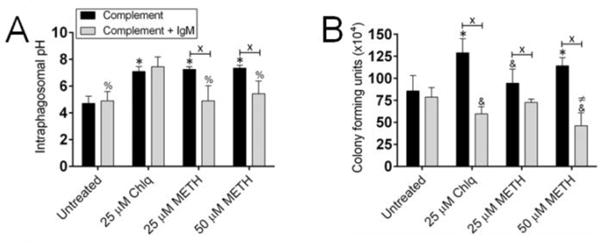
Macrophages were first allowed to phagocytize C. neoformans H99 cells for 2 h. Each well containing interacting cells was gently washed to get rid of fungal cells that were not phagocytize and incubated with growing medium supplemented with either PBS (untreated), Chlq (25 μM), or METH (25 or 50 μM) for 24 h. (A) For phagosomal pH determinations, the pH of phagosomes of J774.16 macrophage-like cells that contain C. neoformans yeast labeled with pH-sensitive and pH-insensitive probes was measured using a spectrofluorometer after treatment with PBS (untreated), Chlq (25 μM), or METH (25 or 50 μM). Bars represent the means of five measurements and error bars denote SDs. Symbols (*, %) denote P-value significance (P<0.0001) calculated using ANOVA and adjusted by use of the Bonferroni correction. * indicates significantly higher pH than in macrophages from untreated groups whereas % indicates significantly lower than in cells incubated with Chlq. Crosses (X) indicate P-value significance (P<0.01) calculated using student’s t-test analysis. The experiments were performed twice with similar results obtained. (B) For killing assay, phagocytic cells were lysed and fungal cells in the supernatant were plated and CFU were counted. Bars represent the means of four wells (three CFU counts per well) and error bars denote SDs. Bars represent the means of four wells (three CFU counts per well) and error bars denote standard deviations. Symbols (*, &, ≠) denote P-value significance (P<0.01) calculated using ANOVA and adjusted by use of the Bonferroni correction. * indicates significantly higher fungal killing than in macrophages from untreated groups. & and ≠ indicate significantly lower cryptococcal killing than in cells incubated with PBS and 25 μM METH, respectively. Crosses (X) indicate P-value significance (P<0.0001) calculated using student’s t-test analysis. For panels A and B, The experiments were performed twice with similar results obtained.
Given that complement + IgM prevented METH-induced intraphagosomal alkalization in macrophage-like cells, it is likely that this environment promotes C. neoformans killing, thus, we investigated this possibility. J774.16 cells incubated with complement and 50 μM METH or 25 μM Chlq demonstrated enhanced phagosomal proliferation of fungi relative to untreated macrophages (P<0.01) (Fig. 3B). Interestingly, macrophages incubated with complement + IgM showed similar increased fungal killing with 50 μM METH- or 25 μM Chlq-treated phagocytic cells displaying a more significant reduction compared to untreated controls (P<0.01). Moreover, complement + IgM significantly inhibited METH or Chlq-induced proliferation of C. neoformans within the phagosome of J774.16 cells relative to cells incubated with complement alone (P<0.0001) (Fig. 3B). Taken together, our findings indicate that IgM maintains the phagosomal acidity in the presence of METH and stimulates the eradication of intracellular C. neoformans.
3.4 Specific IgM to C. neoformans capsular polysaccharide and complement stimulates nitric oxide (NO) production by macrophages after exposure to METH and fungal interaction
The generation of NO by iNOS has been implicated in the antimicrobial activity of activated macrophages against a variety of intracellular pathogens [31]. We investigated the impact of complement or complement + IgM on NO production by J774.16 cells after exposure to METH. NO levels were significantly reduced in the supernatants of complement-incubated leukocytes (Chlq and METH) when compared with untreated controls (P<0.0001) (Fig. 4A). In contrast, NO production ability of phagocytic cells incubated with complement + IgM and METH was not impaired whereas a significant reduction was observed in Chlq-treated J774.16 cells (P<0.0001). A significant reduction in the levels of NO supernatants was observed in METH-treated cells incubated with complement compared to their complement + IgM counterparts (P<0.01). Macrophage-like cells treated with aminoguanidine (AG), an inhibitor of iNOs, showed a substantial reduction in NO production compared to all the other groups regardless of the treatment (Fig. 4A). The expression of iNOS was determined to confirm whether NO production by macrophages was affected by METH after incubation with complement or complement + IgM (Fig. 4B–C). In the complement groups, we observed a significant reduction of iNOS expression in Chlq-treated macrophages when compared with untreated control cells (P<0.0001). Similarly, a dose-dependent decrease of iNOS expression in METH-treated cells was apparent (Fig. 4B) and its quantification demonstrated a significant difference (P<0.0001) (Fig. 4C). We evinced a significant reduction of iNOS expression of macrophages incubated with complement + IgM and treated with 25 μM Chlq and 50 μM METH relative to the untreated and 25 μM METH groups (P<0.0001) (Fig. 4B–C). Complement and complement + IgM groups were compared under all the different conditions and only J774.16 cells incubated with AG and 25 μM METH showed a significant increase difference (P<0.01) (Fig. 4B–C). Since TNF-α mediates the induction of nitric oxide synthase in macrophages [32], we assessed the levels of this pro-inflammatory cytokine in the supernatant of untreated and AG-, Chlq-, and METH-treated cells after interaction with C. neoformans. We found that the amount of TNF-α produced by J774.16 cells was proportional to the levels of NO (data not shown). This result was confirmed by using anti-TNF-α antibodies.
Figure 4. Anti-C. neoformans capsule specific IgM stimulates nitric oxide (NO) production by J774.16 cells after exposure to METH and interaction with C. neoformans.

(A) NO production was quantified using the Griess method after J774.16 cells were treated with aminoguanidine (AG; iNOS inhibitor), PBS (untreated), Chlq (25 μM), or METH (25 or 50 μM). (B) Western blot analysis of inducible nitric oxide synthase (iNOS) of macrophages treated with AG, PBS, Chlq, or METH. (C) Quantitative measurements of individual band intensity in western blot gel in (B) for iNOS. GAPDH was used as a control to determine the relative intensity ratio. For panels A and C, bars represent the means of multiple measurements and error bars denote SDs. Symbols (&, %, ≠, @) denote P-value significance (P<0.0001) calculated using ANOVA and adjusted by use of the Bonferroni correction. &, %, ≠, and @ indicate significantly lower NO production and iNOS expression than in J774.16 cells incubated with PBS, Chlq, 25 and 50 μM METH groups, respectively. Crosses (X) indicate P-value significance (P<0.01) calculated using student’s t-test analysis. The experiments were performed twice with similar results obtained.
4. DISCUSSION
METH abuse can alter biological processes and immune functions necessary for host defense [33, 34]. Antigen presenting cells play major roles as sentinels for first line alerts or as mediators that shape the adaptive immune response. METH at pharmacological concentrations exerts a direct immunosuppressive effect on dendritic cells and macrophages [23, 24]. Also, enhancement of HIV infection [1, 7, 8] and replication of the virus inside of macrophages [35, 36] are excellent examples of how METH can modify the pathogenesis of an infectious disease. Active METH-using HIV-infected persons have higher viral loads than those who never used the drug or recently used the drug [1]. METH use facilitates the acquisition of opportunistic infections in HIV-infected individuals [37, 38]. For instance, 40% of HIV-infected patients with history of METH use in Thailand are susceptible to infection with Mycobacterium tuberculosis [39]. In addition, we have demonstrated that METH enhances cryptococcosis and impairs the host immune response during C. neoformans infection, including the fungus ability to alter its polysaccharide capsule composition, extensively release polysaccharide, form biofilms in tissues, and invade the CNS [14, 15]. Thus, C. neoformans is an ideal model to investigate the effects of METH on the immune system, particularly due to the fungus closed interactions with macrophages [26, 29].
We used J774.16 macrophage-like cells to evaluate the effects of METH in complement-mediated phagocytosis of C. neoformans in the presence of complement alone or complement supplemented with IgM. METH inhibited phagocytosis of the fungus by macrophages in the presence of complement alone. Accumulation of complement-opsonized yeast cells outside and closely to macrophages was observed suggesting the inability of these phagocytic cells to engulf the fungi. The efficacy of complement-mediated phagocytosis of C. neoformans is dependent on the location of C3 in the polysaccharide capsule [19]. When C3 localizes inside of the capsule C. neoformans is poorly phagocytized as opposed when it localizes at the edge of the capsule. Similarly, complement-mediated phagocytosis of C. neoformans depends on the volume of the capsule [19, 40]. For example, fungal cells with large capsule demonstrate low phagocytic indices whereas yeasts with small capsule are easily phagocytized by phagocytes. Cryptococci incubated with METH produce a small capsule because the drug stimulates the release of the polysaccharide material to the extracellular milieu [14]. It is conceivable that complement binds to the polysaccharide released upon stimulation with METH resulting in less number of C3 molecules getting attached to the yeast cells and avoiding recognition by macrophages. Also, C. neoformans has the ability to modify its polysaccharide capsule composition and cell surface charge in the presence of METH [14] which may cause formidable problems to the complement system.
Addition of IgM with complement to C. neoformans cells in the presence of METH resulted in enhanced phagocytosis. A previous study has shown that when encapsulated yeast cells are opsonized simultaneously with complement and antibody molecules, the immunoglobulin binds first and promotes C3 deposition at the edge of the capsule [22]. IgM facilitates C3 localization to the outer most layers of the capsule, modulates the classical complement pathway activation, and provides steric hindrance precluding C3 penetration into the inside of the capsule [22]. This is important because C3 localization determines the phagocytosis efficacy of C. neoformans by macrophages [19, 40]. Localizing C3 at the edge of the capsule may promote phagocytosis through C3-CR3 and C3-CR4 interactions, which does not occur in fungi incubated with complement alone. Since CR3 on the surface of macrophages recognize and bind to C3 attached to the outer edge of the capsular polysaccharide of C. neoformans [19, 20], we evaluated the effect of METH on complement and capsular-specific IgM mediated-expression of CR3 on the surface of these leukocytes. IgM enhances complement-mediated phagocytosis, and a notable increase in CD11b and CD18 expression which is an indication of CR3 involvement in the process and activation of the classical pathway [22]. In addition to the recognition of opsonized cryptococcal cells by CR3, this opsonin-receptor interaction stimulates downstream signaling resulting in the activation of GTPase-RhoA [41], a key protein regulator of actin polymerization during the formation of the phagocytic pocket, thus, increasing fungal engulfment.
Antibodies, especially IgM, agglutinate or aggregate microbes by binding to epitopes on two or more cells simultaneously [42]. The pentameric structure of IgM provides ten Fab binding sites per molecule, making it the most efficient antibody for microbial aggregation. Since we found that anti-capsular IgM enhances complement-mediated phagocytosis of C. neoformans by J774.16 cells in presence of METH, we explored the possibility that IgM may promote fungal aggregation as a mechanism to enhance this process. Indeed, IgM significantly increased yeast cell aggregation and this process was enhanced in presence of complement. Encapsulated pathogens become susceptible to phagocytosis when they are coated with IgM and complement that engages complement receptors on phagocytic cells, triggering microbial uptake. Phagocytosis by binding to complement receptors, which expression is not affected by METH, is particularly important early in the immune response, before isotype switched antibodies have been made. For instance, natural IgM production against C. neoformans is essential for controlling pulmonary infection and increases the phagocytosis of fungi by alveolar macrophages [43]. Capsular polysaccharides belong to the T-cell independent type 2 antigens and therefore can stimulate the early production of IgM antibodies. IgM is effective at activating the complement system and its binding to C. neoformans triggers opsonization of yeast cells by complement and their prompt ingestion and destruction by phagocytes bearing complement receptors.
The fate of microorganisms ingested by macrophages is dependent on phagosome acidification after fusion with lysosomal vacuoles. As a weak base, METH collapses the pH gradient across acidic organelles within macrophages, including lysosomes and associated autophagic organelles [24]. Interestingly, IgM prevents the alkalization of the phagolysosome and promotes the killing of C. neoformans within macrophages. IgM has been shown to modulate early intracellular acidification and fusion of the phagolysosome suggesting that it may neutralize the negative effects of METH [44, 45]. Also, even though C. neoformans is a facultative intracellular microbe, it is likely that IgM impairs the fungus replication and capsular polysaccharide release inside of the phagolysosome allowing leukocytes to keep the integrity of the structure, preventing the release of yeast cells either by lytic or non-lytic exocytosis, and thus, control the infection.
After phagocytosis, macrophages also produce toxic reactive species, such as NO, in response to pathogens within the phagosome. iNOS is important for the control of C. neoformans infection, as evidenced by exacerbation of infection in mice following inhibition of the enzyme or inactivation of the gene [46]. Impaired production of toxic reactive species might create an ideal environment for fungal survival, facilitating intracellular replication and regulation of the phagolysosomal milieu. iNOS catalyzes the production of NO through oxidation of L-arginine on the cytosolic side of the phagosome membrane. NO thus synthesized subsequently diffuses into phagosomes where it can react with the mitochondrial respiratory chain and block electron transport as well as ATP biosynthesis [31]. We have previously shown that METH exposure significantly alters NO generation by murine macrophages [23]. Our data show that NO production is significantly increased in METH-treated J774.16 cells with cryptococci incubated with complement and IgM. In contrast, leukocytes with phagocytized yeast cells opsonized only with complement exhibited a reduction in NO production. Consistent with this, expression of iNOS was also significantly enhanced in phagocytic cells bearing the opsonized fungus with complement and IgM and exposed to METH suggesting the possibility that IgM might stimulate intracellular NO production and elimination of the fungus. Moreover, we observed a high TNF-α production by macrophages which accounts for the increased expression of iNOS since TNF-α directly acts in the activation of the enzyme [32].
In conclusion, METH interferes with macrophage function during interaction with C. neoformans that can enhance the fungus persistence and proliferation. Addition of anti-capsular IgM to complement opsonized fungi enhances the effector functions of macrophages exposed to the drug, highlighting the potential of using passive antibody administration as a therapeutic to treat drug users afflicted with AIDS and/or opportunistic infections. It is likely that capsular- specific IgM levels are lower in drug users as it is the case in HIV-infected [47–49] or other type of immunocompromised individuals such as solid organ transplant recipients [50]. IgM deficiency is associated with increased vulnerability to pulmonary C. neoformans infection and reduced alveolar macrophage phagocytosis of the fungus, which can be restored with natural IgM administration in mice [43]. In fact, it is plausible to design future studies assessing the activity of macrophages and passive administration in the setting of METH abuse and infectious diseases using animal models which may help establish the translational potential of this additive therapy in humans.
HIGHLIGHTS.
IgM enhances macrophage phagocytic function in presence of methamphetamine.
IgM upregulates complement receptor 3 and GTPase-RhoA on drug-treated macrophages.
IgM stimulates killing of microbes within the phagosome of METH-treated macrophages.
IgM prevents METH-induced alkalization of the phagosome.
IgM increases the production of nitric oxide by METH-treated macrophages.
Acknowledgments
L.R.M. was supported by the National Institute of General Medical Sciences of the US NIH under award number R15GM117501.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
L.A., V.V.E., C.M.D-R, and L.R.M. declare no conflict of interest.
References
- 1.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–6. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 2.Urbina JA, Concepcion JL, Caldera A, Payares G, Sanoja C, Otomo T, Hiyoshi H. In vitro and in vivo activities of E5700 and ER-119884, two novel orally active squalene synthase inhibitors, against Trypanosoma cruzi. Antimicrob Agents Chemother. 2004;48(7):2379–87. doi: 10.1128/AAC.48.7.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. J Subst Abuse Treat. 2006;31(2):195–202. doi: 10.1016/j.jsat.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Mihu MR, Roman-Sosa J, Varshney AK, Eugenin EA, Shah BP, Ham Lee H, Nguyen LN, Guimaraes AJ, Fries BC, Nosanchuk JD, Martinez LR. Methamphetamine Alters the Antimicrobial Efficacy of Phagocytic Cells during Methicillin-Resistant Staphylococcus aureus Skin Infection. MBio. 2015;6(6):e01622–15. doi: 10.1128/mBio.01622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlahov D, Sullivan M, Astemborski J, Nelson KE. Bacterial infections and skin cleaning prior to injection among intravenous drug users. Public health reports. 1992;107(5):595–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Meth’s Impact of HIV Epidemic being studied. Drug’s use is growing problem among MSMs. AIDS Alert. 2005;20:79–80. [PubMed] [Google Scholar]
- 7.Jiang J, Wang M, Liang B, Shi Y, Su Q, Chen H, Huang J, Su J, Pan P, Li Y, Wang H, Chen R, Liu J, Zhao F, Ye L, Liang H. In vivo effects of methamphetamine on HIV-1 replication: A population-based study. Drug and alcohol dependence. 2016;159:246–54. doi: 10.1016/j.drugalcdep.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Toussi SS, Joseph A, Zheng JH, Dutta M, Santambrogio L, Goldstein H. Short communication: Methamphetamine treatment increases in vitro and in vivo HIV replication. AIDS Res Hum Retroviruses. 2009;25(11):1117–21. doi: 10.1089/aid.2008.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad K. Addictive drug increases HIV replication and mutation. Lancet Infect Dis. 2002;2(8):456. doi: 10.1016/s1473-3099(02)00360-2. [DOI] [PubMed] [Google Scholar]
- 10.Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, Grant I. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–7. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- 11.Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10(2):185–90. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- 12.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 13.Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38(6):407–17. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- 14.Patel D, Desai GM, Frases S, Cordero RJ, DeLeon-Rodriguez CM, Eugenin EA, Nosanchuk JD, Martinez LR. Methamphetamine enhances Cryptococcus neoformans pulmonary infection and dissemination to the brain. MBio. 2013;4(4) doi: 10.1128/mBio.00400-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eugenin EA, Greco JM, Frases S, Nosanchuk JD, Martinez LR. Methamphetamine alters blood brain barrier protein expression in mice, facilitating central nervous system infection by neurotropic Cryptococcus neoformans. J Infect Dis. 2013;208(4):699–704. doi: 10.1093/infdis/jit117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shorman M, Evans D, Gibson C, Perfect J. Cases of disseminated cryptococcosis in intravenous drug abusers without HIV infection: A new risk factor? Med Mycol Case Rep. 2016;14:17–19. doi: 10.1016/j.mmcr.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulmer GS, Tacker JR. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect Immun. 1975;11(1):73–9. doi: 10.1128/iai.11.1.73-79.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9(6):835–46. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. 2003;33(7):1957–67. doi: 10.1002/eji.200323848. [DOI] [PubMed] [Google Scholar]
- 20.Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16(6):791–802. doi: 10.1016/s1074-7613(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60(11):4534–41. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaragoza O, Casadevall A. Monoclonal antibodies can affect complement deposition on the capsule of the pathogenic fungus Cryptococcus neoformans by both classical pathway activation and steric hindrance. Cell Microbiol. 2006;8(12):1862–76. doi: 10.1111/j.1462-5822.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinez LR, Mihu MR, Gacser A, Santambrogio L, Nosanchuk JD. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J Infect Dis. 2009;200(1):131–41. doi: 10.1086/599328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4(2):e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLeon-Rodriguez CM, Casadevall A. Cryptococcus neoformans: Tripping on Acid in the Phagolysosome. Front Microbiol. 2016;7:164. doi: 10.3389/fmicb.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A. 2002;99(5):3165–70. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61(4):216–20. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- 28.Riviere GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292(3):1042–7. [PubMed] [Google Scholar]
- 29.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68(7):4225–37. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwart W, Griekspoor A, Kuijl C, Marsman M, van Rheenen J, Janssen H, Calafat J, van Ham M, Janssen L, van Lith M, Jalink K, Neefjes J. Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity. 2005;22(2):221–33. doi: 10.1016/j.immuni.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–91. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonseca SG, Romao PR, Figueiredo F, Morais RH, Lima HC, Ferreira SH, Cunha FQ. TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur J Immunol. 2003;33(8):2297–306. doi: 10.1002/eji.200320335. [DOI] [PubMed] [Google Scholar]
- 33.Salamanca SA, Sorrentino EE, Nosanchuk JD, Martinez LR. Impact of methamphetamine on infection and immunity. Front Neurosci. 2014;8:445. doi: 10.3389/fnins.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peerzada H, Gandhi JA, Guimaraes AJ, Nosanchuk JD, Martinez LR. Methamphetamine administration modifies leukocyte proliferation and cytokine production in murine tissues. Immunobiology. 2013;218(8):1063–8. doi: 10.1016/j.imbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Wang Y, Ye L, Li J, Zhou Y, Sakarcan S, Ho W. Modulation of intracellular restriction factors contributes to methamphetamine-mediated enhancement of acquired immune deficiency syndrome virus infection of macrophages. Current HIV research. 2012;10(5):407–14. doi: 10.2174/157016212802138797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. The American journal of pathology. 2008;172(6):1617–24. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pevzner ES, Robison S, Donovan J, Allis D, Spitters C, Friedman R, Ijaz K, Oeltmann JE. Tuberculosis transmission and use of methamphetamines in Snohomish County, WA, 1991–2006. Am J Public Health. 2010;100(12):2481–6. doi: 10.2105/AJPH.2009.162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich LE, Sivakumar P. An atypical presentation of a typical pulmonary pathogen in an immunosuppressed patient. BMJ case reports. 2015;2015 doi: 10.1136/bcr-2015-209911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mankatittham W, Likanonsakul S, Thawornwan U, Kongsanan P, Kittikraisak W, Burapat C, Akksilp S, Sattayawuthipong W, Srinak C, Nateniyom S, Tasaneeyapan T, Varma JK. Characteristics of HIV-infected tuberculosis patients in Thailand. Southeast Asian J Trop Med Public Health. 2009;40(1):93–103. [PubMed] [Google Scholar]
- 40.Kozel TR, Pfrommer GS, Guerlain AS, Highison BA, Highison GJ. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988;56(11):2794–800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asplund MB, Coelho C, Cordero RJ, Martinez LR. Alcohol impairs J774.16 macrophage-like cell antimicrobial functions in Acinetobacter baumannii infection. Virulence. 2013;4(6):467–72. doi: 10.4161/viru.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabrizio K, Manix C, Guimaraes AJ, Nosanchuk JD, Pirofski LA. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clinical and vaccine immunology : CVI. 2010;17(5):713–21. doi: 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. Journal of immunology. 2010;184(10):5755–67. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marches R, Vitetta ES, Uhr JW. A role for intracellular pH in membrane IgM-mediated cell death of human B lymphomas. Proc Natl Acad Sci U S A. 2001;98(6):3434–9. doi: 10.1073/pnas.061028998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouvier G, Benoliel AM, Foa C, Bongrand P. Relationship between phagosome acidification, phagosome-lysosome fusion, and mechanism of particle ingestion. J Leukoc Biol. 1994;55(6):729–34. doi: 10.1002/jlb.55.6.729. [DOI] [PubMed] [Google Scholar]
- 46.Rivera J, Mukherjee J, Weiss LM, Casadevall A. Antibody efficacy in murine pulmonary Cryptococcus neoformans infection: a role for nitric oxide. Journal of immunology. 2002;168(7):3419–27. doi: 10.4049/jimmunol.168.7.3419. [DOI] [PubMed] [Google Scholar]
- 47.Subramaniam K, Metzger B, Hanau LH, Guh A, Rucker L, Badri S, Pirofski LA. IgM(+) memory B cell expression predicts HIV-associated cryptococcosis status. J Infect Dis. 2009;200(2):244–51. doi: 10.1086/599318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramaniam K, French N, Pirofski LA. Cryptococcus neoformans-reactive and total immunoglobulin profiles of human immunodeficiency virus-infected and uninfected Ugandans. Clinical and diagnostic laboratory immunology. 2005;12(10):1168–76. doi: 10.1128/CDLI.12.10.1168-1176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshaw M, Pirofski LA. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clinical and experimental immunology. 1995;99(3):425–32. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jalali Z, Ng L, Singh N, Pirofski LA. Antibody response to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan in patients after solid-organ transplantation. Clinical and vaccine immunology: CVI. 2006;13(7):740–6. doi: 10.1128/CVI.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


