Abstract
The c-Jun N-terminal kinases (JNKs) are ancient and evolutionarily conserved regulators of proliferation, differentiation and cell death responses. Currently, in vitro studies offer conflicting data about whether the JNK pathway augments or represses osteoblast differentiation, and the contribution of the JNK pathway to regulation of bone mass in vivo remains unclear. Here we show that Jnk1-/- mice display severe osteopenia due to impaired bone formation, whereas Jnk2-/- mice display a mild osteopenia only evident in long bones. In order to both confirm that these effects were osteoblast intrinsic and assess whether redundancy with JNK1 masks a potential contribution of JNK2, mice with a conditional deletion of both JNK1 and JNK2 floxed conditional alleles in osteoblasts (Jnk1-2osx) were bred. These mice displayed a similar degree of osteopenia to Jnk1-/- mice due to decreased bone formation. In vitro, Jnk1-/- osteoblasts display a selective defect in the late stages of osteoblast differentiation with impaired mineralization activity. Downstream of JNK1, phosphorylation of JUN is impaired in Jnk1-/- osteoblasts. Transcriptome analysis showed that JNK1 is required for upregulation of several osteoblast-derived proangiogenic factors such as IGF2 and VEGFa. Accordingly, JNK1 deletion results in a significant reduction skeletal vasculature in mice. Taken together, this study establishes that JNK1 is a key mediator of osteoblast function in vivo and in vitro.
Keywords: Bone Formation, JNK, Osteoblasts, JUN, MAPK, Angiogenesis
Introduction
The JNK family of mitogen activated protein kinases (MAPKs) were discovered via their ability to phosphorylate JUN, a component of the AP-1 transcriptional complex, in response to UV irradiation.(1,2) There are three JNK isoforms, of which JNK1 and 2 are widely expressed, whereas JNK3 expression is limited to cardiac and neuronal tissue.(3,4) Subsequent studies have revealed important functions for the JNK pathway in diabetes, ischemic heart disease, stroke, and many models of infection and cancer.(5) In skeletal biology, alterations in JNK activity have been invoked as an explanation for phenotypes with alterations in osteoblast activity, however the role of JNK in vivo remains unclear as in vitro studies offer conflicting reports regarding the role of JNK1 and 2 in osteoblasts.(6) In one study, treatment with a JNK inhibitor was reported to reduce mineralization and expression of mature osteoblast markers, and osteoblast differentiation was enhanced by overexpression of JNK2, leading to the conclusion that JNK2 is critical in late-stage osteoblast differentiation.(7) Conversely, another study showed that inhibition of JNK expression and activity enhanced BMP2-induced osteoblastic differentiation via Runx2 phosphorylation.(8) Given this conflicting data and the fundamental importance of the JNK pathway, further studies are needed to definitively establish the role of the JNK MAPK pathway in osteoblasts.
Materials and Methods
Animals
Jnk1-/-, Jnk2-/-, Jnk1fl/fl, Jnk2fl/fland Osx-Cre mice were described previously.(9,10) All experiments were performed according to protocols approved by the institutional animal care and use committee of Harvard Medical School or Weill Cornell Medical College. All mice were maintained on the C57BL/6 background under specific pathogen free conditions, fed ad libitum chow and housed up to 4 animals per cage on a standard day-night cycle lighting.
Tissue culture, differentiation assays, Western blotting and qPCR
In vitro primary osteoblast cultures, osteoclast cultures, quantitative PCR, Western blotting, ALP and Von Kossa were performed as previously described.(11) Primary antibodies were specific for phospho-JUN, (1:1,000; Cell Signaling) and GAPDH (1:2,000; Sigma), Phospho-SAPK/JNK (Thr183/Tyr185), and SAPK/JNK Antibody (#9251 and #9252 Cell Signaling). Osteoclast culture and RNA isolation from whole bone was performed as previously described.(13) Osteocyte expression analysis was performed in the IDG-SW3 cell line, a generous gift form Dr. Lynda Bonewald.(12)
Micro-CT Analysis, histology, in situ hybridization, immunohistochemistry, and histomorphometry
Micro-CT analysis was conducted on a Scanco Medical Micro-CT 35 system by an investigator blinded to the genotypes of the animals under analysis. Paraffin embedding, in situ hybridization, and immunohistochemistry were performed as previously described.(13) Static and dynamic histomorphometric analyses were performed using the Osteomeasure Analysis System (Osteometrics) as previously described, also by an investigator blinded to the genotypes of the animals being analyzed.(14) Immunofluorescence staining was performed according to a previously published protocol.(15)
RNA sequencing and analysis
Reads were aligned to mm9 mouse transcripts using STAR(version 2.3.0e)(16)using default parameters and resulting bam files sorted and index using samtools. Gene counts were obtained by applying feature counts (version 1.4.3)(17) to sorted bam files, and only unique-mapping reads were used. Genes without any expression counts in any sample were discarded, and in total 11998 genes were used for subsequent analysis. The DESeq2 (version 1.4.5) R package(18) was employed to normalize gene count data, and then detect differentially expressed genes (DEG) with FDR<0.1 and absolute log2 fold-change>0.5.
Results and Discussion
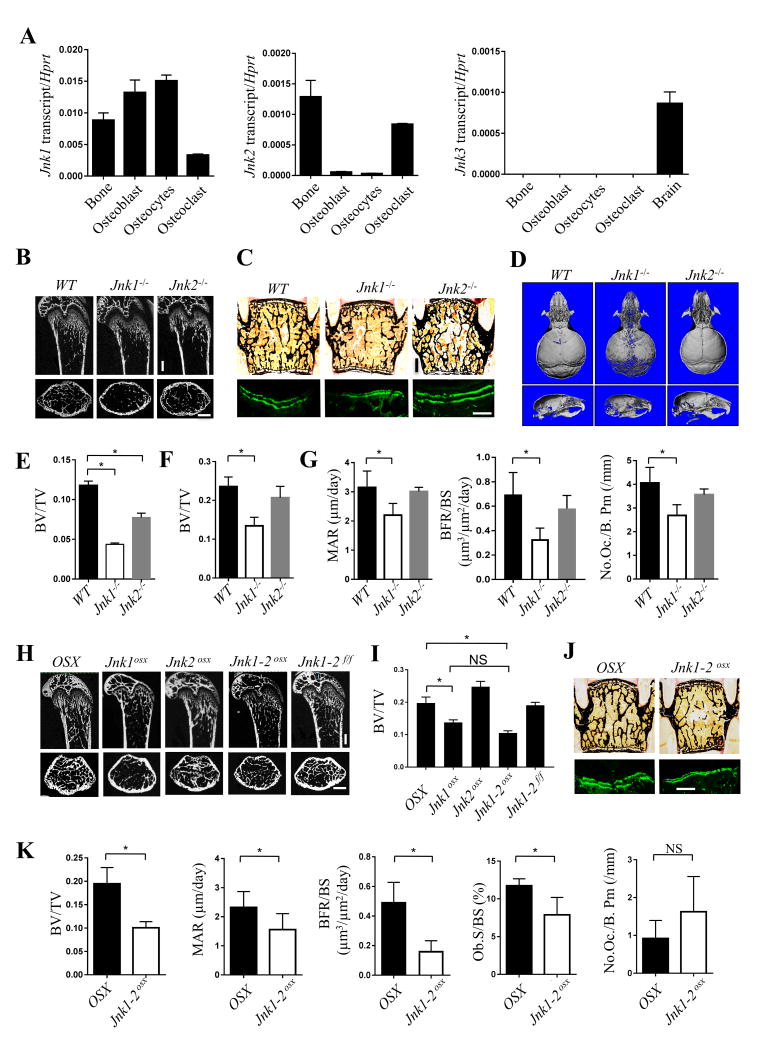
To address the role of JNK in bone formation, we analyzed the expression of JNK1, 2, and 3 in skeletal lineages. Jnk1 showed higher relative expression in osteoblasts than Jnk2, whereas Jnk3 was not expressed in bone cells (Fig. 1A, Supplementary Fig 1). Immunohistochemistry confirmed the presence of JNK1 and JNK2 in osteoblasts in vivo (Supplementary Fig 1E). To assess the role of JNK in osteoblasts in vivo, femoral and vertebral bone mass were examined in Jnk1-/- and Jnk2-/- mice at 6 weeks of age via μCT and histology. Jnk1-/- mice displayed a 50% reduction in trabecular bone volume/total volume in both femurs and vertebrae, whereas Jnk2-/- mice displayed a modest osteopenia in femoral trabeculae and vertebrae (Fig. 1B, C, E and F). Likewise, mineralization of the calvarium was impaired in Jnk1-/- but not Jnk2-/- mice (Fig. 1D). Notably, the pattern of hypomineralization was distinct from that of mice with defects in ERK or p38 MAPK pathway signaling or mice haploinsufficient for Runx2, all of which show impaired mineralization of the frontal bones with corresponding enlargement of the anterior fontanelle.(19,20) In contrast, Jnk1-/- mice displayed hypomineralization of the occipital bone and along the sagittal suture, suggesting that JNK1 displays a distinct function from other MAPKs in osteoblasts. Next, histology and dynamic histomorphometry were performed to characterize the bone phenotypes in Jnk1-/- and Jnk2-/- mice, showing that bone formation parameters, mineral apposition rate and bone formation rate, were significantly decreased in Jnk1-/- but not Jnk2-/- mice. Meanwhile, Jnk1-/- mice also showed a modest reduction in osteoclast numbers (Fig. 1G), consistent with a previous study concluding that JNK1 but not JNK2 is required for osteoclastogenesis.(21)
Figure 1. Jnk1 -/- and Jnk1/2osx mice have low bone mass due to decreased bone formation.
(A) Expression analysis of Jnk1, 2, and 3 (n=4). (B-G) Micro computed tomography analysis (micro-CT) analysis of the trabecular bone in the femur (B and E) and skull (D) and histological (C, upper and F) and histomorphometric (C, lower and G) analysis of the L3 vertebrae in male Jnk1-/- and Jnk2-/- mice at 6-weeks of age (male, n≥6). (H-K) Skeletal analysis of Jnk1osx, Jnk2osx, and Jnk1/2osx mice. Micro-CT analysis of the trabecular bone in the femur (H and I) and histological (J, upper) and histomorphometric (J, lower and K) analysis of the L3 vertebrae of male Jnk1osx, Jnk2osx, and Jnk1/2osx mice in 6-week-old mice (male, n≥6). Bone volume/tissue volume (BV/TV, mineral apposition rate (MAR, μm day-1), bone formation rate/bone surface (BFR/BS, μm3 μm-2 day-1), osteoclast number/bone perimeter (No. Oc./B. Pm) and osteoblast surface/bone surface (Ob.S/BS). Scale bars, 500μm. Error bars, mean ± standard deviation (s.d.) *P < 0.05.
To next confirm if the phenotypes observed in Jnk1-/- mice are osteoblast intrinsic and assess if the modest phenotype of JNK2-deficient mice is due to redundancy with JNK1, mice with deletion of Jnk1 (Jnk1osx), Jnk2 (Jnk2osx), or both Jnk1 and Jnk2 (Jnk1/2osx mice) driven in osteoblasts by the osterix-cre were generated. As seen in Fig 1H and I, both Jnk1osx and Jnk1/2oxs, but not Jnk2osx mice displayed a significant reduction in bone mass in long bones similar to the phenotype of Jnk1-/- mice. Accordingly, histomorphometric analysis showed that all osteoblast but not osteoclast parameters were significantly reduced in Jnk1/2osx mice (Fig. 1J, K). Taken together, these results demonstrate that JNK1 has osteoblast intrinsic functions and that JNK1 is the predominant JNK family MAPK regulating osteoblastogenesis in vivo.
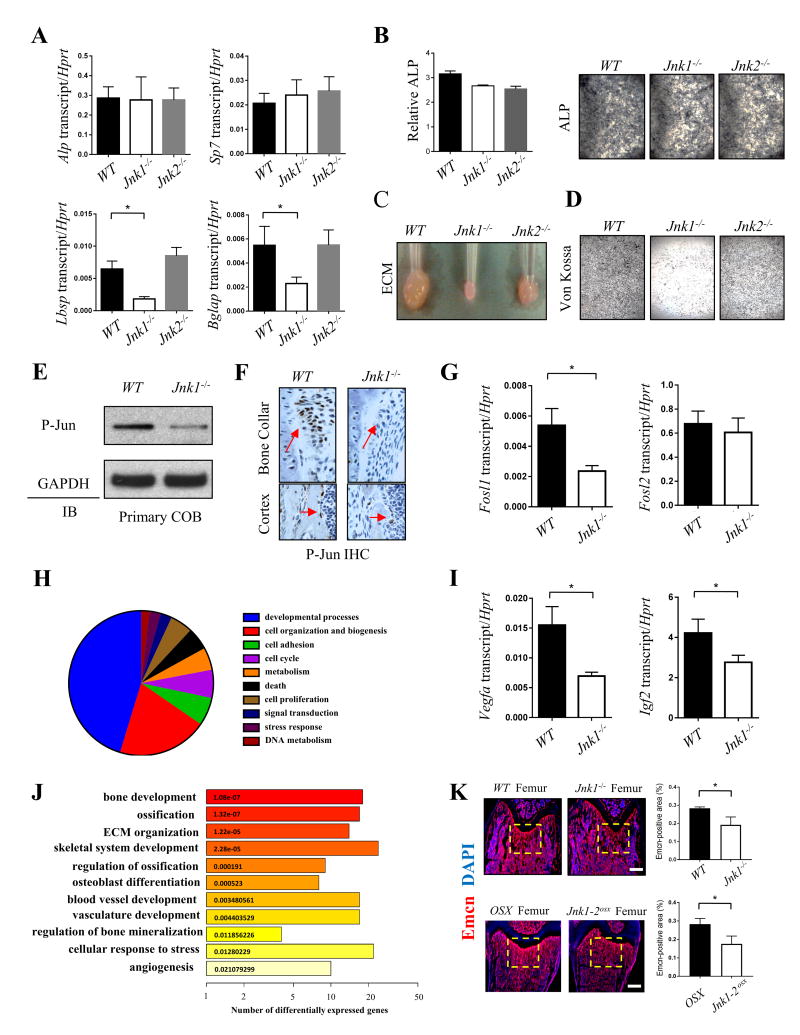
To directly examine the function of JNK1 and JNK2 in osteoblasts in vitro, calvarial osteoblasts (COBs) were isolated and cultured under osteogenic conditions. The expression of transcripts upregulated during early-stage osteoblast differentiation, such as alkaline phosphatase (ALP) and Osterix (Sp7) were not changed in either Jnk1-/- or Jnk2-/- COBs. In contrast, the expression of late-stage osteoblast markers such as osteocalcin (Bglap) and bone sialoprotein (Ibsp) were substantially reduced in Jnk1-/- COBs (Fig. 2A). ALP activity was unchanged or demonstrated a modest reduction in the absence of JNK1 or JNK2 (Fig. 2B). The ability of osteoblasts to perform characteristic late-stage functions, such as secrete extracellular matrix and mineralize was severely impaired in the absence of JNK1 and modestly impaired in the absence of JNK2 (Fig. 2C, D). Similar results were obtained upon analysis of bone marrow stromal cell (BMSC)-derived osteoblasts (Supplemental Fig 2A, B). These results suggest that the functions of JNK1 in the osteoblast lineage are likely restricted to the late stages of osteoblast differentiation. In support of this, JNK1-deficient BMSCs displayed both intact adipocyte differentiation capacity and unaltered proliferation activity during the early stages of osteoblast differentiation (Supplemental Fig 2C-F).
Figure 2. JNK1 is essential for osteoblast function.
(A) Gene expression analysis of Alp, Sp7, Ibsp and Bglap in Jnk1-/- and Jnk2-/- COBs after 7 days of osteoblast differentiation conditions (n=4). (B) ALP activity (left) and representative ALP staining (right) in Jnk1-/- and Jnk2-/- osteoblasts at day 7 of osteogenic induction (n=3). (C and D) Representative non-soluble ECM pellets and Von Kossa staining in differentiated Jnk1-/- and Jnk2-/- COBs at day 14 of osteogenic induction. (E and F) Immunoblotting of phospho-JUN in Jnk1-/- COBs at day 7 of osteogenic induction. (G) and immunohistochemistry for phospho-JUN in Jnk1-/- femurs (bone collar and cortex) in 6-week-old mice. (I) Gene expression analysis of Fosl1, Fosl2, Vegfa and Igf2 in Jnk1-/- COBs at day 7 of osteogenic induction (n=4). (H) CateGOrizer summarizes biological process functions from Gene Ontology (GO) based on DEGs between WT and Jnk1-/- COBs. The fraction of the piechart presents the proportion of genes involving with given function. (J) GO enrichment analysis of DEGs between WT and Jnk1-/- COBs indicated ten significantly overrepresented functions using DAVID. Each bar is colored and labeled according to p-value of enrichment analysis. (K) Longitudinal sections of femurs from WT and Jnk1-/- mice; OSX and Jnk1/2osx mice at 6-weeks of age were subjected to immunofluorescence staining for endomucin (red) and DAPI (blue). Scale bars, 300μm. Error bars, mean ± s.d. *P < 0.05.
To assess the molecular targets of JNK in osteoblasts, we checked phosphorylation levels of a well validated target of JNK, JUN, in Jnk1-/- COBs. JNK phosphorylates the N-terminal domain of JUN to activate activator protein-1 (AP-1) transcriptional activity. While several AP-1 subunits have been identified as important for osteoblast functions, the role of JUN itself in osteoblasts remains incompletely characterized.(22,23) Both immunoblotting and immunohistochemisrty showed that phosphorylation levels of JUN were markedly reduced in Jnk1-/- COBs both in vivo and in vitro (Fig. 2E, F). This confirms a loss of JNK activity in Jnk1-/- COBs and suggests that deletion of JNK1 in osteoblasts may disrupt activation of AP-1 complexes containing JUN. Accordingly, a quantitative PCR analysis of Jnk1-/- COBs showed a significant reduction in transcript levels of the AP-1 target gene Fosl1 (Fra-1), which is itself a key regulator of osteoblast differentiation and ECM production.(22) This confirms that deletion of JNK1 results in impairment in the canonical JNK pathway activity in osteoblasts and raises the possibility that impaired AP-1 activity contributes to the osteopenia observed in Jnk1-/- mice (Fig. 2G).
To identify the set of JNK1-regulated genes in mature osteoblasts, RNA sequencing was performed on Jnk1+/+ and Jnk1-/- COBs cultured under differentiation conditions for 7 days. Interestingly, gene ontology (GO) analysis demonstrated that the set of differentially expressed genes in Jnk1-/- versus Jnk1+/+ COBs is enriched for osteoblast-derived proangiogenic factors in addition to the expected enrichment for genes involved with bone development and mineralization. Accordingly, expression of proangiogenic factors such as VEGFa and IGF2 was markedly reduced in Jnk1-/- COBs (Fig. 2I). To examine if these defects in proangiogenic gene expression correspond to defects in vasculogenesis in vivo, endomucin immunofluorescence staining was performed, showing a decrease in marrow vasculature in Jnk1-/- and Jnk1/2osx mice femurs most evident near the growth plate (Fig. 2K). This is of interest given recent findings that specific subsets of skeletal vasculature may have osteogenic effects on osteoblast lineage cells.(24) Taken together, our study establishes that the JNK pathway, predominantly JNK1, is an important mediator of late stage osteoblast differentiation and mineralization activity in vivo and in vitro and promotes expression of osteoblast-derived proangiogenic factors that regulate skeletal vasculature.
Supplementary Material
Supplementary Figure 1: JNKs expression in bone cells (A, B) Gene expression analysis of Alp, Ctsk, Dmp1 and Sost confirming the differentiation of primary osteoblasts (A, left), bone marrow monocyte-derived osteoclasts (A, right) and the osteocyte cell line-IDG-SW3 (B) used for validation of the culture systems utilized for determination of expression of JNK1, 2, and 3 in Fig 1A. Day 7 in osteoblasts, day 6 in osteoclasts, and day 10 in IDG-SW3 osteocytes were selected as the timepoints for analysis of expression of JNK1, 2, and 3. (C and D) Immunoblotting of JNK1/2 and phospho-JNK1/2 in primary calvarial osteoblasts (C) and bone marrow monocyte-derived osteoclasts (D) during the course of differentiation. (E) Representative immunohistochemistry for JNK1 (left) and JNK2 (right) in 4-week-old WT, Jnk1-/- and Jnk2-/- tibias.
Supplementary Figure 2: JNK1 is essential for osteoblast function not for adipocyte development (A) Quantitative relative ALP activity (left), mineralization capacity (middle) and representative image of Alizarin red staining in mouse bone marrow stromal cells (BMSCs) derived from 6-week-old Jnk1-/-, Jnk2-/- and WT mice after culture under osteogenic conditions (n=5). (B) Gene expression analysis of Sp7 and Ibsp in Jnk1-/- and Jnk2-/- BMSCs after 6 days of culture under osteogenic conditions (n=4). (C) Quantification of adipocyte numbers in the bone marrow of the femur in 6-week-old male Jnk1-/-, Jnk2-/- and WT mice (n=3). (D) Representative image of oil red staining (left) and quantitative adipogenesis capacity (right) in BMSCs derived from 6-week-old Jnk1-/-, Jnk2-/- and WT mice after culture under adipogenic conditions (n=5). (E) Gene expression analysis of Adipoq and Pparg in Jnk1-/-, Jnk2-/- and WT BMSCs after 3 days of culture under adipogenic conditions (n=4). (F) Alamar blue-based cell proliferation assay in primary calvarial osteoblasts (left) or BMSCs (right) derived from Jnk1-/- mice and the littermate controls (n=5). Error bars, mean ± standard deviation (s.d.) *P < 0.05.
Acknowledgments
MBG holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund, support from the Office of the Director of the NIH under award DP5OD021351, support from the Department of Defense under NF150055, and a Junior Investigator Award from the Musculoskeletal Transplant Foundation; JHS holds support from NIAMS of the NIH under R01AR068983 and a pilot project program award from UMCCTS. RX is supported by the Family Friendly Postdoctoral Initiative at Weill Cornell Medical College. We thank the Epigenomics core of Weill Cornell Medical College and Drs. Douglas Ballon and Bin He of the Citigroup Biomedical Imaging Center for their expert assistance.
Footnotes
Conflict of Interest: All authors state that they have no conflicts of interest.
References
- 1.Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–60. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 2.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes & development. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Barrett T, Whitmarsh AJ, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. The EMBO journal. 1996;15:2760–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 5.Weston CR, Davis RJ. The JNK signal transduction pathway. Current opinion in genetics & development. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita M, Ying SX, Zhang GM, et al. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121:101–13. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuguchi T, Chiba N, Bandow K, Kakimoto K, Masuda A, Ohnishi T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:398–410. doi: 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- 8.Huang YF, Lin JJ, Lin CH, Su Y, Hung SC. c-Jun N-terminal kinase 1 negatively regulates osteoblastic differentiation induced by BMP2 via phosphorylation of Runx2 at Ser104. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:1093–105. doi: 10.1002/jbmr.1548. [DOI] [PubMed] [Google Scholar]
- 9.Xu P, Das M, Reilly J, Davis RJ. JNK regulates FoxO-dependent autophagy in neurons. Genes & development. 2011;25:310–22. doi: 10.1101/gad.1984311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 11.Greenblatt MB, Shin DY, Oh H, et al. MEKK2 mediates an alternative beta-catenin pathway that promotes bone formation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1226–35. doi: 10.1073/pnas.1600813113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26:2634–46. doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenblatt MB, Park KH, Oh H, et al. CHMP5 controls bone turnover rates by dampening NF-kappaB activity in osteoclasts. The Journal of experimental medicine. 2015;212:1283–301. doi: 10.1084/jem.20150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda T, Takeda S, Xu R, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–3. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 15.Kusumbe AP, Ramasamy SK, Starsichova A, Adams RH. Sample preparation for high-resolution 3D confocal imaging of mouse skeletal tissue. Nature protocols. 2015;10:1904–14. doi: 10.1038/nprot.2015.125. [DOI] [PubMed] [Google Scholar]
- 16.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 18.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenblatt MB, Shim JH, Zou W, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. The Journal of clinical investigation. 2010;120:2457–73. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bialek P, Kern B, Yang X, et al. A twist code determines the onset of osteoblast differentiation. Developmental cell. 2004;6:423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 21.David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. Journal of cell science. 2002;115:4317–25. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- 22.Eferl R, Hoebertz A, Schilling AF, et al. The Fos-related antigen Fra-1 is an activator of bone matrix formation. The EMBO journal. 2004;23:2789–99. doi: 10.1038/sj.emboj.7600282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozec A, Bakiri L, Jimenez M, Schinke T, Amling M, Wagner EF. Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production. The Journal of cell biology. 2010;190:1093–106. doi: 10.1083/jcb.201002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: JNKs expression in bone cells (A, B) Gene expression analysis of Alp, Ctsk, Dmp1 and Sost confirming the differentiation of primary osteoblasts (A, left), bone marrow monocyte-derived osteoclasts (A, right) and the osteocyte cell line-IDG-SW3 (B) used for validation of the culture systems utilized for determination of expression of JNK1, 2, and 3 in Fig 1A. Day 7 in osteoblasts, day 6 in osteoclasts, and day 10 in IDG-SW3 osteocytes were selected as the timepoints for analysis of expression of JNK1, 2, and 3. (C and D) Immunoblotting of JNK1/2 and phospho-JNK1/2 in primary calvarial osteoblasts (C) and bone marrow monocyte-derived osteoclasts (D) during the course of differentiation. (E) Representative immunohistochemistry for JNK1 (left) and JNK2 (right) in 4-week-old WT, Jnk1-/- and Jnk2-/- tibias.
Supplementary Figure 2: JNK1 is essential for osteoblast function not for adipocyte development (A) Quantitative relative ALP activity (left), mineralization capacity (middle) and representative image of Alizarin red staining in mouse bone marrow stromal cells (BMSCs) derived from 6-week-old Jnk1-/-, Jnk2-/- and WT mice after culture under osteogenic conditions (n=5). (B) Gene expression analysis of Sp7 and Ibsp in Jnk1-/- and Jnk2-/- BMSCs after 6 days of culture under osteogenic conditions (n=4). (C) Quantification of adipocyte numbers in the bone marrow of the femur in 6-week-old male Jnk1-/-, Jnk2-/- and WT mice (n=3). (D) Representative image of oil red staining (left) and quantitative adipogenesis capacity (right) in BMSCs derived from 6-week-old Jnk1-/-, Jnk2-/- and WT mice after culture under adipogenic conditions (n=5). (E) Gene expression analysis of Adipoq and Pparg in Jnk1-/-, Jnk2-/- and WT BMSCs after 3 days of culture under adipogenic conditions (n=4). (F) Alamar blue-based cell proliferation assay in primary calvarial osteoblasts (left) or BMSCs (right) derived from Jnk1-/- mice and the littermate controls (n=5). Error bars, mean ± standard deviation (s.d.) *P < 0.05.