Abstract
Purpose of Review
We have significantly improved hospital mortality from sepsis and critical illness in last 10 years, however over this same period we have tripled the number of “ICU survivors” going to rehabilitation. Further, as up to half the deaths in the first year following ICU admission occur post-ICU discharge, it is unclear how many of these patients ever returned home or a meaningful quality of life (QoL). For those who do survive, recent data reveals many “ICU survivors” will suffer significant functional impairment or Post-ICU Syndrome (PICS). Thus, new innovative metabolic and exercise interventions to address PICS are urgently needed. These should focus on optimal nutrition and lean body mass (LBM) assessment, targeted nutrition delivery, anabolic/anti-catabolic strategies, and utilization of personalized exercise intervention techniques, such as utilized by elite athletes to optimize preparation and recovery from critical care.
Recent findings
New data for novel LBM analysis technique such as CT scan and ultrasound analysis of lean body mass are available showing objective measures of LBM now becoming more practical for predicting metabolic reserve and effectiveness of nutrition/exercise interventions. 13C-breath testing is a novel technique under study to predict infection earlier and predict over- and under-feeding to target nutrition delivery. New technologies utilized routinely by athletes such as muscle glycogen ultrasound also show promise. Finally, the role of personalized cardiopulmonary exercise testing (CPET) to target pre-operative exercise optimization and post-ICU recovery are becoming reality.
Summary
New innovative techniques are demonstrating promise to target recovery from PICS utilizing a combination of objective LBM and metabolic assessment, targeted nutrition interventions, personalized exercise interventions for prehabilitation and post-ICU recovery. These interventions should provide hope that we will soon begin to create more “survivors” and fewer victim's post-ICU care.
Keywords: Lean Body Mass, CT scan, 13C-breath testing, Muscle Glycogen, Quality of Life, Post-Intensive Care Syndrome, Cardiopulmonary Exercise Testing
Introduction
In-hospital mortality following severe sepsis has consistently declined in recent years[1]. However, the same data also reveal that many of these patients are not returning home to functional lives post-ICU, but instead to rehabilitation settings where it is unclear if they ever returned to a meaningful quality of life. In fact, in the same period that in-hospital ICU mortality appears to be declining appears we have tripled the number of patients going to rehabilitation settings [1]. We also know that up to 40% of mortality within the first year of ICU stay occurs following ICU discharge [2]. Unfortunately for those who do survive, nearly half of the survivors will not return to work in the first year post-discharge [3**], often due to post-intensive care syndrome (PICS) and weakness post-ICU[4*]. As a result, many leading experts are calling for future ICU trials to not focus on mortality as the primary endpoint, but rather to focus on Quality of Life QoL [5**], physical function and addressing the epidemic of PICS [1, 6].
Specific to LBM in the ICU, we know a critically ill burn patient can lose as much as a kilogram of LBM a day[7*]. Other critically ill patients also suffer significant LBM loss, much of it in the first 7-10 days of ICU stay[8**]. Patients will gain weight back following ICU stay, but virtually all this weight is fat mass, not functional LBM[9]. This is not surprising, as data from the burn ICU demonstrates the catabolic/hypermetabolic state following injury can persist for up to two years following discharge from hospital and this can markedly hinder recovery of patients LBM and function following injury [7*]. As will be discussed in this review, we desperately need objective methods of screening and quantifying a patient's metabolic, exercise, and LBM reserve prior to anticipated injury/illness (such as surgery/cancer therapy). These should serve as key predictors of pre-operative/pre-illness risk and screen for patients in need of “prehabilitation” prior to a surgical intervention. As this review will describe, interventions such as CT scan LBM analysis and cardiopulmonary exercise testing (CPET) are already being utilized and studied around the world to assess preoperative “metabolic reserve” and “fitness for surgery”. These should be complemented by objective measures of patient's nutritional needs during illness and recovery. Further the effect of nutrition delivery on muscle uptake of nutrients and muscle “fitness” for exercise interventions may be a reality in our ICU's soon [8**, 10*]. Finally, we must improve our post-ICU care “recovery” care and take responsibility for the deficits in strength, function, and cognition we create in our ICU care. We owe it to our ICU patients to continue to use new innovations in objective nutrition delivery to guide nutrition and personalized exercise interventions to guide functional recovery.
Analysis of Lean Body Mass and Relationship to Surgical and ICU Outcome
Baseline skeletal muscle mass[11] and quality[12]** are demonstrated to be predictive of mortality in ICU patients. Muscle wasting and weakness are also major contributors to PICS [4*, 13*]. This is not unsurprising, as these parameters reflect “metabolic reserve”, which is effected by physical activity, nutrition, chronic disease and is tightly linked to function. [3*]. In surgical patients, a rapidly growing body of literature demonstrates low baseline skeletal muscle mass may be an independent risk factor for surgical complications in patients undergoing hepatic [14*] colorectal [15*, 16*], diverticular[17*], and pancreatic[18*] oncological surgery. Muscle mass loss pre-post oncological surgery (just as with ICU patients[19]) is an additional risk factor for complications[20*] and mortality[21**, 22*]. Low muscle contributes to increased length of stay in cardiac surgery[23*] and in patients undergoing transcatheter aortic valve replacement[24*].
A major limitation of these studies is their retrospective nature. Firstly, we are unable to exclude Type II errors: in contrast to the studies above no complications were seen in a patient cohort undergoing endometrial cancer resection [25] and ovarian surgery[26*]. While low muscle mass was associated with mortality in gastric cancer, no association was seen with complications[27*]. Secondly, we are therefore unable to dissect differences between positive and negative studies, i.e. in which surgical procedures does low skeletal muscle mass pose an additional risk for complications?
Prospective Trial Assessment of Muscle Mass
Whilst the evidence from observational studies of baseline muscle mass and quality continues to grow, sufficient data to justify intervention development exists. Primary outcomes should be focused on function and/or complications as opposed to mortality.
Performing a baseline muscle mass assessment in the acutely critically ill patient is challenging. Transfer for CT scans is not without risk, and may be ethically difficult to justify and lead to selection bias. Muscle ultrasound is an attractive emerging technique, and is also able to offer qualitative analysis[28*]. Ultrasound is inexpensive and readily available. Bedside measurements of muscle mass are possible with existing routine ICU ultrasound equipment. Unlike CT however, international consensus does not exist on methodology, with significant differences between techniques[29**].
To advance this science, studying the high-risk surgical patient has two major advantages. First, if the complication and return to work rates can be altered, there are significant gains to be attained for the patient, healthcare providers and society. Secondly methodological difficulties are minimized regarding consent, obtaining muscle mass measurements pre-insult and determining pre-existing functional outcome. A recent prospective observational study demonstrated the ability of pre-operative CT assessment of muscle quality in predicting surgical complications[21**]. In designing interventional trials, caution will be needed with regards to data analysis planning and power calculations. Muscle mass must be considered one of a group of stratification variables in a multi-dimensional fashion[30*]- much like the ICU Nutritional Risk Score or (NUTRIC) score [30*]. We have yet to understand how interdependent muscle mass, muscle quality and functional disability are, and which and how we should either stratify or correct for baseline differences[31**].
Bedside Analysis of Metabolic State and Over-/Under-Feeding: Promise of New Technology
The accurate determination of caloric needs and objective measures of over-/under-feeding over time in critical care has long been a challenge for ICU practitioners and a recent large observational cohort has been conducted on this topic [32*]. Indirect calorimetry, despite its limitations in the hospital setting, has been traditionally utilized for estimating energy needs [32*]. The next evolution of modern indirect calorimetry is described in this issue by De Waele (REF) However, indirect calorimetry has traditionally proven expensive, has significant limitations in the ICU (i.e. cannot be used on CVVH or with FiO2 > 60%), and is not readily available. [33*]
To address this need for objective, bedside metabolic monitoring, new technologies to measure carbon-12 and carbon-13 (13CO2/12CO2) ratios in exhaled breath are showing promise to objectively indicate type of metabolic fuel use and over-/under-feeding over time in an easy to use, non-invasive fashion. This technology is currently under study to for applications related to early infection detection and macronutrient use and caloric need Figure 1A and 1B).
Figure 1.
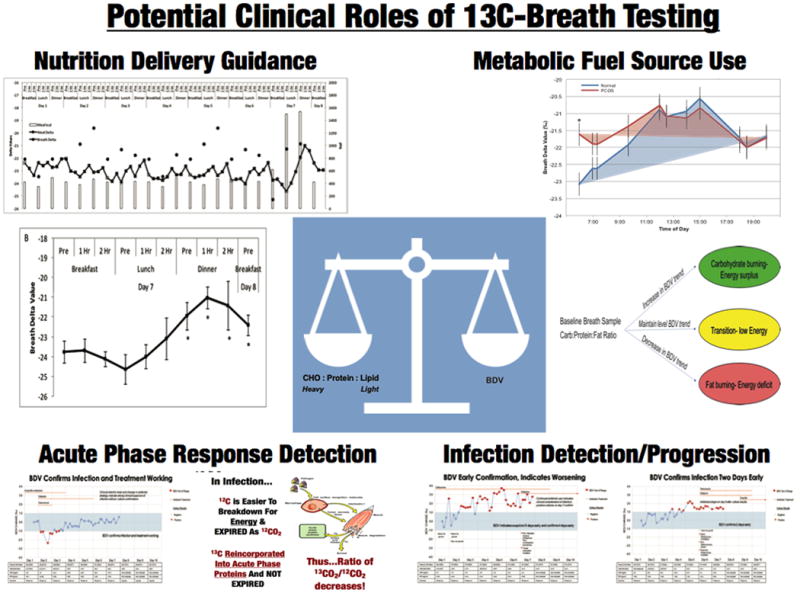
Figure 1 A: Multiple Potential Roles of 13C-Carbon Breath Testing in ICU and Hospitalized Patient. Figure 1B- Hypothetical Example of readout from 13C-Carbon Breath Testing and data that may be able to obtained by future clinicians in ICU. (Figures adapted from Borosi JP et al, J. Anal. At. Spectrom., 2014,29, 599-605 and ref 34, 35)
Carbon-12 and carbon-13 (12C,13C) are naturally-occurring isotopes that are found in exhaled breath. Stable isotopes of carbon isotope exist naturally in the body, however, the isotopic distribution of is not uniform. Because of chemical and enzymatic discrimination against the heavier 13C (i.e. fractionation) in various steps in lipid synthesis body fats are 3-10‰ depleted in 13C compared to body proteins and carbohydrates [36, 37*]. Based on this isotopic difference between the primary metabolic fuel sources it is possible to determine relative differences in metabolic fuel use at the macronutrient level by measuring the isotopic ratio of exhaled breath (Figure 1A and 1B), which now is possible in small ICU-based or bedside devices. Similar to the respiratory exchange ratio that measures rate of oxygen consumption and CO2 production in exhaled breath, the exhaled 13CO2/12CO2 ratio is correlated to ratio of carbohydrate/protein:lipid oxidation [37*, 38]. This technique has been used in wild life ecology studies to determine metabolic fuel usage in birds during different types of flight. For example, humming birds rely primarily on carbohydrate source during hovering flight while migratory birds rely on stored lipids to power long range flights over may thousands of kilometers[39**, 40]. More recently this technique has been applied in humans to understand metabolic fuel usage in health and disease. For example, exhaled 13CO2/12CO2 was a biomarker for metabolic fuel usage in women with polycystic ovarian syndrome shown to have metabolic inflexibility in switching from carbohydrate to lipids during an overnight fast [34]. Similarly, healthy adults during intense exercise (>50%VO2max) increased 13CO2/12CO2 ratio post-exercise indicting increased reliance on carbohydrate for energy [41]. In another study, adults on a healthy weight loss diet (40% energy restriction) demonstrated decreases in 13CO2/12CO2 from pre-diet baseline, indicating increased reliance on body lipids [35]. Ongoing studies are examining the exhaled 13CO2/12CO2 ratio for metabolic fuel usage in our hospital/ICU setting at Duke University and at other U.S. centers for patients requiring total parenteral nutrition (personal communication).
Metabolic Response to ICU- Relationship to Exercise Stress and Measurement
The hypermetabolic response to physiological stress weather at the ICU or running a marathon necessary for cellular energy requirements elicits major alterations in carbohydrate (CHO) metabolism, which in critically ill patients can have an important impact on muscle mass and survival. During exercise, CHOox increases due to the higher rate for ATP synthesis to satisfy muscle contractile demand [42]. (Inigo San-Millan and George Brooks, 2017 personal communication) (Figure 2). Surprisingly, a paucity of information on CHOox rates in ICU patients exists. It is believed though that CHOox can be 4-7mg/kg/min [43] which is ∼2-3 times that of resting levels.
Figure 2.

Carbohydrate (CHO) oxidation (mg/kg/min) during rest, ICU and different exercise intensities. Adapted from [42, 43] Inigo San-Millan and George Brooks, 2017 personal communication). Abbreviations: Wmax- Percentage of Maximal Exercise Work
During both exercise and critical illness, carbohydrates need to be mobilized in an orchestrated manner. During exercise, glycogenolysis for glycolysis is provided from both the liver as well as from skeletal muscle glycogen. Upon ceasing exercise, glycogen stores are repleted via proper nutrition. However, metabolic demand of ICU patients represents, overtime, the most extreme metabolic demand observed in humans. This demand is non-stopping, stretching the limits of “human performance”. Humans are not “trained” to be critically ill which is challenging as the body attempts to mobilize energy stores, driven mainly by cortisol. Therefore, a second phase of carbohydrate metabolism relies on gluconeogenic precursors from skeletal muscle proteolysis. Glycogen depletion can elicit a significant increase in muscle protein breakdown. During physiological stress situations like exercise, protein breakdown accounts for ∼4% of total exercise caloric expenditure under CHO loading. However, under glycogen depletion, protein breakdown is significantly elevated contributing to ∼10% of total caloric expenditure[44]. During exercise post-CHO loading, protein breakdown during exercise accounted for 5.8g/h vs 13.7g/h when muscle glycogen was depleted [44]. Undoubtedly, the repercussions of glycogen depletion on skeletal muscle protein breakdown and catabolism in ICU can be significant and devastating.
New cutting edge ultrasound technology [45] is being utilized routinely by the world's most elite professional athletes (i.e. Tour de France) to directly measure muscle glycogen in a few minutes at the bedside[45]. This technology is utilized by athletes to predict and prevent overtraining and guide nutritional needs during training. Glycogen depletion leads to marked muscle damage and an inability for muscle to recover and become anabolic, as muscle protein must be broken down for energy when energy cannot be obtained from glycogen stores. This leads to ongoing catabolism and inability to recover muscle mass and function[45]. We have previously shown muscle glycogen stores are depleted in ICU patients[10*], even within hours of admission (Figure 3 and 4) . In contrast, even elite endurance athletes do not completely deplete their glycogen stores after a major competition, such as prolonged bike racing or a marathon (Figure 3 and 4). Thus, one might equate being in the ICU as similar to continuously running multiple marathons. It is hoped this ultrasound technique[45] can open new doors in monitoring nutritional and catabolic status in critically ill patients. This technology we hope can guide when patients transition from the acute, catabolic phase of critical illness to a chronic or recovery phase, where increased nutrition delivery and potentially anabolic agents are warranted.
Figure 3.
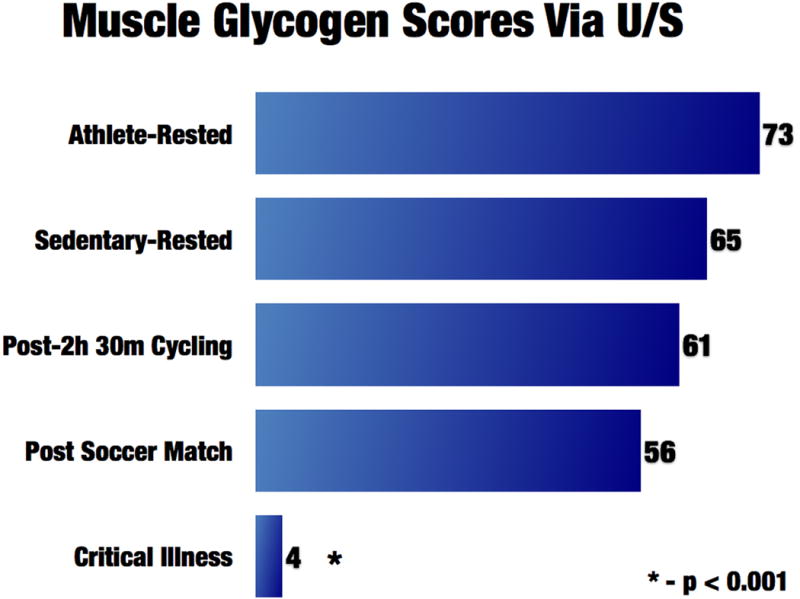
Muscle glycogen scores via ultrasound (from ref. 10)
Figure 4.

Skeletal muscle glycogen content score via ultrasound (from ref. 10)
Personalizing Prehabilitative and Post-ICU Exercise Interventions
Whilst clearly distinct from critical care, the perioperative setting provides interesting data on the relationship between physical fitness and clinical outcomes, along with some intriguing mechanistic insights. This is relevant to critical care because many patients undergoing elective major surgery are subsequently admitted to ICU, electively or in response to postoperative complications. Furthermore, patients undergoing major surgery have long been considered a valuable model for critical illness: many of the same pathophysiological processes occur and reliable pre-morbid physiological data is available – a rare luxury in the ICU.
The relationship between physical fitness and clinical outcomes post-operatively has been known for > 20 years. This relationship demonstrates a consistent association between higher levels of activity and/or physical fitness in almost every aspect of human health and disease. Much of the perioperative literature utilizes cardiopulmonary pulmonary exercise testing (CPET) to measure pre-operative “fitness”. The Early seminal publications originated from Older et al [46], but more recently work in the UK has illuminated the field. A recent systematic review [47**] from 37 studies including 7852 patients concluded CPET was a useful preoperative risk-stratification tool able to predict postoperative outcome in patients across a range of surgical specialties, but further research was needed to justify ability of CPET to predict postoperative outcome. Following this review, the first multi-center perioperative CPET study[48**] evaluated the relationship between CPET measurements and in-hospital morbidity in 703 patients undergoing major elective colorectal surgery in 6 centers. Consistent with previous studies, both anaerobic threshold and peak oxygen consumption (VO2P) were predictive of postoperative morbidity with area under the ROC curve (AUROC) of 0·79 and 0·77 respectively. Multivariable logistic regression model showed low anaerobic threshold and VO2P along with high body mass index (BMI) and non-laparoscopic techniques to be associated with increased odds of in-hospital morbidity. A model comprising all these variables discriminated well between patients with, and without, in-hospital morbidity (AUROC 0·83).
Other approaches to evaluating physical fitness before surgery that have been less well evaluated including 6-minute walk tests and simple stair climbing. Whilst all the indices of physical fitness are correlated to each other, the strength of this relationship is often surprisingly weak[49*] and it is unclear whether alternative approaches will be as effective at predicting adverse outcome as CPET, the technology with the vast majority of data. Data collection for the international multicenter Measuring Exercise Tolerance after Surgery (METS) study [50*] recently completed (target sample size 1723 patients). METS will provide comparative data on CPET, questionnaire based activity assessment (the Duke Activity Status Index), cardiac biomarkers (NT-pro-BNP) and clinician judgment for the prediction of perioperative risk as well as a comparison with 6-minute walk testing in a sub-study of patients.
A recent development has been the observation that pre-surgery cancer therapies, such as neoadjuvant chemo- and chemoradiotherapy, may impact preoperative physical fitness and outcome following surgery. A UK study demonstrated a significant decrement in CPET-derived anaerobic threshold and peak oxygen consumption in neoadjuvant chemotherapy (NAC) patients before elective upper gastrointestinal surgery [51**]. Lower baseline fitness associated with reduced one-year-survival in patients completing NAC and surgery, but not in patients who did not complete NAC. A follow-on study in elective colorectal cancer surgery demonstrated a similar harmful effect on physical fitness that was in turn associated with increased morbidity in the least fit patients [52*]. Larger studies are currently underway in this area.
The advent of the neoadjuvant therapies before surgery brought both the challenge of a reduction in physical fitness, but also an opportunity due to the period after therapy and before surgery typically allowed for patients to recover from these often-debilitating therapies. The result has been studies evaluating effect of exercise interventions preoperatively in these patients, many of which are currently ongoing. An early blinded, non-randomized, trial[53] in elective colorectal surgery demonstrated a six-week structured in-hospital aerobic training program could reverse the effect of neoadjuvant chemoradiotherapy and return patients to baseline fitness levels. A systematic review of prehabilitation for major inter-cavity surgery[54] concluded preoperative aerobic exercise training interventions were feasible, safe and effective in improving physical fitness, but limited evidence for improved clinical outcomes.
At present, data are not available to provide strong recommendations about clinical outcome but several large (>1000 patients) studies are currently ongoing. Whilst improved cardiorespiratory fitness may be the most intuitively obvious benefit of such training, early mechanistic studies suggest that changes in mitochondrial physiology also occur[55] and it may be these will be more relevant if/when clinical outcome benefit. If this is true, it raises the concept of mimicking benefits of exercise by pharmacological means in patients who cannot or will not exercise. Finally, the potential for CPET techniques to be utilized in the ICU setting and in the post-ICU recovery needs to be explored.
Conclusions
The current call for personalizing ICU care is beginning to be addressed in the metabolism and nutrition delivery field [8**] by early studies validating the role of the NUTRIC score in nutrition risk prediction[56*]. This data shows high-malnutrition risk patients may benefit to a greater degree than those with lower risk. The novel assessments, as described in this review, may be key innovations pre-operatively and in facilitating post-ICU recovery. Patients with low muscle quality and quantity via CT scan may have greater and different specific nutritional needs. Preoperative patients with low LBM or poor skeletal muscle quality could be enrolled in prehabilitative exercise/nutrition programs to improve skeletal muscle quality and quantity [57**]. Interventional trials evaluating muscle quality and quantity measures via CT scan and/or ultrasound will also need to be performed to assess targeted methods to optimize patients. Further, in ICU, these techniques need additional research to determine the muscle-level effects of individual nutrition (e.g. protein delivery, anabolic agents[10]) and specific ICU-rehabilitation (e.g. in-bed ergometry, functional electrical muscle stimulation[58]) interventions. Current functional testing (i.e. Medical Research Council sum score, hand-grip strength, walk-testing) are volitional and not muscle specific, and have significant implementation, interpretation, and compliance challenges [59]. Thus, the role of objective measures of LBM, muscle, nutrition need and exercise targeting measurement described here deserve additional study and validation to add an additional “dimension” to our prediction of outcome and personalization of care in the ICU[30].
In conclusion, we must strive to not only continue to evolve technology and therapeutics targeted at improving survival in the ICU, but at the same time focus on innovations to improve our delivery of nutrition, metabolic support, and exercise interventions. These interventions should be implemented throughout the continuity of care- beginning in the “prehabilitation” period (i.e. pre-operatively or pre-cancer therapy period), continue in the hospital and ICU setting, and then intensify in the post-ICU “recovery” phase (Figure 5).
Figure 5.
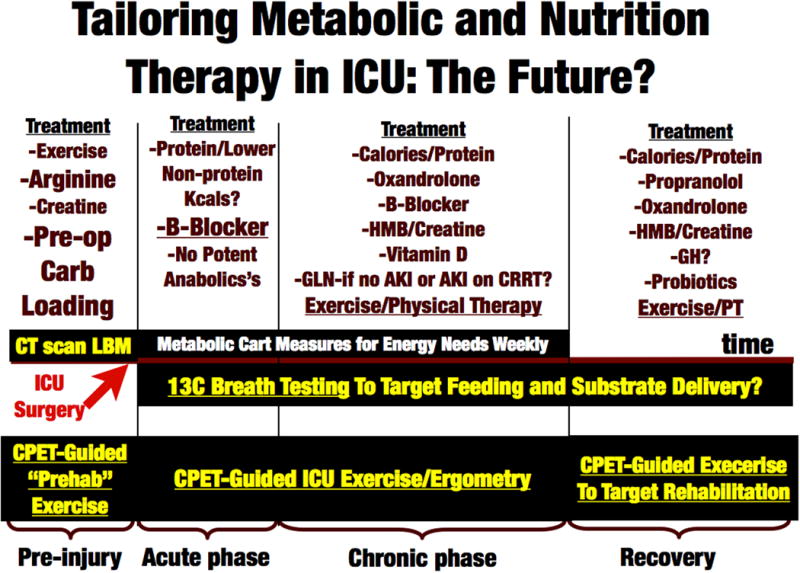
Future of Targeted Metabolic, Lean Body Mass, Exercise and Nutrition Care in ICU. Abbreviations: CPET- Cardiopulmonary Exercise Testing, LBM- Lean Body Mass, GH- Growth Hormone, GLN- Glutamine, HMB- β-Hydroxy β-Methylbutyrate
Key points.
We have significantly improved in-hospital mortality from sepsis and critical illness in last 10 years, however due to Post-ICU Syndrome (PICS) over this same period we have tripled the number of “ICU survivors” going to rehabilitation where it is unclear if they ever return to a meaningful quality of life.
New innovative techniques are demonstrating promise to target recovery from PICS utilizing a combination of objective Lean Body Mass (LBM) and metabolic assessment, targeted nutrition interventions, personalized exercise interventions for prehabilitation and post-ICU recovery.
Admission skeletal muscle mass and quality via CT Scan are predictive of mortality and complications following surgery and critical care and may prove useful to predict pre-illness metabolic reserve and patients in need of pre-illness nutritional optimization.
Novel technique such as 13C-Breath Testing to assess over-/under-feeding and muscle glycogen to evaluate muscle recovery and transition to “recovery phase” are showing promise as objective methods to personalize nutrition and recovery interventions.
Cardiopulmonary Exercise Testing (CPET) is being utilized to predict operative risk and personalize pre-operative (prehabilitative) exercise interventions, with promise to guide post-ICU exercise interventions for PICS.
Acknowledgments
None
Footnotes
Conflicts of Interest: PEW- Is an associate editor of Clinical Nutrition (Elsevier). Has received grant funding related to this work from the NIH NHLBI R34 HL109369, Canadian Institutes of Health Research, Baxter, Fresenius, Lyric Pharmaceuticals, Isomark Inc and Medtronics. Dr. Wischmeyer has served as a consultant to Nestle, Abbott, Fresenius, Baxter, Medtronics, Nutricia, and Lyric Pharmaceuticals, and Takeda for research related to this work. Dr. Wischmeyer has limited ownership shares in Isomark for his consulting work with Isomark, which has otherwise been unpaid in nature. Dr. Wischmeyer has received honoraria or travel expenses for lectures on improving nutrition care in illness from Abbott, Fresenius and Medtronics.
ZP- None Declared
ISM- Is a co-founder and has ownership interest in MuscleSound, and is a member of scientific advisory board of Ascent nutrition.
DB- Daniel E. Butz has an ownership interest in Isomark, LLC, which has licensed technology discussed in this publication.
MG- MPWG is National Specialty Lead, Anaesthesia, Perioperative Medicine and Pain for the UK NIHR CRN, Joint Editor-in-Chief, Extreme Medicine and Physiology and an associate editor of Perioperative Medicine (BioMedCentral Journals) He is also serves on the medical advisory board of Sphere Medical Ltd and is a director, Oxygen Control Systems Ltd and a board member of the Evidence Based Perioperative Medicine (EBPOM) Community Interest Company (a not-for-profit social enterprise). Received honoraria for speaking and / or travel expenses from: Edwards Lifescience (2009 & 2016), Fresenius-Kabi (2008), BOC Medical (Linde Group) (2008), Ely-Lilly Critical Care (2008) and Cortex GmBH (2008 & 2009). MPWG is executive chair (and director) of the Xtreme-Everest Oxygen Research Consortium which has received unrestricted research grant funding paid to my institutions (UoS/UHS/UCL/UCLH) from: John Caudwell, BOC Medical (Linde Group), Ely-Lilly Critical Care, Smiths Medical, Deltex Medical, London Clinic, Rolex, UCLH Special Trustees, Royal Free Special Trustees. MPWG is an elected council member of the Royal College of Anaesthetists and an elected board member, Faculty of Intensive Care Medicine and serves on the board of CPX International.
Financial support: None
References
- 1.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. Jama. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 2.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 3**.Kamdar BB, Huang M, Dinglas VD, Colantuoni E, von Wachter TM, Hopkins RO, Needham DM, National Heart L Blood Institute Acute Respiratory Distress Syndrome N. Joblessness and Lost Earnings After ARDS in a 1-Year National Multicenter Study. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201611-2327OC. New key analysis showing the economic toll on survivors of acute respiratory distress syndrome. Nearly fifty percent of survivors who worked before they were ill were not working after a year, and lost nearly two-thirds of their annual income. The researchers estimated that survivors lost an average of US$27,000 in earnings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Dinglas VD, Aronson Friedman L, Colantuoni E, Mendez-Tellez PA, Shanholtz CB, Ciesla ND, Pronovost PJ, Needham DM. Muscle Weakness and 5-Year Survival in Acute Respiratory Distress Syndrome Survivors. Crit Care Med. 2017;45(3):446–453. doi: 10.1097/CCM.0000000000002208. New key analysis of ARDS patents showing at hospital discharge, greater than one third of ARDS survivors had muscle weakness. Greater strength at discharge and throughout follow-up was associated with improved 5-year survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Iwashyna TJ, Speelmon EC. Advancing a Third Revolution in Critical Care. Am J Respir Crit Care Med. 2016;194(7):782–783. doi: 10.1164/rccm.201603-0619ED. Key Editorial on the next (third) revolution or focus of critical care medicine being to moving away from mortality focused research to quality of life and recovery of pre-ICU function. [DOI] [PubMed] [Google Scholar]
- 6.Needham DM, Feldman DR, Kho ME. The functional costs of ICU survivorship. Collaborating to improve post-ICU disability Am J Respir Crit Care Med. 2011;183(8):962–964. doi: 10.1164/rccm.201012-2042ED. [DOI] [PubMed] [Google Scholar]
- 7*.Stanojcic M, Finnerty CC, Jeschke MG. Anabolic and anticatabolic agents in critical care. Curr Opin Crit Care. 2016;22(4):325–331. doi: 10.1097/MCC.0000000000000330. Key New Review Paper Examining effect of critical illness on metabolism during and after illness. Also examines role of anabolic agents such as oxandolone and anti-catabolic interventions such as propranalol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Wischmeyer PE. Are we creating survivors…or victims in critical care? Delivering targeted nutrition to improve outcomes Curr Opin Crit Care. 2016;22(4):279–284. doi: 10.1097/MCC.0000000000000332. Review Article focused on effect of critical illness on long term patient outcomes and proposes a personalized and targeted nutrition delivery paradigm based latest data and research. [DOI] [PubMed] [Google Scholar]
- 9.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 10*.Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. 2015;19(Suppl 3):S6. doi: 10.1186/cc14724. Review article focused on critical care metabolism and new innovations in nutrition and exercise physiology potentially applicable in recovery from critical care, including initial data on muscle glycogen ultrasound. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, Beishuizen A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18(1):R12. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Twisk JW, Oudemans-van Straaten HM, Weijs PJ. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20(1):386. doi: 10.1186/s13054-016-1563-3. Retrospective CT image analysis showings Low skeletal muscle quality at ICU admission, as assessed by CT-derived skeletal muscle density, is independently associated with higher 6-month mortality in mechanically ventilated patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Mendez-Tellez PA, Shanholtz C, Ciesla ND, Pronovost PJ, Needham DM. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42(10):1557–1566. doi: 10.1007/s00134-016-4530-1. Longitudinal follow-up study of ARDS survivors demonstrating that loss of muscle mass physical disability does not end at hospital discharge. [DOI] [PubMed] [Google Scholar]
- 14*.Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, Iida T, Yagi S, Taura K, Hatano E, et al. Impact of Skeletal Muscle Mass, Muscle Quality, and Visceral Adiposity on Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2017;24(4):1037–1045. doi: 10.1245/s10434-016-5668-3. Retrospective Computer Tomography scan analysis assessing surgical outcomes in liver resection patients. Low skeletal muscle mass and quality were closely related to mortality after surgery. [DOI] [PubMed] [Google Scholar]
- 15*.Boer BC, de Graaff F, Brusse-Keizer M, Bouman DE, Slump CH, Slee-Valentijn M, Klaase JM. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis. 2016;31(6):1117–1124. doi: 10.1007/s00384-016-2538-1. Retrospective Computer Tomography scan analysis assessing surgical outcomes. Skeletal muscle quality is a predictor for overall complications, whereas sarcopenic obesity is a predictor for severe postoperative complications after open colon resection for cancer. Sarcopenia on itself is a predictor for worse overall survival. [DOI] [PubMed] [Google Scholar]
- 16*.Margadant CC, Bruns ER, Sloothaak DA, van Duijvendijk P, van Raamt AF, van der Zaag HJ, Buskens CJ, van Munster BC, van der Zaag ES. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol. 2016;42(11):1654–1659. doi: 10.1016/j.ejso.2016.05.040. Retrospective Computer Tomography scan analysis assessing surgical outcomes. Low muscle density is associated with major postoperative complications in older patients who undergo surgery for colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 17*.Matsushima K, Inaba K, Jhaveri V, Cheng V, Herr K, Siboni S, Strumwasser A, Demetriades D. Loss of muscle mass: a significant predictor of postoperative complications in acute diverticulitis. Journal of Surgical Research. 2017;211:39–44. doi: 10.1016/j.jss.2016.12.002. Retrospective Computer Tomography scan analysis assessing surgical outcomes. Significantly higher rates of postoperative major complications (63% versus 37%, P = 0.027) and surgical site infection (47% versus 19%, P = 0.008) were identified in the LMM group. [DOI] [PubMed] [Google Scholar]
- 18*.Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, Caccialanza R, Gianotti L. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition. 2016;32(11-12):1231–1237. doi: 10.1016/j.nut.2016.04.002. Retrospective Computer Tomography scan analysis assessing surgical outcomes. Sarcopenic obesity is shown to be a strong predictor of major complications after surgery for cancer. [DOI] [PubMed] [Google Scholar]
- 19.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Padhke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. Jama. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 20*.Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Surgery-Related Muscle Loss and Its Association with Postoperative Complications After Major Hepatectomy with Extrahepatic Bile Duct Resection. World J Surg. 2017;41(2):498–507. doi: 10.1007/s00268-016-3732-6. Retrospective Computer Tomography scan analysis assessing surgical outcomes of muscle mass loss following surgery. Post-operative muscle mass loss was found to be significantly associated with postoperative morbidity and mortality. [DOI] [PubMed] [Google Scholar]
- 21**.van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, Weijenberg MP, Dejong CH, Olde Damink SW. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(2):317–326. doi: 10.1002/jcsm.12155. The only prospective Computer Tomography scan analysis assessing surgical outcomes. Low muscle radiation attenuation was associated with reduced survival, and high visceral adiposity was associated with an increase in surgical site infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Fukushima H, Nakanishi Y, Kataoka M, Tobisu KI, Koga F. Postoperative Changes in Skeletal Muscle Mass Predict Survival of Patients With Metastatic Renal Cell Carcinoma Undergoing Cytoreductive Nephrectomy. Clin Genitourin Cancer. 2017;15(2):e229–e238. doi: 10.1016/j.clgc.2016.08.004. Retrospective Computer Tomography scan analysis assessing surgical outcomes. Postoperative changes in skeletal muscle after surgery predicted overall survival for patients. [DOI] [PubMed] [Google Scholar]
- 23*.Zuckerman J, Ades M, Mullie L, Trnkus A, Morin JF, Langlois Y, Ma F, Levental M, Morais JA, Afilalo J. Psoas Muscle Area and Length of Stay in Older Adults Undergoing Cardiac Operations. Ann Thorac Surg. 2017;103(5):1498–1504. doi: 10.1016/j.athoracsur.2016.09.005. Retrospective perioperative Computer Tomography scan analysis showing low lean body mass is a marker of physical frailty associated with increased LOS in older adults undergoing cardiac surgical procedures. [DOI] [PubMed] [Google Scholar]
- 24*.Dahya V, Xiao J, Prado CM, Burroughs P, McGee D, Silva AC, Hurt JE, Mohamed SG, Noel T, Batchelor W. Computed tomography-derived skeletal muscle index: A novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am Heart J. 2016;182:21–27. doi: 10.1016/j.ahj.2016.08.016. Retrospective Computer Tomography study showing skeletal muscle index, a measure of sarcopenia determined from pre-operative CT scans, independently predicts TAVR LOS better than standard frailty testing. [DOI] [PubMed] [Google Scholar]
- 25.Kuroki LM, Mangano M, Allsworth JE, Menias CO, Massad LS, Powell MA, Mutch DG, Thaker PH. Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol. 2015;22(3):972–979. doi: 10.1245/s10434-014-4040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Rutten IJ, Ubachs J, Kruitwagen RF, van Dijk DP, Beets-Tan RG, Massuger LF, Olde Damink SW, Van Gorp T. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43(4):717–724. doi: 10.1016/j.ejso.2016.12.016. Retrospective Computer Tomography scan analysis showing sarcopenia was not predictive of OS or major complications in ovarian cancer patients undergoing primary debulking surgery. However a strong trend towards a survival disadvantage for patients with sarcopenia was seen. [DOI] [PubMed] [Google Scholar]
- 27*.Sakurai K, Kubo N, Tamura T, Toyokawa T, Amano R, Tanaka H, Muguruma K, Yashiro M, Maeda K, Hirakawa K, et al. Adverse Effects of Low Preoperative Skeletal Muscle Mass in Patients Undergoing Gastrectomy for Gastric Cancer. Ann Surg Oncol. 2017 doi: 10.1245/s10434-017-5875-6. Retrospective Computer Tomography scan analysis showing preoperative skeletal muscle index, measured by CT scan, is a useful nutritional determinant that may predict overall survival and cancer-specific survival in patients with gastric cancer who undergo gastrectomy. [DOI] [PubMed] [Google Scholar]
- 28*.Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, Moxham J, Harridge S, Hart N, Montgomery HE. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit Care Med. 2015;43(8):1603–1611. doi: 10.1097/CCM.0000000000001016. Prospective analysis of muscle ultrasound to detect change in muscle quality in critically ill patients. [DOI] [PubMed] [Google Scholar]
- 29**.Puthucheary ZA, McNelly AS, Rawal J, Connolly B, Sidhu PS, Rowlerson A, Moxham J, Harridge SD, Hart N, Montgomery HE. Rectus Femoris Cross-Sectional Area and Muscle Layer Thickness: Comparative Markers of Muscle Wasting and Weakness. Am J Respir Crit Care Med. 2017;195(1):136–138. doi: 10.1164/rccm.201604-0875LE. Prospective analysis of muscle ultrasound methodology to detect change in muscle mass in critically ill patients in addition to muscle strength. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Puthucheary ZA, Wischmeyer P. Predicting critical illness mortality and personalizing therapy: moving to multi-dimensional data. Crit Care. 2017;21(1):20. doi: 10.1186/s13054-016-1597-6. Editorial on developing predictive models focused on assessment of lean body mass and functional outcomes in critical illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Puthucheary ZA, Denehy L. Exercise Interventions in Critical Illness Survivors: Understanding Inclusion and Stratification Criteria. Am J Respir Crit Care Med. 2015;191(12):1464–1467. doi: 10.1164/rccm.201410-1907LE. Randomised Controlled Trial reanalysis on the effect of chronic disease states on response to rehabillitation in critical care. [DOI] [PubMed] [Google Scholar]
- 32*.Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016;20(1):367. doi: 10.1186/s13054-016-1538-4. Retrospective trial utilizing indirect calorimetry showing underfeeding and overfeeding appear to be harmful to ICU patients. A higher caloric intake may also be associated with harm in the form of increased LOS and LOV. The optimal way to define caloric goals should move to more objective measures such as indirect calorimetry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.De Waele E, Honore PM, Spapen HD. New generation indirect calorimeters for measuring energy expenditure in the critically ill: a rampant or reticent revolution? Crit Care. 2016;20(1):138. doi: 10.1186/s13054-016-1315-4. Editorial describing benefits and limitations of indirect calorimetry in the critical care setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whigham LD, Butz DE, Dashti H, Tonelli M, Johnson LK, Cook ME, Porter WP, Eghbalnia HR, Markley JL, Lindheim SR, et al. Metabolic Evidence of Diminished Lipid Oxidation in Women With Polycystic Ovary Syndrome. Current Metabolomics. 2014;2(4):269–278. doi: 10.2174/2213235X01666131203230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whigham LD, Butz DE, Johnson LK, Schoeller DA, Abbott DH, Porter WP, Cook ME. Breath carbon stable isotope ratios identify changes in energy balance and substrate utilization in humans. International journal of obesity. 2014;38(9):1248–1250. doi: 10.1038/ijo.2014.7. [DOI] [PubMed] [Google Scholar]
- 36.Melzer E, Schmidt HL. Carbon isotope effects on the pyruvate dehydrogenase reaction and their importance for relative carbon-13 depletion in lipids. J Biol Chem. 1987;262(17):8159–8164. [PubMed] [Google Scholar]
- 37*.Welch KC, Jr, Peronnet F, Hatch KA, Voigt CC, McCue MD. Carbon stable-isotope tracking in breath for comparative studies of fuel use. Annals of the New York Academy of Sciences. 2016;1365(1):15–32. doi: 10.1111/nyas.12737. Review article describing role of new analytical equipment and refinement of methodology for exhaled breath carbon ratios or 13C-breath testing, combining use of respirometry and stable-isotope tracer techniques, to evaluate metabolism and substrate utilization. [DOI] [PubMed] [Google Scholar]
- 38.Schoeller DA, Brown C, Nakamura K, Nakagawa A, Mazzeo RS, Brooks GA, Budinger TF. Influence of metabolic fuel on the 13C/12C ratio of breath CO2. Biomed Mass Spectrom. 1984;11(11):557–561. doi: 10.1002/bms.1200111103. [DOI] [PubMed] [Google Scholar]
- 39**.McCue MD, Welch KC., Jr (13)C-Breath testing in animals: theory, applications, and future directions. Journal of comparative physiology B, Biochemical, systemic, and environmental physiology. 2016;186(3):265–285. doi: 10.1007/s00360-015-0950-4. Review article describing how 13C-breath testing has been broadly used to study human exercise, nutrition, and pathologies since the 1970s. Describes how recent advances in convenience and affordability of 13C-analyzers in the past decade have led to increased in the use of 13C breath testing. Uses case studies to highlights the myriad applications including analysis of fuel use, energetics and about how and when nutrients are utilized. [DOI] [PubMed] [Google Scholar]
- 40.McCue MD, Sivan O, McWilliams SR, Pinshow B. Tracking the oxidative kinetics of carbohydrates, amino acids and fatty acids in the house sparrow using exhaled 13CO2. The Journal of experimental biology. 2010;213(5):782–789. doi: 10.1242/jeb.039842. [DOI] [PubMed] [Google Scholar]
- 41.Butz DE, Weidmann D, Brownsword R, Cook ME, Schoeller DA, Whigham LD. Immediate biofeedback for energy balance via expired breath delta(13)CO2. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2015;2015:8205–8208. doi: 10.1109/EMBC.2015.7320299. [DOI] [PubMed] [Google Scholar]
- 42.van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. The Journal of physiology. 2001;536(Pt 1):295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009;28(4):387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 44.Lemon PW, Mullin JP. Effect of initial muscle glycogen levels on protein catabolism during exercise. Journal of applied physiology: respiratory, environmental and exercise physiology. 1980;48(4):624–629. doi: 10.1152/jappl.1980.48.4.624. [DOI] [PubMed] [Google Scholar]
- 45.Hill JC, Millan IS. Validation of musculoskeletal ultrasound to assess and quantify muscle glycogen content. A novel approach The Physician and sportsmedicine. 2014;42(3):45–52. doi: 10.3810/psm.2014.09.2075. [DOI] [PubMed] [Google Scholar]
- 46.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116(2):355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 47**.Moran J, Wilson F, Guinan E, McCormick P, Hussey J, Moriarty J. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth. 2016;116(2):177–191. doi: 10.1093/bja/aev454. Most up-to-date comprehensive systematic review in preoperative cardiopulmonary exercise testing which provides a useful overview of this area. [DOI] [PubMed] [Google Scholar]
- 48**.West MA, Asher R, Browning M, Minto G, Swart M, Richardson K, McGarrity L, Jack S, Grocott MP, Perioperative Exercise T, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg. 2016;103(6):744–752. doi: 10.1002/bjs.10112. First multicenter demonstration of the predictive potential of preoperative cardiopulmonary exercise testing in outcome after surgery. [DOI] [PubMed] [Google Scholar]
- 49*.Ribeiro-Samora GA, Montemezzo D, Pereira DAG, Tagliaferri TL, Vieira OA, Britto RR. Could peak oxygen uptake be estimated from proposed equations based on the six-minute walk test in chronic heart failure subjects? Braz J Phys Ther. 2017;21(2):100–106. doi: 10.1016/j.bjpt.2017.03.004. Study evaluating the agreement between the measured peak oxygen uptake (VO2peak) and the VO2peak estimated by four prediction equations based on the six-minute walk test (6MWT) in chronic heart failure patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Wijeysundera DN, Pearse RM, Shulman MA, Abbott TE, Torres E, Croal BL, Granton JT, Thorpe KE, Grocott MP, Farrington C, et al. Measurement of Exercise Tolerance before Surgery (METS) study: a protocol for an international multicentre prospective cohort study of cardiopulmonary exercise testing prior to major non-cardiac surgery. BMJ Open. 2016;6(3):e010359. doi: 10.1136/bmjopen-2015-010359. Protocol for The Measurement of Exercise Tolerance before Surgery (METS) Study: a multicentre prospective cohort study of patients undergoing major elective non-cardiac surgery at 25 participating study sites in Australia, Canada, New Zealand and the UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Jack S, West MA, Raw D, Marwood S, Ambler G, Cope TM, Shrotri M, Sturgess RP, Calverley PM, Ottensmeier CH, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol. 2014;40(10):1313–1320. doi: 10.1016/j.ejso.2014.03.010. First study to demonstrate the harmful effect of neoadjuvant chemotherapy on physical fitness before surgery and highlight the likely relationship with subsequent adverse outcome. [DOI] [PubMed] [Google Scholar]
- 52*.West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, Grocott MP, Jack S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2015;114(2):244–251. doi: 10.1093/bja/aeu318. Pilot study of prehabilitation in after neoadjuvant treatment in preoperative rectal cancer patients. [DOI] [PubMed] [Google Scholar]
- 53.Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, Liberman AS, Stein B, Charlebois P, Feldman LS, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 54.O'Doherty AF, West M, Jack S, Grocott MP. Preoperative aerobic exercise training in elective intra-cavity surgery: a systematic review. Br J Anaesth. 2013;110(5):679–689. doi: 10.1093/bja/aes514. [DOI] [PubMed] [Google Scholar]
- 55.West MA, Loughney L, Lythgoe D, Barben CP, Adams VL, Bimson WE, Grocott MP, Jack S, Kemp GJ. The effect of neoadjuvant chemoradiotherapy on whole-body physical fitness and skeletal muscle mitochondrial oxidative phosphorylation in vivo in locally advanced rectal cancer patients--an observational pilot study. PLoS One. 2014;9(12):e111526. doi: 10.1371/journal.pone.0111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–162. doi: 10.1016/j.clnu.2015.01.015. Study validating use of NUTRIC score identification of critically ill patients most likely to demonstrate improved outcomes from increased nutritional delivery in ICU. [DOI] [PubMed] [Google Scholar]
- 57**.Carli F, Minnella EM. Preoperative functional assessment and optimization in surgical patient. Changing the paradigm. Minerva Anestesiol. 2016 doi: 10.23736/S0375-9393.16.11564-0. Review summarizing evidence that prehabilitation programs, including physical exercise, nutritional optimization and relaxation strategies, can enhance preoperative physiological reserve. Concludes further studies are needed to identify the most appropriate protocols for those patients at risk, and assess the impact of such programs on clinically meaningful surgical outcomes. [DOI] [PubMed] [Google Scholar]
- 58.Parry SM, Berney S, Warrillow S, El-Ansary D, Bryant AL, Hart N, Puthucheary Z, Koopman R, Denehy L. Functional electrical stimulation with cycling in the critically ill: a pilot case-matched control study. J Crit Care. 2014;29(4):695, e691–697. doi: 10.1016/j.jcrc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Hermans G, Van Mechelen H, Bruyninckx F, Vanhullebusch T, Clerckx B, Meersseman P, Debaveye Y, Casaer MP, Wilmer A, Wouters PJ, et al. Predictive value for weakness and 1-year mortality of screening electrophysiology tests in the ICU. Intensive Care Med. 2015;41(12):2138–2148. doi: 10.1007/s00134-015-3979-7. [DOI] [PubMed] [Google Scholar]



