Abstract
Proteome homeostasis, or proteostasis, is essential to maintain cellular fitness and its disturbance is associated with a broad range of human health conditions and diseases. Cells are constantly challenged by various extrinsic and intrinsic insults, which perturb cellular proteostasis and provoke proteotoxic stress. To counter proteomic perturbations and preserve proteostasis, cells mobilize the proteotoxic stress response (PSR), an evolutionarily conserved transcriptional program mediated by heat shock factor 1 (HSF1). The HSF1-mediated PSR guards the proteome against misfolding and aggregation. In addition to proteotoxic stress, emerging studies reveal that this proteostatic mechanism also responds to cellular energy state. This regulation is mediated by the key cellular metabolic sensor AMP-activated protein kinase (AMPK). In this review, we present an overview of the maintenance of proteostasis by HSF1, the metabolic regulation of the PSR, particularly focusing on AMPK, and their implications in the two major age-related diseases—diabetes mellitus and neurodegenerative disorders.
Keywords: Alzheimer’s disease, Dementia, Heat shock response, Insulin resistance, Metabolic stress, Metformin, Molecular chaperone/HSP, PolyQ diseases
Introduction
Under a variety of environment stressors, it is imperative for cells to sustain the internal homeostasis to maintain normal cellular functions. Such stressors include heat shock, heavy metals, acidosis, oxidants, and metabolic poisons. Environmental insults perturb cellular proteome homeostasis, or proteostasis, triggering the heat-shock response (HSR) or proteotoxic stress response (PSR). Cellular proteostasis refers to the delicate, dynamic equilibrium among protein synthesis, folding, and degradation inside cells. Proteotoxic stressors often cause cellular protein damage or conformational changes, leading to protein misfolding. As a means to counter this disturbance and maintain proteostasis, cells markedly produce a group of specialized proteins, named heat-shock proteins (HSPs) or molecular chaperons. The induction of HSPs by proteotoxic stressors, a hallmark of the PSR [1, 2], is transcriptionally regulated by heat shock factors (HSFs), which recognize the heat shock elements (HSEs) located at the promoter regions of HSP genes [3]. In eukaryotes except avian cells, HSF1 has been shown as the master inducer of HSP transcription. In the absence of stress stimuli, HSF1 remains inactive and becomes readily activated upon stress [4].
Metabolic disturbances, including glucose deprivation, hypoxia, ischemia, and metabolic poisons, interfere with mitochondrial production of ATP and provoke metabolic stress. By contrast, growth factors stimulate glucose metabolism to generate ATP and therefore suppress metabolic stress [5, 6]. Impaired glucose metabolism leads to diminished ATP generation and limited biosynthetic precursors, including nucleic acids and fatty acids, which are crucial to cellular growth and homeostasis [7, 8]. Regulation of cellular metabolism by the key energy sensor AMP-activated protein kinase (AMPK) has proven crucial in preserving energy homeostasis, thereby maintaining normal cellular functions for survival of metabolic stress. Aberrant metabolic regulations have been implicated in various human diseases including metabolic syndrome, cancer, and neurodegeneration [9–11]. Like other anabolic processes, chaperone-mediated protein folding also consumes ATP [12, 13]; unsurprisingly, protein misfolding occurs under metabolic stress [1, 14, 15]. Nonetheless, little is known of how metabolic stress impacts protein folding and proteostasis specifically. In this review, we particularly focus on the metabolic regulation of the PSR, through the newly discovered AMPK-HSF1 interactions, and its important implications in both diabetes mellitus (DM) and neurodegenerative disorders.
Heat shock factors (HSFs) and the proteotoxic stress response (PSR)
Maintenance of proteostasis is essential for cell survival under proteotoxic stress. The PSR is a well-characterized molecular mechanism through which chaperones are markedly induced in response to proteotoxic stress to preserve cellular proteostasis. Numerous studies have conclusively indicated that the cellular chaperone network plays a pivotal role in maintaining protein stability, protecting proteins from misfolding and aggregation, regulating assembly of protein complexes, and promoting protein complex translocation [16–18]. The primary transcription factors initiating the PSR are heat shock factors (HSFs). In mammals the HSF family consists of nine members, which exhibit differential functions in regulating cellular proteostasis [19]. Among this family, HSF1 is the master factor controlling the powerful transcriptional response to heat and other proteotoxic stressors [20]. In most tissues HSF1 is constitutively expressed but remains inactive under non-stress conditions. In the absence of stress, HSF1 exists as monomers that are repressed by a protein complex comprising HSPs and co-chaperones in the cytoplasm. Upon challenged by stressors, including heat shock, heavy metals and proteosome inhibitors, HSF1 is released from this inhibitory complex and converted from monomers into trimers with DNA-binding capability. Subsequently, HSF1 timers become phosphorylated, undergo nuclear translocation, and ultimately bind to the heat-shock element (HSE) sequences within the promoters of many HSP genes [21]. This multi-step process of HSF1 activation results in a markedly increased cellular chaperoning capacity to effectively counter proteotoxic stress.
HSF family
Thus far, nine HSF family members—HSF1, 2, 3, 4, 5, X1, X2, Y1, and Y2, have been identified in mammalian cells. Despite many common features, they differ considerably in post-translational modifications, interactions with other proteins, and tissue expression patterns [22, 23]. All HSFs contain the highly conserved N-terminal DNA-binding domain (DBD), a looped helix-turn-helix structure [24, 25]. Upon activation, HSF1 trimers bind to HSEs that consist of several inverted repeats of the pentanucleotide motif nGAAn. Upon withdrawal of stress or under a prolonged stress, the transcriptional activity of HSF1 is attenuated while HSF1 returns to the monomeric state [26]. The domain immediately adjacent to the DBD contains hydrophobic heptad repeats (HR-A and HR-B), which mediate HSF1 trimerization. By contrast, the near C-terminal HR-C domain is thought to constrain HSF1 trimerization [27, 28]. The C-terminal transactivation domain is necessary for the transcription of target genes [29, 30]. In addition, the regulatory domain (RD), located between the HR-A/B and HR-C domain, is responsible for suppressing HSF1 activity under non-stress condition. Intriguingly, the RD domain of HSF1 can act as an intrinsic sensor for heat stress [31], and be targeted by various post-translational modifications [32, 33]. The PSR, triggered by diverse environmental stressors, induces the expression of several classes of molecular chaperones or HSPs, including HSP27, HSP72, and HSP90α. By facilitating the folding, transportation, assembly, and degradation of other proteins, HSPs protect the proteome from the danger of misfolding and aggregation. Therefore, under proteotoxic stress HSPs are essential to proteostasis and cell survival.
In mammals, HSF1, the prototype of HSFs, acts as the master regulator of the PSR. Embryonic fibroblasts derived from Hsf1-deficient mice display no stress-induced Hsp gene transcription [34], indicating the total necessity of HSF1 for the PSR. However, HSF2 can also participate in the PSR through formation of heterotrimers with HSF1 [35–37]. Interestingly, it has been reported that HSF2 maintains the HSP70 gene locus epigenetically at a de-condensed chromatin state [37, 38]. In mitotic cells, inhibition of the binding of HSF2 to the hsp70i promoter promotes cell survival of stress [39]. Congruently, down-regulation of HSF2 promotes the binding of both HSF1 and RNA polymerase II to mitotic chromatins, thereby enhancing stress-induced HSP70 expression [40]. Moreover, HSF4 could also interact with HSF1 to recruit the chromatin remodeling complex SWI/SNF to stress-related genes [41]. By contrast, HSF3, albeit expressed in mice, does not regulate Hsp gene expression [22, 42, 43].
Despite dispensable for the PSR, accumulating evidence indicates that HSF2, HSF3, and HSF4 all have important biological functions. For example, HSF2 is required for normal spermatogenesis [44], and HSF3 regulates the expression of genes during chicken embryonic development [45]. Distinct from HSF2 and HSF3, HSF4 is required for the development of lens [46, 47]. Although HSP genes have been long regarded as the primary transcriptional targets of HSF1, emerging evidence reveals that HSF1 also regulates the expression of numerous non-HSP genes involved in the development and maintenance of brain, germ cells, and immune cells [47–49]. In stark contrast to HSF1, 2, 3, and 4, all of which bind to DNA, the biological functions of HSF5 and sex chromosome-linked HSFX1, X2, Y1, and Y2 still remain largely unknown.
Regulation of HSF1
Activation of HSF1 is a complex, multi-step process, wherein post-translational modifications, including phosphorylation, sumoylation, and acetylation, play a key role. Following exposure to heat, HSF1 trimers are heavily phosphorylated. At least 12 phosphorylation sites, either stimulatory or inhibitory, have been recognized on HSF1 [20, 50]. For example, phosphorylation at Ser326 is critical to HSF1 activation by heat stress [51]. Phosphorylation at Ser230 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) and phosphorylation at Ser320 by protein kinase A (PKA) also induce HSF1 activation [52, 53]. By contrast, phosphorylation at Ser307 by ERK and Ser303 by glycogen synthase kinase 3 (GSK3) repress HSF1 activity under non-stress conditions [54]. Recently, phosphorylation at Ser121 by AMPK was shown to inhibit HSF1 activation [55]. Furthermore, through AMPK activation, the metabolic stressor metformin induces Ser121 phosphorylation and thereby impairs the DNA-binding activity of HSF1 [55] (Fig. 1). Importantly, emerging evidence has uncovered that key oncogenic and tumor-suppressing signaling pathways intimately regulate HSF1. For example, the well-known tumor suppressor neurofibromatosis type I (NF1) negatively regulates HSF1 and its mediated PSR [56]. Loss of NF1 alone suffices to activate HSF1 through hyper-activation of oncogenic RAS/MAPK signaling [56]. In light of the important role of RAS/MAPK signaling in activating HSF1, surprisingly, it is MEK, rather than ERK, that mediates HSF1 activation by directly phosphorylating Ser326 [57]. Ser326 phosphorylation is crucial to the nuclear translocation, DNA binding, as well as stability of HSF1 [57]. Congruently, clinically relevant MEK inhibitors inactivate and deplete HSF1, provoking global protein destabilization and ubiquitination, aggregation, and amyloidogenesis in human melanoma cells, similarly to HSF1 knockdown [57]. Given that hyper-activation of the RAS/MAPK signaling cascade occurs in one-third of all human cancers [58], it is not surprising that constitutive HSF1 activation is widespread in human malignancies and of significant prognostic value [59].
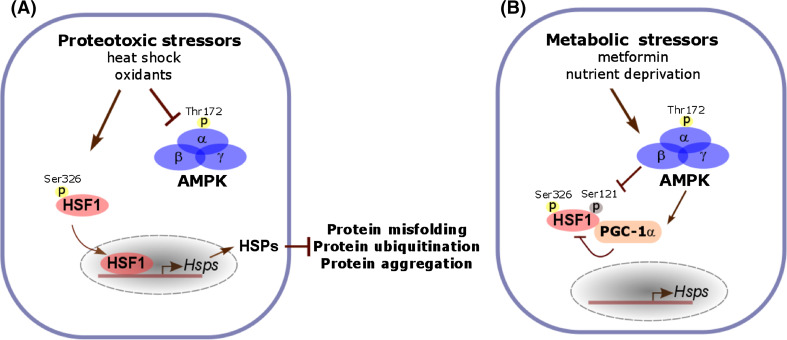
Fig. 1.
Suppression of the HSF1-mediated PSR by metabolic stressors. a Proteotoxic stressors activate HSF1 and its mediated PSR. Under proteotoxic stress, Ser326 phosphorylation is a key post-translational modification activating HSF1. In addition, heat stress also inactivates AMPK and blocks its mediated Ser121 phosphorylation, a modification inhibitory to HSF1 activation. Under proteotoxic stress, induced HSP expression through the PSR plays a critical role in preserving cellular proteostasis and enhance survival. b In contrast to proteotoxic stressors, metabolic stressors, including metformin and nutrient deprivation, suppresses HSF1 activation through AMPK activation. Activated AMPK phosphorylates HSF1 directly at Ser121 to impair the nuclear translocation and DNA binding of HSF1. In addition, AMPK can suppress the transcriptional activity of HSF1 indirectly through PGC-1α. Upon phosphorylated and activated by AMPK, PGC-1α acts as a transcriptional repressor by physically interacting with HSF1. Through suppression of the PSR, metabolic stressors exacerbate the disruption of proteostasis by proteotoxic stressors
Sumoylation also plays a notable role in regulating HSF1 activity. For example, phosphorylation at Ser303 leads to sumoylation at Lys298 on HSF1, thereby suppressing its transcriptional activity [60, 61]. Moreover, sumoylation could mediate protein–protein interactions by providing a docking site for proteins containing small ubiquitin-like modifier (SUMO)-interacting motif [62, 63].
Another modification influencing HSF1 is acetylation. Acetylation at Lys80 causes HSF1 dissociation from chromatins and subsequently diminishes HSF1 activity [64]. Interestingly, sirtuin 1 (SIRT1) serves as a deacetylase for HSF1 at Lys80 and thereby maintains the DNA-binding competent state of HSF1 [64]. Acetylation of HSF1 is enhanced by pharmacological inhibition of SIRT1 and reduced by overexpression of SIRT1, respectively [64]. In contrast to impaired DNA binding, acetylation enhances the stability of HSF1 proteins. By acetylating multiple lysine residues, the acetyltransferase p300 protects HSF1 from proteasome-mediated degradation [65].
Protein–protein interactions, in addition to post-translational modifications, regulate HSF1 as well. For example, HSF1 is repressed by its own transcriptional targets HSPs, thereby not only maintaining HSF1 at the inactive state under non-stress conditions but also constituting a negative feedback mechanism to attenuate HSF1 activity during stress recovery [66]. In support of this, HSP90-HSF1 interactions mitigate the translocation of HSF1 into the nucleus and impair HSP72 induction by hyperthermia in the rat myocardial infraction model [67]. By contrast, ATF1-HSF1 interactions recruit the chromatin-remodeling factor BRG1 and histone acetyltransferases, both p300 and CREB-binding protein (CBP), to assemble a potent HSF1 transcription complex [68].
Transcription-independent action of HSF1
It has been widely recognized that HSF1 promotes cellular and organismal survival of proteotoxic stress and prolongs lifespan; in stark contrast to these beneficial effects, its surprising pro-oncogenic role has just begun to emerge recently [56, 69, 70]. In models for diverse types of cancer, including malignant peripheral nerve sheath tumor, mammary carcinoma, melanoma, and hepatocellular carcinoma, the pro-oncogenic role of HSF1 has been demonstrated [56, 57, 71].
The underlying mechanisms, unsurprisingly, are diverse, given the large transcriptional network HSF1 regulates. It has been shown that HSF1 augments the oncogenic RAS signaling cascade, suppresses oncogene-induced cell death and senescence, promotes cellular migration and epithelial-mesenchymal transition (EMT), as well as enhances lipogenesis [72]. Of note, new evidence further indicates that HSF1 plays a critical role in preserving proteostasis and suppressing amyloidogenesis to promote oncogenesis [57]. Canonically, all these multifaceted effects of HSF1 have been ascribed to its eminent transcriptional action.
Unexpectedly, a new study reports that through a transcription-independent mechanism, HSF1 preserves mTROC1 integrity and supports robust protein translation by suppressing c-Jun N-terminal kinase (JNK), thereby promoting stress resistance and growth [73]. JNK, acting as a cellular sensor of proteotoxic stress, constitutively associates with mammalian target of rapamycin complex 1 (mTORC1). Upon rapid activation by proteotoxic stress, JNK phosphorylates both regulatory-associated protein of mTOR (RAPTOR) and mTOR directly, leading to mTORC1 dissociation and subsequent translation inhibition. Importantly, HSF1 physically interacts with and sequesters JNK apart from mTORC1, thereby maintaining protein synthesis and cellular growth [73]. Of note, HSF1 exerts this effect independently of its transcriptional regulation, highlighting a new mode of action of this ancient cytoprotective factor.
Metabolic control of the PSR in diabetes mellitus
Impacts of metabolic states on proteostasis
Although less appreciated, metabolic disturbance can impact proteostasis. For example, metabolic dysregulation in diabetes induces protein aggregation in pancreatic β-cells [74, 75]. In rodents, the metabolic disturbance induced by high-fat diets or associated with diabetes promotes β-amyloid deposits in the brain [76, 77].
It has been well recognized that an array of signaling pathways, including mTORC1 and AMPK, sense the cellular energy state and respond to metabolic changes closely. While AMPK senses fluctuations in the intracellular AMP:ATP ratio, mTORC1 senses the availability of nutrients such as amino acids. Activated by nutrients, mTORC1 controls cellular growth by regulating protein translation and autophagy [78]. Interestingly, it has been shown that metabolic stress activates AMPK, which, in turn, inhibits mTORC1 [79]. AMPK directly phosphorylates RAPTOR, a key binding partner of mTOR, at two sites, Ser722 and Ser792, which subsequently induces 14–3–3 binding to RAPTOR [79]. Therefore, metabolic stress, through AMPK activation, impairs the mTORC1-mediated protein synthesis [80].
Metabolic states can also inflict protein damage. Metabolic syndrome, including obesity, hyperglycemia, hyercholesterolemia, hypertriglyceridemia, and insulin resistance, greatly increases the risk of developing type 2 diabetes (T2D) [81]. Of note, metabolic syndrome is frequently accompanied by oxidative stress, owing to impaired mitochondrial ATP production and subsequent induction of reactive oxygen species (ROS) [82]. Accumulation of ROS is detrimental to cells by damaging cellular macromolecules including lipids, nucleic acids and proteins. Protein oxidation by ROS alters the conformations, solubility, and stability of proteins [83], provoking proteotoxic stress. For example, high glucose-induced oxidative stress results in protein misfolding and aggregation in obese Zucker rats [74]. Inevitably, oxidative stress activates HSF1 and its mediated PSR [84]. Thus, metabolic dysregulation, at least in part through oxidative damage, deteriorates protein quality and disrupts proteostasis.
Metabolic regulation of HSF1 and the PSR
Intriguingly, recent studies have revealed that the PSR, one of the key protein quality-control machineries, is also implicated in metabolic syndrome and DM. For example, in diabetic monkeys, HSF1, HSP70, and HSP90 proteins are all diminished in the liver; by contrast, their expression is elevated in the pancreas [85], likely reflecting the compensatory mechanism to restore proteostasis in this tissue. Interestingly, dietary or calorie restriction, a metabolic intervention effectively suppressing age-related diseases, including cardiovascular diseases, cancer, neurodegeneration, and T2D, and prolonging lifespan, also regulates HSF1 [86, 87]. In calorie-restricted cells, the age-related diminishment of HSF1 DNA binding is reversed [87]. Contrary to calorie restriction, amino acid deprivation impairs HSF1 DNA-binding activity and suppresses the expression of HSP mRNAs [88]. Similarly, depletion of glutathione also suppresses the HSF1 activation by heat shock [89]. Moreover, under fasting HSF1 activity in mouse livers is low; however, re-feeding markedly increases HSF1 activity and HSP expression in the liver [71]. Taken together, accumulating evidence reveals a complex HSF1 regulation by the cellular metabolic state. Despite these paradoxical findings, it remains possible that the ultimate impacts of nutrients on HSF1 may depend on the severity of nutrient inaccessibility.
Imbalanced energy intake and expenditure is closely associated with metabolic diseases including DM. Two key players in sensing cellular nutritional status and preserving energy homeostasis are insulin signaling and mitochondria. Metabolic signaling has been implicated in regulating HSF1 and the PSR. For example, it was proposed that insulin signaling inhibits HSF1 activation [90]. Stimulation of insulin-like growth factor receptor (IGFR in mammals, DAF-2 in C. elegans) leads to activation of PI3K/AKT signaling and subsequent phosphorylation of the transcriptional factor Forkhead Box O (FOXO), impairing its nuclear translocation. Importantly, FOXO is required to cooperate with HSF1 in co-regulating a subset of target genes including small hsp genes [91]. Furthermore, in C. elegans insulin/IGF-1 signaling can negatively regulate HSF1 through DDL1 (homologue of human CCDC53), which forms a repressive protein complex with HSF1 [92]. In addition, activation of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α), a key regulator of mitochondrial biogenesis, has also been reported to inhibit HSF1. Through physical interaction, PGC-1α represses the transcriptional action of HSF1 directly [93]. In further support of the metabolic regulation of HSF1, other key metabolic sensors including SIRT1 and AMPK are able to modify HSF1 as well. AMPK phosphorylates and SIRT1 deacetylates HSF1, respectively [54, 63]. Congruently, high-fat diets impair AMPK-dependent phosphorylation of PGC-1α and increase the expression and activity of SIRT1, thereby activating HSF1 [94]. Thus, it is conceivable that metabolic dysregulation associated with DM likely affects the HSF1-mediated PSR, contributing to disruption of proteostasis.
Protective roles of HSF1 and HSPs in DM
Ample evidence has implicated dysregulation of the chaperone network in the pathogenesis of DM. For example, HSP72 expression induced by hyperthermia is markedly impaired in rats developing streptozotocin (STZ)-induced T2D [95]. Similarly, exercise fails to induce the expression of HSF1 and HSP72 in the skeletal muscle of diabetic rats [96]. In diabetes patients, HSPs are also markedly diminished, correlating with insulin resistance [97, 98].
Importantly, reduced HSP72 expression impairs insulin-stimulated glucose uptake in DM patients [99]. Moreover, Hsp72 knockout mice display exacerbated obesity, insulin resistance, and lipid accumulation in the skeletal muscle [100]. Mechanistically, deletion of Hsp72 reduces oxygen uptake and fatty acid oxidation rate in primary myocytes [100]. Conversely, restoring HSP72 expression improves insulin resistance and glucose metabolism. In mice, transgenic HSP72 overexpression protects against high-fat diet or obesity-induced insulin resistance, which is tightly associated with suppressed JNK phosphorylation [101]. Furthermore, enhanced HSP72 expression increases mitochondrial oxidation and ameliorates insulin resistance in the skeletal muscles in mice [102]. Also, by suppressing the aggregation and amyloidogenesis of human islet amyloid polypeptide (h-IAPP), a peptide hormone co-secreted with insulin, HSP72 overexpression protects pancreatic β-cells from toxicity [103]. Moreover, BGP-15, a small-molecule stimulant of HSP72, has been shown to improve insulin sensitivity, suppress inflammation, increase mitochondrial activity, and restore metabolic homeostasis in Goto-Kakizaki (GK) rats, a non-obese T2D model [102, 104, 105]. Importantly, BGP-15 significantly improves insulin sensitivity in insulin-resistant patients and displays no adverse effects [104]. Furthermore, heat shock in combination with mild electrical stimulation, which induces Hsp72 expression, markedly improves insulin sensitivity and glucose homeostasis in db/db mice [106]. Similarly, treatment with ADAPT-232, an adaptogen known to induce HSF1 and HSP72 expression, notably rescues growth retardation in a transgenic C. elegans model expressing h-IAPP [103]. In addition to HSP72, HSP27 also plays a beneficial role in diabetes. In mice, transgenic HSP27 overexpression antagonizes cytokine-induced islet apoptosis and mitigates STZ-induced T2D [107].
Congruent with the effects of HSPs in diabetes, expression of a constitutively active HSF1 in pancreatic β-cells enhances glucose-driven insulin secretion, elevating serum insulin levels and reducing blood glucose levels in neonatal STZ-induced diabetic rats [108]. These effects are correlated with activation of glucokinase and neuronal nitric oxide synthase [108]. As the pivotal regulator of HSP expression under stress, HSF1 is subjected to multi-layers of regulation, including negative feedback control by its own transcriptional targets, HSPs, and kinase-mediated phosphorylation events [109]. Interestingly, ERK, GSK3β, and JNK kinases, all of which are associated with insulin resistance, have been shown to phosphorylate and suppress HSF1 [110] (Fig. 2). By inhibiting GSK3β to activate HSF1, physical activity/exercise, a lifestyle intervention well known to reduce the incidence of T2D, induces the expression of HSPs [111]. Furthermore, mild heat treatment has been shown to decrease fasting plasma glucose levels in T2D patients and prevent insulin resistance in high-fat diet-fed rats [112, 113]. Similarly, a recent study demonstrated that activation of HSF1 by the natural compound celastrol regulates energy expenditure in mice fed high-fat diets [114]. Through stimulation of PGC-1α signaling, celastrol-induced HSF1 activation enhances mitochondrial function, regulates white fat browning, and prevent obesity, insulin resistance, and hepatic steatosis [114].
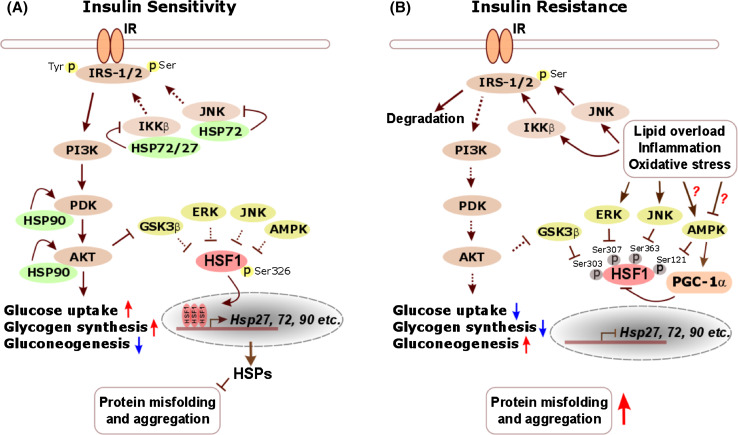
Fig. 2.
The HSF1-mediated PSR antagonizes insulin resistance and preserves proteostasis. a In insulin-sensitive cells, insulin signaling mobilizes AKT, which subsequently leads to inactivation of GSK3β [196], a negative regulator of HSF1. In addition, other HSF1 suppressors, including ERK, JNK and AMPK, also remain at a normal or latent state. As a consequence, the HSF1-mediated PSR is operational to induce abundant HSP expression. Importantly, HSP27 and HSP72 suppress both IKKβ and JNK [197–199], two key kinases that inhibit insulin receptor substrate-1/2 (IRS-1/2) through direct serine phosphorylation and thereby impede insulin signaling [200]. Moreover, HSP90 can stabilize and enhance the activation of both PDK and AKT [201, 202], two essential components within the insulin signaling cascade. Thus, in addition to preserving cellular proteostasis, the HSF1-mediated PSR maintains robust insulin signaling, enhancing glucose uptake and glycogen synthesis but suppressing gluconeogenesis. b In insulin-resistant cells, activated IKKβ and JNK, owing to lipid overload, inflammation, and oxidative stress, markedly phosphorylate IRS-1/2, causing their dissociation from IR and proteasomal degradation [203, 204]. Impaired insulin signaling further leads to enhanced GSK3β activity. In addition, inflammation and oxidative stress closely associated with the insulin-resistant state also activate ERK and JNK. However, it still remains controversial whether AMPK is activated under insulin resistance. Whereas lipid overload can suppress AMPK [205], oxidative stress is reported to cause its activation [206]. Collectively, these negative regulators deactivate HSF1 and its mediated PSR, depleting cellular HSPs to further exacerbate the impairment of insulin signaling and disruption of proteostasis
Collectively, a large body of evidence has revealed important roles of HSF1 and HSPs in both proteostasis and energy metabolism, thus supporting the contribution of their dysregulation to the pathogeneses of DM and further suggesting them as valuable therapeutic targets.
AMPK: a key mediator of the metabolic stress response
How does metabolic dysregulation impact the PSR? It has been widely recognized that AMPK plays a pivotal role in sensing cellular energy state and initiating the metabolic stress response (MSR). AMPK is a heterotrimeric protein consisting of α, β, and γ subunits that are encoded by seven individual genes in total. There are two α isoforms (α1 and α2), two β isoforms (β1 and β2), and three γ isoforms (γ1, γ2, and γ3). The N terminus of the α subunit contains a serine/threonine kinase domain that is activated by upstream kinases. The C-terminal domain of the β subunits serves as a linker to connect the C-terminal domain of the α subunits and the N-terminal domain of the γ subunits, and acts as a glycogen sensor [115, 116]. The γ subunits contain regulatory adenine nucleotide-binding sites and four tandem repeats known as cystathionine-b-synthase (CBS) domains. Through these CBS domains, the γ subunits are able to bind AMP, ATP, or ADP, thereby sensing the cellular energy status. Phosphorylation of Thr172 on the α1 subunit, a key modification activating AMPK, is mediated by the tumor suppressor liver kinase B1 (LKB1/STK11) or Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) [117, 118].
AMPK is activated by elevated intracellular AMP:ATP ratio. Under energy stress, increased intracellular AMPs leads to ATP replacement from the exchangeable sites on the γ subunits, causing a modest increase in AMPK Thr172 phosphorylation [119]. The ATP replacement also suppresses de-phosphorylation of Thr172, further enhancing AMPK activity [119]. Pharmacological metabolic stressors including metformin, arsenite, and antimycin A, or pathological conditions including ischemia and hypoxia, can also activate AMPK through depletion of ATP [120–123]. In addition, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), an adenosine analog, is widely used as a pharmacological activator of AMPK. Following uptake mediated by the adenosine transporter, inside cells AICAR is converted by the adenosine kinase into mono-phosphorylated forms, which mimic AMP [124].
Energy homeostasis is essential for cellular survival of metabolic stress. A large body of evidence has pinpointed a critical role of AMPK in preserving energy homeostasis. AMPK is known to regulate lipid metabolism in numerous tissues [117, 118]. Through direct phosphorylation, AMPK inactivates two key lipogenic enzymes, acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), the rate limiting enzymes for fatty acid and cholesterol synthesis, respectively [125]. In addition to lipogenesis, protein synthesis is another major ATP-consuming process. Under metabolic stress, AMPK is activated to inhibit both the lipogenesis, mediated by ACC and HMGCR, and the protein synthesis, mediated by mTORC1. mTORC1, sensitive to the inhibition by rapamycin, is a protein complex comprised of mTOR, DEPTOR, mLST8, PRAS40, and RAPTOR. mTORC1 phosphorylates ribosomal S6 kinase (p70-S6K) and eukaryotic translation initiation factor 4E (elF4E)-binding protein 1 (4EBP1), both of which are key players in controlling protein translation [126]. In the past decade, several studies have revealed that mTORC1 is regulated by the tumor-suppressing LKB1-AMPK signaling pathway. Indirectly, AMPK inhibits mTORC1 activity through activation of the tumor suppressors tuberous sclerosis complex 1 and 2 (TSC1 and TSC2). Through their GTPase activity, TSC1 and TSC2 inactivate the small G protein Ras homologue enriched in brain (RHEB), a key activator of mTORC1 [127]. AMPK activates TSC2 though phosphorylation of both Thr1227 and Ser1345 [128], subsequently inactivating RHEB and suppressing mTORC1. Moreover, AMPK is able to inhibit mTORC1 activity by phosphorylating RAPTOR directly [79].
In addition to key enzymes and kinases, AMPK also regulates various transcription factors or co-activators to mediate metabolic reprogramming and enhance cellular survival under metabolic stress. For example, AMPK activates FOXO3α to enhance stress resistance, glucose metabolism, and cell survival [129]. By contrast, AMPK inhibits the lipogenic transcription factor SREBP-1c, another means to suppress lipogenesis [130]. AMPK also phosphorylates and stabilizes TP53 through inhibition of the SIRT1-mediated TP53 deacetylation [131]. A prominent regulator of mitochondrial biogenesis and function is PGC-1α. Overexpression of PGC-1α in cultured cells promotes energy expenditure and increase cardiac mitochondrial biogenesis [132, 133]. AMPK phosphorylates PGC-1 directly to enhance its transcriptional activity and thereby promote mitochondrial biogenesis [134]. In mice expressing a dominant-negative AMPK transgene, mitochondrial biogenesis cannot be induced by energy deprivation in the skeletal muscle [135]. Taken together, by orchestrating a systemic cellular response to metabolic stress, AMPK activation reduces ATP consumption but enhances ATP production, thereby restoring energy homeostasis.
Implications of the AMPK-mediated HSF1 suppression in DM
Emerging studies have begun to shed light on the previously unappreciated link between metabolic stress and the HSF1-mediated PSR. A recent study revealed that metabolic stress, provoked by metformin or nutrient deprivation, inactivates the HSF1-mediated PSR through AMPK [55]. Upon activation, AMPK phosphorylates HSF1 at Ser121 directly, which impairs its nuclear translocation and DNA binding and subsequently renders cells vulnerable to proteotoxic stress [55]. Intriguingly, heat stress suppresses AMPK and its mediated MSR [55]. Moreover, AMPK can also suppress the PSR indirectly through PGC-1α. Another recent study showed that both PGC-1α and HSF1, through physical associations, are co-localized on several HSP gene promoters [93]. Importantly, PGC-1α acts as a repressor of the HSF1-mediated HSP transcription [93]. Thus, under metabolic stress AMPK is able to suppress the activation of HSF1 and its mediated PSR, both directly and indirectly.
Metformin, a metabolic stressor that potently activates AMPK, is a first-line medicine to treat T2D and prescribed to over 120 million people worldwide [136]. Instead of acting on LKB1 or AMPK directly, metformin mobilizes AMPK by inhibiting complex I of the mitochondrial electron transport chain, thereby causing cellular energetic stress [136]. Beyond lowering blood glucose levels by improving insulin sensitivity [137], metformin also prevents massive accumulation of autophagic vacuoles and thereby alleviates β-cell death in T2D patients [138]. Congruently, a long-term follow-up study reveals that metformin treatment reduces the mortality of T2D patients by 36 % [139].
Undoubtedly, metformin exerts a wide array of beneficial metabolic effects on T2D; however, emerging evidence reveals that metformin is also able to suppress the HSF1-mediated PSR through AMPK activation [55]. Thus, through disruption of proteostasis, metformin may not protect pancreatic β-cells, especially those suffering from IAPP amyloidogenesis, or even exacerbate their failure in T2D. Importantly, this new finding further suggests that activation of HSF1 and its mediated PSR, in combination with metformin, may represent a more effective therapeutic strategy for T2D.
Metabolic control of the PSR in neurodegenerative disorders
Disruption of proteostasis in neurodegenerative disorders
It has been well recognized that disruption of proteostasis is causally associated with aging and age-related diseases, particularly neurodegenerative disorders, in humans. Neurodegenerative disorders, including Huntington’s disease (HD) and Alzheimer’s disease (AD), are often characterized by protein misfolding, aggregation, and amyloidogenesis. Impaired cellular stress responses and diminished capacity of cellular machineries to clear misfolded and aggregated proteins likely contribute to severe disruption of proteostasis in these neurodegenerative disorders [140].
Protective roles of HSF1 and HSPs in neurodegenerative disorders
HD is an autosomal dominant neurodegenerative disease caused by expansion of the CAG trinucleotides, encoding for glutamine, in the first exon of the HTT gene [141, 142]. Huntingtin proteins with the polyglutamine (polyQ) tract are prone to misfolding and aggregation, resulting in neuronal toxicity [143]. The clinical symptoms of HD include the progressive movement disorders, dementia, cognitive impairment, and a shorten lifespan. In various HD models, it has been shown that HSF1 suppresses the aggregation of polyQ proteins; by contrast, loss of HSF1 accelerates its accumulation [144]. Overexpression of a constitutively active HSF1 in R6/2 mice, a widely used transgenic HD model, reduces polyQ aggregation and rescues body weight loss [145]. Furthermore, activation of HSF1 by the small molecule HSF1A ameliorates polyQ misfolding and protects neuronal precursor cells from toxicity in a Drosophila model of polyQ-mediated neurodegeneration [146]. Interestingly, nuclear factor of activated T cells (NFAT) appears to be required for the HSF1-mediated suppression of polyQ aggregation. Deletion of NFAT exacerbates polyQ aggregation and shortens the lifespan of R6/2 mice [147]. Mechanistically, NFAT and HSF1 cooperate to induce the expression of PDZ domain containing 3 (PDZD3) and αB-Crystallin/HSPB5, two important players in preventing polyQ aggregation [147].
Another polyQ disease is spinal and bulbar muscular atrophy (SBMA), an adult-onset motor neuron disease caused by the expression of CAC repeats in the gene coding androgen receptor (AR) [148–150]. Heterozygous deletion of Hsf1 increases accumulation of pathogenic AR in both neural and non-neural tissues, and aggravates neurodegeneration in AR-97Q mice, a popular transgenic model for SBMA [151]. Conversely, lentiviral delivery of Hsf1 into the motor cortex and striatum suppresses AR accumulation and alleviates neurodegeneration in these mice [151].
Worldwide nearly 44 million people are afflicted with AD, one of the most devastating neurodegenerative disorders. AD is characterized by the progressive loss of cholinergic neurons, leading to behavioral, motor and cognitive impairments [152]. Amyloids are protein aggregates that are enriched for β-sheet structures and resistant to degradation. Deposition of Aβ peptides, called amyloid plaque, is the primary pathological feature of AD and results in impaired synaptic activity and neuronal damage [153]. Aβ peptides are generated from the amyloid precursor protein (APP) through β- and γ-secretase cleavages [154]. Another key pathological feature of AD is aggregation of the microtube-associated protein Tau, also known as neurofibrillary tangle, in the brain [155]. Hyper-phosphorylation of Tau proteins results in increased Tau aggregation and microtubule destabilization, causing neurodegeneration [156, 157]. In a mouse AD model expressing the human APP transgene, Aβ accumulates to form insoluble amyloid plaques in the brain; and HSF1 suppresses the formation of Aβ amyloids and ameliorates cognitive deficits [158]. Furthermore, in the Samaritan Alzheimer’s rat model in which Aβ peptides are infused directly into the ventricles of the brain, lentiviral delivery of HSF1 into the cerebella markedly reverses the reduction in the number of Purkinje cell bodies [159]. These beneficial effects of HSF1 are believed to be mediated primarily through HSP expression. Congruently, HSP70 has been shown to protect against neurodegeneration in the central nervous system [160–162]. HSP70 not only suppresses the toxicity of Aβ accumulation by interfering with Aβ homeostasis, but also blocks Aβ self-assembly and thereby suppresses the production of toxic Aβ [163, 164]. Also, HSP70 promotes the clearance of Aβ by up-regulating the insulin-degrading enzyme (IDE) [165]. Moreover, HSP70 can interact with Tau proteins, thereby blocking its aggregation and promoting its degradation [166, 167]. In addition to HSP70, HSP90 also assists Tau degradation via the proteasomal and autophagic-lysosomal pathways [166, 168]. Moreover, HSP90 binds misfolded Aβ to prevent it from aggregating [169, 170].
Implications of the AMPK-mediated HSF1 suppression in neurodegenerative disorders
In neurodegenerative disorders, cellular energy homeostasis is frequently disrupted. For example, in HD and AD mitochondrial biogenesis is impaired [171]. In the brain, AMPK is activated by ischemia, hypoxia, and glucose shortage, all of which provoke metabolic stress and are associated with AD [172–174].
Recent studies have implicated AMPK in neurodegenerative disorders directly, including HD and AD. The oxidative stress, induced by the mutant HTT with polyQ expansion, activates AMPKα1 and causes neurotoxicity in striatal progenitor cells and in the striatum of R6/2 HD mice [175]. Congruently, alleviation of oxidative stress suppresses AMPK activation and mitigates the neurotoxicity in mice, suggesting a causative role of AMPK activation in the progression of HD [175]. AD is frequently associated with aberrant energy metabolism, including reduced glucose uptake, mitochondrial dysfunction, impaired cholesterol metabolism, and disrupted calcium homeostasis [176–179]. It has been shown that AMPK, activated by the aggregation of Aβ peptides, phosphorylates Tau proteins directly at Thr231 and Ser296/404, interrupting the binding of Tau to microtubules and causing Tau aggregation in primary mouse neurons [180, 181]. Moreover, in transgenic mice expressing a mutant human APP, AMPK, activated by elevated intracellular calcium levels, phosphorylates Tau proteins, leading to dendritic spine loss and inducing AD [181]. Furthermore, AMPK activation is reported to induce cell death in primary cortical neurons by mediating glutamate release [182]. Also, AMPK activation leads to hippocampal neuronal death under drastic dietary restriction [183]. Of note, AMPK activation may also aggravate neurodegeneration through disruption of neuronal proteostasis (Fig. 3). A recent study revealed that metabolic stressors including metformin stimulate the AMPK-mediated HSF1 inactivation [55]. Moreover, another study showed that metformin activates AMPK to up-regulate β-secretase, inducing the generation of Aβ peptides both extraneuronally and intraneuronally [184]. It was also shown that metformin aggravates tauopathy in mice by enhancing Tau protein aggregation [185]. Importantly, it has been reported that in patients with diabetes metformin use is associated with increased risk of cognitive impairment [186]. Conversely, both pharmacological and genetic inhibition of AMPK signaling alleviate the Aβ-induced impairments in hippocampal synaptic plasticity in mice [187]. Together, these findings support a causative role of AMPK activation in the pathogeneses of AD and other neurodegenerative disorders, and further imply that AMPK inhibition may be a promising therapeutic strategy to improve neuronal proteostasis and antagonize neurodegeneration. Importantly, it also suggests that patients afflicted with neurodegenerative disorders should be cautious to take metformin.
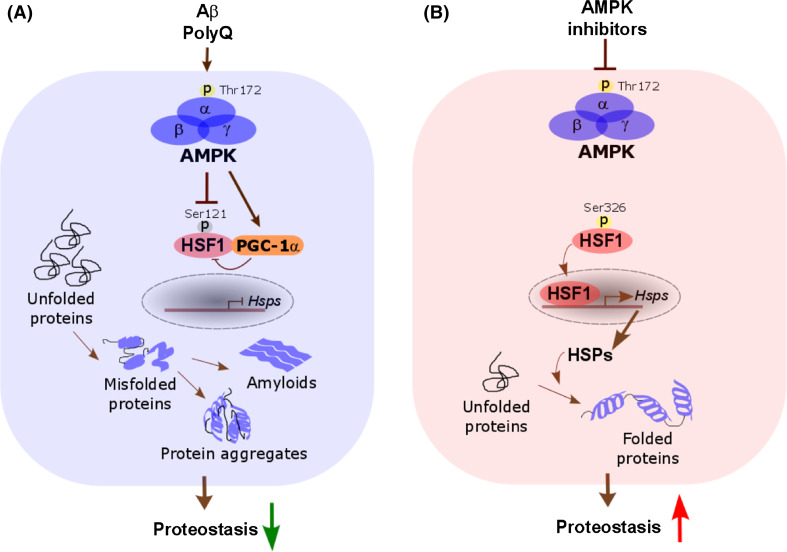
Fig. 3.
AMPK activation may disrupt neuronal proteostasis to promote neurodegeneration. a In neurodegenerative disorders, accumulation of protein aggregates and amyloids in neurons causes AMPK activation, which, in turn, may inactivate the HSF1-mediated PSR. This HSF1 inactivation leads to increased protein aggregates and amyloids, exacerbating neurotoxicity and neurodegeneration. b By contrast, AMPK inhibition may improve neuronal proteostasis by enhancing the HSF1-mediated PSR, thus representing a promising anti-neurodegeneration therapeutic strategy
However, AMPK is also reported to inhibit Tau phosphorylation in vitro in the rat cortical neuron model [188, 189]. In addition, acetylation of Tau protein inhibits its ubiquitination and proteasomal degradation, causing tauopathy [190]. By deacetylating Tau, SIRT1 prevents accumulation of phosphorylated Tau proteins [190]. Interestingly, the expression of SIRT1 is diminished in AD patients’ brains [191]. Thus, through activation of SIRT1, AMPK could enhance the proteasomal degradation of Tau proteins and thereby prevent its aggregation. Furthermore, mitochondrial dysfunction, a crucial contributing factor in the pathogenesis of AD, is highly correlated with metabolic stress in AD patients. Mitochondria are enriched in synapses, which are highly metabolically active organelles and maintain the normal functions of neurons. Accumulation of Aβ amyloids interferes with the electron transport through the mitochondrial membrane, leading to mitochondrial dysfunction [192]. On the contrary, SIRT1, by sensing basal NAD+ levels, protects cells from mitochondrial dysfunction. Activation of SIRT1 promotes mitochondrial biogenesis and subsequently antagonizes metabolic stress [193]. Importantly, studies using Sirt1 knockout mice have shown that SIRT1 plays an important role in activating AMPK and improving mitochondrial functions [193]. Therefore, activated SIRT1-AMPK signaling can rescue mitochondrial dysfunction and ultimately prevent neural injury. In line with a protective role of AMPK, rats treated with AICAR, an AMPK activator, display mitigated AD-like pathologies and improved spatial memory [194]. Furthermore, an epidemiological study revealed that long-term metformin usage is associated with mitigated cognitive decline and reduced risk of dementia in T2D patients [195], suggesting a protective role of metformin.
Taken together, contradictory evidence also exists implying the beneficial roles of AMPK and metformin in AD and other neurodegenerative disorders. Despite ample evidence strongly implicating AMPK in neurodegenerative disorders, its precise action still remains controversial. In light of the widespread usage of metformin worldwide, this question is of great importance to public health and warrants extensive investigations.
Summary and perspective
The evidence presented in this review illuminates an intimate connection between metabolic state and proteostasis, with a special emphasis on the regulation of HSF1 by AMPK. While HSF1 senses proteotoxic stress and plays a pivotal role in preserving cellular proteostasis by mediating the PSR, AMPK senses metabolic stress and acts as a key player in preserving cellular energy homeostasis by mediating the MSR. Aberrancies in both proteome and energy homeostasis are closely associated with age-related diseases, including cancer, T2D, and neurodegenerative disorders. Sharply contrasting with its pro-oncogenic role, intriguingly, proteostasis protects against T2D and neurodegeneration. Thus, the newly discovered metabolic regulation of the PSR not only helps to better elucidate the pathogeneses of these diseases but also may have important implications in therapeutic interventions.
Acknowledgments
We sincerely apologize to those authors whose work could not be cited in this review due to space limitations. C. D. was supported by The Jackson Laboratory Cancer Center Support Grant 3P30CA034196, Grants 1DP2OD007070 and R21CA184704 from the NIH, and the New Scholar Award AS-NS-0599-09 from the Ellison Medical Foundation.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- AD
Alzheimer’s disease
- AICAR
5-Aminoimidazole-4-carboxamide ribonucleoside
- AMPK
AMP-activated protein kinase
- APP
Amyloid precursor protein
- AR
Androgen receptor
- AKT
v-Akt murine thymoma viral oncogene homolog
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CAMKK
Ca2+/calmodulin-dependent protein kinase kinase β
- DM
Diabetes mellitus
- EIF4EBP1
Eukaryotic translation initiation factor 4E (elF4E)-binding protein 1
- ERK
Extracellular signal-regulated kinase
- GSK3β
Glycogen synthase kinase 3 β
- HD
Huntington disease
- HSE
Heat shock element
- HSF
Heat shock factor
- HSP
Heat shock proteins
- HSR
Heat shock response
- IAPP
Islet amyloid polypeptide
- IDE
Insulin-degrading enzyme
- IKKβ
Inhibitor of nuclear factor kappa-B kinase subunit beta
- IR
Insulin receptor
- IRS-1/2
Insulin receptor substrate-1/2
- JNK
c-Jun N-terminal kinase
- LKB1
Liver kinase B1
- mTORC1
Mammalian target of rapamycin complex 1
- MSR
Metabolic stress response
- PDK
Phosphoinositide-dependent kinase
- PGC-1α
Peroxisome proliferator–activated receptor gamma coactivator 1-alpha
- PI3K
Phosphoinositide 3-kinase
- polyQ
Polyglutamine
- PSR
Proteotoxic stress response
- STZ
Streptozotocin
- SUMO
Small ubiquitin-like modifier
References
- 1.Gidalevitz T, Prahlad V, Morimoto RI. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes M, Xiao H, Lis JT. Fine structure analyses of the Drosophila and Saccharomyces heat shock factor-heat shock element interactions. Nucleic Acids Res. 1994;22(2):167–173. doi: 10.1093/nar/22.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. Faseb J. 2001;15(7):1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21(17):5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman BJ, Rathmell JC. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb Perspect Biol. 2012;4(9):a008763. doi: 10.1101/cshperspect.a008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem. 2008;283(52):36344–36353. doi: 10.1074/jbc.M803580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26(2):69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carro EM. Therapeutic approaches of leptin in Alzheimer’s disease. Recent Pat CNS Drug Discov. 2009;4(3):200–208. doi: 10.2174/157488909789104848. [DOI] [PubMed] [Google Scholar]
- 12.Welch WJ. The role of heat-shock proteins as molecular chaperones. Curr Opin Cell Biol. 1991;3(6):1033–1038. doi: 10.1016/0955-0674(91)90125-I. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki S, Kobayashi M, Yoda M, Sakaguchi Y, Katsuma S, Suzuki T, Tomari Y. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell. 2010;39(2):292–299. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in beta-cells. Diabetes Obes Metab. 2010;12(Suppl 2):99–107. doi: 10.1111/j.1463-1326.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14(9):1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 16.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 17.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125(3):443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 19.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 21.Dai C, Dai S, Cao J. Proteotoxic stress of cancer: implication of the heat-shock response in oncogenesis. J Cell Physiol. 2012;227(8):2982–2987. doi: 10.1002/jcp.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto M, Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010;277(20):4112–4125. doi: 10.1111/j.1742-4658.2010.07827.x. [DOI] [PubMed] [Google Scholar]
- 23.Akerfelt M, Vihervaara A, Laiho A, Conter A, Christians ES, Sistonen L, Henriksson E. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J Biol Chem. 2010;285(45):34469–34476. doi: 10.1074/jbc.M110.157552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuister GW, Kim SJ, Orosz A, Marquardt J, Wu C, Bax A. Solution structure of the DNA-binding domain of Drosophila heat shock transcription factor. Nat Struct Biol. 1994;1(9):605–614. doi: 10.1038/nsb0994-605. [DOI] [PubMed] [Google Scholar]
- 25.Littlefield O, Nelson HC. A new use for the ‘wing’ of the ‘winged’ helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999;6(5):464–470. doi: 10.1038/8269. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12(5):654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993;259(5092):230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- 28.Peteranderl R, Rabenstein M, Shin YK, Liu CW, Wemmer DE, King DS, Nelson HC. Biochemical and biophysical characterization of the trimerization domain from the heat shock transcription factor. Biochemistry. 1999;38(12):3559–3569. doi: 10.1021/bi981774j. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Barlev NA, Westergaard O, Jakobsen BK. Identification of the C-terminal activator domain in yeast heat shock factor: independent control of transient and sustained transcriptional activity. EMBO J. 1993;12(13):5007–5018. doi: 10.1002/j.1460-2075.1993.tb06194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisniewski J, Orosz A, Allada R, Wu C. The C-terminal region of Drosophila heat shock factor (HSF) contains a constitutively functional transactivation domain. Nucleic Acids Res. 1996;24(2):367–374. doi: 10.1093/nar/24.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newton EM, Knauf U, Green M, Kingston RE. The regulatory domain of human heat shock factor 1 is sufficient to sense heat stress. Mol Cell Biol. 1996;16(3):839–846. doi: 10.1128/MCB.16.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green M, Schuetz TJ, Sullivan EK, Kingston RE. A heat shock-responsive domain of human HSF1 that regulates transcription activation domain function. Mol Cell Biol. 1995;15(6):3354–3362. doi: 10.1128/MCB.15.6.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y, Guettouche T, Fenna M, Boellmann F, Pratt WB, Toft DO, Smith DF, Voellmy R. Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J Biol Chem. 2001;276(49):45791–45799. doi: 10.1074/jbc.M105931200. [DOI] [PubMed] [Google Scholar]
- 34.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273(13):7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 35.He H, Soncin F, Grammatikakis N, Li Y, Siganou A, Gong J, Brown SA, Kingston RE, Calderwood SK. Elevated expression of heat shock factor (HSF) 2A stimulates HSF1-induced transcription during stress. J Biol Chem. 2003;278(37):35465–35475. doi: 10.1074/jbc.M304663200. [DOI] [PubMed] [Google Scholar]
- 36.Loison F, Debure L, Nizard P, le Goff P, Michel D, le Drean Y. Up-regulation of the clusterin gene after proteotoxic stress: implication of HSF1-HSF2 heterocomplexes. Biochem J. 2006;395(1):223–231. doi: 10.1042/BJ20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostling P, Bjork JK, Roos-Mattjus P, Mezger V, Sistonen L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. 2007;282(10):7077–7086. doi: 10.1074/jbc.M607556200. [DOI] [PubMed] [Google Scholar]
- 38.Kroeger PE, Sarge KD, Morimoto RI. Mouse heat shock transcription factors 1 and 2 prefer a trimeric binding site but interact differently with the HSP70 heat shock element. Mol Cell Biol. 1993;13(6):3370–3383. doi: 10.1128/MCB.13.6.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing H, Wilkerson DC, Mayhew CN, Lubert EJ, Skaggs HS, Goodson ML, Hong Y, Park-Sarge OK, Sarge KD. Mechanism of hsp70i gene bookmarking. Science. 2005;307(5708):421–423. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]
- 40.Elsing AN, Aspelin C, Bjork JK, Bergman HA, Himanen SV, Kallio MJ, Roos-Mattjus P, Sistonen L. Expression of HSF2 decreases in mitosis to enable stress-inducible transcription and cell survival. J Cell Biol. 2014;206(6):735–749. doi: 10.1083/jcb.201402002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu N, Hu Y, Mivechi NF. Heat shock transcription factor (Hsf)-4b recruits Brg1 during the G1 phase of the cell cycle and regulates the expression of heat shock proteins. J Cell Biochem. 2006;98(6):1528–1542. doi: 10.1002/jcb.20865. [DOI] [PubMed] [Google Scholar]
- 42.Fujimoto M, Hayashida N, Katoh T, Oshima K, Shinkawa T, Prakasam R, Tan K, Inouye S, Takii R, Nakai A. A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol Biol Cell. 2010;21(1):106–116. doi: 10.1091/mbc.E09-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanabe M, Nakai A, Kawazoe Y, Nagata K. Different thresholds in the responses of two heat shock transcription factors, HSF1 and HSF3. J Biol Chem. 1997;272(24):15389–15395. doi: 10.1074/jbc.272.24.15389. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, Ying Z, Jin X, Tu N, Zhang Y, Phillips M, Moskophidis D, Mivechi NF. Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis. 2004;38(2):66–80. doi: 10.1002/gene.20005. [DOI] [PubMed] [Google Scholar]
- 45.Kawazoe Y, Tanabe M, Sasai N, Nagata K, Nakai A. HSF3 is a major heat shock responsive factor duringchicken embryonic development. Eur J Biochem. 1999;265(2):688–697. doi: 10.1046/j.1432-1327.1999.00762.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17(1):469–481. doi: 10.1128/MCB.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakai A. Heat shock transcription factors and sensory placode development. BMB Rep. 2009;42(10):631–635. doi: 10.5483/BMBRep.2009.42.10.631. [DOI] [PubMed] [Google Scholar]
- 48.Izu H, Inouye S, Fujimoto M, Shiraishi K, Naito K, Nakai A. Heat shock transcription factor 1 is involved in quality-control mechanisms in male germ cells. Biol Reprod. 2004;70(1):18–24. doi: 10.1095/biolreprod.103.020065. [DOI] [PubMed] [Google Scholar]
- 49.Fan R, Wang C, Wang Y, Ren P, Gan P, Ji H, Xia Z, Hu S, Zeng Q, Huang W, Jiang Y, Huang X. Enhanced antitumoral efficacy and immune response following conditionally replicative adenovirus containing constitutive HSF1 delivery to rodent tumors. J Transl Med. 2012;10:101. doi: 10.1186/1479-5876-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vihervaara A, Sistonen L. HSF1 at a glance. J Cell Sci. 2014;127(Pt 2):261–266. doi: 10.1242/jcs.132605. [DOI] [PubMed] [Google Scholar]
- 51.Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmberg CI, Hietakangas V, Mikhailov A, Rantanen JO, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto RI, Eriksson JE, Sistonen L. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J. 2001;20(14):3800–3810. doi: 10.1093/emboj/20.14.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Murshid A, Prince T, Calderwood SK. Protein kinase A regulates molecular chaperone transcription and protein aggregation. PLoS One. 2011;6(12):e28950. doi: 10.1371/journal.pone.0028950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knauf U, Newton EM, Kyriakis J, Kingston RE. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10(21):2782–2793. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- 55.Dai S, Tang Z, Cao J, Zhou W, Li H, Sampson S, Dai C. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015;34(3):275–293. doi: 10.15252/embj.201489062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai C, Santagata S, Tang Z, Shi J, Cao J, Kwon H, Bronson RT, Whitesell L, Lindquist S. Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin Invest. 2012;122(10):3742–3754. doi: 10.1172/JCI62727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Z, Dai S, He Y, Doty RA, Shultz LD, Sampson SB, Dai C. MEK guards proteome stability and inhibits tumor-suppressive amyloidogenesis via HSF1. Cell. 2015;160(4):729–744. doi: 10.1016/j.cell.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150(3):549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anckar J, Hietakangas V, Denessiouk K, Thiele DJ, Johnson MS, Sistonen L. Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol. 2006;26(3):955–964. doi: 10.1128/MCB.26.3.955-964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23(8):2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8(12):947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 63.Brunet Simioni M, De Thonel A, Hammann A, Joly AL, Bossis G, Fourmaux E, Bouchot A, Landry J, Piechaczyk M, Garrido C. Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene. 2009;28(37):3332–3344. doi: 10.1038/onc.2009.188. [DOI] [PubMed] [Google Scholar]
- 64.Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raychaudhuri S, Loew C, Korner R, Pinkert S, Theis M, Hayer-Hartl M, Buchholz F, Hartl FU. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell. 2014;156(5):975–985. doi: 10.1016/j.cell.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 66.Bharadwaj S, Ali A, Ovsenek N. Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 in vivo. Mol Cell Biol. 1999;19(12):8033–8041. doi: 10.1128/MCB.19.12.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marunouchi T, Araki M, Murata M, Takagi N, Tanonaka K. Possible involvement of HSP90-HSF1 multichaperone complex in impairment of HSP72 induction in the failing heart following myocardial infarction in rats. J Pharmacol Sci. 2013;123(4):336–346. doi: 10.1254/jphs.13109FP. [DOI] [PubMed] [Google Scholar]
- 68.Takii R, Fujimoto M, Tan K, Takaki E, Hayashida N, Nakato R, Shirahige K, Nakai A. ATF1 modulates the heat shock response by regulating the stress-inducible heat shock factor 1 transcription complex. Mol Cell Biol. 2015;35(1):11–25. doi: 10.1128/MCB.00754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130(6):1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li D, Yallowitz A, Ozog L, Marchenko N. A gain-of-function mutant p53-HSF1 feed forward circuit governs adaptation of cancer cells to proteotoxic stress. Cell Death Dis. 2014;5:e1194. doi: 10.1038/cddis.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin X, Moskophidis D, Mivechi NF. Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell Metab. 2011;14(1):91–103. doi: 10.1016/j.cmet.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang S, Tu K, Fu Q, Schmitt DC, Zhou L, Lu N, Zhao Y. Multifaceted roles of HSF1 in cancer. Tumour Biol. 2015;36(7):4923–4931. doi: 10.1007/s13277-015-3674-x. [DOI] [PubMed] [Google Scholar]
- 73.Su KH, Cao J, Tang Z, Dai S, He Y, Sampson SB, Benjamin IJ, Dai C. HSF1 critically attunes proteotoxic stress sensing by mTORC1 to combat stress and promote growth. Nat Cell Biol. 2016;18(5):527–539. doi: 10.1038/ncb3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56(4):930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 75.Dancso B, Spiro Z, Arslan MA, Nguyen MT, Papp D, Csermely P, Soti C. The heat shock connection of metabolic stress and dietary restriction. Curr Pharm Biotechnol. 2010;11(2):139–145. doi: 10.2174/138920110790909704. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Liu H, Yang J, Liu X, Lu S, Wen T, Xie L, Wang G. Increased amyloid beta-peptide (1–40) level in brain of streptozotocin-induced diabetic rats. Neuroscience. 2008;153(3):796–802. doi: 10.1016/j.neuroscience.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 77.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7(4):321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 78.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 79.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aryal P, Kim K, Park PH, Ham S, Cho J, Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014;281(20):4644–4658. doi: 10.1111/febs.12969. [DOI] [PubMed] [Google Scholar]
- 81.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibson GE, Starkov A, Blass JP, Ratan RR. Beal MF (2010) Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta. 1802;1:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niforou K, Cheimonidou C, Trougakos IP. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014;2:323–332. doi: 10.1016/j.redox.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoo HJ, Im CN, Youn DY, Yun HH, Lee JH. Bis is induced by oxidative stress via activation of HSF1. Korean J Physiol Pharmacol. 2014;18(5):403–409. doi: 10.4196/kjpp.2014.18.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14(3):291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez FP, Moinuddin SS, ul ain Shamim Q, Joseph DJ, Morisaki J, Zhou X. Longevity pathways: HSF1 and FoxO pathways, a new therapeutic target to prevent age-related diseases. Curr Aging Sci. 2012;5(2):87–95. doi: 10.2174/1874609811205020087. [DOI] [PubMed] [Google Scholar]
- 87.Heydari AR, You S, Takahashi R, Gutsmann A, Sarge KD, Richardson A. Effect of caloric restriction on the expression of heat shock protein 70 and the activation of heat shock transcription factor 1. Dev Genet. 1996;18(2):114–124. doi: 10.1002/(SICI)1520-6408(1996)18:2<114::AID-DVG4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 88.Hensen SM, Heldens L, van Enckevort CM, van Genesen ST, Pruijn GJ, Lubsen NH. Heat shock factor 1 is inactivated by amino acid deprivation. Cell Stress Chaperones. 2012;17(6):743–755. doi: 10.1007/s12192-012-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rokutan K, Hirakawa T, Teshima S, Honda S, Kishi K. Glutathione depletion impairs transcriptional activation of heat shock genes in primary cultures of guinea pig gastric mucosal cells. J Clin Invest. 1996;97(10):2242–2250. doi: 10.1172/JCI118665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barna J, Princz A, Kosztelnik M, Hargitai B, Takacs-Vellai K, Vellai T. Heat shock factor-1 intertwines insulin/IGF-1, TGF-beta and cGMP signaling to control development and aging. BMC Dev Biol. 2012;12:32. doi: 10.1186/1471-213X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 92.Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148(1–2):322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minsky N, Roeder RG. Direct link between metabolic regulation and the heat-shock response through the transcriptional regulator PGC-1alpha. Proc Natl Acad Sci USA. 2015;112(42):E5669–E5678. doi: 10.1073/pnas.1516219112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gueant JL, Elakoum R, Ziegler O, Coelho D, Feigerlova E, Daval JL, Gueant-Rodriguez RM. Nutritional models of foetal programming and nutrigenomic and epigenomic dysregulations of fatty acid metabolism in the liver and heart. Pflugers Arch. 2014;466(5):833–850. doi: 10.1007/s00424-013-1339-4. [DOI] [PubMed] [Google Scholar]
- 95.Yamagishi N, Nakayama K, Wakatsuki T, Hatayama T. Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci. 2001;69(22):2603–2609. doi: 10.1016/S0024-3205(01)01337-6. [DOI] [PubMed] [Google Scholar]
- 96.Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol (1985) 2004;97(2):605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- 97.Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- 98.Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51(4):1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- 99.Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17(3):293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, Soleymani T, Daraei P, Sitz D, Vergnes L, Wanagat J, Reue K, Febbraio MA, Hevener AL. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 2014;63(5):1488–1505. doi: 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63(6):1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosas PC, Nagaraja GM, Kaur P, Panossian A, Wickman G, Garcia LR, Al-Khamis FA, Asea AA. Hsp72 (HSPA1A) prevents human islet amyloid polypeptide aggregation and toxicity: a new approach for type 2 diabetes treatment. PLoS One. 2016;11(3):e0149409. doi: 10.1371/journal.pone.0149409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Koranyi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41(5):374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- 105.Literati-Nagy B, Tory K, Peitl B, Bajza A, Koranyi L, Literati-Nagy Z, Hooper PL, Vigh L, Szilvassy Z. Improvement of insulin sensitivity by a novel drug candidate, BGP-15, in different animal studies. Metab Syndr Relat Disord. 2014;12(2):125–131. doi: 10.1089/met.2013.0098. [DOI] [PubMed] [Google Scholar]
- 106.Kondo T, Sasaki K, Matsuyama R, Morino-Koga S, Adachi H, Suico MA, Kawashima J, Motoshima H, Furukawa N, Kai H, Araki E. Hyperthermia with mild electrical stimulation protects pancreatic beta-cells from cell stresses and apoptosis. Diabetes. 2012;61(4):838–847. doi: 10.2337/db11-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai T, Patel-Chamberlin M, Natarajan R, Todorov I, Ma J, LaPage J, Phillips L, Nast CC, Becerra D, Chuang P, Tong L, de Belleroche J, Wells DJ, Wang Y, Adler SG. Heat shock protein 27 overexpression mitigates cytokine-induced islet apoptosis and streptozotocin-induced diabetes. Endocrinology. 2009;150(7):3031–3039. doi: 10.1210/en.2008-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uchiyama T, Tomono S, Utsugi T, Ohyama Y, Nakamura T, Tomura H, Kawazu S, Okajima F, Kurabayashi M. Constitutively active heat shock factor 1 enhances glucose-driven insulin secretion. Metabolism. 2011;60(6):789–798. doi: 10.1016/j.metabol.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 109.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12(24):3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 110.Kline MP, Morimoto RI. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol. 1997;17(4):2107–2115. doi: 10.1128/MCB.17.4.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geiger PC, Gupte AA. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exerc Sport Sci Rev. 2011;39(1):34–42. doi: 10.1097/JES.0b013e318201f236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 113.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58(3):567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma X, Xu L, Alberobello AT, Gavrilova O, Bagattin A, Skarulis M, Liu J, Finkel T, Mueller E. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1alpha transcriptional axis. Cell Metab. 2015;22(4):695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 115.Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315(5819):1726–1729. doi: 10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]
- 116.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449(7161):496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 117.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26(16):5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Racioppi L, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem. 2012;287(38):31658–31665. doi: 10.1074/jbc.R112.356485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472(7342):230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4(4):315–324. doi: 10.1016/S0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- 121.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10(20):1247–1255. doi: 10.1016/S0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 122.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273(3):1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 123.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51(8):2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 124.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 125.Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248(1):378–380. [PubMed] [Google Scholar]
- 126.van Veelen W, Korsse SE, van de Laar L, Peppelenbosch MP. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene. 2011;30(20):2289–2303. doi: 10.1038/onc.2010.630. [DOI] [PubMed] [Google Scholar]
- 127.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13(15):1259–1268. doi: 10.1016/S0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 128.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 129.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 130.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13(4):376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM, Hardie DG, Ng IO, Ching YP. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012;72(17):4394–4404. doi: 10.1158/0008-5472.CAN-12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 133.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106(7):847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moses AC, Young SC, Morrow LA, O’Brien M, Clemmons DR. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45(1):91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 138.Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, Marselli L, Masiello P, Marchetti P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52(6):1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 139.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 140.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 141.The Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 142.Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5(10):958–963. doi: 10.1038/sj.embor.7400250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parekh-Olmedo H, Wang J, Gusella JF, Kmiec EB. Modified single-stranded oligonucleotides inhibit aggregate formation and toxicity induced by expanded polyglutamine. J Mol Neurosci. 2004;24(2):257–267. doi: 10.1385/JMN:24:2:257. [DOI] [PubMed] [Google Scholar]
- 144.Chafekar SM, Duennwald ML. Impaired heat shock response in cells expressing full-length polyglutamine-expanded huntingtin. PLoS One. 2012;7(5):e37929. doi: 10.1371/journal.pone.0037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, Nakai A. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280(41):34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 146.Neef DW, Turski ML, Thiele DJ. Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease. PLoS Biol. 2010;8(1):e1000291. doi: 10.1371/journal.pbio.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hayashida N, Fujimoto M, Tan K, Prakasam R, Shinkawa T, Li L, Ichikawa H, Takii R, Nakai A. Heat shock factor 1 ameliorates proteotoxicity in cooperation with the transcription factor NFAT. EMBO J. 2010;29(20):3459–3469. doi: 10.1038/emboj.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18(7):671–680. doi: 10.1212/WNL.18.7.671. [DOI] [PubMed] [Google Scholar]
- 149.Sobue G, Hashizume Y, Mukai E, Hirayama M, Mitsuma T, Takahashi A. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain. 1989;112(Pt 1):209–232. doi: 10.1093/brain/112.1.209. [DOI] [PubMed] [Google Scholar]
- 150.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 151.Kondo N, Katsuno M, Adachi H, Minamiyama M, Doi H, Matsumoto S, Miyazaki Y, Iida M, Tohnai G, Nakatsuji H, Ishigaki S, Fujioka Y, Watanabe H, Tanaka F, Nakai A, Sobue G. Heat shock factor-1 influences pathological lesion distribution of polyglutamine-induced neurodegeneration. Nat Commun. 2013;4:1405. doi: 10.1038/ncomms2417. [DOI] [PubMed] [Google Scholar]
- 152.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 154.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12(1):1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739(2–3):198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 156.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2(7):a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takalo M, Salminen A, Soininen H, Hiltunen M, Haapasalo A. Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 158.Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18(10):1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 159.Jiang YQ, Wang XL, Cao XH, Ye ZY, Li L, Cai WQ. Increased heat shock transcription factor 1 in the cerebellum reverses the deficiency of Purkinje cells in Alzheimer’s disease. Brain Res. 2013;1519:105–111. doi: 10.1016/j.brainres.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 160.Guzhova I, Margulis B. Hsp70 chaperone as a survival factor in cell pathology. Int Rev Cytol. 2006;254:101–149. doi: 10.1016/S0074-7696(06)54003-3. [DOI] [PubMed] [Google Scholar]
- 161.Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem. 2006;281(44):33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 162.Wacker JL, Huang SY, Steele AD, Aron R, Lotz GP, Nguyen Q, Giorgini F, Roberson ED, Lindquist S, Masliah E, Muchowski PJ. Loss of Hsp70 exacerbates pathogenesis but not levels of fibrillar aggregates in a mouse model of Huntington’s disease. J Neurosci. 2009;29(28):9104–9114. doi: 10.1523/JNEUROSCI.2250-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, Sobue G, Matsushima T, Suzuki T, Mizushima T. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011;31(14):5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24(7):1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar P, Ambasta RK, Veereshwarayya V, Rosen KM, Kosik KS, Band H, Mestril R, Patterson C, Querfurth HW. CHIP and HSPs interact with beta-APP in a proteasome-dependent manner and influence Abeta metabolism. Hum Mol Genet. 2007;16(7):848–864. doi: 10.1093/hmg/ddm030. [DOI] [PubMed] [Google Scholar]
- 166.Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, O’Leary J, Morgan D, Lee DC, Shults CL, Rousaki A, Weeber EJ, Zuiderweg ER, Gestwicki JE, Dickey CA. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29(39):12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Patterson KR, Ward SM, Combs B, Voss K, Kanaan NM, Morfini G, Brady ST, Gamblin TC, Binder LI. Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport. Biochemistry. 2011;50(47):10300–10310. doi: 10.1021/bi2009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thompson AD, Scaglione KM, Prensner J, Gillies AT, Chinnaiyan A, Paulson HL, Jinwal UK, Dickey CA, Gestwicki JE. Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation. ACS Chem Biol. 2012;7(10):1677–1686. doi: 10.1021/cb3002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kakimura J, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohama S. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. Faseb J. 2002;16(6):601–603. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- 170.Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Doyu M, Sobue G. Modulation of Hsp90 function in neurodegenerative disorders: a molecular-targeted therapy against disease-causing protein. J Mol Med (Berl) 2006;84(8):635–646. doi: 10.1007/s00109-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 171.Duan W, Jiang M, Jin J. Metabolism in HD: still a relevant mechanism? Mov Disord. 2014;29(11):1366–1374. doi: 10.1002/mds.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17(1):45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 173.Gadalla AE, Pearson T, Currie AJ, Dale N, Hawley SA, Sheehan M, Hirst W, Michel AD, Randall A, Hardie DG, Frenguelli BG. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem. 2004;88(5):1272–1282. doi: 10.1046/j.1471-4159.2003.02253.x. [DOI] [PubMed] [Google Scholar]
- 174.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280(21):20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 175.Ju TC, Chen HM, Chen YC, Chang CP, Chang C, Chern Y. AMPK-alpha1 functions downstream of oxidative stress to mediate neuronal atrophy in Huntington’s disease. Biochim Biophys Acta. 2014;1842(9):1668–1680. doi: 10.1016/j.bbadis.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 176.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3(11):862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 177.Martins IJ, Berger T, Sharman MJ, Verdile G, Fuller SJ, Martins RN. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;111(6):1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 178.Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72(6):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Galindo MF, Ikuta I, Zhu X, Casadesus G, Jordan J. Mitochondrial biology in Alzheimer’s disease pathogenesis. J Neurochem. 2010;114(4):933–945. doi: 10.1111/j.1471-4159.2010.06814.x. [DOI] [PubMed] [Google Scholar]
- 180.Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J. 2011;434(3):503–512. doi: 10.1042/BJ20101485. [DOI] [PubMed] [Google Scholar]
- 181.Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78(1):94–108. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nakatsu Y, Kotake Y, Hino A, Ohta S. Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicol Appl Pharmacol. 2008;230(3):358–363. doi: 10.1016/j.taap.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 183.Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem. 2005;280(51):42142–42148. doi: 10.1074/jbc.M507607200. [DOI] [PubMed] [Google Scholar]
- 184.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA. 2009;106(10):3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barini E, Antico O, Zhao Y, Asta F, Tucci V, Catelani T, Marotta R, Xu H, Gasparini L. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener. 2016;11:16. doi: 10.1186/s13024-016-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36(10):2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid beta. J Neurosci. 2014;34(36):12230–12238. doi: 10.1523/JNEUROSCI.1694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009;380(1):98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Greco SJ, Hamzelou A, Johnston JM, Smith MA, Ashford JW, Tezapsidis N. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and beta-amyloid in neurons. Biochem Biophys Res Commun. 2011;414(1):170–174. doi: 10.1016/j.bbrc.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marques-Aleixo I, Rocha-Rodrigues S, Santos-Alves E, Coxito PM, Passos E, Oliveira PJ, Magalhaes J, Ascensao A. In vitro salicylate does not further impair aging-induced brain mitochondrial dysfunction. Toxicology. 2012;302(1):51–59. doi: 10.1016/j.tox.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 193.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, Liu LB, Wu K, Liu R, Wang JZ, Zhou XW. AMPK activation ameliorates Alzheimer’s disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer’s disease model in rats. J Alzheimers Dis. 2015;43(3):775–784. doi: 10.3233/JAD-140564. [DOI] [PubMed] [Google Scholar]
- 195.Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41(1):61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- 196.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 197.Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278(37):35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- 198.Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18(12):1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gabai VL, Yaglom JA, Volloch V, Meriin AB, Force T, Koutroumanis M, Massie B, Mosser DD, Sherman MY. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol Cell Biol. 2000;20(18):6826–6836. doi: 10.1128/MCB.20.18.6826-6836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2002;277(12):10346–10353. doi: 10.1074/jbc.M106736200. [DOI] [PubMed] [Google Scholar]
- 202.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277(42):39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 203.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277(2):1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 204.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50(1):24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 205.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45(4):276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31(17):3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]