The level of evidence for the use of weekly cisplatin chemoradiation in locally advanced squamous cell carcinoma of the head and neck is limited. This systematic review of the literature compares the efficacy, toxicity, and compliance of low‐dose cisplatin given once a week during external beam irradiation with the standard, three‐weekly high‐dose treatment schedule.
Keywords: Head and neck cancer, Radiotherapy, Cisplatin, Toxicity, Survival
Abstract
Background.
Three‐weekly high‐dose cisplatin (100 mg/m2) is considered the standard systemic regimen given concurrently with postoperative or definitive radiotherapy in locally advanced squamous cell carcinoma of the head and neck (LA‐SCCHN). However, due to unsatisfactory patient tolerance, various weekly low‐dose schedules have been increasingly used in clinical practice. The aim of this meta‐analysis was to compare the efficacy, safety, and compliance between these two approaches.
Materials and Methods.
We systematically searched literature for prospective trials of patients with LA‐SCCHN who received postoperative or definitive conventionally fractionated concurrent chemoradiation. Radiation doses were usually 60–66 gray (Gy) in the postoperative setting and 66–70 Gy in the definitive setting. Standard, three‐weekly high‐dose cisplatin (100 mg/m2, 3 doses) was compared with the weekly low‐dose protocol (≤50 mg/m2, ≥6 doses). The primary endpoint was overall survival. Secondary outcomes comprised response rate, acute and late adverse events, and treatment compliance.
Results.
Fifty‐two studies with 4,209 patients were included in two separate meta‐analyses according to the two clinical settings. There was no difference in treatment efficacy as measured by overall survival or response rate between the chemoradiation settings with low‐dose weekly and high‐dose three‐weekly cisplatin regimens. In the definitive treatment setting, the weekly regimen was more compliant and significantly less toxic with respect to severe (grade 3–4) myelosuppression (leukopenia p = .0083; neutropenia p = .0024), severe nausea and/or vomiting (p < .0001), and severe nephrotoxicity (p = .0099). Although in the postoperative setting the two approaches were more equal in compliance and with clearly less differences in the cisplatin‐induced toxicities, the weekly approach induced more grade 3–4 dysphagia (p = .0026) and weight loss (p < .0001).
Conclusion.
In LA‐SCCHN, current evidence is insufficient to demonstrate a meaningful survival difference between the two dosing regimens. Prior to its adoption into routine clinical practice, the low‐dose weekly approach needs to be prospectively compared with the standard three‐weekly high‐dose schedule.
Implications for Practice.
Given concurrently with conventional radiotherapy in locally advanced head and neck cancer, high‐dose three‐weekly cisplatin has often been replaced with weekly low‐dose infusions to increase compliance and decrease toxicity. The present meta‐analysis suggests that both approaches might be equal in efficacy, both in the definitive and postoperative settings, but differ in toxicity. However, some toxicity data can be influenced by unbalanced representation, and the conclusions are not based on adequately sized prospective randomized studies. Therefore, low‐dose weekly cisplatin should not be used outside clinical trials but first prospectively studied in adequately sized phase III trials versus the high‐dose three‐weekly approach.
Introduction
For the past 3 decades, cisplatin has been used in the management of locally advanced squamous cell carcinoma of the head and neck (LA‐SCCHN) to enhance the tumoricidal activity of irradiation. Among various proposed treatment schedules, differing in frequency, dose, and route of administration, there is level 1 evidence for a significant improvement in locoregional control and/or overall survival achieved by three‐weekly high‐dose intravenous cisplatin given concurrently with conventional external beam radiotherapy compared with radiotherapy alone. The supporting data originate from four large randomized phase III trials investigating the role of cisplatin in both the definitive and postoperative settings [1], [2], [3], [4]. Despite the indisputable benefits, high rates of severe acute and late adverse events remain areas of concern. Due to unacceptable systemic and mucosal toxicities reported in these studies, up to almost 40% of patients could not tolerate all three planned cycles of high‐dose cisplatin. The registered percentages of severe acute myelosuppression and mucositis ranged from 16–47 and from 30–44, respectively. Moreover, a 2013 update of the Intergroup Radiation Therapy Oncology Group 91‐11 trial, evaluating the contribution of cisplatin added to radiation therapy for larynx preservation, revealed a 10‐year cumulative rate of grade 3–5 late toxicity of 33%. Alarmingly, a substantially higher incidence of deaths unrelated to larynx cancer occurred in the concomitant study arm of that latter study [5].
Consequently, there have been growing efforts to reduce the treatment‐related complications without compromising high anticancer activity. Apart from refinements in radiation delivery techniques, the modifiable parameters of single‐agent regimens include peak dose, cumulative dose, and the corresponding dose intensity calculated usually per week. Prolongation of infusion (to 8 or 24 hours) or giving the total cisplatin dose of 100 mg/m2 over 5 consecutive days in a 21‐day cycle, used as a standard in testicular cancer, results in less toxicity [6]. Furthermore, in a randomized trial in patients with non‐small cell lung cancer comparing low‐dose daily cisplatin or weekly cisplatin during radiation versus radiation alone, the daily schedule proved to be superior in terms of local control and survival [7]. Recently, a systematic review pointed out a significant association between total cisplatin dose and overall survival in SCCHN [8]. Here, although the optimal exposition to cisplatin has not been defined yet, cumulative doses of above 200 mg/m2 seem to produce sufficient therapeutic effect, particularly in human papillomavirus‐negative tumors [9], [10], [11], [12]. Mathematically, splitting high‐dose cisplatin into several lower‐dose infusions may provide adequate active drug exposure, but it is currently unclear whether such adjustments reduce damage to healthy tissues and are biologically effective enough in SCCHN. The observed final half‐life values of the free platinum species, ranging from 26.0–78.8 minutes, after cisplatin infusions of different durations generate uncertainty about the optimal time interval between cisplatin administration and the radiation [13]. However, considering that the cytotoxic effects of cisplatin probably result from induction of apoptosis following replication disruption, the long‐term stability of its DNA‐adducts, found to be at least 3 days at 37°C in in vitro studies, may represent a more acceptable rationale for translational applications [14], [15]. Further studies seem warranted in that respect.
Without any support from large comparative phase III trials, weekly low‐dose cisplatin regimens have gradually gained clinical acceptance, replacing the standard, three‐weekly schedule at some institutions. The theoretical background for such a quiet paradigm shift relies on the assumptions that low‐dose cisplatin given weekly compared with high‐dose cisplatin given three‐weekly has a superior capacity to (a) increase treatment compliance while maintaining dose intensity and avoiding unscheduled interruptions of radiotherapy [16], [17]; (b) reduce chemotherapy‐related acute and late side effects without jeopardizing outcome [18]; (c) facilitate dose adjustments according to changes in patient's condition and enable timely discontinuation of treatment if unexpected toxicity develops [19], [20]; (d) enhance radiosensitization of the tumor and possibly also decrease the risk of developing radio‐resistance [18], [21], [22], [23]; (e) demonstrate a similar survival benefit over radiotherapy alone in non‐nasopharyngeal SCCHN as seen in nasopharyngeal and uterine cervical cancers treated with weekly cisplatin‐based chemoradiation [24], [25]; and (f) lower costs and optimize logistical requirements for outpatient administration [26]. However, a need for caution before adopting low‐dose weekly cisplatin as a treatment standard was expressed at the 2015 Annual Meeting of the American Society of Clinical Oncology, based on the data presented from the Longitudinal Oncology Registry of Head And Neck Carcinoma by Wong et al., suggesting that a significantly higher total cumulative dose with the high‐dose regimen versus low‐dose weekly regimen could be reached with potential impact on overall survival [27].
As outlined above, the level of evidence for the use of weekly cisplatin in LA‐SCCHN is limited at this time. Nevertheless, in view of the increasing number of individual studies with small sample sizes and equivocal results, a systematic review is warranted to justify or oppose the strengthening position of this administration schedule in clinical practice. To address this issue, we performed a systematic review and meta‐analysis of aggregate data from prospective trials and compared the efficacy, toxicity, and compliance of low‐dose cisplatin given once a week during external beam irradiation with the standard, three‐weekly schedule for treatment of LA‐SCCHN.
Materials and Methods
Search Strategy and Selection Criteria
This systematic review and meta‐analysis complies with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines [28]. A comprehensive search from the National Library of Medicine for full‐text articles published in print or online up to December 1, 2015, was carried out to identify prospective studies of patients with LA‐SCCHN (stage III–IVB) who received concurrent chemoradiation either in the definitive setting as first‐line treatment with curative intent or postoperatively after curative resection of treatment‐naïve tumors. Trials employing only conventionally fractionated radiotherapy schedules were eligible. The conventional fractionation of definitive radiotherapy consists of standard 2 gray (Gy) daily fractions delivered from Monday to Friday over 7–7.5 weeks to a total dose of about 70 Gy, which is reduced to 60–66 Gy given over 6–6.5 weeks in the adjuvant setting. We compared two concurrent single‐agent cisplatin regimens. In the standard, three‐weekly high‐dose protocol, cisplatin at a dose of 100 mg/m2 was administered on days 1, 22, and 43 (alternatively 2, 23, 44), whereas the weekly schedules were defined by a dose not exceeding 50 mg/m2 and given for at least six treatment cycles.
The following keywords and their combinations were used for the computer‐aided literature search: “cisplatin,” “head and neck/oral cavity/pharynx/larynx,” “chemoradiotherapy/chemoradiation,” and “radiotherapy/radiation” (for detailed search strategy see supplemental online Appendix A). The language was restricted to English. After screening the results by title and abstract, studies of potential relevance were retrieved for full‐text assessment. Articles were excluded if they met any of the following criteria: (a) retrospective studies; (b) updates and additional investigations of previously reported patient populations, which did not add substantial new information on efficacy or safety; (c) altered fractionated radiotherapy and/or induction chemotherapy being part of the treatment protocol in more than 25% of study subjects; (d) cisplatin administered at different time intervals (e.g., daily), doses (e.g., 75 or 80 mg/m2), or by any route other than intravenously (e.g., intra‐arterially); (e) neither efficacy nor toxicity data (according to standard scoring systems and scales) reported; (f) incomplete specification of treatment schedule or more than 25% of subjects received an alternative treatment schedule (e.g., in terms of fractionation or cisplatin dose); (g) head and neck cancer subsites other than oral cavity, oropharynx, larynx, and hypopharynx (e.g. nasopharynx, salivary glands) were designated as primary tumor locations and/or presence of recurrent tumors in more than 50% of the study population; (h) cisplatin was combined with other drugs (i.e., antineoplastic agents or hypoxic modifiers) in more than 25% of study subjects; (i) chemoradiotherapy was delivered in combination with hyperthermia.
Data Extraction
The specific data items extracted from eligible studies included (a) study characteristics (first author, year of publication, study design, study arms in the case of a randomized trial); (b) the accrual period; (c) study population (number of patients in the intention‐to‐treat population, number of patients started on planned therapy, number of oropharyngeal cancer cases); (d) information on radiotherapy (planned and given total doses to the primary tumor, compliance, duration); (e) characteristics of the weekly and three‐weekly cisplatin administration (treatment schedule, planned and given cumulative dose, proportion of patients who received all planned cycles and those who received a cumulative dose of at least 200 mg/m2); (f) adverse events (number of patients evaluable for acute toxicity, selected acute grade 3–4 side effects during chemoradiotherapy, toxic deaths, number of patients evaluable for late toxicity, selected late side effects); (g) 30‐day mortality; (h) response rate (number of evaluable patients, overall response, complete response); and (i) survival outcome (number of evaluable patients, disease‐free survival, locoregional control, overall survival, duration of follow‐up). Where insufficient data were provided in the published reports, we attempted to retrieve them directly from the investigators.
In studies in which data were available from both an independent review and the investigators, we opted for the investigator‐based assessment unless otherwise stated. If information on nausea and vomiting were provided separately, the higher of these two figures was quoted and included in the meta‐analysis. If the rate of neutropenia equaled zero, the rate of febrile neutropenia was also deemed zero. If the rate of hematologic toxicity equaled zero, the rates of anemia, thrombocytopenia, neutropenia, and febrile neutropenia were also deemed zero. In both meta‐analyses, the numbers of patients eligible for response, toxicity, or survival were used for the statistical testing. Toxic deaths and 30‐day mortality were linked to populations eligible for acute toxicity.
Outcomes
The primary outcome measure was overall survival. Secondary outcomes comprised overall and complete response rates, grade 3–5 acute adverse events, grade 1–4 late toxicity, and treatment compliance.
Statistical Analysis
Separate meta‐analyses were performed for the two settings, that is, the postoperative and definitive chemoradiation groups. To assess differences in study characteristics, compliance, and early and late toxicity between weekly and three‐weekly regimens, a random effects meta‐analysis for proportions was used. Overall proportions and their 95% confidence intervals (CI) were reported. The p values were calculated based on the between subgroups heterogeneity statistic.
Furthermore, multivariate random‐effects models were used for joint analysis of the survival proportions reported at multiple time points [29]. No individual patient data were available, so survival probabilities and their standard errors were extracted for each of the included studies based on reported numbers and Kaplan‐Meier curves. To be able to analyze survival probabilities using linear modelling, a ln(–ln) transformation of the probabilities was performed. Together with a natural log‐transformation of the covariate (i.e., ln[year]), this allows interpretation of the covariate effects as hazard ratios in a Cox model. The fixed effects in the model were ln(year), group (weekly versus three‐weekly), and interaction ln(year) x group. A study‐specific intercept and slope ln(year) were added as random effects. Overall mean estimated survival curves are plotted.
All statistical analyses were performed using R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org) and SAS software version 9.4 (SAS Institute Inc, Cary NC USA, http://www.sas.com).
Results
The flow diagram for study selection is detailed in Figure 1. Fifty‐two prospective trials with 4,209 patients, treated with radiation and single‐agent concurrent cisplatin, were included in two separate meta‐analyses comparing weekly versus three‐weekly cisplatin schedules in the settings of postoperative (three vs. six studies, respectively, and two relevant study updates) and definitive radiotherapies (14 vs. 25 studies; two relevant study updates) [1], [2], [3], [4], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]. There were 34 randomized trials (excluding the study updates), of which 11 compared chemoradiotherapy with radiotherapy; five explored targeted agents (lapatinib, gefitinib, erlotinib, panitumumab) combined with either cisplatin‐based chemoradiotherapy or radiotherapy alone; four explored other cytotoxic drugs (vinorelbin, paclitaxel, 5‐fluorouracil) combined with either chemoradiotherapy or radiotherapy alone; seven investigated supportive care drugs, measures, or radiosensitizers (trolamine emulsion, Lactobacillus brevis CD2 lozenges, tirapazamine, palifermin, low‐level laser therapy, bioadhesive chlorhexidine gel) added to chemoradiotherapy; four compared different chemoradiation schedules (concurrent vs. sequential, conventional vs. altered fractionation) and routes of cisplatin administration (intravenous vs. intra‐arterial); and in two studies, induction chemotherapy was tested prior to chemoradiation [1], [2], [3], [4], [30], [37], [40], [42], [43], [44], [46], [47], [49], [50], [51], [55], [57], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]. Only one small randomized trial compared weekly versus three‐weekly chemoradiation for postoperative LA‐SCCHN, and the data were used in both meta‐analyses accordingly [33]. The supplemental online Tables 1–12 summarize efficacy and safety outcomes as well as characteristics of all trials included in the meta‐analyses. In addition, they contain information on the applied adjuvant systemic treatments and neck dissections from six studies in which definitive chemoradiation was given first.
Figure 1.
Flow chart of study selection.
Postoperative Chemoradiotherapy
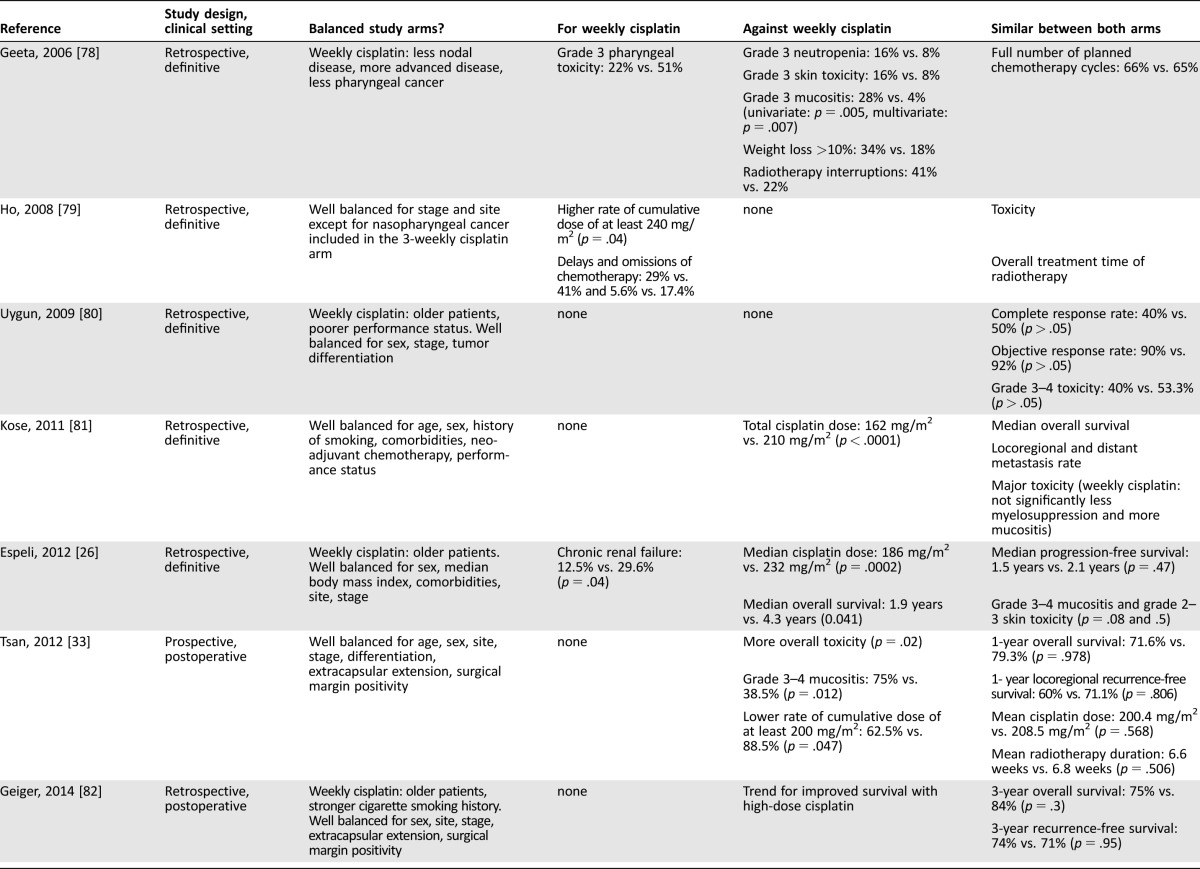
Between the weekly and three‐weekly arms, no differences in study characteristics concerning the number of oropharyngeal cancer cases, radiotherapy completion (as prescribed), and proportions of patients who received all planned cycles of cisplatin (71% vs. 64%, respectively) or a cumulative dose of at least 200 mg/m2 could be observed in the postoperative setting. The studies did not provide sufficient data for analysis of radiotherapy completion without interruption. Regarding toxicity, acute grade 3–4 dysphagia and weight loss occurred significantly more often in the weekly (54%, 95% CI 35–73 and 21%, 95% CI 11–36, respectively) versus three‐weekly arms (20%, 95% CI 12–32, p = .0026 and 3%, 95% CI 1–5, p < .0001, respectively). Grade 3–4 acute mucositis and/or stomatitis (51% vs. 37%, respectively) and other acute and late adverse events were comparable between both schedules (Table 1). No significant effects of group or interaction between group and time could be demonstrated in the overall survival analysis (Fig. 2; supplemental online Appendix B).
Table 1. Postoperative concurrent chemoradiotherapy—compliance and toxicity.
Bolded values indicate parameters with significant differences between the two arms.
Abbreviations: CI, confidence interval; CRT, chemoradiation; N/A, not available; RT, radiotherapy.
Figure 2.
Overall survival analysis comparing weekly and three‐weekly cisplatin given concurrently with postoperative radiotherapy.
Definitive Chemoradiotherapy
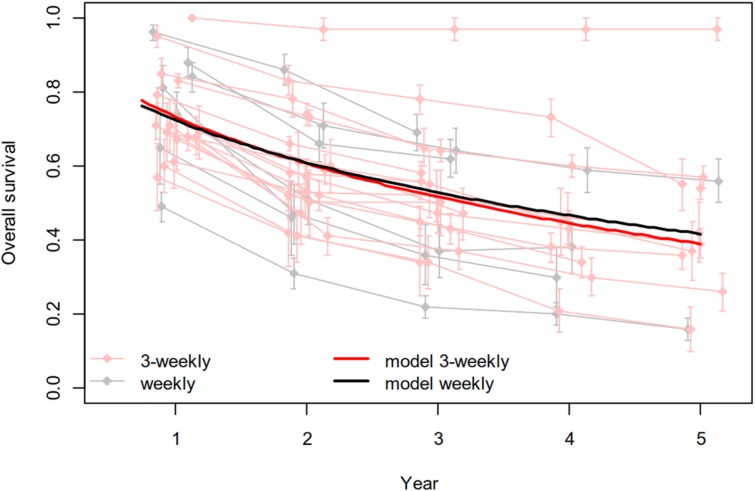
The weekly cisplatin regimen, given concurrently with definitive conventional radiotherapy, was associated with a significantly higher proportion of patients who received all planned chemotherapy cycles (88%, 95% CI 79–93 vs. 71%, 95% CI 66–75 in the three‐weekly schedule, p = .0017). Thereby, it is important that in the studies in which this was indicated, the 200 mg/m2 cumulative dose was reached in the great majority of patients (>90%) with both regimens. Cisplatin given once weekly produced fewer grade 3–4 acute adverse events, and this reached statistical significance for leukopenia (1%, 95% CI 0–10 vs. 19%, 95% CI 12–28, p = .0083, respectively), neutropenia (5%, 95% CI 2–12 vs. 18%, 95% CI 14–24, p = .0024, respectively), nausea and/or vomiting (3%, 95% CI 1–6 vs. 16%, 95% CI 12–20, p < .0001, respectively), and nephrotoxicity (1%, 95% CI 0–3 vs. 5%, 95% CI 4–7, p = .0099, respectively) but not for mucositis and/or stomatitis (25% vs. 42%, respectively; Table 2). Overall and complete response rates were similar between the two groups (89% vs. 80% and 58% vs. 60%, respectively). Despite a disproportionate number of oropharyngeal cancer cases (36%, 95% CI 26–47 vs. 49%, 95% CI 42–56 in the weekly vs. three‐weekly arms, respectively, p = .0395), no differences were found in overall survival, and even adding this factor to the model of survival analysis had no effect on the results (Fig. 3; supplemental online Appendix B).
Table 2. Definitive concurrent chemoradiotherapy—compliance and toxicity.
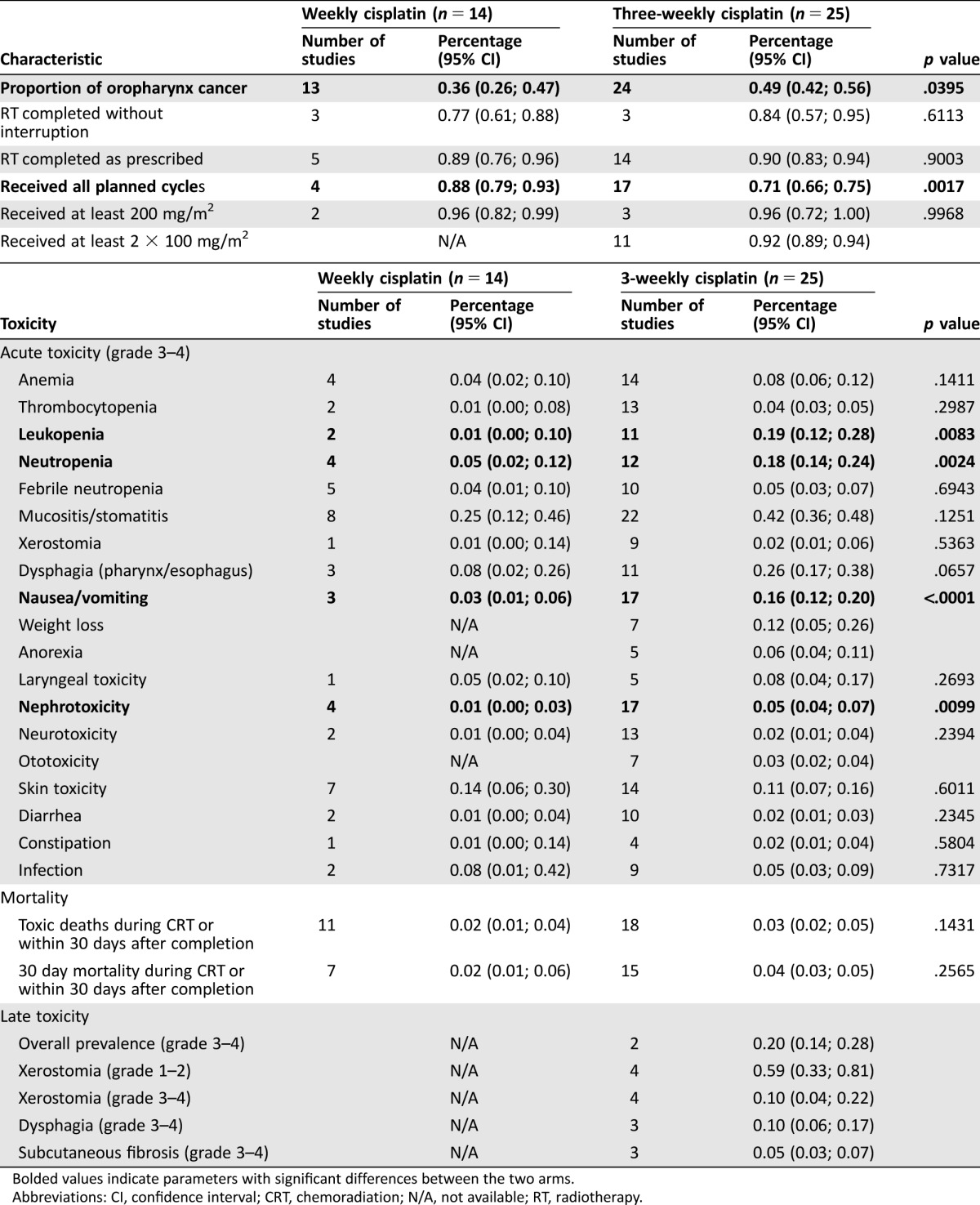
Bolded values indicate parameters with significant differences between the two arms.
Abbreviations: CI, confidence interval; CRT, chemoradiation; N/A, not available; RT, radiotherapy.
Figure 3.
Overall survival analysis comparing weekly and three‐weekly cisplatin given concurrently with definitive radiotherapy.
Discussion
To our knowledge, this is the first systematic review stratified by study type and clinical setting that compares compliance, efficacy, and safety between two of the most frequently used cisplatin‐based concurrent chemoradiotherapy schedules in LA‐SCCHN: the standard, high‐dose three‐weekly regimen, and its alternative, a low‐dose weekly regimen. We performed two meta‐analyses of altogether 52 prospective trials addressing this issue separately in postoperative and definitive settings. Based on our results, no differences in overall survival were detected, neither in the definitive chemoradiation group nor in the postoperative chemoradiation group. In the definitive setting, both cisplatin schedules also yielded comparable response rates. However, significant differences were noted when looking at treatment adherence and toxicity data. While compliance (proportion of patients who received all planned chemotherapy cycles) with the three‐weekly regimen was significantly lower than with the low‐dose weekly regimen in the definitive chemoradiation setting (71% vs. 88%, respectively), that was not so evident in the postoperative one. This is in line with toxicity outcomes, where more robust data could be collected for the definitive group. Compared with the weekly regimen, the three‐weekly cisplatin induced significantly more severe (grade 3–4) toxicity when given in the definitive setting in terms of myelosuppression (leukopenia p = .0083; neutropenia p = .0024), nausea and vomiting (p < .0001), and renal toxicity (p = .0099). In the postoperative group, the low‐dose weekly approach induced more severe dysphagia (p = .0026) and weight loss (p < .0001) than the high‐dose three‐weekly approach. The more severe dysphagia was observed in a single study, in which patients receiving this low weekly dose did not receive hydration routinely [33].
Although both regimens might be equal in efficacy and differ to some extent in toxicity, the findings should be interpreted with caution, because they are not based on adequately sized prospective randomized studies. More specifically, there are several limitations that could have biased our results. First, randomized trials comparing weekly with three‐weekly cisplatin schedules are strikingly lacking. Therefore, we also included non‐randomized and uncontrolled trials as well as randomized trials containing at least one eligible arm of interest. These studies differed in patient populations, endpoints, and other characteristics as outlined in the supplemental online Tables. Consequently, we compared groups that were not prospectively intended to be compared. This led to several statistical challenges as discussed in the Cochrane handbook (chapter 13) [77]. Potential biases, such as selection bias (in terms of baseline differences between groups), confounding, and reporting biases, are likely to be more pronounced in non‐randomized or uncontrolled studies compared with randomized trials. To minimize these biases, we focused exclusively on prospective trials and took into account the proportion of prognostically favorable oropharyngeal cancer.
Second, only a small number of studies were available for some analyses. In addition, several aspects of toxicity and mortality were compared between the two groups without correction for multiple testing of the p values. Therefore, one should be aware that type I error is increased, and the results should be judged with caution, especially in cases of only borderline significance. Moreover, the recruitment periods extended from 1981–2013. Considering advances in diagnostics during the last 30 years, some patients with distant metastases could have been under‐staged in the early trials. Enrollment of such unrecognized cases with poor prognosis in protocols for locally advanced disease could have influenced treatment outcomes. Besides that, changes in the toxicity scales over the past 3 decades need to be acknowledged when interpreting our results.
Another important point is the heterogeneity in the weekly schedules. Whereas three‐weekly cisplatin given three‐times at a dose of 100 mg/m2 did not allow almost any variation, the peak dose levels and number of cycles ranged from 20 mg/m2 to 50 mg/m2 and from six to nine in the weekly regimens, respectively. Thus, performing a joint analysis might constitute a potential source of bias. Finally, we had to deal with inconsistent reporting of compliance, survival, and side effects, of which particularly late toxicity suffered from under‐reporting. We found significant differences in proportions of patients who received all planned chemotherapy. However, most of the studies did not provide sufficient data on dose reductions and time delays. Similarly, different author groups used different definitions of disease‐free survival and locoregional control, which prevented us from merging the collected data here. Of note, selected patients (supplemental online Appendix B) received some kind of adjuvant treatment, which might have had an impact on the outcome data.
Conclusion
Despite these limitations, we came to the following conclusions. First, neither a meaningful difference nor a favoring trend in overall survival could be demonstrated between the two chemoradiation protocols. Keeping in mind the lack of support for low‐dose weekly cisplatin in controlled trials, we are concerned about its indiscriminate and premature adoption in routine clinical practice. The second conclusion is that there seems to be a considerable level of uncertainty regarding the benefit of the third dose of cisplatin in the high‐dose three‐weekly concurrent regimen, which is backed up by observations of other investigators [9], [10], [11]. Only 64%–71% of the patients attained the target number of three high‐dose cisplatin applications during conventional radiotherapy. We assume that the third cisplatin dose is responsible for the increased number of acute adverse events in the definitive chemoradiation group. The reason why this negative effect was seen less in the postoperative setting is even more speculative. However, in that setting a numerically lower percentage of patients received three cycles than in the definitive chemoradiation group (64% vs. 71%, respectively), and the preceding surgical intervention may have played a role in this. Undoubtedly, the radiation dose in the postoperative setting is lower than in the definitive one (60–66 Gy vs. 66–70 Gy, respectively), but this will have no bearing on the typical cisplatin‐induced toxicities.
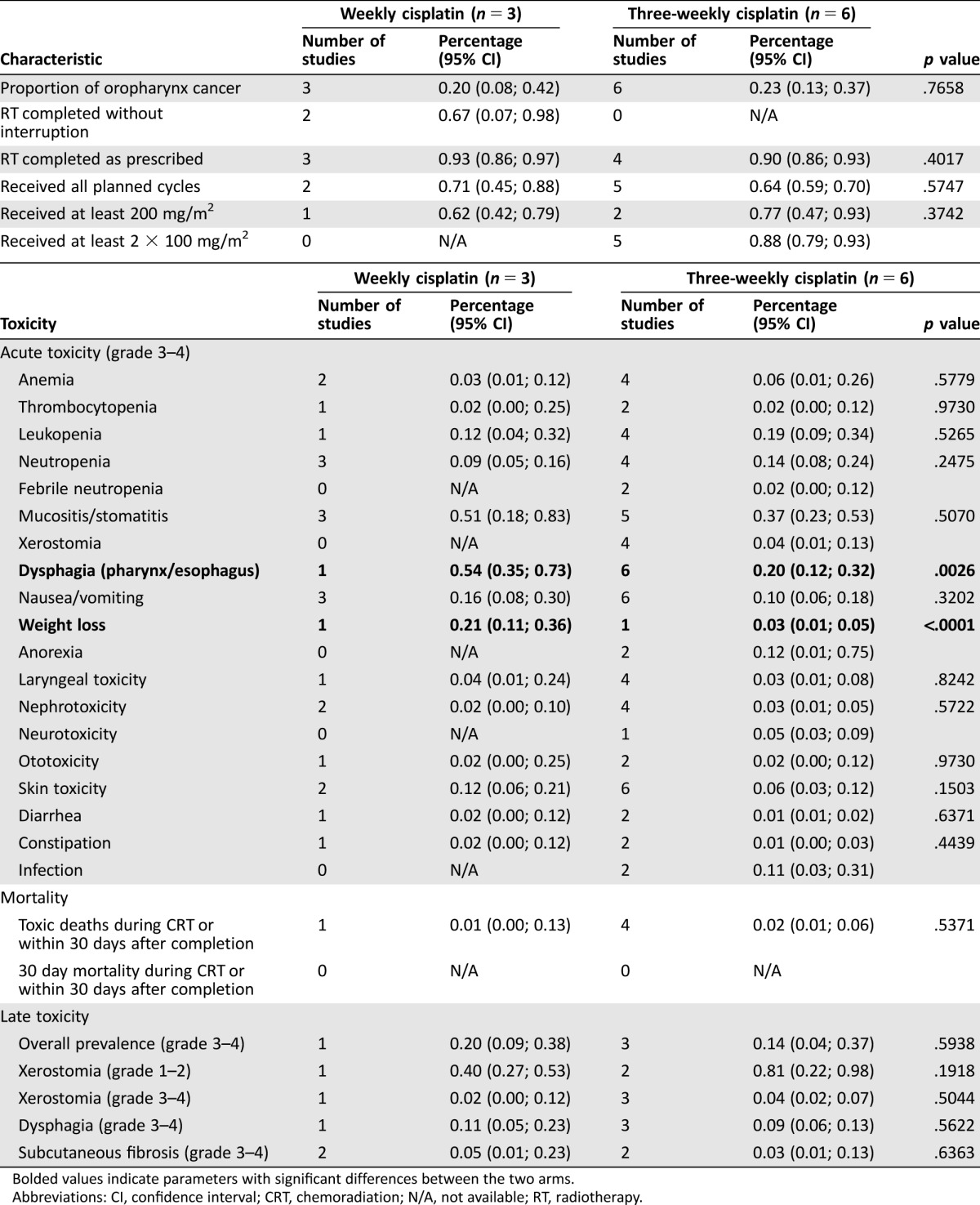
As published in 2012, Tsan et al. randomized 55 postoperative high‐risk SCCHN cases of the oral cavity between weekly and three‐weekly regimens, demonstrating superiority of the latter scheme both in terms of compliance and toxicity [33]. Additionally, six studies retrospectively investigating the same comparison yielded outcomes favoring neither of these two schedules (Table 3) [26], [78], [79], [80], [81], [82].
Table 3. Studies comparing weekly versus three‐weekly concomitant cisplatin.
As for now, three randomized trials are ongoing. A phase II/III study of the Japanese Clinical Oncology Group (protocol JCOG#1008) evaluates the non‐inferiority of concurrent chemoradiotherapy with weekly (7 × 40 mg/m2) versus three‐weekly cisplatin (3 × 100 mg/m2) in SCCHN patients in the postoperative setting [83]. The results are planned to be meta‐analyzed with the data obtained from a phase III trial of the Italian and Portuguese Clinical Oncology Groups, which, apart from a lower cumulative dose of weekly cisplatin (6 × 40 mg/m2), adopts the same protocol. Lastly, a non‐inferiority phase III study that is being performed in India (Tata Memorial Hospital in Mumbai) compares weekly 30 mg/m2 with three‐weekly 100 mg/m2 cisplatin in a similar clinical setup, but both in postoperative and definitive settings.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
See the related article, “Commentary on “Weekly Low‐Dose Versus Three‐Weekly High‐Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non‐Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta‐Analysis of Aggregate Data” by A. Dimitrios Colevas, on pages 1022–1023 of this issue.
Author Contributions
Conception/design: Petr Szturz, Kristien Wouters, David Adelstein, Jan B. Vermorken
Collection and/or assembly of data: Petr Szturz, Kristien Wouters, Jan B. Vermorken
Data analysis and interpretation: Petr Szturz, Kristien Wouters, Jan B. Vermorken
Manuscript writing: Petr Szturz, Kristien Wouters, David Adelstein, Jan B. Vermorken
Final approval of manuscript: Petr Szturz, Kristien Wouters, Naomi Kiyota, Makoto Tahara, Kumar Prabhash, Vanita Noronha, Ana Castro, Lisa Licitra, David Adelstein, Jan B. Vermorken
Disclosures
Naomi Kiyota: ONO, Eisai, Bayer, Bristol‐Myers Squibb (C/A), ONO, Eisai, Boehringer Ingelheim, AstraZeneca (RF), Bayer, Bristol‐Myers Squibb (H); Makoto Tahara: Merck Sharpe & Dohme, Bayer, AstraZeneca, Pfizer, Ono, Bristol‐Myers Squibb (C/A), Eisai, Boehringer Ingelheim, Novartis, NanoCarrier, Merck Sharp & Dohme, Ono (RF), Ono, Eisai, Bayer, Bristol‐Myers Squibb (H); Ana Castro: Merck Serono, Roche (C/A), Merck Serono, Pfizer (RF), Merck Serono, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, Amgen, Celgene, Pfizer (ET), Merck Serono, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, Amgen, Celgene, Janssen, Astellas, Pfizer (H), Merck Serono, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche (SAB); Lisa Licitra: EISAI, Bristol‐Myers Squibb, Merck Sharp & Dohme, Merck‐Serono, Boehringer Ingelheim, Debiopharm, SOBI, Novartis, AstraZeneca, Bayer, Roche, Amgen (C/A); Jan B. Vermorken: Merck KGaA, Amgen (C/A), Merck KGaA, Sanofi (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Adelstein DJ, Li Y, Adams GL et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–98. [DOI] [PubMed] [Google Scholar]
- 2. Forastiere AA, Goepfert H, Maor M et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091–2098. [DOI] [PubMed] [Google Scholar]
- 3. Bernier J, Domenge C, Ozsahin M et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 4. Cooper JS, Pajak TF, Forastiere AA et al. Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–1944. [DOI] [PubMed] [Google Scholar]
- 5. Forastiere AA, Zhang Q, Weber RS et al. Long‐term results of RTOG 91‐11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartmann JT, Kollmannsberger C, Kanz L et al. Platinum organ toxicity and possible prevention in patients with testicular cancer. Int J Cancer 1999;83:866–869. [DOI] [PubMed] [Google Scholar]
- 7. Schaake‐Koning C, van den Bogaert W, Dalesio O et al. Radiosensitization by cytotoxic drugs. The EORTC experience by the Radiotherapy and Lung Cancer Cooperative Groups. Lung Cancer 1994;10(suppl 1):S263–S270. [DOI] [PubMed] [Google Scholar]
- 8. Strojan P, Vermorken JB, Beitler JJ et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck 2016;38(suppl 1):E2151–E2158. [DOI] [PubMed] [Google Scholar]
- 9. Ang KK. Concurrent radiation chemotherapy for locally advanced head and neck carcinoma: Are we addressing burning subjects? J Clin Oncol 2004;22:4657–4659. [DOI] [PubMed] [Google Scholar]
- 10. Ghi MG, Paccagnella A, Floriani I et al. Concomitant chemoradiation in locally advanced head and neck squamous cell carcinoma: A literature‐based meta‐analysis on the platinum concomitant chemotherapy. J Clin Oncol 2011;29:5534a. [Google Scholar]
- 11. Otty Z, Skinner MB, Dass J et al. Efficacy and tolerability of weekly low‐dose cisplatin concurrent with radiotherapy in head and neck cancer patients. Asia Pac J Clin Oncol 2011;7:287–292. [DOI] [PubMed] [Google Scholar]
- 12. Spreafico A, Huang SH, Xu W et al. Impact of cisplatin dose intensity on human papillomavirus‐related and ‐unrelated locally advanced head and neck squamous cell carcinoma. Eur J Cancer 2016;67:174–182. [DOI] [PubMed] [Google Scholar]
- 13. Vermorken JB, van der Vijgh WJ, Klein I et al. Pharmacokinetics of free and total platinum species after rapid and prolonged infusions of cisplatin. Clin Pharmacol Ther 1986;39:136–144. [DOI] [PubMed] [Google Scholar]
- 14. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol 2014;740:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson NP, Hoeschele JD, Rahn RO. Kinetic analysis of the in vitro binding of radioactive cis‐ and trans‐dichlorodiammineplatinum(II) to DNA. Chem Biol Interact 1980;30:151–169. [DOI] [PubMed] [Google Scholar]
- 16. Traynor AM, Richards GM, Hartig GK et al. Comprehensive IMRT plus weekly cisplatin for advanced head and neck cancer: The University of Wisconsin experience. Head Neck 2010;32:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medina JA, Rueda A, de Pasos AS et al. A phase II study of concomitant boost radiation plus concurrent weekly cisplatin for locally advanced unresectable head and neck carcinomas. Radiother Oncol 2006;79:34–38. [DOI] [PubMed] [Google Scholar]
- 18. Kurihara N, Kubota T, Hoshiya Y et al. Pharmacokinetics of cisdiamminedichloroplatinum (II) given as low‐dose and high‐dose infusions. J Surg Oncol 1996;62:135–138. [DOI] [PubMed] [Google Scholar]
- 19. Newlin HE, Amdur RJ, Riggs CE et al. Concomitant weekly cisplatin and altered fractionation radiotherapy in locally advanced head and neck cancer. Cancer 2010;116:4533–4540. [DOI] [PubMed] [Google Scholar]
- 20. Beckmann GK, Hoppe F, Pfreundner L et al. Hyperfractionated accelerated radiotherapy in combination with weekly cisplatin for locally advanced head and neck cancer. Head Neck 2005;27:36–43. [DOI] [PubMed] [Google Scholar]
- 21. Bartelink H, Kallman RF, Rapacchietta D et al. Therapeutic enhancement in mice by clinically relevant dose and fractionation schedules of cis‐diamminedichloroplatinum (II) and irradiation. Radiother Oncol 1986;6:61–74. [DOI] [PubMed] [Google Scholar]
- 22. Marcu L, Bezak E, Olver I. Scheduling cisplatin and radiotherapy in the treatment of squamous cell carcinomas of the head and neck: A modelling approach. Phys Med Biol 2006;51:3625–3637. [DOI] [PubMed] [Google Scholar]
- 23. Myint WK, Ng C, Raaphorst GP. Examining the non‐homologous repair process following cisplatin and radiation treatments. Int J Radiat Biol 2002;78:417–424. [DOI] [PubMed] [Google Scholar]
- 24. Chan AT, Leung SF, Ngan RK et al. Overall survival after concurrent cisplatin‐radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2005;97:536–539. [DOI] [PubMed] [Google Scholar]
- 25. Rose PG, Bundy BN, Watkins EB et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–1153. [DOI] [PubMed] [Google Scholar]
- 26. Espeli V, Zucca E, Ghielmini M et al. Weekly and 3‐weekly cisplatin concurrent with intensity‐modulated radiotherapy in locally advanced head and neck squamous cell cancer. Oral Oncol 2012;48:266–271. [DOI] [PubMed] [Google Scholar]
- 27. Wong SJ, Li L, Hess LM et al. Utilization and outcomes of low dose versus high dose cisplatin in head and neck cancer patients receiving concurrent radiation. J Clin Oncol 2015;33:6019a. [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arends LR, Hunink MG, Stijnen T. Meta‐analysis of summary survival curve data. Stat Med 2008;27:4381–4396. [DOI] [PubMed] [Google Scholar]
- 30. Bachaud JM, David JM, Boussin G et al. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced squamous cell carcinoma of the head and neck: Preliminary report of a randomized trial. Int J Radiat Oncol Biol Phys 1991;20:243–246. [DOI] [PubMed] [Google Scholar]
- 31. Bachaud JM, Cohen‐Jonathan E, Alzieu C et al. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int J Radiat Oncol Biol Phys 1996;36:999–1004. [DOI] [PubMed] [Google Scholar]
- 32. Rampino M, Ricardi U, Munoz F et al. Concomitant adjuvant chemoradiotherapy with weekly low‐dose cisplatin for high‐risk squamous cell carcinoma of the head and neck: A phase II prospective trial. Clin Oncol (R Coll Radiol) 2011;23:134–140. [DOI] [PubMed] [Google Scholar]
- 33. Tsan DL, Lin CY, Kang CJ et al. The comparison between weekly and three‐weekly cisplatin delivered concurrently with radiotherapy for patients with postoperative high‐risk squamous cell carcinoma of the oral cavity. Radiat Oncol 2012;7:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Sarraf M, Pajak TF, Byhardt RW et al. Postoperative radiotherapy with concurrent cisplatin appears to improve locoregional control of advanced, resectable head and neck cancers: RTOG 88‐24. Int J Radiat Oncol Biol Phys 1997;37:777–782. [DOI] [PubMed] [Google Scholar]
- 35. Cooper JS, Zhang Q, Pajak TF et al. Long‐term follow‐up of the RTOG 9501/intergroup phase III trial: Postoperative concurrent radiation therapy and chemotherapy in high‐risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2012;84:1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiyota N, Tahara M, Okano S et al. Phase II feasibility trial of adjuvant chemoradiotherapy with 3‐weekly cisplatin for Japanese patients with post‐operative high‐risk squamous cell carcinoma of the head and neck. Jpn J Clin Oncol 2012;42:927–933. [DOI] [PubMed] [Google Scholar]
- 37. Harrington K, Temam S, Mehanna H et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high‐risk patients with resected squamous cell carcinoma of the head and neck: A phase III, randomized, double‐blind, placebo‐controlled study. J Clin Oncol 2015;33:4202–4209. [DOI] [PubMed] [Google Scholar]
- 38. Glaser MG, Leslie MD, O'Reilly SM et al. Weekly cisplatinum concomitant with radical radiotherapy in the treatment of advanced head and neck cancer. Clin Oncol (R Coll Radiol) 1993;5:286–289. [DOI] [PubMed] [Google Scholar]
- 39. Asif R, Chandra K, Chopra V et al. Concurrent cisplatin and radiotherapy in advanced head and neck cancer. Indian J Otolaryngol Head Neck Surg 2003;55:94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarkar SK, Patra NB, Goswami J et al. Comparative study of efficacy and toxicities of cisplatin vs vinorelbine as radiosensitisers in locally advanced head and neck cancer. J Laryngol Otol 2008;122:188–192. [DOI] [PubMed] [Google Scholar]
- 41. Jain RK, Kirar P, Gupta G et al. A comparative study of low dose weekly paclitaxel versus cisplatin with concurrent radiation in the treatment of locally advanced head and neck cancers. Indian J Cancer 2009;46:50–53. [DOI] [PubMed] [Google Scholar]
- 42.Devleena, Majumdar A, Poddar S et al. Comparison of vinorelbine with cisplatin in concomitant chemoradiotherapy in head and neck carcinoma. Indian J Med Paediatr Oncol 2010;31:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Essa HH, Azzam M. Concurrent chemoradiation in locally advanced head and neck cancers: A comparative study of weekly Paclitaxel versus Cisplatin‐based regimen. J Egypt Natl Canc Inst 2010;22:165–173. [PubMed] [Google Scholar]
- 44. Sharma A, Mohanti BK, Thakar A et al. Concomitant chemoradiation versus radical radiotherapy in advanced squamous cell carcinoma of oropharynx and nasopharynx using weekly cisplatin: A phase II randomized trial. Ann Oncol 2010;21:2272–2277. [DOI] [PubMed] [Google Scholar]
- 45. Kanotra SP, Kanotra S, Gupta A et al. Chemoradiation in advanced head and neck cancers: A comparison of two radiosensitizers, paclitaxel and cisplatin. Indian J Otolaryngol Head Neck Surg 2011;63:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quon H, Leong T, Haselow R et al. Phase III study of radiation therapy with or without cis‐platinum in patients with unresectable squamous or undifferentiated carcinoma of the head and neck: An intergroup trial of the Eastern Cooperative Oncology Group (E2382). Int J Radiat Oncol Biol Phys 2011;81:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abbas H, Bensadoun RJ. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer 2012;20:185–190. [DOI] [PubMed] [Google Scholar]
- 48. Agrawal S, Awasthi R, Singh A et al. An exploratory study into the role of dynamic contrast‐enhanced (DCE) MRI metrics as predictors of response in head and neck cancers. Clin Radiol 2012;67:e1–e5. [DOI] [PubMed] [Google Scholar]
- 49. Sharma A, Rath GK, Chaudhary SP et al. Lactobacillus brevis CD2 lozenges reduce radiation‐ and chemotherapy‐induced mucositis in patients with head and neck cancer: A randomized double‐blind placebo‐controlled study. Eur J Cancer 2012;48:875–881. [DOI] [PubMed] [Google Scholar]
- 50. Majumder D, Choudhury K, Das P et al. Different fractionation schedules of radiotherapy in locally advanced head and neck malignancy: A prospective randomized study to compare the results of treatment and toxicities of different protocols. South Asian J Cancer 2013;2:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghosh‐Laskar S, Kalyani N, Gupta T et al. Conventional radiotherapy versus concurrent chemoradiotherapy versus accelerated radiotherapy in locoregionally advanced carcinoma of head and neck: Results of a prospective randomized trial. Head Neck 2016;38:202–207. [DOI] [PubMed] [Google Scholar]
- 52. Al‐Sarraf M, Pajak TF, Marcial VA et al. Concurrent radiotherapy and chemotherapy with cisplatin in inoperable squamous cell carcinoma of the head and neck. An RTOG Study. Cancer 1987;59:259–265. [DOI] [PubMed] [Google Scholar]
- 53. Marcial VA, Pajak TF, Mohiuddin M et al. Concomitant cisplatin chemotherapy and radiotherapy in advanced mucosal squamous cell carcinoma of the head and neck. Long‐term results of the Radiation Therapy Oncology Group study 81‐17. Cancer 1990;66:1861–1868. [DOI] [PubMed] [Google Scholar]
- 54. Fountzilas G, Skarlos D, Kosmidis P et al. Radiation therapy and concurrent cisplatin administration in locally advanced head and neck cancer. A Hellenic Co‐operative Oncology Group study. Acta Oncol 1994;33:825–830. [DOI] [PubMed] [Google Scholar]
- 55. Pinnarò P, Cercato MC, Giannarelli D et al. A randomized phase II study comparing sequential versus simultaneous chemo‐radiotherapy in patients with unresectable locally advanced squamous cell cancer of the head and neck. Ann Oncol 1994;5:513–519. [DOI] [PubMed] [Google Scholar]
- 56. Forastiere AA, Zhang Q, Weber RS et al. Long‐term results of RTOG 91‐11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fountzilas G, Ciuleanu E, Dafni U et al. Concomitant radiochemotherapy vs radiotherapy alone in patients with head and neck cancer: A Hellenic Cooperative Oncology Group Phase III Study. Med Oncol 2004;21:95–107. [DOI] [PubMed] [Google Scholar]
- 58. de Castro G Jr, Snitcovsky IM, Gebrim EM et al. High‐dose cisplatin concurrent to conventionally delivered radiotherapy is associated with unacceptable toxicity in unresectable, non‐metastatic stage IV head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2007;264:1475–1482. [DOI] [PubMed] [Google Scholar]
- 59. Zenda S, Onozawa Y, Tahara M et al. Feasibility study of single agent Cisplatin and concurrent radiotherapy in Japanese patients with squamous cell carcinoma of the head and neck: Preliminary results. Jpn J Clin Oncol 2007;37:725–729. [DOI] [PubMed] [Google Scholar]
- 60. Herchenhorn D, Dias FL, Ferreira CG et al. Impact of previous tracheotomy as a prognostic factor in patients with locally advanced squamous cell carcinoma of the larynx submitted to concomitant chemotherapy and radiation. ORL J Otorhinolaryngol Relat Spec 2008;70:381–388. [DOI] [PubMed] [Google Scholar]
- 61. Prades JM, Lallemant B, Garrel R et al. Randomized phase III trial comparing induction chemotherapy followed by radiotherapy to concomitant chemoradiotherapy for laryngeal preservation in T3M0 pyriform sinus carcinoma. Acta Otolaryngol 2010;130:150–155. [DOI] [PubMed] [Google Scholar]
- 62. Rasch CR, Hauptmann M, Schornagel J et al. Intra‐arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer 2010;116:2159–2165. [DOI] [PubMed] [Google Scholar]
- 63. Rischin D, Peters LJ, O'Sullivan B et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): A phase III trial of the Trans‐Tasman Radiation Oncology Group. J Clin Oncol 2010;28:2989–2995. [DOI] [PubMed] [Google Scholar]
- 64. Gregoire V, Hamoir M, Chen C et al. Gefitinib plus cisplatin and radiotherapy in previously untreated head and neck squamous cell carcinoma: A phase II, randomized, double‐blind, placebo‐controlled study. Radiother Oncol 2011;100:62–69. [DOI] [PubMed] [Google Scholar]
- 65. Le QT, Kim HE, Schneider CJ et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo‐controlled study. J Clin Oncol 2011;29:2808–2814. [DOI] [PubMed] [Google Scholar]
- 66. Gautam AP, Fernandes DJ, Vidyasagar MS et al. Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients ‐ A triple blinded randomized controlled trial. Radiother Oncol 2012;104:349–354. [DOI] [PubMed] [Google Scholar]
- 67. Antunes HS, Herchenhorn D, Small IA et al. Phase III trial of low‐level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother Oncol 2013;109:297–302. [DOI] [PubMed] [Google Scholar]
- 68. Harrington K, Berrier A, Robinson M et al. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: Rationale for future randomised trials in human papilloma virus‐negative disease. Eur J Cancer 2013;49:1609–1618. [DOI] [PubMed] [Google Scholar]
- 69. Martins RG, Parvathaneni U, Bauman JE et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: A randomized phase II trial. J Clin Oncol 2013;31:1415–1421. [DOI] [PubMed] [Google Scholar]
- 70. Rishi A, Ghoshal S, Verma R et al. Comparison of concomitant boost radiotherapy against concurrent chemoradiation in locally advanced oropharyngeal cancers: A phase III randomised trial. Radiother Oncol 2013;107:317–324. [DOI] [PubMed] [Google Scholar]
- 71. Hitt R, Grau JJ, López‐Pousa A et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 2014;25:216–225. [DOI] [PubMed] [Google Scholar]
- 72. Nguyen‐Tan PF, Zhang Q, Ang KK et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long‐term report of efficacy and toxicity. J Clin Oncol 2014;32:3858–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rodriguez CP, Adelstein DJ, Rybicki LA et al. Randomized phase III study of 2 cisplatin‐based chemoradiation regimens in locally advanced head and neck squamous cell carcinoma: Impact of changing disease epidemiology on contemporary trial design. Head Neck 2015;37:1583–1589. [DOI] [PubMed] [Google Scholar]
- 74. Diaz‐Sanchez RM, Pachón‐Ibáñez J, Marín‐Conde F et al. Double‐blind, randomized pilot study of bioadhesive chlorhexidine gel in the prevention and treatment of mucositis induced by chemoradiotherapy of head and neck cancer. Med Oral Patol Oral Cir Bucal 2015;20:e378–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mesía R, Henke M, Fortin A et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous‐cell carcinoma of the head and neck (CONCERT‐1): A randomised, controlled, open‐label phase 2 trial. Lancet Oncol 2015;16:208–220. [DOI] [PubMed] [Google Scholar]
- 76. Takácsi‐Nagy Z, Hitre E, Remenár É et al. Docetaxel, cisplatin and 5‐fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III‐IV unresectable head and neck cancer: Results of a randomized phase II study. Strahlenther Onkol 2015;191:635–641. [DOI] [PubMed] [Google Scholar]
- 77. Reeves BC, Deeks JJ, Higgins JPT et al. Chapter 13: Including non‐randomised studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from http://www.handbook.cochrane.org. Accessed on April 13, 2017.
- 78. Geeta SN, Padmanabhan TK, Samuel J et al. Comparison of acute toxicities of two chemotherapy schedules for head and neck cancers. J Cancer Res Ther 2006;2:100–104. [DOI] [PubMed] [Google Scholar]
- 79. Ho KF, Swindell R, Brammer CV. Dose intensity comparison between weekly and 3‐weekly cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: A retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol 2008;47:1513–1518. [DOI] [PubMed] [Google Scholar]
- 80. Uygun K, Bilici A, Karagol H et al. The comparison of weekly and three‐weekly cisplatin chemotherapy concurrent with radiotherapy in patients with previously untreated inoperable non‐metastatic squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2009;64:601–605. [DOI] [PubMed] [Google Scholar]
- 81. Kose F, Besen A, Sumbul T et al. Weekly cisplatin versus standard three‐weekly cisplatin in concurrent chemoradiotherapy of head and neck cancer: The Baskent University experience. Asian Pac J Cancer Prev 2011;12:1185–1188. [PubMed] [Google Scholar]
- 82. Geiger JL, Lazim AF, Walsh FJ et al. Adjuvant chemoradiation therapy with high‐dose versus weekly cisplatin for resected, locally‐advanced HPV/p16‐positive and negative head and neck squamous cell carcinoma. Oral Oncol 2014;50:311–318. [DOI] [PubMed] [Google Scholar]
- 83. Kunieda F, Kiyota N, Tahara M et al. Randomized phase II/III trial of post‐operative chemoradiotherapy comparing 3‐weekly cisplatin with weekly cisplatin in high‐risk patients with squamous cell carcinoma of head and neck: Japan Clinical Oncology Group Study (JCOG1008). Jpn J Clin Oncol 2014;44:770–774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.