Abstract
Lessons Learned.
Percutaneous thermal ablation combined with in situ granulocyte‐macrophage colony‐stimulating factor cytokine therapy was technically feasible and well tolerated.
No significant clinical or immunologic responses were seen.
Background.
Melanoma tumor‐derived heat‐shock proteins (HSPs) and HSP‐peptide complexes can elicit protective antitumor responses. The granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) chemokine can also promote uptake and processing by professional antigen presenting cells (APCs). On this basis, we designed a pilot study of percutaneous thermal ablation as a means to induce heat‐shock protein vaccination plus GM‐CSF to determine safety and preliminary antitumor activity of this combination.
Materials and Methods.
This study was designed to assess overall safety of percutaneous ablation combined with GM‐CSF for unresectable, metastatic melanoma including uveal and mucosal types. All patients received heat‐shock therapy (42°C for 30 minutes), then received one of three treatments: (a) intralesional GM‐CSF (500 mcg standard dose); (b) radiofrequency ablation (RFA) + GM‐CSF; or (c) cryoablation plus GM‐CSF. The primary endpoint of the study was the induction of endogenous HSP70 and melanoma‐specific cytotoxic T lymphocytes (CTL).
Results.
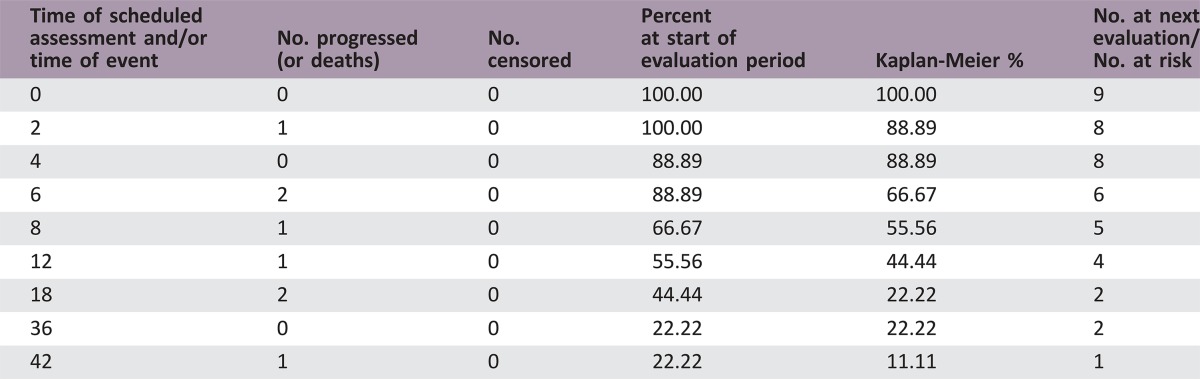
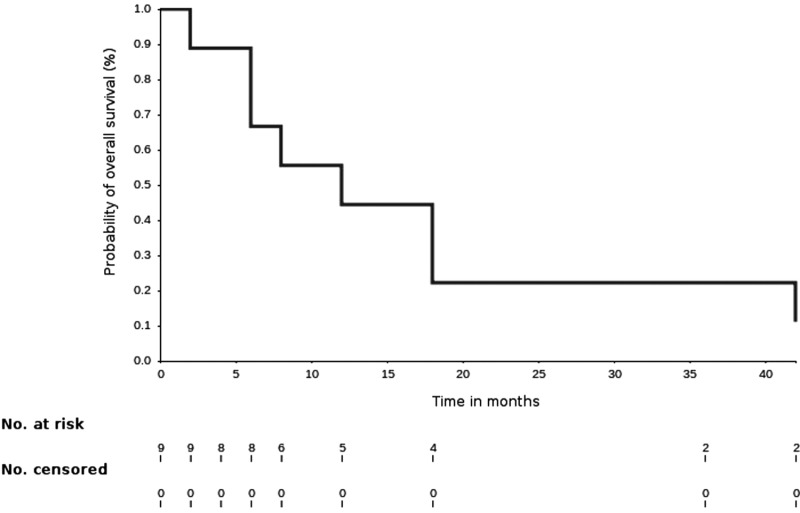
Nine patients (three per study arm) were enrolled. No dose‐limiting toxicity was observed as specified per protocol. All patients developed progressive disease and went on to receive alternative therapy. Median overall survival (OS) was 8.2 months (95% confidence interval [CI] 2–17.2). The study was not powered to detect a difference in clinical outcome among treatment groups.
Conclusion.
Percutaneous thermal ablation plus GM‐CSF was well tolerated, technically feasible, and demonstrated an acceptable adverse event profile comparable to conventional RFA and cryoablation. While HSP70 was induced following therapy, the degree of HSP70 elevation was not associated with clinical outcome or induced CTL responses. While percutaneous thermal ablation plus GM‐CSF combinations including checkpoint inhibitors could be considered in future studies, the use of GM‐CSF remains experimental and for use in the context of clinical trials.
Abstract
经验总结
• 经皮热消融联合原位粒细胞‐巨噬细胞集落刺激因子细胞因子疗法在技术上可行、耐受性良好。
• 没有观察到明显的临床或免疫应答。
摘要
背景.黑色素瘤衍生的热休克蛋白(HSP)和HSP‐肽复合物可以引发保护性抗肿瘤反应。粒细胞‐巨噬细胞集落刺激因子(GM‐CSF)趋化因子也可促进专职抗原呈递细胞(APC)的摄取和加工处理。在此基础上, 我们设计了一项经皮热消融初步研究, 据此诱导热休克蛋白疫苗与GM‐CSF, 以确定这种联合的安全性和初步抗肿瘤效果。
材料和方法.本研究旨在评估经皮消融联合GM‐CSF用于不可切除的转移性黑色素瘤(包括葡萄膜和粘膜型)的总体安全性。所有患者首先接受热休克治疗(42°C, 30分钟), 然后接受以下三种治疗方法之一:(a)病灶内GM‐CSF(500 mcg标准剂量);(b)射频消融(RFA)+GM‐CSF;或(c)冷冻消融联合GM‐CSF。研究的主要终点是诱导内源性HSP70和黑色素瘤特异性细胞毒性T淋巴细胞(CTL)。
结果.入组了九名患者(每个研究组三名)。按照方案规定, 未观察到剂量限制性毒性。所有患者均发生了疾病进展, 并继续接受替代治疗。中位总生存期(OS)为8.2个月[95%置信区间(CI)2‐17.2]。该研究的把握度不足以检出治疗组间临床结局的差异。
结论.经皮热消融联合GM‐CSF具有良好的耐受性且在技术上可行, 其不良事件特征被证实可接受并且与常规RFA和冷冻消融相似。HSP70在治疗后被诱导, HSP70升高程度与临床结局或诱导的CTL应答无关。虽然在未来的研究中可以考虑联合使用经皮热消融与GM‐CSF(包括检查点抑制剂), 但GM‐CSF的使用仍然是实验性的, 并且是在临床试验范围内使用。
Discussion
Metastatic melanoma has historically carried poor prognosis [1], but new approaches using combination immunotherapy [2], molecularly targeted agents [3], and radiation therapy [4] are rapidly changing the outlook of the disease. Estimates for 2017 predict approximately 87,110 new cases of melanoma and 9,730 deaths from the disease [5]. Objective response rates (ORR) in previously untreated patients on anti‐programmed death receptor‐1 (PD‐1) therapy approach 40%; still more than half of patients fail to respond [6], [7]. For uveal melanoma patients, response rates are significantly worse, with an ORR of 3.6% and median PFS and OS of 2.6 months and 7.6 months, respectively [8].
Multimodality and multidisciplinary management of metastatic disease includes the use of percutaneous thermal ablation, a modality associated with durable local control and oncologic outcomes comparable to surgery [9]. In this study, we sought to evaluate whether in situ melanoma vaccination could be achieved by three local directed therapies combined with intralesional GM‐CSF. We show that heat‐shock therapy, RFA, and cryoablation are all associated with an increase in HSP70 levels following therapy; however, we did not detect significant induction of anti‐melanoma T‐cell responses, a pre‐specified endpoint of the study.
Therapeutic strategies that help generate an immunostimulatory tumor microenvironment may help inform clinical approaches to treat patients with refractory disease. Response to current checkpoint inhibitor strategies depends on antitumor T cells expressing PD‐1 and correlates with programmed death‐ligand 1 (PD‐L1) expression on tumor cells. Here we have shown that thermal ablation therapy for melanoma induces expression of HSP70, a melanoma tumor‐associated antigen and alarmin molecule with immunological adjuvant activity. Several studies have shown that heat‐shock protein complexes containing tumor‐derived proteins are released and can be processed by antigen presenting cells [10], [11]. In this study, cryoablation was associated with the most significant plasma HSP70 elevations, although it is not clear that this is necessary or sufficient for effective in situ vaccination. However, local expression of GM‐CSF by the modified oncolytic herpes simplex virus talimogene laherparepvec has shown promise [12]. The cellular immunologic responses to viruses, as well as differences in local tumor dose of GM‐CSF expressed by oncolytic viruses, may in part explain some of the difference in observed outcomes between the two approaches.
Further refinement of this in situ vaccination therapy strategy is still required prior to further development of the approach. Testing of lower doses of GM‐CSF in combination with checkpoint inhibition may prove more fruitful, particularly in patients with uveal melanoma, for whom responses to checkpoint inhibitors are low and the presence of liver‐predominant metastatic disease can be successfully targeted for percutaneous ablation therapy.
Trial Information
- Disease
Melanoma
- Stage of Disease/Treatment
Metastatic/Advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase I
- Type of Study ‐ 2
Null
- Primary Endpoint
Tolerability
- Primary Endpoint
Correlative endpoint
- Secondary Endpoint
Deliverability
- Secondary Endpoint
Toxicity
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
Correlative endpoints: Plasma HSP70 levels and doubling of anti‐melanoma CTLs
- Investigator's Analysis
Drug tolerable, efficacy indeterminant
Drug Information for Phase I Thermal Ablation
- Drug 1
- Generic/Working name
Sargramostim/GM‐CSF
- Trade name
Leukine
- Company name
Sanofi
- Drug type
Cytokine
- Drug class
Immune therapy
- Dose
500 Micrograms (mcg) per flat dose
- Route
Intra‐tumoral
- Schedule of administration
Once
Patient Characteristics for Phase I Thermal Ablation
- Number of patients, male
3
- Number of patients, female
6
- Stage
IV
- Age
Median (range): 63 (45–81)
- Number of prior systemic therapies
Median (range): 2 (0–4)
- Performance Status: ECOG
-
0 — 9
1 —
2 —
3 —
unknown —
- Cancer Types or Histologic Subtypes
-
Mucosal melanoma: 3
Ocular melanoma: 3
Cutaneous melanoma: 2
Acral‐lentiginous melanoma: 1
Primary Assessment Method for Phase I Thermal Ablation
- Assessment
- Number of patients screened
13
- Number of patients enrolled
11
- Number of patients evaluable for toxicity
9
- Number of patients evaluated for efficacy
9
- Response assessment PD
n = 9 (100%)
- (Median) duration assessments OS
8.2 months, CI: 2–17.2
- Kaplan‐Meier time units
Months
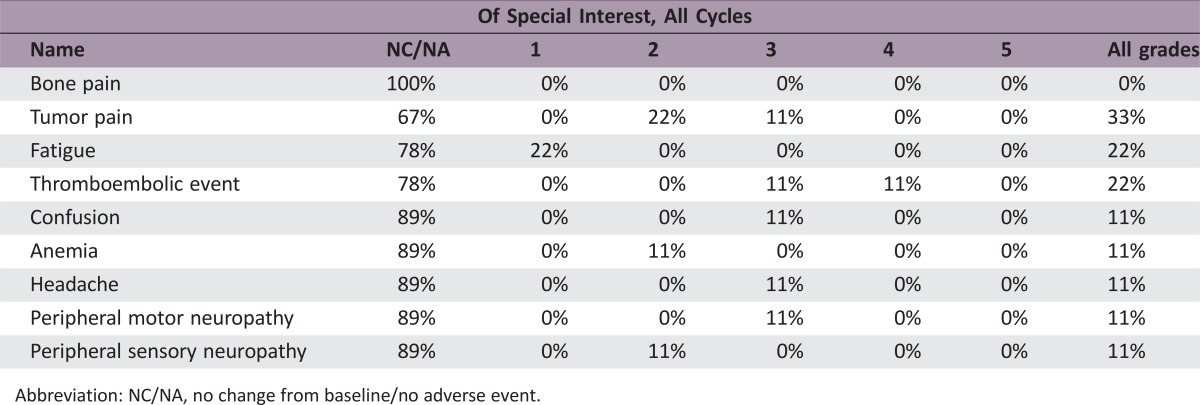
Phase I Thermal Ablation Adverse Events
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Drug tolerable, efficacy indeterminant
Heat‐shock proteins (HSPs), also known as molecular chaperones, form an intracellular network of molecular machinery that maintain the proteome. Mammalian HSPs are classified according to molecular weight into families: HSP110, HSP90, HSP70, HSP60, HSP40, and small HSPs (HSP27 and others). Humans encode at least 13 members of the HSP70 family that use the chemical energy provided by ATP hydrolysis to orchestrate cotranslational folding of nascent polypeptide chains [13], [14].
HSP70 family members can bind apoptotic protease activation factor 1 (APAF‐1) and Bax proteins in the cytoplasm and inhibit apoptosome formation and proapoptotic activity, respectively [15]. Client proteins for the HSP70 chaperone include mutated BRAF as well as activated phosphorylated focal adhesion kinase (p‐FAK, Y397), associated with melanoma invasiveness and metastasis [16]. Extracellular HSP70 peptide complexes released from tumor cells can also activate anti‐melanoma T cells in vitro [11] and, in vivo, are bound by and internalized via scavenger receptors, such as CD91 and LOX‐1, on the surface of antigen presenting cells [17], [18], [19], [20]. In this way, antigen presenting cells (APCs) such as dendritic cells receive tumor antigens that can be processed and presented on major histocompatibility complex class I and class II molecules to CD8 positive and CD4 positive T cells. This process has been termed HSP vaccination, and there is long‐standing interest in the use of HSP peptide complexes in tumor vaccination strategies [21].
In the present study, we combined percutaneous ablation therapy and intralesional granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) to couple the DC‐stimulatory activity of GM‐CSF with the antigenic stimulation of the ablation procedure. We show that the combination was well tolerated and that ablation therapy increases plasma HSP70 levels, demonstrating effective antigen mobilization by the procedure. We also demonstrate feasibility of using intralesional cytokine therapy for in situ vaccination following ablation therapy. Studies combining HSP70 vaccination with interleukin 2 (IL‐2), or IL‐7/IL‐12/IL‐15 show improved T cell cytotoxic and proliferative capacity [22], and use of intralesional cytokine combinations as well as other investigational agents (i.e., CpG oligodeoxynucleotides, TLR‐agonists) may increase the potency of the approach [23].
Several study limitations are inherent to the small pilot nature of the study. Although a flat 500 mcg dose of GM‐CSF was chosen for purposes of this feasibility study, there is a need to define the optimal dose of GM‐CSF for HSP vaccination. Furthermore, design of this trial did not permit an assessment of the tumor microenvironment, and future studies would benefit from analysis of therapy‐induced changes in the expression of HSPs and checkpoint ligands in biopsy specimens. The chaperone‐based vaccination approach we employed was well tolerated, but it was insufficient to produce clinically relevant immunologic activation. Further rational development of HSP vaccination using percutaneous thermal ablation appears likely to require checkpoint inhibition, which has already shown evidence of synergy in murine metastatic melanoma models [24].
Heat‐shock proteins play an important role in melanoma tumorigenesis and HSP peptide vaccines have shown promising immunostimulatory activity in pre‐clinical and human clinical trials [25]. Future studies exploring combinations with immune checkpoint inhibitors with/without GM‐CSF are warranted and will likely translate into improved outcomes for patients managed with percutaneous ablation therapy for metastatic melanoma.
Figures and Tables
Figure 1.
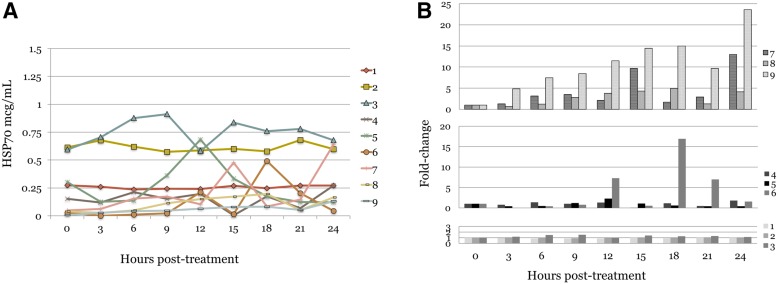
Plasma HSP70 levels. Baseline circulating HSP70 was detectable in all patients. (A): Individual patient levels of plasma HSP70 over 24 hours shown for heat shock therapy (HST), RFA, and cryoablation cohorts. (B): HSP70 fold‐change above baseline level measurements for patients receiving cryoablation (top, arm C), RFA (middle, arm B), and HST (lower, arm A), with sustained elevations of HSP70 seen in patients receiving cryoablation therapy.
Abbreviations: HSP70, 70 kilodalton heat‐shock protein; RFA, radiofrequency ablation.
Figure 2.
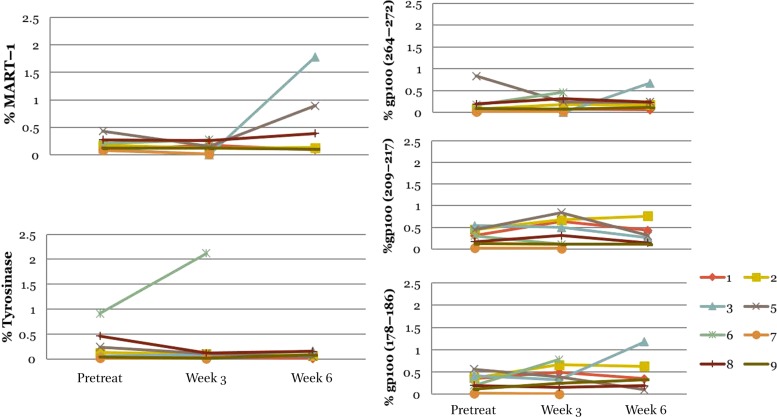
Major histocompatibility complex‐tetramer testing identified cluster of differentiation 8 positive (CD8+) cytotoxic T lymphocytes (CTLs) for each tumor antigen. Pre‐specified criteria for positive tetramer results was >0.02% tetramer positive CD8+ CTLs if baseline negative, or >2‐fold increase in the baseline detectable number. We detected low baseline levels of peripheral CD8+ CTLs against known melanoma antigens MART‐1, GP100, and Tyrosinase (7 of 8 evaluable patients). There was no significant induction of CD8+ CTL responses to melanoma antigens following treatment.
Abbreviations: GP100, glycoprotein 100; MART‐1, melanoma antigen recognized by T cells 1.
Acknowledgments
We wish to thank Jacob B. Allred for statistical input, and William J. Charboneau for procedural assistance and expertise.
Footnotes
ClinicalTrials.gov Identifier: NCT00568763
Sponsor(s): Mayo Clinic
Principal Investigators: Svetomir N. Markovic, James M. Heun, Evidio Domingo‐Musibay
IRB Approved: Yes
Disclosures
Matthew Callstrom: Neuwave Medical, Medtronic, Thermedical (C/A), Galil Medical (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 1995;181:193–201. [PubMed] [Google Scholar]
- 2. Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau PK, Ascierto PA, McArthur G. Melanoma: The intersection of molecular targeted therapy and immune checkpoint inhibition. Curr Opin Immunol 2016;39:30–38. [DOI] [PubMed] [Google Scholar]
- 4. Morris ZS, Guy EI, Francis DM et al. In situ tumor vaccination by combining local radiation and tumor‐specific antibody or immunocytokine treatments. Cancer Res 2016;76:3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 6. Ribas A, Hamid O, Daud A et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–1609. [DOI] [PubMed] [Google Scholar]
- 7. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 8. Algazi AP, Tsai KK, Shoushtari AN et al. Clinical outcomes in metastatic uveal melanoma treated with PD‐1 and PD‐L1 antibodies. Cancer 2016;122:3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doussot A, Nardin C, Takaki H et al. Liver resection and ablation for metastatic melanoma: A single center experience. J Surg Oncol 2015;111:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bendz H, Ruhland SC, Pandya MJ et al. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem 2007;282:31688–31702. [DOI] [PubMed] [Google Scholar]
- 11. Castelli C, Ciupitu AM, Rini F et al. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res 2001;61:222–227. [PubMed] [Google Scholar]
- 12. Andtbacka RH, Ross M, Puzanov I et al. Patterns of clinical response with talimogene laherparepvec (T‐VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann Surg Oncol 2016;23:4169–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harper JW, Bennett EJ. Proteome complexity and the forces that drive proteome imbalance. Nature 2016;537:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kampinga HH, Hageman J, Vos MJ et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009;14:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murphy ME. The HSP70 family and cancer. Carcinogenesis 2013;34:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Budina‐Kolomets A, Webster MR, Leu JI et al. HSP70 inhibition limits FAK‐dependent invasion and enhances the response to melanoma treatment with BRAF inhibitors. Cancer Res 2016;76:2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delneste Y, Magistrelli G, Gauchat J et al. Involvement of LOX‐1 in dendritic cell‐mediated antigen cross‐presentation. Immunity 2002;17:353–362. [DOI] [PubMed] [Google Scholar]
- 18. Theriault JR, Mambula SS, Sawamura T et al. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett 2005;579:1951–1960. [DOI] [PubMed] [Google Scholar]
- 19. Basu S, Binder RJ, Ramalingam T et al. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001;14:303–313. [DOI] [PubMed] [Google Scholar]
- 20. Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol 2006;177:8604–8611. [DOI] [PubMed] [Google Scholar]
- 21. Shevtsov M, Multhoff G. Heat shock protein‐peptide and HSP‐based immunotherapies for the treatment of cancer. Front Immunol 2016;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, Blasczyk R, et al. Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood. 2009;113:3008–3016. [DOI] [PubMed] [Google Scholar]
- 23. Sluijter BJ, van den Hout MF, Koster BD et al. Arming the melanoma sentinel lymph node through local administration of CpG‐B and GM‐CSF: Recruitment and activation of BDCA3/CD141(+) dendritic cells and enhanced cross‐presentation. Cancer Immunol Res 2015;3:495–505. [DOI] [PubMed] [Google Scholar]
- 24. Geng H, Zhang GM, Xiao H et al. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD‐1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer 2006;118:2657–2664. [DOI] [PubMed] [Google Scholar]
- 25. Murshid A, Gong J, Stevenson MA et al. Heat shock proteins and cancer vaccines: Developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines 2011;10:1553–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]