A retrospective analysis of patients with a diagnosis of colorectal cancer was conducted. Patients with liver metastases were re‐evaluated by a liver‐specific multidisciplinary team, and the resulting treatment decisions were compared with the original management. Improvement in resection rates were evaluated, and results are presented here.
Keywords: Colorectal cancer, Liver metastases, Extrahepatic metastases, Hepatobiliary multidisciplinary teams, Imaging
Abstract
Background.
Assessing patients with colorectal cancer liver metastases (CRCLM) by a liver multidisciplinary team (MDT) results in higher resection rates and improved survival. The aim of this study was to evaluate the potentially improved resection rate in a defined cohort if all patients with CRCLM were evaluated by a liver MDT.
Patients and Methods.
A retrospective analysis of patients diagnosed with colorectal cancer during 2008 in the greater Stockholm region was conducted. All patients with liver metastases (LM), detected during 5‐year follow‐up, were re‐evaluated at a fictive liver MDT in which previous imaging studies, tumor characteristics, medical history, and patients’ own treatment preferences were presented. Treatment decisions for each patient were compared to the original management. Odds ratios (ORs) and 95% confidence intervals were estimated for factors associated with referral to the liver MDT.
Results.
Of 272 patients diagnosed with LM, 102 patients were discussed at an original liver MDT and 69 patients were eventually resected. At the fictive liver MDT, a further 22 patients were considered as resectable/potentially resectable, none previously assessed by a hepatobiliary surgeon. Factors influencing referral to liver MDT were age (OR 3.12, 1.72–5.65), American Society of Anaesthesiologists (ASA) score (OR 0.34, 0.18–0.63; ASA 2 vs. ASA 3), and number of LM (OR 0.10, 0.04–0.22; 1–5 LM vs. >10 LM), while gender (p = .194) and treatment at a teaching hospital (p = .838) were not.
Conclusion.
A meaningful number of patients with liver metastases are not managed according to best available evidence and the potential for higher resection rates is substantial.
Implications for Practice.
Patients with liver metastatic colorectal cancer who are assessed at a hepatobiliary multidisciplinary meeting achieve higher resection rates and improved survival. Unfortunately, patients who may benefit from resection are not always properly referred. In this study, the potential improved resection rate was assessed by re‐evaluating all patients with liver metastases from a population‐based cohort, including patients with extrahepatic metastases and accounting for comorbidity and patients’ own preferences towards treatment. An additional 12.9% of the patients were found to be potentially resectable. The results highlight the importance of all patients being evaluated in the setting of a hepatobiliary multidisciplinary meeting.
摘要
背景. 由肝脏多学科专家组(MDT)对结直肠癌肝转移(CRCLM)患者进行评估后, 患者的切除率提高且生存期有所改善。本研究旨在评估若一个规定队列中的所有CRCLM患者均接受肝脏MDT评价时的潜在切除率提高水平。
患者和方法. 针对2008年大斯德哥尔摩地区诊断为结直肠癌的患者进行了一项回顾性分析。在5年随访期间检出肝转移(LM)的所有患者均由本研究的肝脏MDT重新进行评价, 评价时提供既往影像学研究结果、肿瘤特征、病史和患者本人的治疗偏好。将每例患者的治疗决策与原始治疗方案进行比较。估算与转诊肝脏MDT相关的各项因素的比值比(OR)和95%置信区间。
结果. 在诊断为LM的272例患者中, 102例最初由肝脏MDT进行了会诊, 其中69例最终行切除术。本研究的肝脏MDT评估后认为, 另有22例患者也属于可切除/潜在可切除患者, 而这些患者之前未由肝胆外科医生进行评估。转诊肝脏MDT的影响因素包括年龄(OR 3.12, 1.72‐5.65)、美国麻醉医师协会(ASA)评分(OR 0.34, 0.18‐0.63;ASA 2 vs. ASA 3)和LM数量(OR 0.10, 0.04‐0.22;1‐5 LM vs. >10 LM), 而性别(p=0.194)和在教学医院接受治疗(p=0.838)并非影响因素。
结论. 大量肝转移患者未遵循最佳可用证据接受治疗, 切除率的潜在提高空间相当大。
Introduction
Surgical resection of colorectal cancer liver metastases (CRCLM) is a potentially curative treatment with reported 5‐year survival rates of 36%–55% and a 10‐year survival rate of 26% [1], [2]. The criteria for resectability are constantly evolving and tumor factors such as number, size, and distribution of metastases are no longer used as absolute criteria. At present, the only absolute contraindications, as proposed by Adam et al., are the inability to achieve a R0 hepatic resection, preserving a liver remnant >30%, or the presence of unresectable extrahepatic disease [3]. Initially, unresectable liver metastases can be rendered resectable by combinations of tumor‐directed interventions and future liver remnant manipulation [4], [5]. Improvement in imaging technology, surgical and interventional techniques, and oncologic therapy have increased the observed resection rate to approximately 25%, as reported in population‐based materials [6].
Assessment of patients with CRCLM by a dedicated liver multidisciplinary team (MDT) has been shown to result in higher resection rates and improved disease‐free survival (DFS) and overall survival (OS) [7], [8], [9], [10], [11]. In general, regulatory practices concerning referral to MDT conferences varies widely, from mandatory referral of all patients to guideline‐dictated referral of selected patients and referral at the discretion of referring physicians. Nonmandatory referral, especially in the absence of guidelines, leaves room for personal interpretations and opinions to influence decisions, a practice that potentially can deprive patients of proper assessment. Discrepancies in how medical oncologists and surgeons assess resectability and indications for preoperative chemotherapy are well documented [10], [12]. It is not uncommon for physicians and medical oncologists to judge bilateral hepatic disease and large tumor size as contraindications for surgery [13]. Furthermore, differences in referral rates between hospitals in the same geographic region have been reported [8], [14], [15], [16]. The aim of this study was to evaluate the potentially improved resection rate if all patients with CRCLM in a well‐defined population‐based cohort were evaluated by a liver‐specific MDT.
Patients and Methods
Patient Cohort
Ethical approval for the study was obtained from the Regional Ethical Review Board in Stockholm. The greater Stockholm region (counties of Stockholm and Gotland) is serviced by nine regional hospitals with all hepatic intervention performed within the framework of a dedicated hepato‐pancreatico‐biliary (HPB) unit. Each hospital has a weekly colorectal cancer (CRC) MDT conference with medical oncologists, pathologists, diagnostic radiologists, and colorectal surgeons present. Referrals to the weekly liver MDT conference usually originate from the CRC MDT conferences. All patients diagnosed with CRC in the greater Stockholm region from January 1, 2008, to December 31, 2008, were identified through the National Quality Registry for Colorectal Cancer Treatment. The register has a validated coverage of over 99% and includes all patients with adenocarcinoma of the colon and the rectum [17]. The quality registry further contains the unique personal identification number assigned to all Swedish citizens, which was used to identify each patient in hospital electronic patient records.
Clinical Review
An extensive review of all patient records was performed to identify all patients with liver metastases in the defined cohort. Patient and tumor characteristics, surgical and oncological treatment of primary tumors, as well as metastatic disease and time of death in deceased patients, were recorded. Furthermore, patient comorbidities, World Health Organization performance status, American Society of Anaesthesiologists (ASA) score and relevant laboratory test results were documented. Recorded patient preferences related to treatment options were documented. Synchronous metastases were defined as liver metastases detected prior to, or at resection of, the primary tumor, and in nonresected patients, as detected prior to or concurrently with the primary tumor. Treatment decisions for patients, which divided patients with liver metastases into resectable, potentially resectable, or unlikely to ever become resectable groups, were documented, and it was noted whether the treatment plan for each patient originated from discussion at a liver MDT conference or not.
Radiological Evaluation
For each patient, all available thoraco‐abdominal imaging studies, including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography‐CT were reviewed by a HPB radiologist (N.K.). The images were assessed for the location, number, and size of liver and lung metastases, as well as the presence and extent of extrahepatic metastatic disease. In cases with US examinations, the original radiological report was used for documentation of lesions when the archived imaging material did not allow a proper secondary assessment.
Fictive MDT Conference
All patients with CRCLM were presented at a fictive liver MDT conference with four specialist liver surgeons, three medical oncologists, one diagnostic radiologist, and one presenting physician (J.E.). All participants, except the presenting physician, were blinded to whether the patient had originally been discussed at a liver MDT, the original treatment decision, and what treatment the patient eventually had. Patient age, gender, medical history, existing extrahepatic disease, and, if known, the patients’ own preference towards treatment of their metastases were presented at the fictive MDT. The radiologist demonstrated all available images up to the time point where the patient would have been referred to the liver MDT. For each patient, the combined imaging portfolio was classified according to a radiological classification that was devised for grading the quality and clinical applicability of the imaging for the specific patient, as defined in Table 1. The term “diagnostic” assesses the nature and quality of the imaging as being sufficient to enable a complete assessment and planning. For example, a CT examination without intravenous contrast, in which there are innumerable metastases in all liver segments, was classified as “poor” technique but still “diagnostic,” as the information derived from the images was sufficient for making a definitive treatment decision for that particular patient.
Table 1. Radiology classification system.
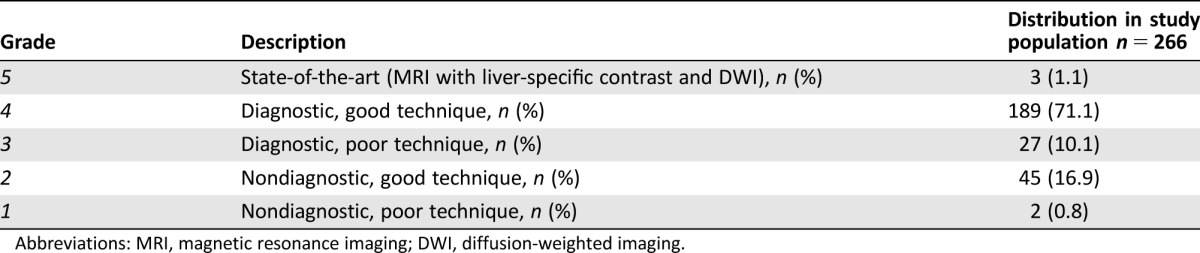
Abbreviations: MRI, magnetic resonance imaging; DWI, diffusion‐weighted imaging.
Based on the European Society for Medical Oncology Clinical Practice Guidelines for Metastatic colorectal cancer, the fictive MDT classified patients into one of three defined groups:
group 0: primarily technically R0‐resectable liver metastases
group 1: potentially resectable metastatic disease with curative intention
group 2: disseminated disease, technically “never”/unlikely resectable [18].
Patients with unresectable extrahepatic metastases were classified as group 2, regardless of the extent of their liver metastases. A treatment plan for each patient was decided on in consensus, stating the intention of oncological treatment (neo‐adjuvant, conversion, adjuvant, palliative) and specifying possible surgical interventions (extent and nature of surgery and local thermal ablation).
Statistical Methods
Baseline characteristics were assessed by medians (min, max) for non‐normally distributed continuous variables and percentages for categorical variables. Differences in proportions were tested with Fisher's exact test or Pearson's χ2 test. Logistic regression was used to calculate adjusted odds ratios and 95% confidence interval (CI) for factors predictive of referral to the liver MDT conference. Variables with p < .15 in the univariate analysis were included in the multivariate model. Cohen's Kappa for interrater agreement was used to determine the overall agreement between original and fictive liver MDT conference, analysing resectable/potentially resectable vs. unresectable, and κ > .7 was considered acceptable. Patient survival curves were created using the Kaplan‐Meier method and differences in survival estimates were assessed using the log‐rank method. All data analyses were performed using the statistical software package STATA 13 (StataCorp, College Station, Texas 77845) and results were regarded as statistically significant if p < .05.
Results
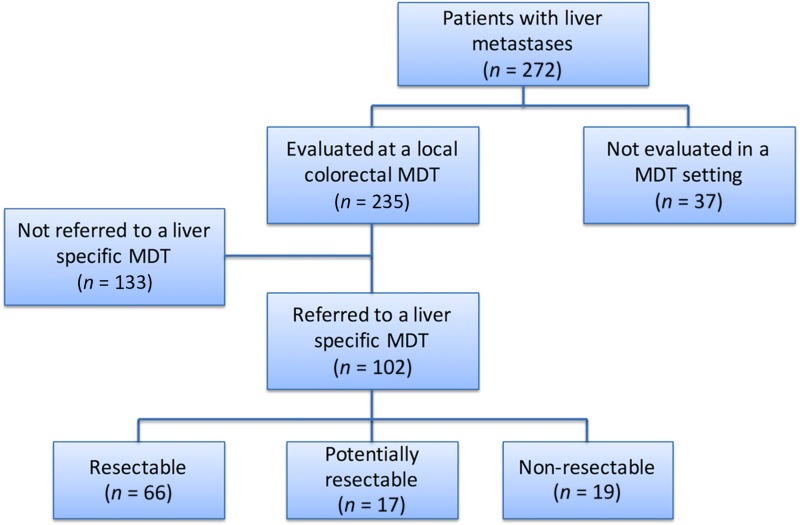
A total of 1,026 patients were identified in the registry as being diagnosed with CRC during the study period, of whom 272 (26.5%) developed liver metastases during a median follow‐up of 5.3 years. Of these, 102 (37.5%) patients with liver metastases were referred to and discussed at a liver MDT conference, of whom 66 were considered primarily resectable, 17 potentially resectable, and 19 as unresectable, as presented in Figure 1. Of the 272 patients with CRCLM, 69 (25.4%) were eventually treated with curative intent, 63 with liver resection, of whom four patients had simultaneous local thermal ablation therapy, and six patients were treated with thermal ablation alone.
Figure 1.
Decision‐making process at the original liver MDT. Flow chart showing the result of the original decision‐making process.
Abbreviation: MDT, multidisciplinary team.
Patient and tumor characteristics of all patients with CRCLM are shown in Table 2 for patients that either were referred to a liver MDT or were not. Median age was significantly lower in those referred to the liver MDT conference compared with those who were not (p < .001). Referral rates did not differ significantly between genders (p = .194). Patients with synchronous detection of liver metastases (LM) and a higher tumor burden were less likely to be referred (p = .034 and p < .001, respectively). In logistic regression of factors that could influence the probability of referral to the liver MDT conference, a more advanced age, higher ASA score, and total number of LM remained significant in the multivariate analysis, while gender and treatment at a teaching hospital did not (Table 3). Of the 170 patients not referred to the liver MDT conference, 55 were considered by the local colorectal team to have inoperable liver disease, 26 as having inoperable extrahepatic disease, 42 as having a combination of too extensive liver and extrahepatic metastases, and an additional 10 were not referred for a variety of other reasons. Thirty‐seven patients were not discussed at a CRC MDT at all.
Table 2. Patient and tumor characteristics for patients with liver metastases.
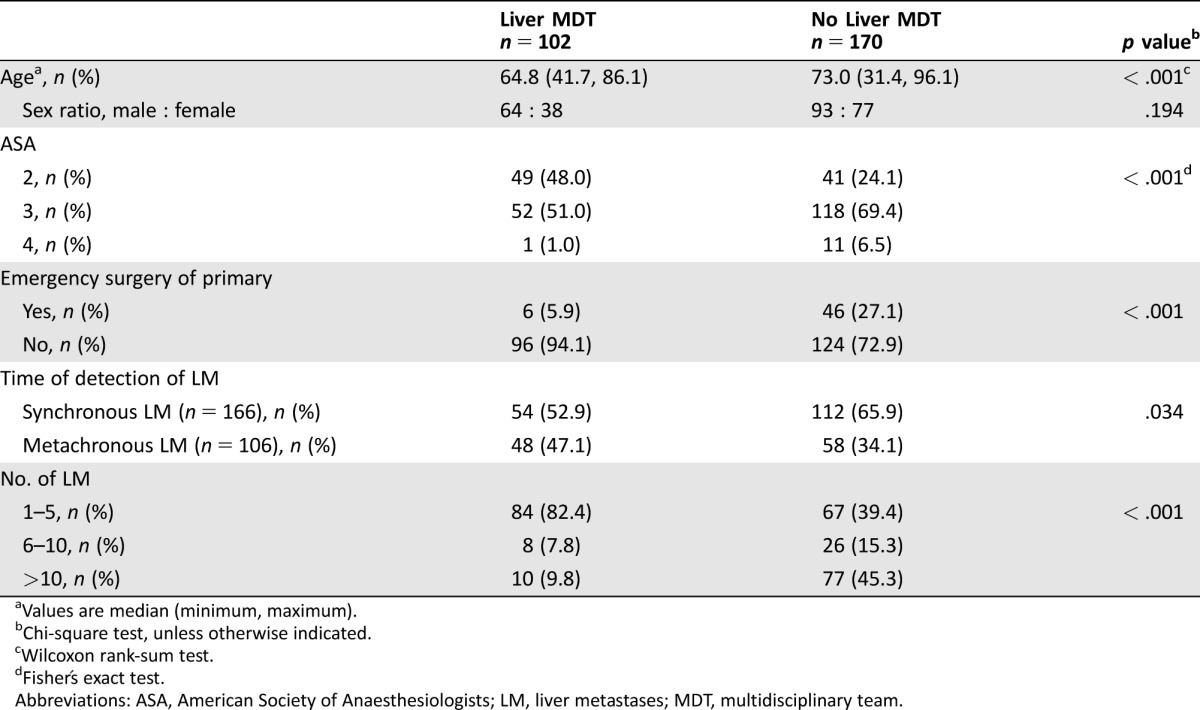
Values are median (minimum, maximum).
Chi‐square test, unless otherwise indicated.
Wilcoxon rank‐sum test.
Fisher's exact test.
Abbreviations: ASA, American Society of Anaesthesiologists; LM, liver metastases; MDT, multidisciplinary team.
Table 3. Univariate and multivariate logistic regression analysis of factors associated with the probability of referral to a liver MDT conference.
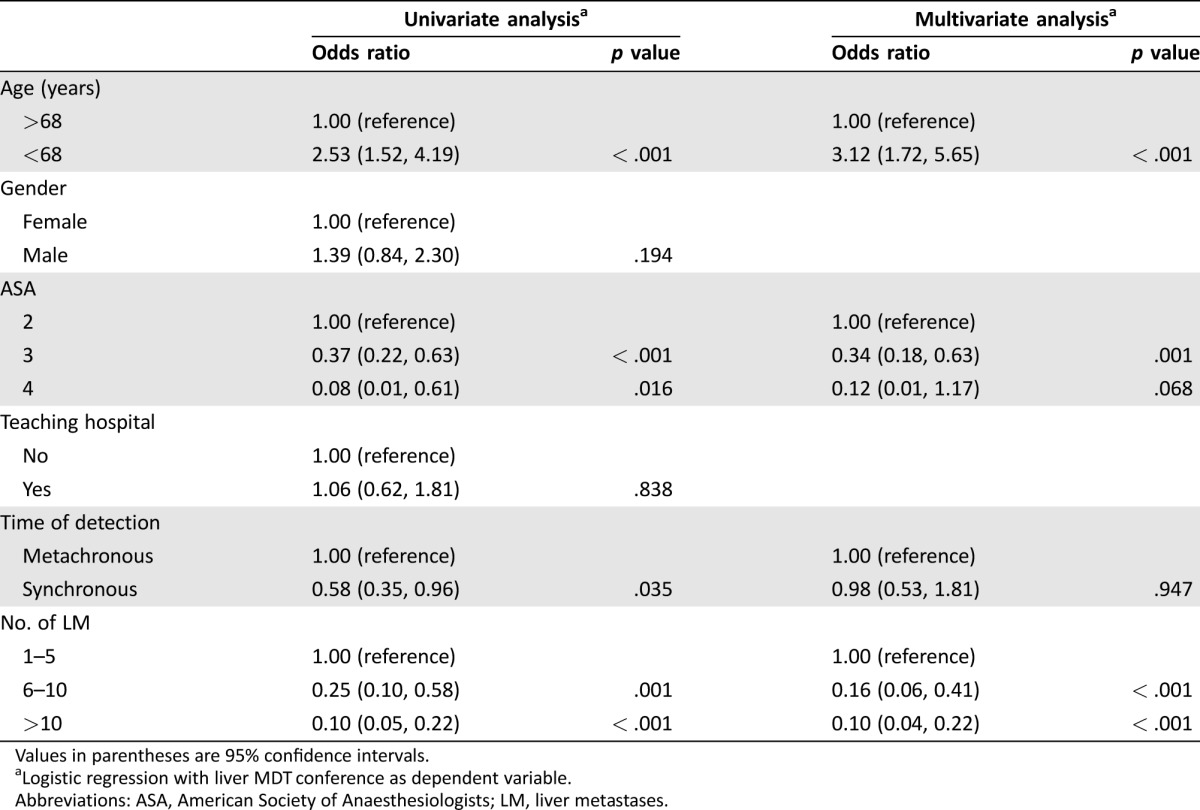
Values in parentheses are 95% confidence intervals.
Logistic regression with liver MDT conference as dependent variable.
Abbreviations: ASA, American Society of Anaesthesiologists; LM, liver metastases.
Results from the Fictive Liver MDT
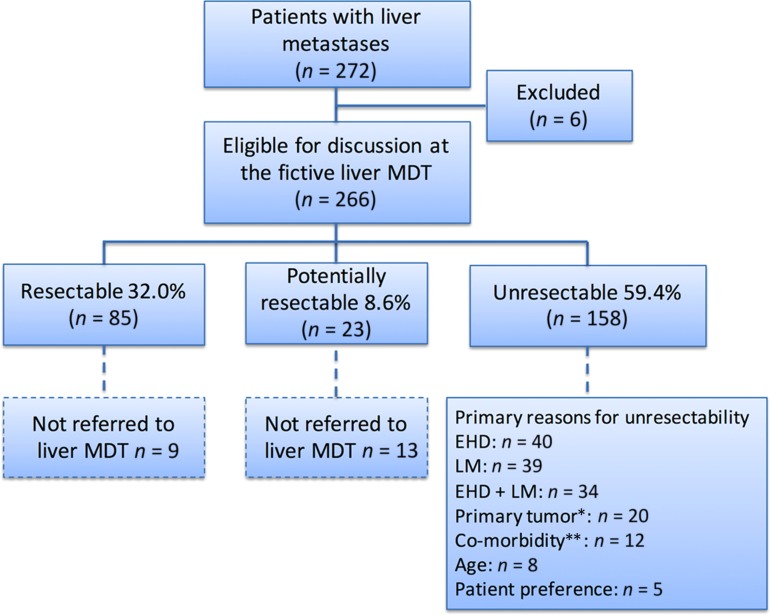
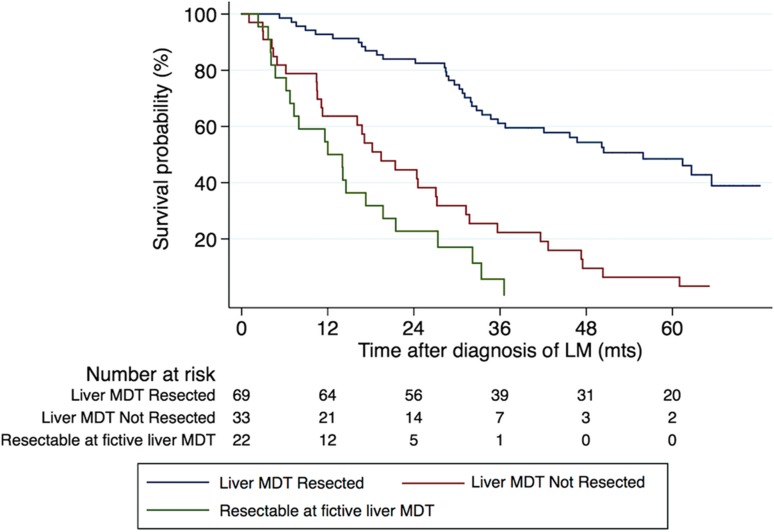
All but six patients, where imaging for re‐evaluation could not be retrieved, were discussed at the fictive liver MDT conference. Of the 266 patients, 85 (32.0%) were considered resectable, 23 as potentially resectable, and the remaining 158 (59.4%) as unresectable (Fig. 2). Primary reasons for unresectability are outlined in Figure 2. Twenty‐two patients who were not originally discussed at a liver MDT conference were assessed as being either primarily resectable (n = 9) or potentially resectable (n = 13) at the fictive liver MDT conference. A detailed analysis of the 22 patients showed that two were originally assessed by a medical oncologist only, three were assessed by a colorectal surgeon outside a colorectal MDT, and the remaining 17 were discussed at a local colorectal MDT without a specialist hepatobiliary surgeon being present. For sixteen of these 22 patients it was stated that they were not referred for assessment by a hepatobiliary surgeon due to extensive hepatic metastatic disease. Twelve patients had fewer than eight metastases and no extrahepatic disease, while the remaining four had multiple LM (10, 13, 15, and 23 metastases, respectively) but more than two tumor‐free segments. An additional two patients were not referred to a liver MDT due to extrahepatic disease. One patient had an abdominal wall metastasis and the other had peritoneal carcinomatosis (Peritoneal Cancer Index 2) in addition to two LM. One patient was not referred because of advanced age. In three patients, the reasond for not referring were not stated in the medical record. The 22 patients had a median OS of 12 months compared to 55.9 months for patients originally discussed at a liver MDT and resected, and 19.4 months for patients originally discussed at a liver MDT and not resected (log rank p < .001) (Fig. 3). Age (p = .005), emergency surgery of the primary tumor (p = .002), and proportion of patients with more than 5 LM (p < .001) differed significantly between those actually referred to the liver MDT and the 22 patients assessed as potentially resectable at the fictive liver MDT conference, while gender distribution (p = .375), synchronous detection of LM (p = .361), and ASA score (p = .523) did not. Actual treatment decisions among those with resectable or potentially resectable LM made at the original liver MDT and treatment decisions during the fictive liver MDT were the same in 95.1% of patients (Cohen's Kappa 0.83). The original and fictive liver MDT conferences disagreed on the management of five patients. One patient, assessed as potentially resectable with local ablation at the original liver MDT conference, was not assessed as even potentially resectable at the fictive liver MDT. On the contrary, four patients were evaluated as potentially resectable at the fictive liver MDT, but not at the original liver MDT conference. The motivations for the decisions at the original MDT were bilateral disease, a too‐small FLR, one complicated located LM, and the last patient was accessed as unresectable because of extrahepatic disease (a single lung metastasis and a single metastasis of the abdominal wall).
Figure 2.
Decision‐making process at the fictive liver MDT. Flow chart showing the decisions made at the fictive liver MDT conference and primary reasons for unresectability outlined.
Abbreviations: MDT, multidisciplinary team. EHD, extrahepatic disease. LM, liver metastases.
*Unresectable or unresectable local recurrence.
**Severe comorbidity omitting resection.
Figure 3.
Survival depending on management by a liver MDT or not. Kaplan‐Meier curves showing overall survival for patients discussed at the liver‐specific MDT conference and who ultimately underwent resection, those who were referred but did not undergo resection, and the 22 patients assessed as resectable or potentially resectable at the fictive liver MDT. Liver MDT and resection (n = 69) versus liver MDT and no resection (n = 33) (3‐ and 5‐year OS 61.1% and 48.5% vs. 22.3% and 6.4%) p < .001 (log‐rank test). Liver MDT and no resection (n = 33) vs. resectable or potentially resectable at fictive liver MDT (n = 22) (3‐year OS 22.3% vs. 5.7%), p = .057 (log‐rank test). Abbreviations: LM, liver metastases; MDT, multidisciplinary team; mts, months.
The liver imaging itself was nondiagnostic for 47 (17.7%) patients, and in a further 27 (10.1%) patients, the available imaging was performed with inadequate technique or poor quality, but other patient‐related factors made decision making on treatment strategy possible (Table 1).
Referral Rates
Referral rates of patients with LM to the liver MDT ranged from 28.6 to 48.6%, excluding two hospitals that had less than 10 patients with LM (p = .505). There were no significant differences in referral rates between teaching and nonteaching hospitals (p = .882), not even if all hospitals were included in the analysis (p = .554).
Discussion
Improved outcomes in terms of DFS and OS in patients with CRCLM managed in the setting of dedicated liver MDT conferences are well documented [7], [9], [11]. In the present population‐based study, 22 patients from the cohort of patients with CRCLM treated palliative were assessed as resectable or potentially resectable at a fictive MDT conference, of which none had previously been discussed at a liver MDT conference. These 22 patients had a higher age, a higher proportion of emergency surgery of the primary tumor, and a larger number of metastases as compared to patients originally referred to the liver MDT, but they did not differ significantly regarding ASA score or proportion of synchronous presentation of LM. However, it is possible that there may have been other factors not clearly identified in this study that would have limited the number of these patients who eventually would have undergone surgery. Survival for this group of patients was worse than for patients who were treated with palliative intent in which the decision was made in a liver MDT conference, suggesting grossly nonoptimal management. This re‐evaluation of all patients with liver metastases; taking extrahepatic disease, comorbidity, and patients’ own treatment preference into account, indicate that nearly 40% could have been candidates for curative‐intended surgical treatment. This is an optimistic number, but considering that criteria for resection of LM and extrahepatic disease are moving targets, resection rates in this order might be reached in a not too distant future. This is in part supported by other population‐based studies in which an increase in resection rates was observed over time to above 30% in patients diagnosed during 2007 [6].
Previously published studies on the subject are limited to re‐evaluation of patients with liver‐only metastases, only palliative‐treated patients, or hypothetic decisions based on imaging only, not considering patient‐related factors that might preclude surgical resection. Jones et al. assessed decision making by nonliver surgeons and found that almost two‐thirds of patients with tumors deemed unresectable by nonspecialists were considered potentially resectable by a panel of experienced specialist liver surgeons [16]. However, the study was limited to patients treated with palliative intent, and patients with extrahepatic disease were excluded from re‐evaluation. Young et al. showed that management decisions differed between colorectal and liver specialists in almost 50% of patients [14]. In a study by Thillai et al., a third of patients with liver‐limited metastatic CRC were never referred to a specialist HPB meeting [19]. In a 13‐year‐old study conducted in the same geographic area as the present study, 3.9% of the patients with liver metastases ultimately underwent liver surgery [15]. The same study estimated the highest possible resection rate to be 17%, as opposed to the actual resection rate of 25.4% in the present study, reflecting that major progress has been made over the last decade regarding the management of patients with CRCLM.
Several authors have demonstrated variations in referral practice between different hospitals and regions [8], [14]. In the present study, 37.5% of patients were referred with seemingly large variations between hospitals, although statistically nonsignificant. In a Swedish nationwide study by Norén et al., the resection rates of synchronous liver metastases varied considerably between different regions in Sweden and were lower in regions outside of the Stockholm area [20]. The present study further showed that predictors associated with referral to liver MDT were age, ASA score and number of liver metastases and number of liver metastases. Patients not referred to the liver MDT had a significantly higher median age and proportion of patient with higher ASA score, which could explain the reluctance to refer. Ksienski et al. have presented data in which a significant proportion of patients were not referred because of advanced age, despite studies proving the benefit of liver surgery among the elderly [21], [22].
As complex surgeries are often centralized to regional high‐volume centers, up‐to‐date knowledge on new treatment strategies can be perceived as too aggressive and over‐optimistic by general surgeons. Furthermore, the importance of a hepatobiliary oncologist present in the MDT, to initiate neoadjuvant chemotherapy when indicated, should not be underestimated. Lack of expertise in local community hospitals and timely access to specialty care and treatment have been identified as the main factors that contributed to why current management of CRCLM does not reflect the best evidence [10].
In the present study, the available imaging material was deemed nondiagnostic in 17.7% of patients. Jones et al. presented similar results in which a significant number of treatment decisions were based on examinations of inadequate quality that were too poor to allow an accurate assessment [16]. The combination of diffusion‐weighted MRI and hepatobiliary‐specific contrast‐enhanced MRI has been shown to be the optimal method for the evaluation of patients with CRCLM and is of particular importance in patients where curative treatment is planned [23], [24].
This study has a number of limitations. First, it is limited by its retrospective nature of a 2008 cohort in which 61.0% of the patients had synchronous metastases. This might be considered as an “outdated” cohort, and actual referral rates and resection rates today are likely higher. Shifting trends in treatment, both surgical and oncological, in recent years could therefore not be commented on. The participants at the fictive MDT‐conferences are coauthors and were not blinded to the hypothesis of the study. There is the possibility that the participants decided in favor of a particular treatment more often than in a real situation. This could result in an over‐estimation of the importance of a hepatobiliary surgeon being present. To minimize the expectation bias, the constellation at the different occasions for the fictive liver MDT conferences were varied (apart from the presenting physician and the diagnostic radiologist). Consistency in decision making compared with the original conference was 95.1% with interrater agreement κ > .7 (κ = .83).
Continuous education and updating of health care professionals involved in the care of CRCLM patients will most likely increase referral rate and ultimately improve outcome. Furthermore, standardized routines should be followed/established stipulating minimum imaging requirements. Lastly there should be clear policies in place directing referrals to liver MDTs. A policy of referral of all patients with CRCLM would be optimal but might not be practical and affordable. If a model of selective referral is applied, there should be clear and liberal guidelines that at regular intervals are disseminated to colorectal and general surgeons as well as medical oncologists.
Conclusion
The patient cohort presented in this study originates from a quite homogenous population, served by a health care system that is perceived as offering equal access to all patients regardless of socioeconomic standing and geographical factors. Despite this, a meaningful number of patients with CRCLM were not treated optimally and the potential of an improved resection rate is substantial. A resection rate of around 40% is estimated based on re‐evaluation of this population‐based cohort, which underscores the importance of managing patients in the setting of a dedicated liver MDT conference.
Acknowledgment
The study was funded by the Karolinska Institutet, Department of Clinical Sciences Danderyd Hospital and the Department of Surgery and Urology, Danderyd Hospital, Sweden.
Footnotes
For Further Reading: Katsunori Imai, Marc‐Antoine Allard, Carlos Castro Benitez et al. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors?. The Oncologist 2016;21:887–894.
Implications for Practice: In this study, the optimal cutoff point of early recurrence was determined to be 8 months after surgery based on the minimum p value approach, and its prognostic impact was demonstrated mainly in patients who received preoperative chemotherapy. Five factors, including age, number of preoperative chemotherapy lines, response to last‐line chemotherapy, number of tumors, and carbohydrate antigen 19–9 concentrations, were identified as predictors of early recurrence. Salvage surgery for recurrence significantly improved survival, even in patients with early recurrence. For better selection of patients who could truly benefit from surgery and should also receive strong postoperative chemotherapy, the accurate preoperative prediction of early recurrence is crucial.
Author Contributions
Conception/Design: Jennie Engstrand, Nikolaos Kartalis, Cecilia Strömberg, Mats Broberg, Anna Stillström, Tobias Lekberg, Eduard Jonas, Jacob Freedman, Henrik Nilsson
Provision of study material or patients: Jennie Engstrand, Nikolaos Kartalis, Cecilia Strömberg, Mats Broberg, Anna Stillström, Tobias Lekberg, Eduard Jonas, Jacob Freedman, Henrik Nilsson
Collection and/or assembly of data: Jennie Engstrand, Nikolaos Kartalis
Data analysis and interpretation: Jennie Engstrand, Nikolaos Kartalis, Henrik Nilsson
Manuscript writing: Jennie Engstrand, Nikolaos Kartalis, Henrik Nilsson, Eduard Jonas, Jacob Freedman
Final approval of manuscript: Jennie Engstrand, Nikolaos Kartalis, Cecilia Strömberg, Mats Broberg, Anna Stillström, Tobias Lekberg, Eduard Jonas, Jacob Freedman, Henrik Nilsson
Disclosures
The authors indicated no financial relationships.
References
- 1. Landreau P, Drouillard A, Launoy G et al. Incidence and survival in late liver metastases of colorectal cancer. J Gastroenterol Hepatol 2015;30:82–85. [DOI] [PubMed] [Google Scholar]
- 2. Nanji S, Cleary S, Ryan P et al. Up‐front hepatic resection for metastatic colorectal cancer results in favorable long‐term survival. Ann Surg Oncol 2013;20:295–304. [DOI] [PubMed] [Google Scholar]
- 3. Adam R, De Gramont A, Figueras J et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. The Oncologist 2012;17:1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schadde E, Ardiles V, Slankamenac K et al. Alpps offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional‐staged hepatectomies: Results of a multicenter analysis. World J Surg 2014;38:1510–1519. [DOI] [PubMed] [Google Scholar]
- 5. Engstrand J, Nilsson H, Jansson A et al. A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases ‐ A safety and feasibility study of a new concept. Eur J Surg Oncol 2014;40:1488–1493. [DOI] [PubMed] [Google Scholar]
- 6. Hackl C, Neumann P, Gerken M et al. Treatment of colorectal liver metastases in germany: A ten‐year population‐based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014;14:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lordan JT, Karanjia ND, Quiney N et al. A 10‐year study of outcome following hepatic resection for colorectal liver metastases ‐ The effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol 2009;35:302–306. [DOI] [PubMed] [Google Scholar]
- 8. Tiernan J, Briggs CD, Irving GR et al. Evaluation of the introduction of a standardised protocol for the staging and follow‐up of colorectal cancer on resection rates for liver metastases. Ann R Coll Surg Engl 2010;92:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vigano L, Langella S, Ferrero A et al. Single synchronous resectable colorectal liver metastasis: Monocentric management in tertiary referral hpb centre improves survival outcomes HPB 2011;13:48. [DOI] [PubMed] [Google Scholar]
- 10. Wei AC, Sandhu L, Devitt KS et al. Practice patterns for the management of hepatic metastases from colorectal cancer: A mixed methods analysis. Ann Surg Oncol 2013;20:1567–1574. [DOI] [PubMed] [Google Scholar]
- 11. Lan YT, Jiang JK, Chang SC et al. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int J Colorectal Dis 2016;31:403–411. [DOI] [PubMed] [Google Scholar]
- 12. Homayounfar K, Bleckmann A, Helms HJ et al. Discrepancies between medical oncologists and surgeons in assessment of resectability and indication for chemotherapy in patients with colorectal liver metastases. Br J Surg 2014;101:550–57. [DOI] [PubMed] [Google Scholar]
- 13. Krell RW, Reames BN, Hendren S et al. Surgical referral for colorectal liver metastases: A population‐based survey. Ann Surg Oncol 2015;22:2179–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young AL, Adair R, Culverwell A et al. Variation in referral practice for patients with colorectal cancer liver metastases. Br J Surg 2013;100:1627–1632. [DOI] [PubMed] [Google Scholar]
- 15. Sjovall A, Jarv V, Blomqvist L et al. The potential for improved outcome in patients with hepatic metastases from colon cancer: A population‐based study. Eur J Surg Oncol 2004;30:834–841. [DOI] [PubMed] [Google Scholar]
- 16. Jones RP, Vauthey JN, Adam R et al. Effect of specialist decision‐making on treatment strategies for colorectal liver metastases. Br J Surg 2012;99:1263–1269. [DOI] [PubMed] [Google Scholar]
- 17. Kodeda K, Nathanaelsson L, Jung B et al. Population‐based data from the swedish colon cancer registry. Br J Surg 2013;100:1100–1107. [DOI] [PubMed] [Google Scholar]
- 18. Van Cutsem E, Cervantes A, Nordlinger B et al. Metastatic colorectal cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(Suppl 3):iii1–iii9. [DOI] [PubMed] [Google Scholar]
- 19. Thillai K, Repana D, Korantzis I et al. Clinical outcomes for patients with liver‐limited metastatic colorectal cancer: Arguing the case for specialist hepatobiliary multidisciplinary assessment. Eur J Surg Oncol 2016;42:1331–1336. [DOI] [PubMed] [Google Scholar]
- 20. Noren A, Eriksson HG, Olsson LI. Selection for surgery and survival of synchronous colorectal liver metastases; a nationwide study. Eur J Cancer 2016;53:105–114. [DOI] [PubMed] [Google Scholar]
- 21. Ksienski D, Woods R, Speers C et al. Patterns of referral and resection among patients with liver‐only metastatic colorectal cancer (mcrc). Ann Surg Oncol 2010;17:3085–3093. [DOI] [PubMed] [Google Scholar]
- 22. Booth CM, Nanji S, Wei X et al. Management and outcome of colorectal cancer liver metastases in elderly patients: A population‐based study. JAMA Oncol 2015;1:1111–1119. [DOI] [PubMed] [Google Scholar]
- 23. Vilgrain V, Esvan M, Ronot M et al. A meta‐analysis of diffusion‐weighted and gadoxetic acid‐enhanced mr imaging for the detection of liver metastases. Eur Radiol 2016;26:4595–4615. [DOI] [PubMed] [Google Scholar]
- 24. Zech CJ, Korpraphong P, Huppertz A et al. Randomized multicentre trial of gadoxetic acid‐enhanced mri versus conventional mri or ct in the staging of colorectal cancer liver metastases. Br J Surg 2014;101:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]