The aim of this study was to determine aspects of health‐related quality of life and clinicopathological characteristics before starting chemotherapy for platinum‐resistant/refractory ovarian cancer that were associated with early cessation of chemotherapy, survival time, and death within 30 days of chemotherapy.
Keywords: Patient‐reported outcomes, Prognosis, Platinum‐resistant ovarian cancer, Quality of life
Abstract
Background.
Clinicians and patients often overestimate the benefits of chemotherapy, and overall survival (OS), in platinum resistant/refractory ovarian cancer (PRROC). This study sought to determine aspects of health‐related quality of life and clinicopathological characteristics before starting chemotherapy that were associated with stopping chemotherapy early, shortened survival, and death within 30 days of chemotherapy.
Materials and Methods.
This study enrolled women with PRROC before starting palliative chemotherapy. Health‐related quality of life was measured with EORTC QLQ‐C30/QLQ‐OV28. Chemotherapy stopped within 8 weeks of starting was defined as stopping early. Logistic regression was used to assess univariable and multivariable associations with stopping chemotherapy early and death within 30 days of chemotherapy; Cox proportional hazards regression was used to assess associations with progression‐free and OS.
Results.
Low baseline global health status (GHS), role function (RF), physical function (PF), and high abdominal/gastrointestinal symptom (AGIS) were associated with stopping chemotherapy early (all p < .007); low PF and RF remained significant after adjusting for clinicopathological factors (both p < .0401). Most who stopped chemotherapy early had Eastern Cooperative Oncology Group Performance Score 0–1 at baseline (79%); PF, RF, and GHS remained independently significant predictors of stopping chemotherapy early in this subgroup. Death within 30 days of chemotherapy occurred in 14%. Low GHS, RF, and PF remained significantly associated with death within 30 days of chemotherapy after adjusting for clinicopathological factors (all p < .012).
Conclusion.
Women with low GHS, RF, or PF before starting chemotherapy were more likely to stop chemotherapy early, with short OS. Self‐ratings of GHS, RF, and PF could improve patient‐clinician communication regarding prognosis and help decision‐making in women considering chemotherapy for PRROC.
Implications for Practice.
Measuring aspects of health‐related quality of life when considering further chemotherapy in platinum resistant/refractory ovarian cancer (PRROC) could help identify women with a particularly poor prognosis who are unlikely to benefit from chemotherapy and could therefore be spared unnecessary treatment and toxicity in their last months of life. Self‐ratings of global health status, role function, and physical function could improve patient‐clinician communication regarding prognosis and help decision‐making in women considering chemotherapy for PRROC.
Introduction
Ovarian cancer is the leading cause of death in women with gynecological cancers in the Western world [1]. The 5‐year survival rate for women with ovarian cancer in the U.S. is 45% [2]. Most women with advanced ovarian cancer initially respond to platinum based chemotherapy, but the majority relapse, with a median overall survival (OS) time after recurrence of 2 years [3], [4], [5], [6]. Duration of survival and clinical benefit of further treatment are associated with multiple factors, including response to initial treatment, number of lines of prior chemotherapy, and time to progression after chemotherapy [7]. Most women with recurrent ovarian cancer are offered further chemotherapy aimed at palliating disease‐related symptoms, maintaining or improving quality of life, delaying time to progression, and prolonging survival. Women who progress within 6 months of completing chemotherapy are classified as “platinum resistant,” have a median survival of 12 months, and response rates to second line chemotherapy of 10%–30% [8], [9], [10], [11], [12]. Women who progress during chemotherapy, or within 4 weeks of their last dose, are classified as “platinum refractory,” have a median survival of 3–5 months, and response rates to chemotherapy of <10% [13]. Ultimately, most women with advanced ovarian cancer develop disease that is platinum resistant/refractory. A major challenge for clinicians is the identification of patients with a short survival time who are unlikely to benefit from further chemotherapy, and for whom palliative care without chemotherapy would be a better option.
There is significant heterogeneity among women meeting the definition for platinum resistant/refractory ovarian cancer (PRROC). This heterogeneity is reflected in highly variable rates of tumor response, disease control, progression free survival (PFS) at 6 months, and OS times reported in over 5,000 women with PRROC treated in phase 2 and 3 clinical trials over the last 15 years [14]. This variability may be partly explained by differences in both the criteria used to categorize ovarian cancer as being platinum resistant, refractory, or sensitive, and the eligibility criteria in individual clinical trials, particularly the number of lines of prior chemotherapy.
Historically, the concepts of, and definitions for, platinum resistant and refractory were developed to help predict the likelihood of response to second‐line chemotherapy in patients with recurrent disease [11], [15]. However, over time, these definitions have been more generally applied to all patients with recurrent disease, including those who progress after multiple lines of therapy, not just after first‐line chemotherapy. The methods for diagnosing and defining recurrence are also variable, and include rising serum CA125 alone, symptoms, tumor progression on conventional imaging, or progression on PET imaging. Furthermore, clinical trial reports rarely distinguish women with primary platinum resistance after first‐line treatment from those with secondary resistance after two or more lines of platinum‐based chemotherapy. Clinical trial reports rarely document the proportions of women with recurrences that are symptomatic or asymptomatic. Given the short PFS and OS times of many women with PRROC, there is a need to better identify the subset who are unlikely to benefit from further chemotherapy, in keeping with the ASCO Choosing Wisely recommendations for advanced solid tumors [16]. Chemotherapy within the last 30 days of life is generally accepted to be undesirable, but there is little guidance to help clinicians identify patients likely to die within 30 days. There is evidence that many patients want information about their likely disease trajectory and the potential benefits of further chemotherapy [17]. This can help patients make more informed decisions and receive care that is better aligned with their goals and preferences.
Health‐related quality of life (HRQL) has long been recognized as an important consideration in cancer treatment and a key patient‐rated outcome (PRO) for cancer clinical trials [18]. Multiple studies and systematic reviews have demonstrated overwhelming evidence that HRQL is a powerful prognostic factor in a range of advanced cancers [19], [20], [21], [22], [23], [24], yet its application in clinical decision‐making seems limited.
The aim of this study was to determine aspects of HRQL and clinicopathological characteristics before starting chemotherapy for PRROC that were associated with early cessation of chemotherapy, survival time, and death within 30 days of chemotherapy.
Materials and Methods
The Gynecologic Cancer InterGroup Symptom Benefit Study (GCIG SBS) is a prospective, observational, cohort study, led by ANZGOG and the GCIG Symptom Benefit Committee and coordinated by the NHMRC Clinical Trials Centre, that enrolled women from collaborating GCIG clinical trials groups in 11 countries. The target population was women about to start chemotherapy for PRROC that had progressed on or within 6 months of platinum‐based chemotherapy. Key eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–3, ability to complete HRQL questionnaires independently, and a life expectancy of at least 3 months.
The type, schedule, and duration of chemotherapy, and all aspects of supportive care, were at the treating physician's discretion according to their usual institutional practice.
The trial was registered on the Australian New Zealand Clinical Trials Registry (ANZCTR: 12607000603415). The study was performed in accordance to the NHMRC Statement on Ethical Conduct in Research Involving Humans and the Declaration of Helsinki. Ethical approval was obtained at all participating sites and all participants provided signed, written, informed consent.
Assessments by Treating Physicians
Treating physicians assessed participants at baseline and before each cycle of chemotherapy. They recorded baseline characteristics, including the presence of (a) symptoms (ascites and cramping abdominal pain or intermittent/incomplete bowel obstruction); (b) platinum resistant or refractory disease; and (c) laboratory test values including serum levels of hemoglobin, LDH, CA125, CA125 velocity (difference between two most recent CA125 levels before commencement of chemotherapy divided by number of days between measurements), C‐reactive protein (CRP), albumin, platelets, neutrophils, and lymphocytes. Tumor response was assessed every 6–8 weeks using the same method of assessment throughout the study, at the discretion of the treating physician, and response was recorded according to CA125, Response Evaluation Criteria in Solid Tumors (RECIST), and/or clinical assessment.
Patient‐Rated Outcomes
Participants completed HRQL questionnaires at baseline (within 2 weeks before their first cycle of chemotherapy), and then every 3–4 weeks before each subsequent cycle of chemotherapy, until disease progression. The questionnaires at baseline included the European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ C‐30) and Ovarian Module (OV28). The QLQ‐C30 includes multi‐item scales for global health status and five functional domains (physical, role, cognitive, emotional, and social) all scored from 0 (worst) to 100 (best) and six single items for symptoms scored from 0 (least) to 100 (worst). The OV28 includes seven multi‐item scales for abdominal/gastrointestinal symptoms (AGIS), peripheral neuropathy, hormonal, body image, attitude to disease/treatment, chemotherapy side effects, and sexuality rated from 0 (worst) to 100 (least).
Statistical Analysis
Cut points for dichotomizing the QLQ‐C30 subscales were as recommended by Diouf et al. [25]. Cut‐points for the OV28 AGIS were the lower and upper quartiles from a previous study in platinum resistant ovarian cancer [26]. Sensitivity analyses included the minimum p value approach to selecting optimal cut points based on our own data [27]. Associations between dichotomized HRQL baseline scores (low global health status [GHS] <50/100, low role function [RF] <67/100, low physical function [PF] <58/100, high AGIS >44) with stopping chemotherapy early, OS, and death within 30 days of chemotherapy were assessed. Overall survival and progression‐free survival were analyzed using time‐to‐event methods. A multivariable Cox model was developed to determine independent predictors of OS. Candidate variables for multivariable analyses were those with p < .05 in univariable analysis, and were selected for inclusion in the final model using backward elimination. Selected clinical variables were used in all adjusted analyses.
Logistic regression was used to assess univariable and multivariable associations of HRQL with stopping chemotherapy early and death within 30 days of chemotherapy. Cox proportional hazards regression models were used to assess univariable and multivariable associations of HRQL with OS. Kaplan‐Meier curves of survival by HRQL category were constructed.
Results
These analyses are based on the 545 of 570 participants with PRROC in the GCIG SBS who completed baseline HRQL questionnaires and who were recruited from 100 sites in 11 countries. Baseline characteristics are summarized in Table 1. The median age was 63 years (range 23–89) and the median number of previous lines of chemotherapy was 2 (range 1–8). Almost all participants had a good performance status (ECOG 0 or 1, 87%). The median PFS was 3.6 months (95% confidence interval [CI] 3.0–3.9) and median OS was 11.1 months (95% CI 9.4–12.0).
Table 1. Baseline characteristics of patients who had baseline health‐related quality of life questionnaire completion.
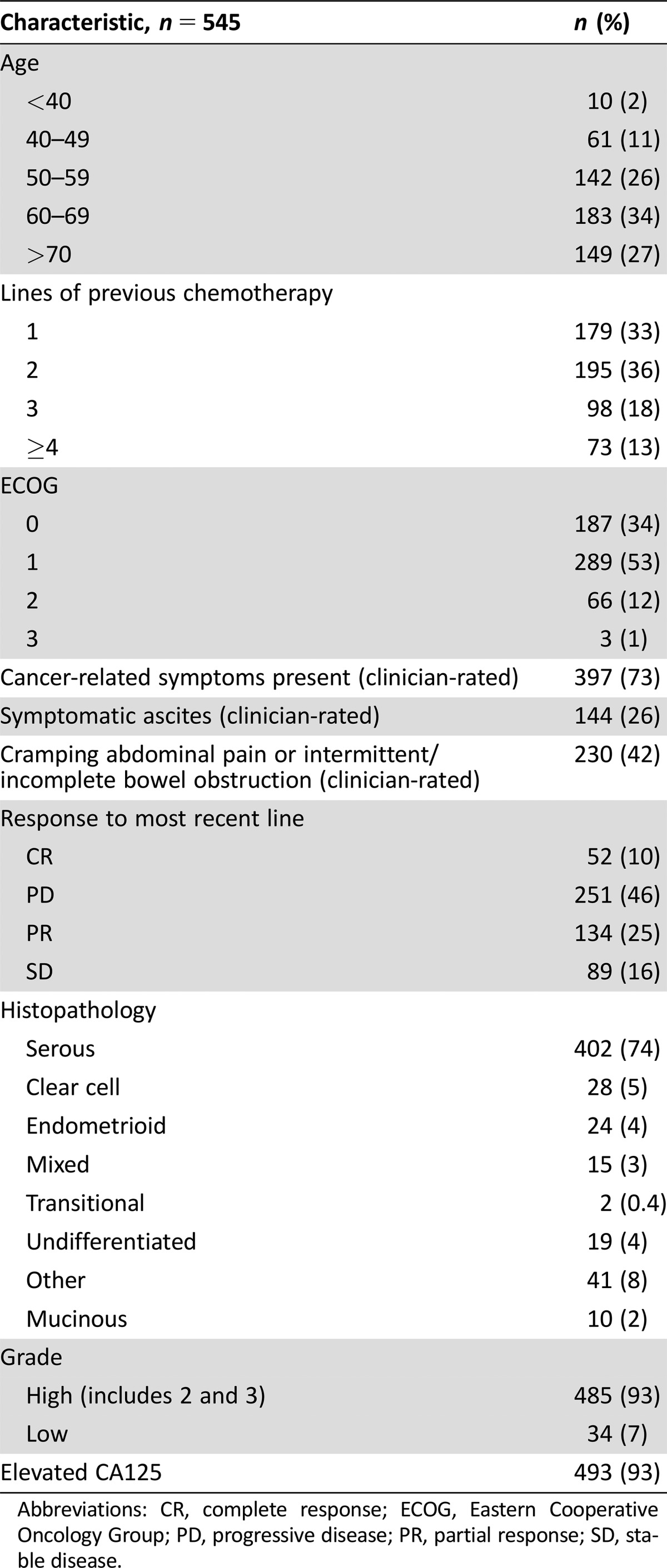
Abbreviations: CR, complete response; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease; PR, partial response; SD, stable disease.
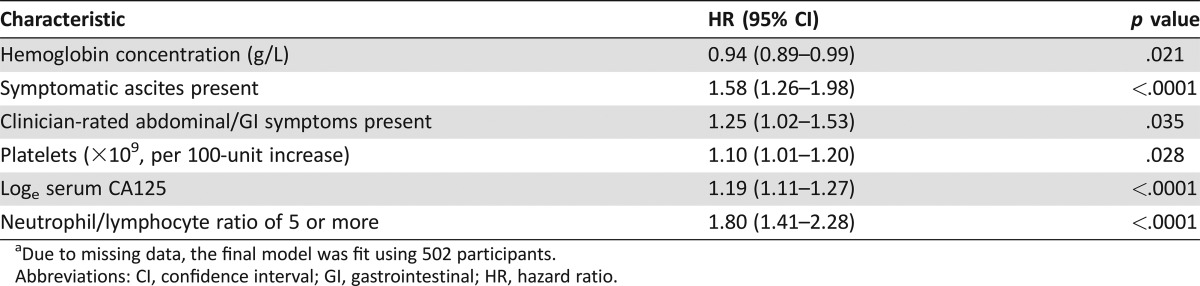
Clinicopathological characteristics independently associated with OS in a multivariable analysis are shown in Table 2. The presence of ascites, loge serum CA125 concentration, and a neutrophil:lymphocyte ratio of 5 or more were strongly associated with OS; platelet count, the presence of abdominal symptoms according to the clinician, and hemoglobin concentration had weaker associations with OS.
Table 2. Clinicopathological characteristics at baseline associated with overall survival in multivariable analysis (n = 502)a.
Due to missing data, the final model was fit using 502 participants.
Abbreviations: CI, confidence interval; GI, gastrointestinal; HR, hazard ratio.
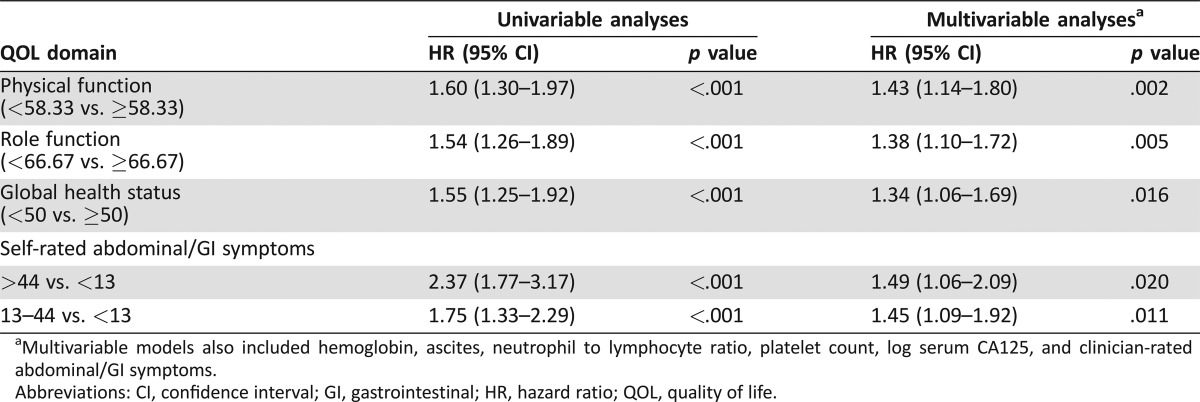
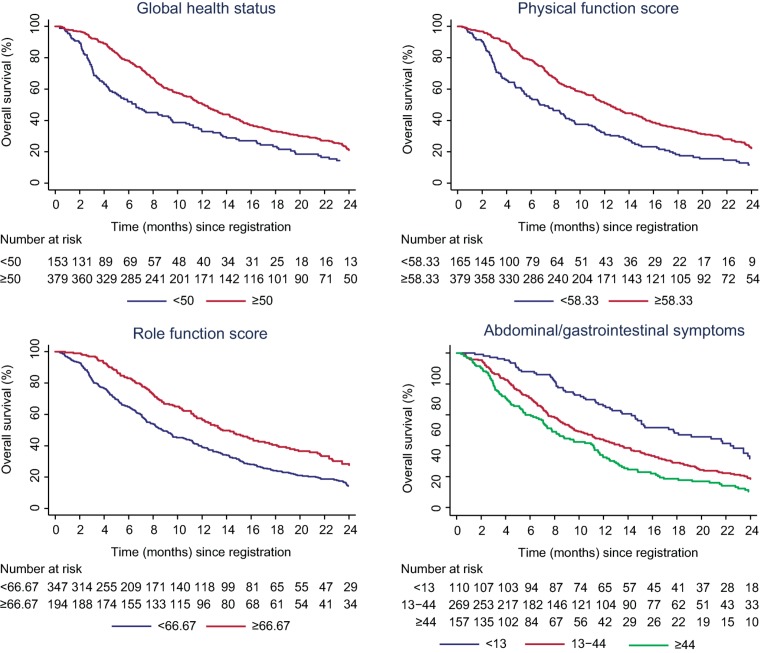
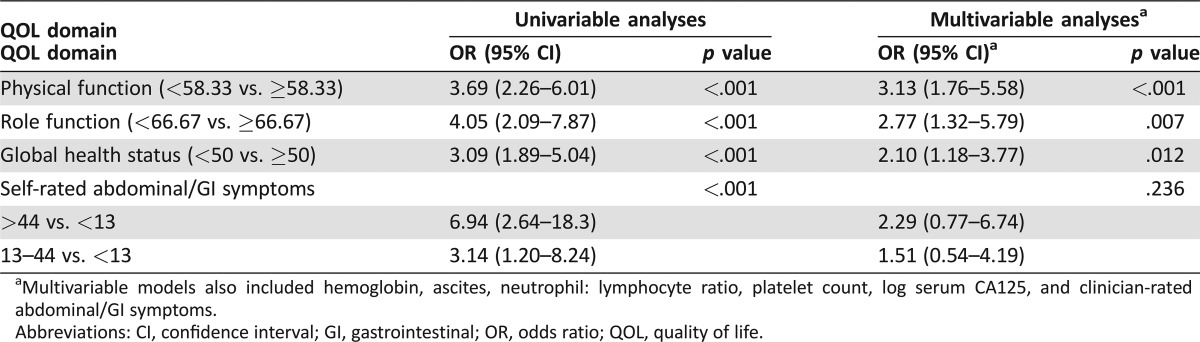
Univariable analyses of baseline HRQL (n = 545) showed that PF, RF, GHS, and AGIS were significantly associated with OS (all p < .001; Table 3).
Table 3. Univariable and multivariable analysis of baseline health‐related quality of life domains as predictors of overall survival (n = 545).
Multivariable models also included hemoglobin, ascites, neutrophil to lymphocyte ratio, platelet count, log serum CA125, and clinician‐rated abdominal/GI symptoms.
Abbreviations: CI, confidence interval; GI, gastrointestinal; HR, hazard ratio; QOL, quality of life.
Physical function, RF, GHS, and AGIS remained independently significant predictors of OS in a multivariable model that also accounted for the clinicopathological factors (Table 3).
Both PF and GHS negatively correlated with ECOG PS but the association was moderate (Spearman's rank‐correlation coefficient, −0.37, p < .001; −0.32, p < .001, respectively; Fig. 2). Baseline PF, RF and AGIS remained significant independent predictors of OS in a multivariable analysis adjusted for clinicopathological factors in the 87% of participants with good PS (ECOG 0–1), that is, after excluding participants with poor PS (ECOG 2–3).
Figure 2.
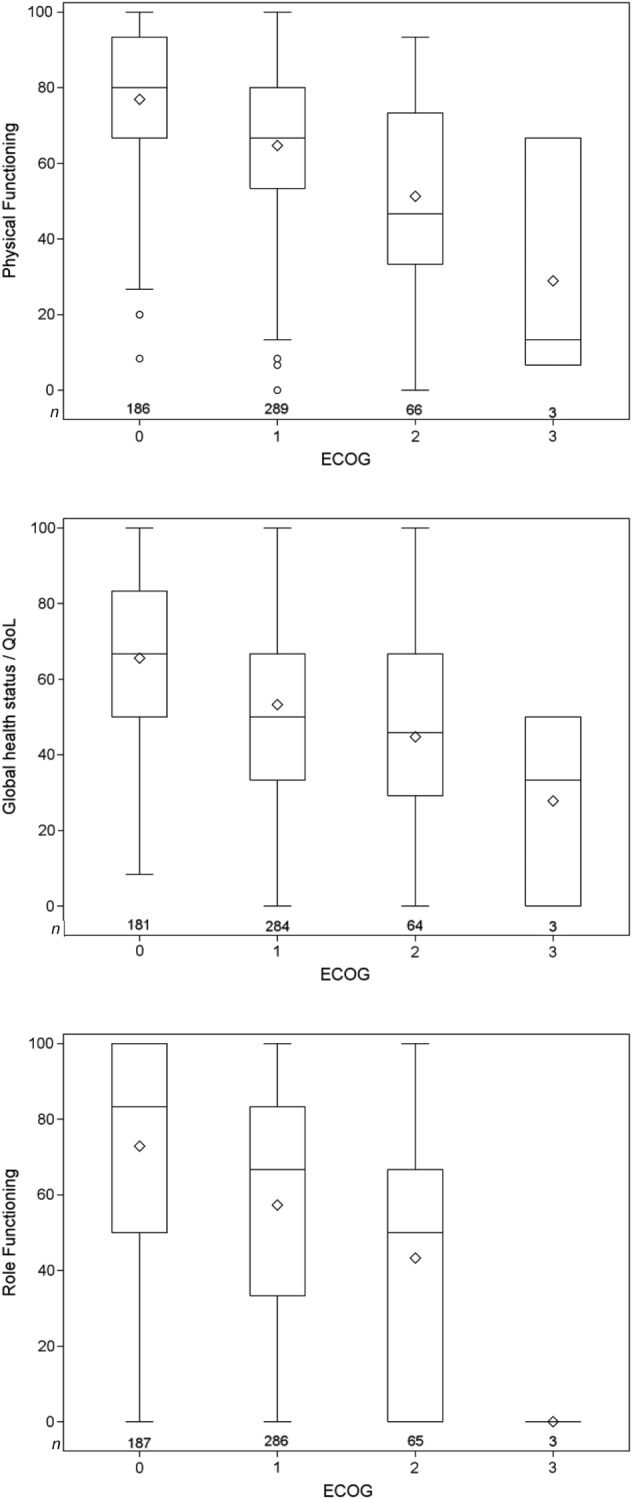
Distribution of health‐related quality of life by ECOG performance status.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; QOL, quality of life.
Figure 1.
Kaplan‐Meier curves for overall survival by quality of life domain.
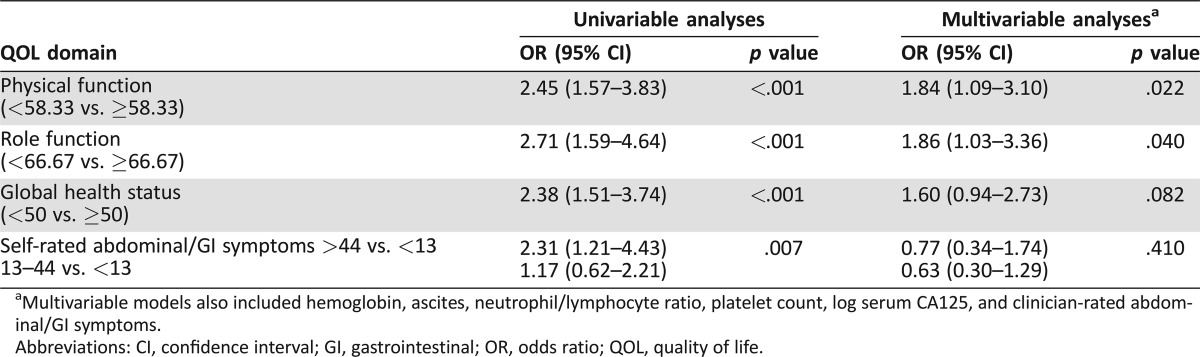
One hundred and ten out of five hundred seventy (19%) women stopped chemotherapy early (within 8 weeks of starting). The reported reasons for stopping chemotherapy (percentage of all stopping) were (a) disease progression (46%), (b) patient preference (12%), (c) adverse events (7%), (d) clinician preference (6%), (e) other (11%), and (f) death (18%). Median PFS in women stopping chemotherapy early was 1.2 months (95% CI 1.0–1.4) and median OS was 2.9 months (95% CI 2.5–4.2). Low GHS, RF, PF, and high AGIS were all significantly associated with stopping chemotherapy early (all p < .007; Table 4). After adjusting for clinicopathological factors, PF and RF remained significant predictors of stopping chemotherapy early (both p < .0401; Table 4).
Table 4. Univariable and multivariable analysis of health‐related quality of life as predictors of early stopping of chemotherapy within 8 weeks (n = 545).
Multivariable models also included hemoglobin, ascites, neutrophil/lymphocyte ratio, platelet count, log serum CA125, and clinician‐rated abdominal/GI symptoms.
Abbreviations: CI, confidence interval; GI, gastrointestinal; OR, odds ratio; QOL, quality of life.
Most of the participants who stopped chemotherapy early had good PS at baseline (ECOG 0 or 1 in 87/110, 79%). Physical function, RF, and GHS, but not AGIS, remained significantly associated with stopping chemotherapy early in analyses confined to women with good PS (ECOG 0 or 1, 87% of participants), that is, excluding those with poor PS (ECOG 2 or 3, 13% of participants).
Eighty‐two (14%) participants died within 30 days of chemotherapy. Low GHS, PF, RF, and AGIS at baseline were all significantly associated with dying within 30 days of chemotherapy (all p < .0001; Table 5). After adjusting for clinicopathological factors, low GHS, PF, and RF, but not AGIS, remained significant predictors of death within 30 days of chemotherapy (all p < .012; Table 5).
Table 5. Univariable and multivariable analysis of health‐related quality of life as predictors of death within 30 days of chemotherapy (n = 545).
Multivariable models also included hemoglobin, ascites, neutrophil: lymphocyte ratio, platelet count, log serum CA125, and clinician‐rated abdominal/GI symptoms.
Abbreviations: CI, confidence interval; GI, gastrointestinal; OR, odds ratio; QOL, quality of life.
Sixty‐five percent of participants (357/549) did not receive the predicted number of cycles of chemotherapy as recorded by the clinician before starting chemotherapy. The median number of cycles expected by clinicians was 6, which would equate to 18–24 weeks of treatment depending on a 3–4 weekly cycle. The median treatment duration was 13.9 weeks.
Clinicians’ assessments of ascites and abdominal/GI symptoms were highly correlated with the patients’ ratings of AGIS. There was strong evidence that mean patient‐rated AGIS was higher in patients in which the clinician reported AGIS. Mean score was 42 in 227 patients in which the clinician did report the symptoms and 27 in 309 patients in which the clinician did not report the symptoms (difference in means 15, 95% CI 11–19, p < .001). Patient‐rated AGIS at baseline remained a statistically significant predictor of OS in sensitivity analyses accounting for physicians’ ratings of symptomatic ascites, but not physicians’ ratings of abdominal/GI symptoms (AGIS >44, hazard ratio (HR) 1.59, 95% CI 1.15–2.21, p = .005; and AGIS 13–44, HR 1.49, 95% CI 1.12–1.97, p = .006).
Sensitivity analyses using the minimum p value approach [27] for defining optimal cut‐points resulted in similar cut‐points to those recommended by Diouf et al. [25] with similar results and conclusions, supporting the validity of these cut‐points in this population.
Discussion
Almost 20% of patients with PRROC stopped chemotherapy within 8 weeks and had a median OS of 2.9 (2.5–4.2) months. The majority of patients who stopped chemotherapy early were reported to have good ECOG PS (ECOG 0 or 1) before starting chemotherapy, and 53% of them were expected to receive six or more cycles of chemotherapy. Low GHS, RF, PF, and high AGIS were all significantly associated with stopping chemotherapy early in univariable analysis (all p < .008); low RF and PF remained independently significant predictors of stopping chemotherapy early after accounting for clinicopathological factors. Low GHS, RF, PF, and high AGIS were also significant predictors of shorter OS time in both univariable and multivariable analyses. Low GHS, RF, and PF were also significant predictors of death within 30 days of chemotherapy. Almost two thirds of participants with PRROC did not have the expected duration of treatment predicted by their treating clinicians before starting chemotherapy.
Baseline hemoglobin concentration, ascites, neutrophil:lymphocyte ratio of 5 or more, platelet count, serum CA125, and the clinician‐rated presence of abdominal/GI symptoms were independent predictors of OS, as were participants’ self‐ratings of PF, RF, GHS, and AGIS. These variables remained significant predictors of OS in analyses restricted to participants with good performance status (ECOG 0 or 1). Health‐related quality of life and ECOG PS do not necessarily reflect similar aspects of a patient's level of functioning, as measures of HRQL address different functional aspects in patients compared with ECOG PS and has been reported to be more predictive of prognosis than ECOG PS [28]. In this study, higher ECOG PS only moderately correlated with poorer PF and GHS. This study showed that specific measures of HRQL (PF, RF, GHS) were independent predictors of poor prognosis even in patients reported by clinicians to have an ECOG PS of 0–1. This does not negate the potential value of ECOG PS in the clinic, but rather strengthens the case for incorporating a measure of HRQL as well to help predict prognosis and make treatment recommendations. Low PF, GHS, RF, and high AGIS were more closely associated with poorer survival than ECOG PS in patients with PRROC in this study.
The American Society of Clinical Oncology (ASCO) “Choosing Wisely” campaign was developed to improve the quality and value of oncology care, and included a recommendation to “avoid cancer‐directed therapy in patients with low performance status (3 or 4), no benefit from prior evidence‐based interventions, not eligible for a clinical trial, and no strong evidence supporting the clinical value of further anti‐cancer treatment.” [16]. It is generally accepted that chemotherapy is best avoided in the last month of life, and in many centers this is considered an indicator of quality of care. However, a recent analysis of 28,000 people with advanced cancer found that 75% received aggressive management in the last 30 days of life, with approximately 28% receiving chemotherapy in the last 30 days of life, and approximately one‐third died in hospital [29]. We found that 14% of participants with PRROC had chemotherapy in their last month of life, and surmise that this proportion might be higher outside of the clinical research setting. Low baseline HRQL also predicted for dying within 30 days of chemotherapy; this information could be helpful in clinical practice. Furthermore, almost one in five participants with PRROC stopped chemotherapy within 8 weeks, mainly because of rapid progression, despite most of them rated by clinicians as having good PS before starting chemotherapy.
Baseline self‐ratings of key aspects of HRQL (GHS, RF, PF, and AGIS) were more closely associated with stopping chemotherapy early, and with OS time, than was performance status rated by clinicians. These aspects of HRQL can be easily measured before considering palliative chemotherapy with the EORTC QLQ C30 and OV28, which is freely available, well validated, and can be implemented in clinical practice [30]. The findings of this study could help clinicians, patients, and families making decisions by supporting important discussions about the likelihood of benefit from further chemotherapy. We are not arguing against offering further chemotherapy to all patients with PRROC, or that patients cannot opt for additional treatment even if likelihood of benefit is very low. However, provision of this important information might temper expectations and allow patients to make more informed decisions.
PRROC comprises heterogeneous subgroups of patients with substantial variability in likelihood of response to chemotherapy, progression free time, and OS time. The definitions of platinum‐resistant and platinum‐refractory disease are crude and applied inconsistently, both in routine practice and in clinical trials. Studies rarely report on how recurrence was diagnosed, for example, according to CA125, RECIST, or symptomatic progression, limiting the interpretation of trials and application of their results in clinical practice.
A subset of women with PRROC derive significant clinical benefit from chemotherapy, and have favorable outcomes. However, there is also a subset of women with PRROC for whom further chemotherapy is futile. Identifying these women, who are likely to progress rapidly and have a very short survival time, is a major challenge. Previous attempts to develop prognostic indices [31], [32] have not resulted in widespread clinical application. Clinicopathological factors that have been used to predict response to chemotherapy, and prognosis, in recurrent ovarian cancer include PS, response to prior therapy, number of lines of prior chemotherapy, breast cancer susceptiblity gene (BRCA) mutation status, platinum‐refractory versus platinum‐resistant disease, tumor volume, number of metastatic sites, histological subtype, and grade [33], [34], [35]. In addition, markers of inflammation and inflammatory response such as C‐reactive protein, hemoglobin, white cell count, platelet count, lactate dehydrogenase, and alkaline phosphatase have also been associated with prognosis [36], [37], [38], [39], [40]. The findings of this study support the incorporation of patients’ ratings of HRQL, together with independently significant clinicopathologic factors, in a prognostic index. The development and validation of a prognostic index that is suitable for clinical application, and able to stratify patients in clinical trials, is clearly desirable given the large numbers of women with recurrent ovarian cancer who are treated with chemotherapy.
The main strengths of this study are its prospective design, international participation, and large sample size providing sufficient power to simultaneously assess multiple potential prognostic variables. Completion of HRQL questionnaires and data collection at baseline were high (96%). A limitation of this study is that it was not population‐based and so it is unknown how closely the results would reflect those that would be seen in the general community. However, the broad and inclusive eligibility criteria and use of “real‐world” clinical settings should enhance the applicability of the results to routine clinical practice in centers similar to those included in this study. This study's results require corroboration in independent cohorts of women with PRROC.
Conclusion
Self‐ratings of low GHS, RF, PF, and high AGIS were significantly associated with stopping chemotherapy early, and with shorter OS, in patients with PRROC after accounting for conventional clinicopathological factors, including PS. Low GHS, RF, and PF were also independent predictors of death within 30 days of chemotherapy. Measuring aspects of HRQL when considering further chemotherapy in PRROC could help identify women with a particularly poor prognosis who are unlikely to benefit from chemotherapy and could therefore be spared unnecessary treatment and toxicity in their last months of life.
Acknowledgements
We wish to acknowledge Dr. Eriko Aotani and her contribution to this study and dedicate this to her memory. This study was funded by the Australian National Health and Medical Research Council (GNT1063012). In the U.K., this NIHR study was jointly funded by Target Ovarian Cancer and the Cancer Research U.K. and UCL Cancer Trials Centre (Programme Grant C444/A15953). The Cancer Research U.K. and UCL Cancer Trials Centre coordinated U.K. participation in the study and AL was assisted by the National Institute for Health Research (NIHR) UCLH/UCL Biomedical Research Centre, which is supported by the Department of Health. We thank Ms. Rhana Pike for assistance with formatting of figures. Some of the data contained in the manuscript have been previously presented as an oral presentation at ASCO 2016.
Deceased.
Author Contributions
Conception/design: Martin R. Stockler, Madeleine King, Michael Friedlander
Provision of study material or patients: Michael Friedlander
Collection and/or assembly of data: Florence Joly, Rachel O'Connell, Anne Lanceley, Felix Hilpert, Luke Buizen, Aikou Okamoto, Eriko Aotani, Sandro Pignata, Amit Oza, Elisabeth Avall‐Lundqvist, Jonathan S. Berek, Florian Heitz, Amanda Feeney, Dominique Berton‐Rigaud, Michael Friedlander
Data analysis and interpretation: Felicia T. Roncolato, Florence Joly, Rachel O'Connell, Anne Lanceley, Felix Hilpert, Luke Buizen, Aikou Okamoto, Eriko Aotani, Sandro Pignata, Amit Oza, Elisabeth Avall‐Lundqvist, Jonathan S. Berek, Florian Heitz, Amanda Feeney, Dominique Berton‐Rigaud, Martin R. Stockler, Madeleine King, Michael Friedlander
Manuscript writing: Felicia T. Roncolato, Martin R. Stockler, Michael Friedlander
Final approval of manuscript: Felicia T. Roncolato, Florence Joly, Rachel O'Connell, Anne Lanceley, Felix Hilpert, Luke Buizen, Aikou Okamoto, Eriko Aotani, Sandro Pignata, Amit Oza, Elisabeth Avall‐Lundqvist, Jonathan S. Berek, Florian Heitz, Amanda Feeney, Dominique Berton‐Rigaud, Martin R. Stockler, Madeleine King, Michael Friedlander
Disclosures
Felix Hilpert: Roche (C/A, H); Martin R. Stockler: Amgen, Astellas, Astra Zeneca, Bayer, Bristo‐Myers Squibb, Merck, Pfizer, Roche (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Colombo N, Van Gorp T, Parma G et al. Ovarian cancer. Crit Rev Oncol Hematol 2006;60:159–179. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results program. 2012. Available at https://seer.cancer.gov/. Accessed May 25, 2017.
- 3. Fung‐Kee‐Fung M, Oliver T, Elit L et al. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol 2007;14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cantu M, Buda A, Parma G et al. Randomized controlled trial of single‐agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first‐line platinum‐based regimens. J Clin Oncol 2002;20:1232–1237. [DOI] [PubMed] [Google Scholar]
- 5. Ferrandina G, Ludovisi M, Lorusso D et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 2008;26:890–896. [DOI] [PubMed] [Google Scholar]
- 6. Parmar MK, Ledermann JA, Colombo N et al. Paclitaxel plus platinum‐based chemotherapy versus conventional platinum‐based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO‐OVAR‐2.2 trial. Lancet 2003;361:2099–2106. [DOI] [PubMed] [Google Scholar]
- 7. Markman M, Bookman MA. Second‐line treatment of ovarian cancer. The Oncologist 2000;5:26–35. [DOI] [PubMed] [Google Scholar]
- 8. Herzog TJ, Pothuri B. Ovarian cancer: A focus on management of recurrent disease. Nat Clin Pract Oncol 2006;3:604–611. [DOI] [PubMed] [Google Scholar]
- 9. Rustin G, Nelstrop A, Tuxen M et al. Defining progression of ovarian carcinoma during follow‐up according to CA 125: A North Thames Ovary Group Study. Ann Oncol 1996;7:361–364. [DOI] [PubMed] [Google Scholar]
- 10. Blackledge G, Lawton F, Redman C et al. Response of patients in phase II studies of chemotherapy in ovarian cancer: Implications for patient treatment and the design of phase II trials. Br J Cancer 1989;59:650–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Markman M, Rothman R, Hakes T et al. Second‐line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991;9:389–393. [DOI] [PubMed] [Google Scholar]
- 12. Gordon AN, Fleagle JT, Guthrie D et al. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001;19:3312–3322. [DOI] [PubMed] [Google Scholar]
- 13. Davis A, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol Oncol 2014;133:624–631. [DOI] [PubMed] [Google Scholar]
- 14. Grunewald T, Tang M, Chen J et al. Where have we gone wrong? Phase II trials (Ph2t) do not inform the results of phase III trials (Ph3t) in platinum resistant ovarian cancer (PROC). ASCO Meeting Abstracts 2016;34:5559a.
- 15. Gore M, Fryatt I, Wiltshaw E et al. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol 1990;36:207–211. [DOI] [PubMed] [Google Scholar]
- 16. Schnipper LE, Smith TJ, Raghavan D et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol; 30:1715–1724. [DOI] [PubMed] [Google Scholar]
- 17. Temel JS, Shaw AT, Greer JA. Challenge of prognostic uncertainty in the modern era of cancer therapeutics. J Clin Oncol 2016;34:3605–3608. [DOI] [PubMed] [Google Scholar]
- 18. Osoba D. Health‐related quality‐of‐life assessment in clinical trials of supportive care in oncology. Support Care Cancer 2000;8:84–88. [DOI] [PubMed] [Google Scholar]
- 19. Gotay CC, Kawamoto CT, Bottomley A et al. The prognostic significance of patient‐reported outcomes in cancer clinical trials. J Clin Oncol 2008;26:1355–1363. [DOI] [PubMed] [Google Scholar]
- 20. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quinten C, Coens C, Mauer M et al. Baseline quality of life as a prognostic indicator of survival: A meta‐analysis of individual patient data from EORTC clinical trials. Lancet Oncol 2009;10:865–871. [DOI] [PubMed] [Google Scholar]
- 22. Quinten C, Martinelli F, Coens C et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014;120:302–311. [DOI] [PubMed] [Google Scholar]
- 23. Grande GE, Farquhar MC, Barclay SI et al. Quality of life measures (EORTC QLQ‐C30 and SF‐36) as predictors of survival in palliative colorectal and lung cancer patients. Palliat Support Care 2009;7:289–297. [DOI] [PubMed] [Google Scholar]
- 24. Gourgou‐Bourgade S, Bascoul‐Mollevi C, Desseigne F et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23–29. [DOI] [PubMed] [Google Scholar]
- 25. Diouf M, Bonnetain F, Barbare JC et al. Optimal cut points for quality of life questionnaire‐core 30 (QLQ‐C30) scales: Utility for clinical trials and updates of prognostic systems in advanced hepatocellular carcinoma. The Oncologist 2015;20:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roncolato F, Gibbs E, Lee C et al. Quality of life (QOL) to predict overall survival (OS) in women with platinum‐resistant ovarian cancer (PROC). ASCO Meeting Abstracts 2016;34:5575a.
- 27. Faraggi D, Simon R. A simulation study of cross‐validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med 1996;15:2203–2213. [DOI] [PubMed] [Google Scholar]
- 28. Osoba D. Health‐related quality of life and cancer clinical trials. Ther Adv Med Oncol 2011;3:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen RC, Falchook AD, Tian F et al. Aggressive care at the end‐of‐life for younger patients with cancer: Impact of ASCO's Choosing Wisely campaign. ASCO Meeting Abstracts 2016;34:LBA10033a.
- 30. Aaronson N, Elliott T, Greenhalgh J et al. ISOQOL: User's guide to implementing patient‐reported outcomes. ISOQOL 2015. Available at http://www.isoqol.org/UserFiles/2015UsersGuide-Version2.pdf. Accessed May 25, 2017.
- 31. Hoskins P, Tu D, James K et al. Factors predictive of survival after first relapse or progression in advanced epithelial ovarian carcinoma: A prediction tree analysis‐derived model with test and validation groups. Gynecol Oncol 1998;70:224–230. [DOI] [PubMed] [Google Scholar]
- 32. Clark T, Stewart M, Altman D et al. A prognostic model for ovarian cancer. Br J Cancer 2001;85:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eisenhauer E, Vermorken J, Van Glabbeke M. Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: A multivariate analysis of 704 patients. Ann Oncol 1997;8:963–968. [DOI] [PubMed] [Google Scholar]
- 34. Chan JK, Teoh D, Hu JM et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 2008;109:370–376. [DOI] [PubMed] [Google Scholar]
- 35. Pignata S, Ferrandina G, Scarfone G et al. Activity of chemotherapy in mucinous ovarian cancer with a recurrence free interval of more than 6 months: Results from the SOCRATES retrospective study. BMC Cancer 2008;8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahmoud FA, Rivera NI. The role of C‐reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 2002;4:250–255. [DOI] [PubMed] [Google Scholar]
- 37. Kodama J, Miyagi Y, Seki N et al. Serum C‐reactive protein as a prognostic factor in patients with epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol 1999;82:107–110. [DOI] [PubMed] [Google Scholar]
- 38. Gungor T, Kanat‐Pektas M, Sucak A et al. The role of thrombocytosis in prognostic evaluation of epithelial ovarian tumors. Arch Gynecol Obstet 2009;279:53–56. [DOI] [PubMed] [Google Scholar]
- 39. Di Maio M, Pisano C, Tambaro R et al. The prognostic role of pre‐chemotherapy hemoglobin level in patients with ovarian cancer. Front Biosci 2006;11:1585–1590. [DOI] [PubMed] [Google Scholar]
- 40. Hefler LA, Concin N, Hofstetter G et al. Serum C‐reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 2008;14:710–714. [DOI] [PubMed] [Google Scholar]