Abstract
Lessons Learned.
Cobimetinib and duligotuzumab were well tolerated as single agents and in combination with other agents.
The cobimetinib and duligotuzumab combination was associated with increased toxicity, most notably gastrointestinal, and limited efficacy in the patient population tested.
Background.
KRAS‐mutant tumors possess abnormal mitogen‐activated protein kinases (MAPK) pathway signaling, leading to dysregulated cell proliferation. Cobimetinib blocks MAPK signaling. The dual‐action antibody duligotuzumab (MEHD7945A) inhibits ligand binding to both epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 3 (HER3). Blockade of EGFR/HER3 and inhibition of mitogen‐activated protein kinase (MEK) in KRAS‐mutant tumors may provide additive benefit.
Methods.
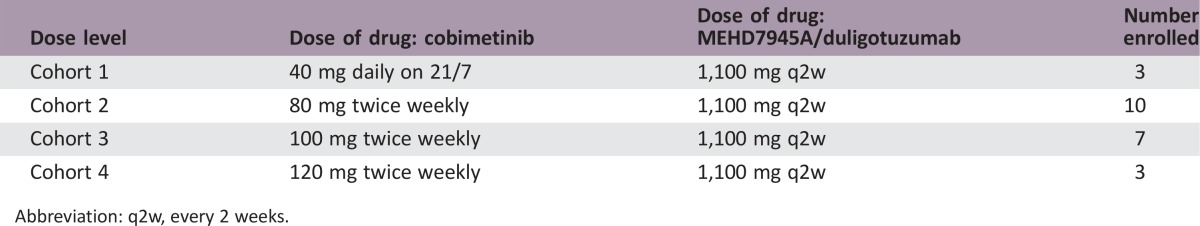
Patients with KRAS‐mutant solid tumors were eligible for this phase Ib dose‐escalation study with a planned expansion phase. Duligotuzumab was given intravenously (IV) at 1,100 mg every 2 weeks (q2w), while cobimetinib was given orally in a standard 3 + 3 design to identify the recommended phase II dose (RP2D). The primary objective was to evaluate the safety and tolerability of this combination.
Results.
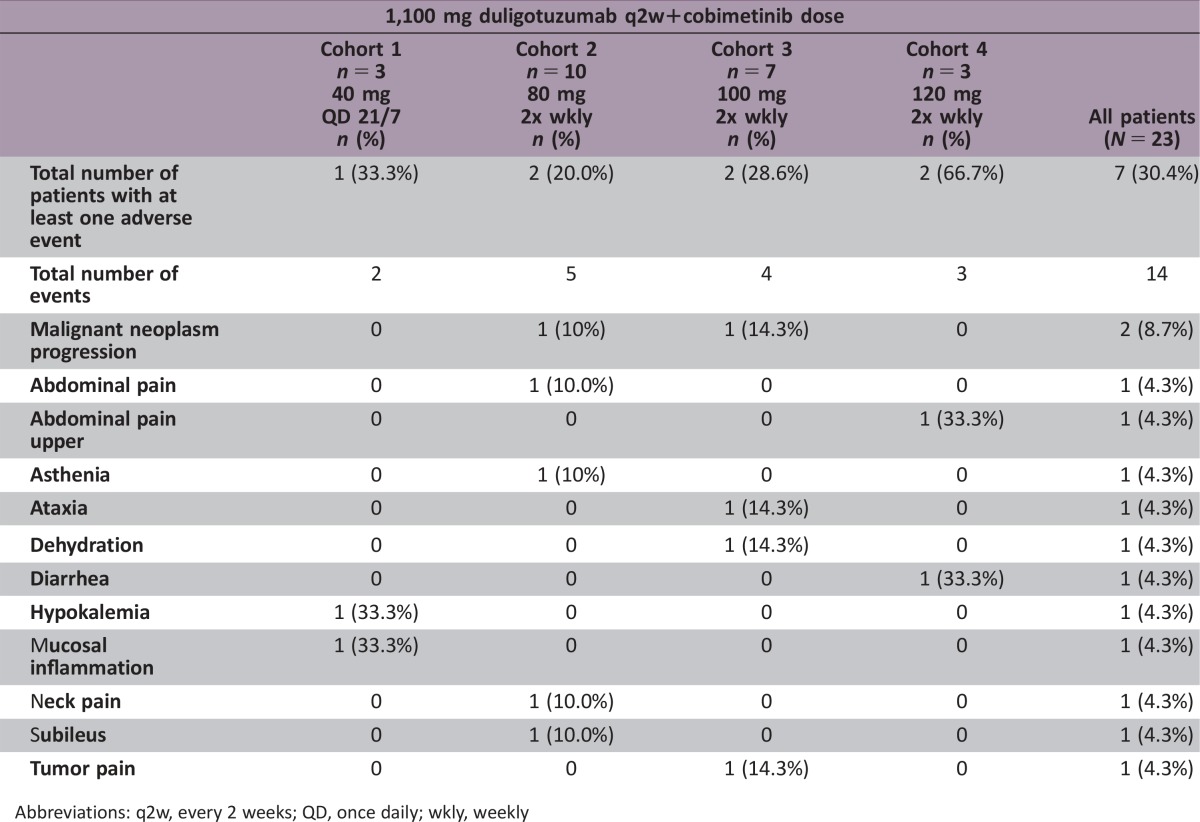
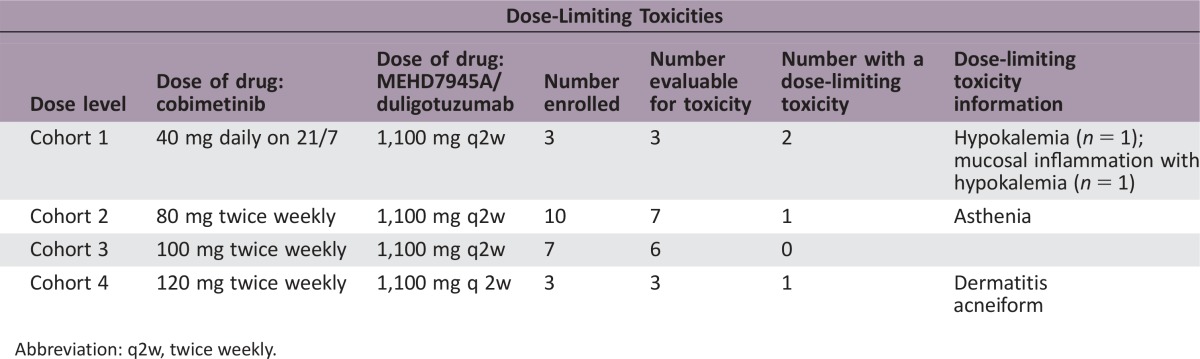
Twenty‐three patients were enrolled. Dose‐limiting toxicities (DLTs) included grade 4 hypokalemia and grade 3 mucosal inflammation, asthenia, and dermatitis acneiform. Seventy percent of patients experienced grade 3 or worse adverse events (AEs). Five (22%) and 12 (52%) patients missed at least 1 dose of duligotuzumab and cobimetinib, respectively, and 9 (39%) patients required a cobimetinib dose reduction. Three (13%) patients discontinued due to an AE. Best response was limited to 9 patients with stable disease and 13 patients with progressive disease.
Conclusion.
Given the limited tolerability and efficacy of this combination, the study did not proceed to expansion stage and closed for enrollment.
Abstract
经验总结
• Cobimetinib和Duligotuzumab单药治疗或与其他药物联合治疗时均耐受良好。
Cobimetinib与Duligotuzumab联用在本试验患者人群中导致毒性增加(尤其是胃肠道毒性), 且疗效有限。
摘要
背景. KRAS突变肿瘤中的丝裂原活化蛋白激酶(MAPK)通路信号传导异常, 从而导致细胞增殖失调。Cobimetinib可阻断MAPK信号传导。双重作用抗体Duligotuzumab(MEHD7945A)可同时抑制配体与表皮生长因子受体(EGFR)和人表皮生长因子受体3(HER3)的结合。在KRAS突变肿瘤中, 阻断EGFR/HER3并抑制丝裂原活化蛋白激酶(MEK)可能会产生叠加获益。
方法. KRAS突变实体瘤患者有资格参加本项Ib期剂量递增研究(包含一个计划扩展期)。Duligotuzumab以1 100 mg的剂量每2周一次(q2w)静脉注射(IV)给药, 而Cobimetinib以标准3 + 3设计口服给药, 据此确定二者的II期推荐剂量(RP2D)。主要研究目的是评估这一联合治疗方案的安全性和耐受性。
结果. 本研究入组了23例患者。剂量限制性毒性(DLT)包括4级低钾血症和3级黏膜炎症、乏力和痤疮样皮炎。70%的患者出现了≥3级不良事件(AE)。分别有5例(22%)和12例(52%)患者漏用至少1剂Duligotuzumab和Cobimetinib, 9例(39%)患者需要降低Cobimetinib剂量。3例(13%)患者因AE而停药。最佳疗效仅限于9例患者疾病稳定, 13例患者疾病进展。
结论. 鉴于该联合治疗方案的耐受性和疗效有限, 研究未继续进入扩展期并终止了患者招募。
Discussion
Dysregulated human epidermal growth receptor (HER)‐family signaling plays an important role in tumorigenesis [1], [2]. The mitogen‐activated protein kinases (MAPK) pathway is activated by mutations in KRAS, NRAS, and BRAF oncogenes, which have been identified in multiple cancers. MAPK pathway inhibition promotes EGFR activation by releasing EGFR from extracellular signal regulated kinases (ERK)‐dependent negative feedback [3], [4], [5], [6] and/or inducing EGFR‐ligand expression [7]. Furthermore, MEK inhibition results in MYC‐dependent transcriptional upregulation of HER3 [8]. Thus, combining duligotuzumab with cobimetinib in RAS‐mutant tumors may overcome resistance observed with either approach alone.
This phase Ib dose escalation trial enrolled 23 patients with KRAS mutant cancers, 65% with metastatic colorectal cancer. DLTs are summarized below. The study was revised to twice weekly (2x wkly) dosing of cobimetinib with the same fixed dose of duligotuzumab, and it was determined that cohort 4 dosing exceeded the maximum tolerated dose (MTD), and cohort 3 dosing was the RP2D.
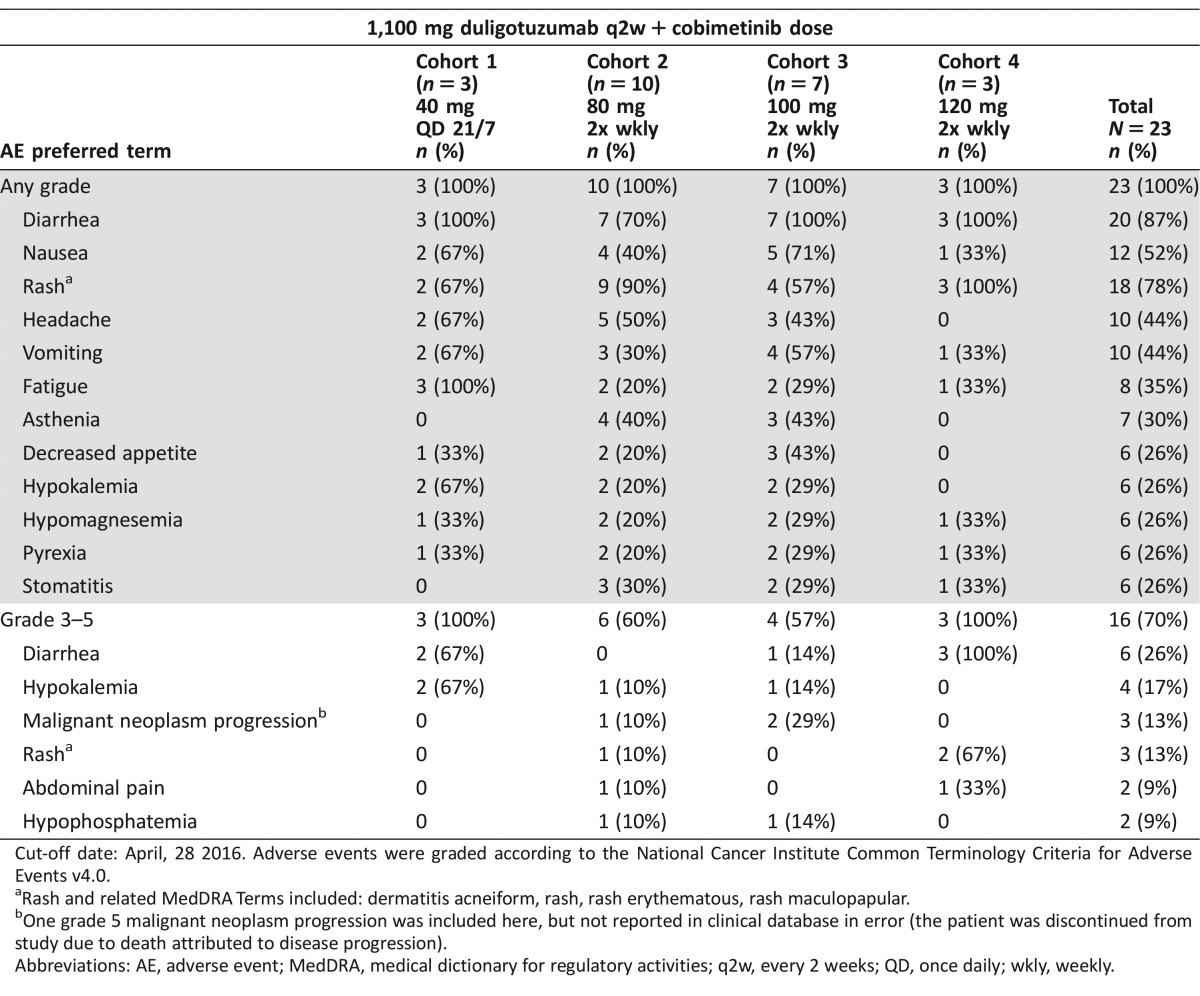
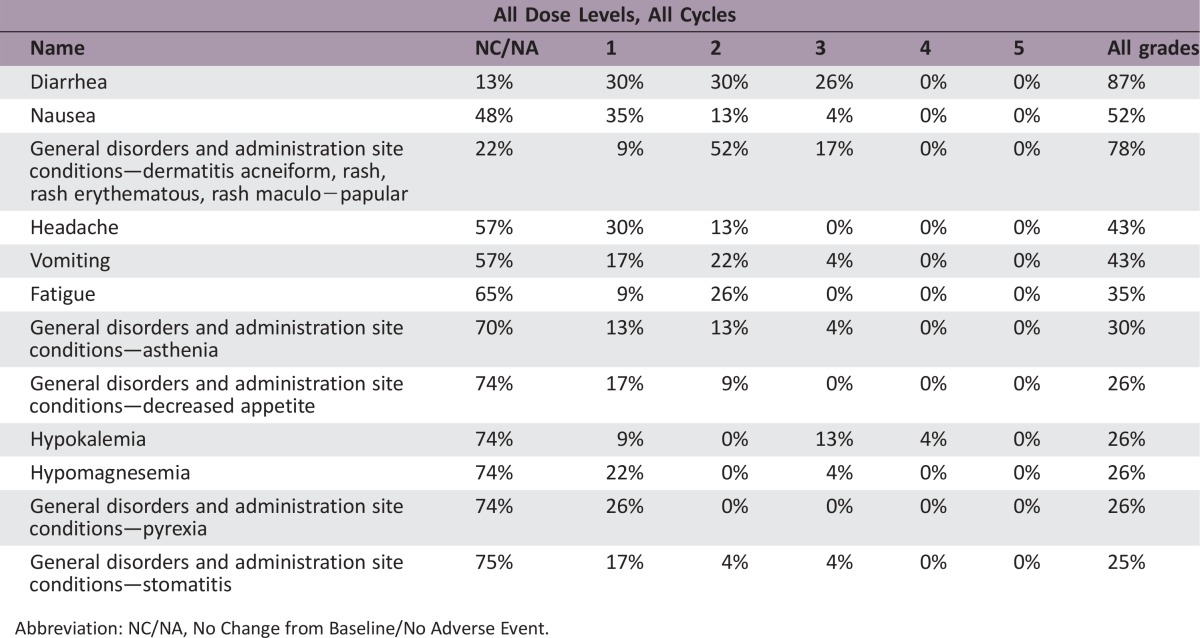
Overall, while most AEs were manageable, combination therapy required frequent dose holds and/or reductions (Table 1). A comparison of the AE profile of the combination of cobimetinib and duligotuzumab versus the previously characterized AE profiles of the respective single agents showed that AEs occurred at a higher frequency (≥10%) versus either single agents [9].
Table 1. All AEs in >25% patients regardless of causality, and all grade 3–4 AEs in ≥ 2 patients.
Cut‐off date: April, 28 2016. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Rash and related MedDRA Terms included: dermatitis acneiform, rash, rash erythematous, rash maculopapular.
One grade 5 malignant neoplasm progression was included here, but not reported in clinical database in error (the patient was discontinued from study due to death attributed to disease progression).
Abbreviations: AE, adverse event; MedDRA, medical dictionary for regulatory activities; q2w, every 2 weeks; QD, once daily; wkly, weekly.
Duligotuzumab and cobimetinib pharmacokinetic (PK) findings were consistent with the PK observed in the respective single‐agent studies, suggesting that there was no interaction.
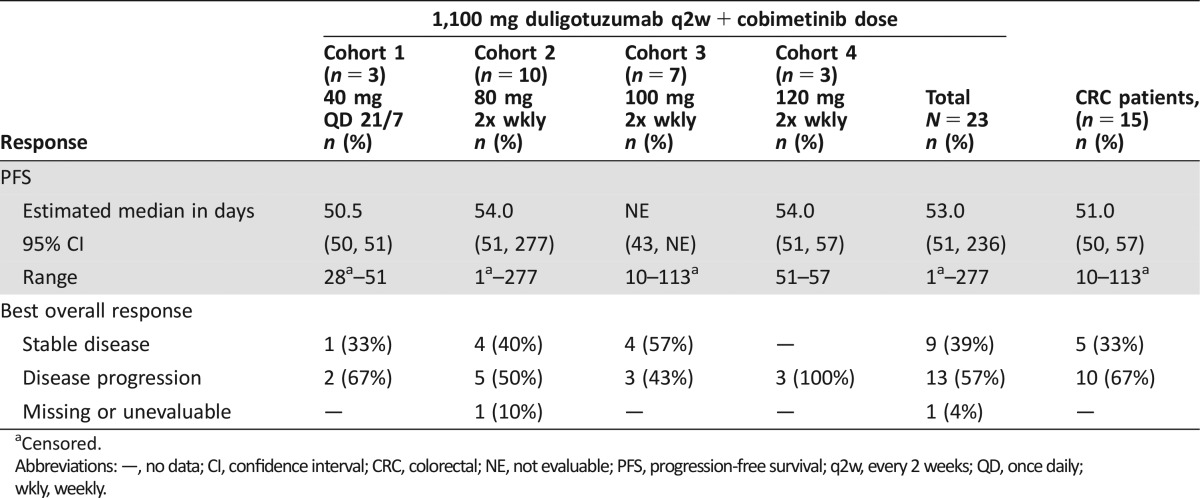
In 23 evaluable patients, the best Response Evaluation Criteria In Solid Tumors (RECIST v1.1) response was stable disease in 9 patients (39%), with 4/9 experiencing stable disease beyond 4 cycles (Table 2). Among 15 patients with colorectal cancer (CRC), 5 had stable disease, with 2/5 experiencing stable disease beyond 4 cycles. Upon evaluation of past treatment history for the 4 patients experiencing stable disease beyond 4 cycles, all patients had atypical extended times on prior systemic therapy when compared with historical average time on those standard therapies, indicating that these may be atypical patients who had more indolent disease.
Table 2. Efficacy summary.
Censored.
Abbreviations: —, no data; CI, confidence interval; CRC, colorectal; NE, not evaluable; PFS, progression‐free survival; q2w, every 2 weeks; QD, once daily; wkly, weekly.
Due to limited efficacy, safety and tolerability of the combination, dose expansion was not pursued, and the combination of duligotuzumab and cobimetinib is no longer being developed in solid tumors.
Trial Information
- Disease
Advanced cancer/solid tumor only
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
More than 2 prior regimens
- Type of Study ‐ 1
Phase I
- Type of Study ‐ 2
Prospective, open‐label, dose finding
- Primary Endpoint
Safety
- Secondary Endpoint
Pharmacodynamic
- Additional Details of Endpoints or Study Design
- Primary Endpoint: Safety and tolerability of duligotuzumab plus cobimetinib
- Primary Endpoint: Identify DLTs, MTD, and RP2D dose and schedule
- Secondary Endpoint: Pharmacokinetics, tumor assessment
- Investigator's Analysis
Level of activity did not meet planned endpoint
Drug Information for Phase I Single Arm
- Drug 1
- Generic/Working name
Cobimetinib
- Trade name
Cotellic
- Company name
Genentech, Inc.
- Drug type
Small molecule
- Drug class
MEK
- Dose
milligrams (mg) per flat dose
- Route
oral (po)
- Drug 2
- Generic/Working name
MEHD7945A/duligotuzumab
- Trade name
n/a
- Company name
Genentech, Inc.
- Drug type
Biological
- Drug class
EGFR
- Dose
milligrams (mg) per flat dose
- Route
IV
Dose‐Escalation Table
Abbreviation: q2w, every 2 weeks.
Patient Characteristics for Phase I Single Arm
- Number of patients, male
15
- Number of patients, female
8
- Stage
n/a
- Age
Median (range): 58 (38–77)
- Number of prior systemic therapies
Median (range): 4 (1–7)
- Performance Status: ECOG
-
0 — 11
1 — 11
2 — 1
3 — 0
unknown — 0
- Cancer Types or Histologic Subtypes
-
Colon 10
Rectum 5
Lung 3
Pancreas 2
Salivary gland 1
Anus 1
Cervix 1
Primary Assessment Method for Phase I Single Arm
- Assessment
- Number of patients enrolled
23
- Number of patients evaluable for toxicity
23
- Number of patients evaluated for efficacy
23
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 0 (0%)
- Response assessment SD
n = 9 (39%)
- Response assessment PD
n = 13 (57%)
- Response assessment OTHER
n = 1 (4%)
- (Median) duration assessments PFS
53 days, confidence interval (CI): 51–236
Phase I Single Arm Adverse Events
Abbreviation: NC/NA, No Change from Baseline/No Adverse Event.
Serious Treatment‐Emergent Adverse Events Regardless of Relationship to Study Treatments by Frequency of Preferred Term, Safety‐Evaluable Patients
Abbreviations: q2w, every 2 weeks; QD, once daily; wkly, weekly
Abbreviation: q2w, twice weekly.
Pharmacokinetics/Pharmacodynamics
- Duligotuzumab and cobimetinib PK consistent with single agent studies suggesting no interaction.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Toxicity
- Investigator's Assessment
Level of activity did not meet planned endpoint
The mitogen‐activated protein kinases (MAPK) signaling pathway is a key intracellular signaling network that regulates cellular proliferation and differentiation. Abnormal activation leads to tumorigenesis by contributing to uncontrolled proliferation, invasion, metastasis and diminished apoptosis. The mitogen‐activated protein kinases, MEK1 and MEK2, are key signaling hubs for inhibition of the MAPK signaling pathway because they directly phosphorylate the extracellular signal regulated kinases, ERK1 and ERK2, which directly translocate into the nucleus to activate multiple transcription factors. The MAPK pathway is activated by mutations in the KRAS, NRAS, and BRAF oncogenes, which have been identified in multiple cancers such as pancreatic adenocarcinomas (90%), colorectal adenocarcinomas (30%–50%), and non‐small cell lung cancers (30%) [10].
The epidermal growth factor receptor (EGFR) family consists of four members: EGFR, human epidermal growth receptor 2 (HER2), HER3, and HER4. Ligand binding induces the formation of homodimers and heterodimers and the activation of the intrinsic kinase activities of these receptors. The two major signaling pathways activated by the human epidermal growth receptor (HER)‐family dimers are the MAPK pathway and the phosphatidylinositol‐4,5‐bisphosphate 3‐kinase/protein kinase B (PI3K/AKT) pathway, which control a variety of cellular processes including cell proliferation, differentiation, and cell survival. Dysregulation of the HER‐family signaling pathways plays an important role in tumorigenesis [1], [11], and two members of the HER family, EGFR (HER1) and HER2, have been successfully targeted for the treatment of cancer of epithelial origin [12], [13], [14]. HER3 is implicated in uncontrolled cell growth and requires heterodimerization with a fully functional kinase in order to signal [12], [15], [16], [17], [18].
The extensive crosstalk among the HER‐family receptors likely contributes to emerging reports that blockade of a particular signaling pathway can lead to activation of compensatory mechanisms such as negative feedback loops and, consequently, upregulates parallel pathways [2], [19]. These findings led to the hypothesis that inhibiting the signaling potential of more than one of the HER‐family receptors may offer an opportunity for superior efficacy as well as potentially overcoming resistance to currently available EGFR and other directed therapies.
Activation of alternative pathways has been observed following MEK inhibition, suggesting that blocking upstream receptor tyrosine kinases when inhibiting downstream effectors of oncogenic RAS may be more effective as treatment for RAS mutant tumors. Inhibition of the MAPK pathway can promote EGFR activation and resultant signaling via the PI3K pathway by releasing EGFR from ERK‐dependent negative feedback [3], [4], [5], [6], and/or inducing EGFR‐ligand expression [7]. Given these observations, blockade of EGFR/HER3 during inhibition of downstream signaling modulators of the MAPK pathway in RAS‐mutant tumors may increase the responsiveness of such tumors and overcome or prevent the resistance observed with either approach alone.
Cobimetinib is a reversible selective inhibitor of MEK1 and MEK2 that is approved in combination with vemurafenib for the treatment of advanced BRAF mutated melanoma. Cobimetinib has been studied in over 1,000 cancer patients and generally is well tolerated. The main adverse events (AEs) were diarrhea, fatigue, rash, nausea, vomiting, and peripheral edema.
Duligotuzumab is a humanized monoclonal dual specific antibody that targets both HER3 and EGFR and inhibiting ligand binding to either receptor. Preclinical studies show that duligotuzumab prevents HER3 and EGFR receptor activation leading to inhibition of downstream signaling of AKT and ERK. Duligotuzumab has been studied in four other clinical trials in phase I and II, either as a single agent or in combination with chemotherapy. Common adverse events (>20%) associated with duligotuzumab monotherapy were rash, headache, diarrhea, pyrexia, nausea and vomiting, decreased appetite, paronychia, chills, dry skin, and fatigue.
In preclinical studies, the combination of duligotuzumab and cobimetinib enhanced inhibition of activation of ERK1 and ERK2 and AKT in various cancer cell lines and inhibited tumor growth in two CRC xenograft models.
This phase Ib study aimed to study the combination of cobimetinib and duligotuzumab in a variety of KRAS mutant tumors and had two components: a dose escalation and dose expansion stage. In the dose escalation stage, 23 patients were enrolled (table of patient characteristics). The dose escalation stage initially consisted of a fixed dose of duligotuzumab with three cohorts of cobimetinib at 40 mg daily on a 21‐day out of a 28‐day cycle (21/7), 60 mg daily on 21/7, and 80 mg daily on 21/7. At the first dose level (Cohort 1), all 3 patients experienced gastrointestinal toxicity, with 2/3 patients experiencing dose‐limiting toxicities (DLTs) of hypokalemia associated with diarrhea, and thus the maximum tolerated dose (MTD) on the 21/7 schedule was exceeded at the lowest dose level. The protocol was modified where duligotuzumab was given at the optimal fixed dose of 1,100 mg in conjunction with 3 subsequent dosing schedules of cobimetinib 80–120 mg twice weekly.
Ten patients were enrolled in Cohort 2 (80 mg twice weekly) with 1/7 DLT of asthenia. All 10 patients experienced an AE, and 70% experienced diarrhea. Seven patients were enrolled in Cohort 3 (100 mg twice weekly) with no DLTs, but all patients experienced diarrhea, with 1 patient experiencing grade ≥3 diarrhea. Three patients were enrolled in Cohort 4 (120 mg twice weekly), with 1 DLT of dermatitis acneiform and all patients experiencing grade ≥3 diarrhea. It was determined that Cohort 4 exceeded the MTD and the recommended phase II dose (RP2D) was determined to be 1,100 mg intravenous (IV) duligotuzumab every 2 weeks in combination with 100 mg cobimetinib orally twice weekly.
Five (22%) and 12 (52%) patients missed at least 1 dose of duligotuzumab and cobimetinib, respectively, and 9 (39%) patients required a cobimetinib dose reduction. Three (13%) patients discontinued due to an AE.
Among the 23 patients treated in the dose escalation stage, the best overall response was stable disease in 9 (39%) patients, disease progression in 13 (57%) patients, and unevaluable in 1 (4%) patients. Fifteen of the 23 patients had metastatic colorectal cancer. Among those patients, 5 (33%) had best response of stable disease and 10 (67%) had disease progression. None of the patients experienced a partial response. Time on study treatment was median 6.4 weeks (range 0–36.4). Evaluation of past cancer treatment history for the 4 patients experiencing stable disease beyond 4 cycles suggested these may be atypical patients who had more indolent disease.
Pharmacokinetic studies showed no interaction between cobimetinib and duligotuzumab. Cobimetinib pharmacokinetics were highly variable; the average exposure (area under the curve) was similar in Cohorts 1–3 but was higher than expected in Cohort 4 at Cycle 1, Day 15, though driven by high concentrations in 1 patient.
Because of the generally poor tolerability of the combination and limited efficacy seen in this limited patient population, the dose expansion was not pursued and the study was halted.
Acknowledgments
The authors thank the patients and investigators who participated in this study. Writing assistance provided by Genentech, Inc.
Footnotes
ClinicalTrials.gov Identifier: NCT01986166
Sponsor(s): Genentech, Inc.
Principal Investigator: Christopher Lieu
IRB Approved: Yes
Disclosures
Jodran Berlin: Genentech/Roche (C/A, H, RF); Andrew Ko: Seattle Genetics, New Beta Innovations (C/A), Roche/Genentech, Merrimack, Merck, BMS, Halozyme, Aduro Biotech, Prism Bio Ltd, Abgenomics (RF); Andres Cervantes: Roche (C/A, H), Roche, Genentech (RF); Patricia LoRusso: Genentech (C/A, RF); Gail Eckhardt: Genentech (RF); Amy Kapp: Genentech, Inc. (E), Roche (O/I); Bruce McCall: Genentech/Roche (E, OI); Andrea Pirzkall: Genentech/Roche (OI); Anne Uyei: Genentech (E), Roche (OI); Josep Tabernero: Amgen, Bayer, Boehringer Ingelheim, Celgene, Chugai, Genentech, Lilly, MSD, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Symphogen, Taiho, Takeda (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Sergina NV, Moasser MM. The HER family and cancer: Emerging molecular mechanisms and therapeutic targets. Trends Mol Med 2007;13:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carracedo A, Ma L, Teruya‐Feldstein J et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K‐dependent feedback loop in human cancer. J Clin Invest 2008;118:3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turke AB, Song Y, Costa C et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res 2012;72:3228–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mirzoeva OK, Das D, Heiser LM et al. Basal subtype and MAPK/ERK kinase (MEK)‐phosphoinositide 3‐kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res 2009;69:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diep CH, Munoz RM, Choudhary A et al. Synergistic effect between erlotinib and MEK inhibitors in KRAS wild‐type human pancreatic cancer cells. Clin Cancer Res 2011;17:2744–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young A, Lou D, McCormick F. Oncogenic and wild‐type Ras play divergent roles in the regulation of mitogen‐activated protein kinase signaling. Cancer Discov 2013;3:112–123. [DOI] [PubMed] [Google Scholar]
- 7. Sunaga N, Kaira K, Imai H et al. Oncogenic KRAS‐induced epiregulin overexpression contributes to aggressive phenotype and is a promising therapeutic target in non‐small‐cell lung cancer. Oncogene 2013;32:4034–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun C, Hobor S, Bertotti A et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transciptional induction of ERBB3. Cell Rep 2014;7:86–93. [DOI] [PubMed] [Google Scholar]
- 9. Fayette J, Wirth L, Oprean C et al. Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study). Front Oncol 2016;6:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson L, Mercer K, Greenbaum D et al. Somatic activation of the K‐ras oncogene causes early onset lung cancer in mice. Nature 2001;410:1111–1116. [DOI] [PubMed] [Google Scholar]
- 11. Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 2009;21:177–184. [DOI] [PubMed] [Google Scholar]
- 12. Holbro T, Civenni, G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res 2003;284:99–110. [DOI] [PubMed] [Google Scholar]
- 13. Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene 2007;26:6577–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160–1174. [DOI] [PubMed] [Google Scholar]
- 15. Riese DJ 2nd, van Raaij TM, Plowman GD et al. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol 1995;15:5770–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinkas‐Kramarski R, Soussan L, Waterman H et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 17. Jura N, Shan Y, Cao X et al. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci USA 2009;106:21608–21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi F, Telesco SE, Liu Y et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 2010;107:7692–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sergina NV, Rausch M, Wang D et al. Escape from HER‐family tyrosine kinase inhibitor therapy by the kinase‐inactive HER3. Nature 2007;445:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]