This review article reports the clinical management of potential toxicities and drug interactions seen with the use of CDK4/6 inhibitors in breast cancer, with a focus on palbociclib and ribociclib, and summarizes practical management strategies for the oncologist.
Keywords: Breast cancer, Cyclin‐dependent kinases, Drug toxicity
Abstract
Aberrations of the cell cycle are pervasive in cancer, and selective cell cycle inhibition of cancer cells is a target of choice for a number of novel cancer therapeutics. Cyclin‐dependent kinases (CDKs) are key regulatory enzymes that control cell cycle transitions and the commitment to cell division. Palbociclib and ribociclib are both orally active, highly selective reversible inhibitors of CDK4 and CDK6 that are approved by the U.S. Food and Drug Administration (FDA) for hormone receptor‐positive metastatic breast cancer in combination with specific endocrine therapies. A third oral CDK4/6 inhibitor, abemaciclib, received Breakthrough Therapy designation status from the FDA and is also being developed in breast cancer. The most common adverse events associated with palbociclib and ribociclib are hematologic, particularly neutropenia. However, the neutropenia associated with CDK4/6 inhibitors is distinct from chemotherapy‐induced neutropenia in that it is rapidly reversible, reflecting a cytostatic effect on neutrophil precursors in the bone marrow. Most hematologic abnormalities seen with CDK4/6 inhibitors are not complicated and are adequately managed with standard supportive care and dose adjustments when indicated. Cytopenias are less prevalent with abemaciclib, although fatigue and gastrointestinal toxicity is more common with this agent. This review focuses on the clinical management of potential toxicities and drug interactions seen with the use of CDK4/6 inhibitors in breast cancer, with a focus on palbociclib and ribociclib, and summarizes practical management strategies for an oncologist.
Implications for Practice.
The emergence of modern cyclin‐dependent kinase (CDK) inhibitors has changed the treatment paradigm for metastatic hormone receptor (HR)‐positive breast cancer. Palbociclib, ribociclib, and abemaciclib are highly selective reversible inhibitors of CDK4 and CDK6. Palbociclib is U.S. Food and Drug Administration (FDA)‐approved in the first‐ and second‐line settings in combination with endocrine therapy for HR‐positive metastatic breast cancer. Ribociclib is FDA‐approved in the first‐line setting. Abemaciclib has received FDA Breakthrough Therapy designation status. This review focuses on the clinical management of potential toxicities and drug interactions seen with the use of CDK4/6 inhibitors in breast cancer.
摘要
癌症中细胞周期发生改变的情况非常常见, 许多新癌症疗法的目标是选择性抑制癌细胞的细胞周期。细胞周期蛋白依赖性激酶(CDK)是控制细胞周期变化和限定细胞分裂的关键调节酶。Palbociclib和ribociclib均为口服活性的CDK4与CDK6高选择性可逆抑制剂, 已获美国食品药品监督管理局批准用于联合特定内分泌疗法治疗激素受体阳性转移性乳腺癌。第三种口服CDK4/6抑制剂是abemaciclib, 其已经被FDA授予”突破性疗法认定”资格, 目前正被开发用于治疗乳腺癌。与palbociclib和ribociclib相关的最常见不良事件是血液学事件, 尤其是中性粒细胞减少症。然而, 与CDK4/6抑制剂相关的中性粒细胞减少症与化疗诱导的中性粒细胞减少症的不同之处在于前者快速可逆, 反映了其对骨髓中中性粒细胞前体的细胞生长抑制作用。与CDK4/6抑制剂相关的大多数血液学异常并不复杂, 可以通过标准支持性治疗和调整剂量(如必要)进行充分管理。虽然abemaciclib诱发疲乏和胃肠道毒性的情况更为常见, 但导致血细胞减少的情况并不常见。本综述主要论述使用CDK4/6抑制剂(主要为palbociclib和ribociclib)治疗乳腺癌的潜在毒性的临床管理和药物相互作用, 并总结肿瘤医师的实际管理策略。
Introduction
At a fundamental level, abnormal cellular division and migration represents the hallmark of cancer. Cyclin‐dependent kinases (CDKs), including CDK1, CDK2, CDK4, and CDK6, are key regulatory enzymes that control cell cycle transitions and the commitment to cell division [1], [2], [3], [4]. Cyclin‐dependent kinase 4 and CDK6 regulate the transition from the G1 to S phase, CDK2 regulates transition through the S phase, and CDK1 regulates the transition from the G2 to the M phase [5]. In animals and humans, much of the control over cell cycle entry is determined by CDK4 and CDK6 [6], [7].
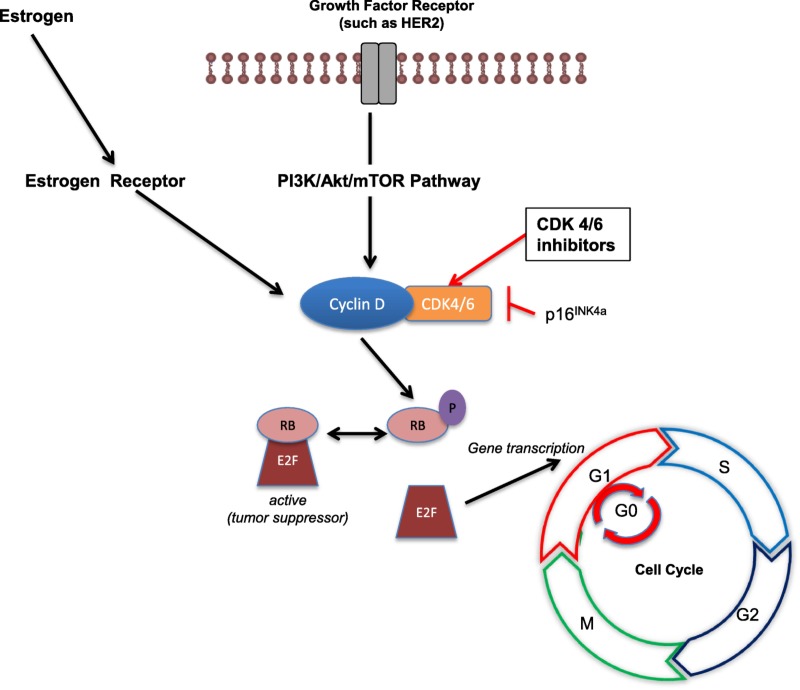
Transition from G1 to the S phase, a critical phase in cell division, is controlled by the tumor suppressor retinoblastoma (Rb) protein [8]. Retinoblastoma is a key negative regulator of the cell cycle because it prevents premature cell division by binding the E2F transcription factors to inhibit G1/S transition [8]. Inactivation of Rb, which occurs via sequential phosphorylation of the protein, allows cellular division to proceed [9]. During G1, growth signals allow cyclin D to complex with either CDK4 or CDK6, leading to phosphorylation of Rb, which releases E2F to drive expression of genes required for S‐phase entry and progression through the cell cycle (Fig. 1) [8].
Figure 1.
Role of cyclin‐dependent kinase 4/6 inhibitors in halting cellular division.
Abbreviations: Akt, protein kinase B; CDK, cyclin‐dependent kinase; E2F, E2 factor; G, growth; HER2, human epidermal growth factor receptor 2; M, mitosis; mTOR, mechanistic target of rapamycin; P, phosphate; PI3K, phosphoinositide 3‐kinase; RB, retinoblastoma tumor suppressor protein; S, synthesis.
Cyclin‐dependent kinase 4 and CDK6, both serine/threonine kinases, are structurally related proteins with many biochemical and biological similarities [6]. In terms of therapeutics, CDK4 and CDK6 are considered functionally equivalent in their ability to phosphorylate Rb, and inhibition of both kinases is likewise of considerable interest [10]. This review will focus on clinical management of potential toxicities and drug interactions seen with the use of CDK4/6 inhibitors in breast cancer and summarize practical management strategies for an oncologist.
Dysregulation of the CDK4/6–Rb pathway in breast cancer
The CDK4/6–Rb axis is critical to cell cycle entry, and therefore it is to be expected that the vast majority of cancers disrupt this axis to promote growth [2], [11]. The majority of estrogen receptor‐positive (ER+) and human epidermal growth factor receptor 2 (HER2)‐positive breast cancers maintain functioning Rb, and, potentially, susceptibility to CDK4/6 inhibitors [12], [13], [14]. Cyclin D1 amplification occurs in an estimated 15% of breast cancers, particularly ER+ breast cancer [15]. Loss of proteins in the INK4 and Cip/Kip families, as well as amplification of CDK4 and CDK6, have also been noted in breast cancer [16], [17]. In particular, in ER+ breast cancer, estrogen has been shown to increase the rate of progression from the G1 to the S phase, where the estrogen effector is the cyclin D1‐CDK4/6–Rb complex [18], [19], [20]. Binding of estrogen to ER‐alpha drives cyclin D1 transcription, with activation of CDK4/6 and phosphorylation of Rb leading to subsequent cell cycling [21], [22], [23]. Similarly, HER2‐induced cellular proliferation is mediated via the CDK4/6–Rb axis, and knockdown of cyclin‐D in mice makes them resistant to tumors induced by the neu oncogene [24].
CDK4/6 Inhibitor Development in Breast Cancer
The development of selective inhibitors of both CDK4 and CDK6 has markedly changed the perception of CDKs as therapeutic targets in cancer after underwhelming results and unacceptable toxicity were seen with pan‐CDK inhibitors such as flavopiridol (alvocidib) in the early 2000s [17]. Palbociclib is an orally active pyridopyrimidine that is a potent first‐in‐class, highly selective reversible inhibitor of CDK4 and CDK6 [25]. As expected based on the biology of the G1–S transition, the effects of palbociclib are dependent on the presence of a functional Rb protein, and no activity was seen in Rb‐deficient cells [10], [25]. Parallel drug discovery efforts resulted in the development of the drugs abemaciclib and ribociclib [26], [27], [28], [29], [30], [31]. Abemaciclib and ribociclib are both orally bioavailable highly selective small molecule reversible inhibitors of CDK4/6. The selectivity of all three compounds is theorized to reflect binding to the specialized ATP‐binding pocket of CDK4 and CDK6 with specific interactions with residues in the ATP‐binding cleft [17].
Finn and colleagues tested palbociclib in vitro on molecularly characterized human breast cancer cell lines and found that sensitivity to palbociclib varied based on molecular phenotype. Estrogen receptor‐positive cell lines with luminal features were found to be the most sensitive and basal cell lines were found to be resistant [32]. The combination of palbociclib with tamoxifen was tested in vitro in ER+ human breast cancer cell lines and demonstrated a synergistic interaction [32]. Similar to results seen with tamoxifen, the combination of palbociclib and trastuzumab was synergistic in sensitive HER2‐amplified cell lines [32]. Furthermore, combination studies carried out in cell lines and primary tumor explants have illustrated that CDK4/6 inhibition with palbociclib provides a complementary mechanism of action to ado‐trastuzumab emtansine, and efficiently suppresses the proliferation of residual HER2‐positive tumor cell populations that survive ado‐trastuzumab emtansine [33]. These preclinical observations led to the development of several clinical trials evaluating the combination of CDK4/6 inhibitors with endocrine therapy, and, more recently, with HER2‐directed therapies.
Clinical Experience
Select phase II and phase III studies of CDK4/6 inhibitors in breast cancer are outlined in Table 1.
Table 1. Select phase II and III studies of cyclin‐dependent kinase 4/6 inhibitors in breast cancer.
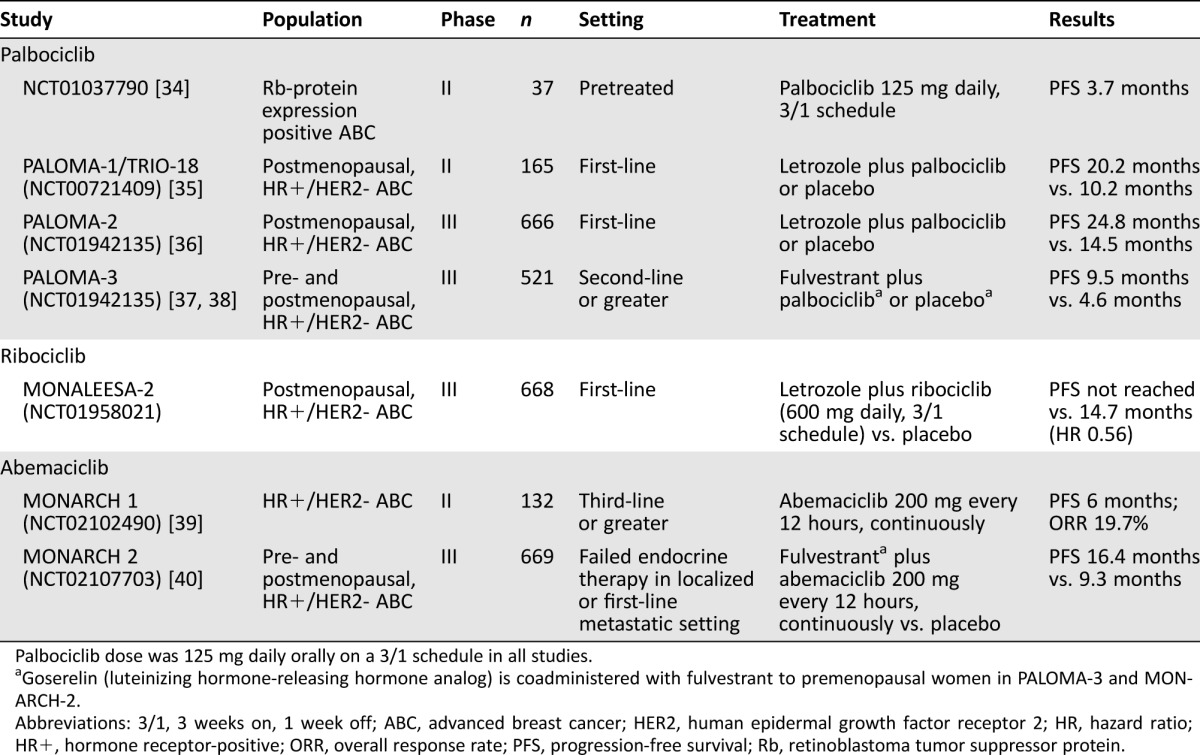
Palbociclib dose was 125 mg daily orally on a 3/1 schedule in all studies.
Goserelin (luteinizing hormone‐releasing hormone analog) is coadministered with fulvestrant to premenopausal women in PALOMA‐3 and MONARCH‐2.
Abbreviations: 3/1, 3 weeks on, 1 week off; ABC, advanced breast cancer; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; HR+, hormone receptor‐positive; ORR, overall response rate; PFS, progression‐free survival; Rb, retinoblastoma tumor suppressor protein.
Palbociclib.
Early trials studying palbociclib in advanced solid tumors demonstrated encouraging results in breast cancer [34], [40], [41]. The PALOMA‐1 trial was the basis for the U.S. Food and Drug Administration (FDA) granting palbociclib accelerated approval in February 2015 after it had received Breakthrough Therapy designation in April 2013 [42]. In this trial, 165 previously untreated patients with metastatic HR‐positive/HER2‐negative breast cancer were randomized to palbociclib plus letrozole or letrozole alone. Palbociclib significantly increased progression‐free survival (PFS) compared with letrozole alone, with a median PFS of 10.2 months for the letrozole group and 20.2 months for the palbociclib plus letrozole group (hazard ratio [HR] 0.488; one‐sided p < .001) [35]. A phase 3 confirmatory trial, PALOMA‐2 (NCT01942135), confirmed these findings with a median PFS of 24.8 versus 14.5 months, respectively, for patients treated with palbociclib or placebo in addition to letrozole (HR 0.58; p < .001) [36]. Initial overall survival (OS) results from PALOMA‐1 demonstrate a statistically non‐significant trend towards an improvement in OS in the palbociclib group (37.5 months vs. 33.3 months, respectively, HR 0.837, p = .280) [44].
The PALOMA‐3 trial was a double‐blind placebo‐controlled, randomized, phase 3 study of palbociclib added to the ER down‐regulator fulvestrant in previously treated HR‐positive, HER2‐negative metastatic breast cancer patients [37]. Both pre‐ and postmenopausal women were included in the study. The addition of palbociclib improved the median PFS as compared with fulvestrant alone, from 4.6 months to 9.5 months (HR 0.46; p < .001) [37], [38]. Based on these results, the FDA also approved palbociclib for use in combination with fulvestrant for disease progression following endocrine therapy. A number of ongoing studies are exploring palbociclib in a number of disease settings for breast cancer, including adjuvant and neoadjuvant studies, and in combination with other targeted agents [43].
Ribociclib.
Ribociclib received FDA Breakthrough Therapy designation in August 2016 and full approval in March 2017 based primarily on the phase III MONALEESA‐2 (NCT01958021) trial exploring ribociclib in combination with letrozole as first‐line therapy in women with HR‐positive/HER2‐negative metastatic breast cancer [44]. The trial met its primary endpoint, with the median duration of PFS not reached in the ribociclib group (95% confidence interval [CI], 19.3 to not reached) versus 14.7 months (95% CI, 13.0–16.5) in the placebo group (HR, 0.56; 95% CI, 0.43–0.72; p = 3.29 × 10−6 for superiority) [44]. For HR‐positive/HER2‐negative metastatic breast cancer, MONALEESA‐7 (NCT02278120) is a phase 3 study exploring ribociclib in combination with endocrine therapy (plus the gonadotropin‐releasing hormone agonist goserelin) in premenopausal women, and MONALEESA‐3 (NCT02422615) is a phase 3 study in men and postmenopausal women exploring fulvestrant with or without ribociclib in both the first‐ and/or second‐line metastatic setting. A number of other ongoing studies are exploring ribociclib in various disease settings for breast cancer and in combination with other targeted agents [43].
Abemaciclib.
Abemaciclib received FDA Breakthrough Therapy designation in October 2015. Among heavily pretreated HR‐positive breast cancer patients (n = 47) enrolled in a phase 1 study of abemaciclib monotherapy, the overall response rate was 31%, with 61% of patients achieving either response or stable disease lasting ≥6 months [45]. This study suggested abemaciclib may have benefit as monotherapy [45]. In the phase 2 single‐arm study, MONARCH 1 (NCT02102490), women with metastatic HR‐positive/HER2‐negative breast cancer with disease progression following both antiestrogen therapy and at least one, but no more than two, lines of chemotherapy in the metastatic setting, received abemaciclib as monotherapy [39]. The confirmed objective response rate was 19.7% and median PFS was 6.0 months [39]. The MONARCH 3 (NCT02246621) and MONARCH 2 (NCT02107703) trials are both large phase 3, randomized, double‐blind, placebo‐controlled trials further evaluating the combination of abemaciclib plus endocrine therapy. Recent results from MONARCH 2 exploring the addition of abemaciclib to fulvestrant demonstrated a median PFS of 16.4 months for abemaciclib plus fulvestrant compared with 9.3 months in the placebo plus fulvestrant group (HR: 0.553; p < .001) [40].
Pharmacology and Dosing Schedule
Palbociclib.
In all three PALOMA trials, palbociclib was dosed at 125 mg daily for 3 weeks followed by 1 week off based on earlier work determining the recommended phase II dose (RP2D) [40], [41]. The recommended initial dose of palbociclib is a 125 mg capsule taken orally once daily, with food, for 21 consecutive days, followed by 7 days off treatment, constituting a total cycle of 28 days [46]. Absorption and drug exposure were found to be low in the fasted state in a portion of the population, which was increased when administered with food. Therefore, taking palbociclib on an empty stomach could reduce drug levels and may compromise effectiveness in a subset of patients [46]. The FDA approval for palbociclib for first‐line treatment of metastatic HR‐positive/HER2‐negative breast cancer was initially in combination with letrozole 2.5 mg once daily, but has been updated to include any of the three approved aromatase inhibitors (letrozole, anastrozole, or exemestane) [48].
Absorption and drug exposure were found to be low in the fasted state in a portion of the population, which was increased when administered with food. Therefore, taking palbociclib on an empty stomach could reduce drug levels and may compromise effectiveness in a subset of patients.
In vitro and in vivo studies indicated that palbociclib undergoes hepatic metabolism in humans and is primarily metabolized by CYP3A and sulfotransferase (SULT) enzyme SULT2A1 [46]. Concomitant use of strong CYP3A inhibitors should be avoided, but if patients must be coadministered a strong CYP3A inhibitor, the recommendation is to reduce the palbociclib dose to 75 mg once daily [46]. If the strong inhibitor is discontinued, the dose of palbociclib can be increased (after 3–5 half‐lives of the inhibitor) to the dose used prior to the initiation of the strong CYP3A inhibitor [46]. Additionally, coadministration of a strong CYP3A inducer (rifampin) decreased the plasma exposure of palbociclib in healthy subjects by 85%, and therefore it is recommended to avoid coadministration of strong CYP3A inducers [46]. In vivo, palbociclib is a weak, time‐dependent inhibitor of CYP3A. Lastly, the dose of a sensitive CYP3A substrate with a narrow therapeutic index may need to be reduced because palbociclib could increase its exposure (Table 2) [46].
Table 2. Potential drug‐drug interactions with palbociclib and ribociclib [46], [47], [48].
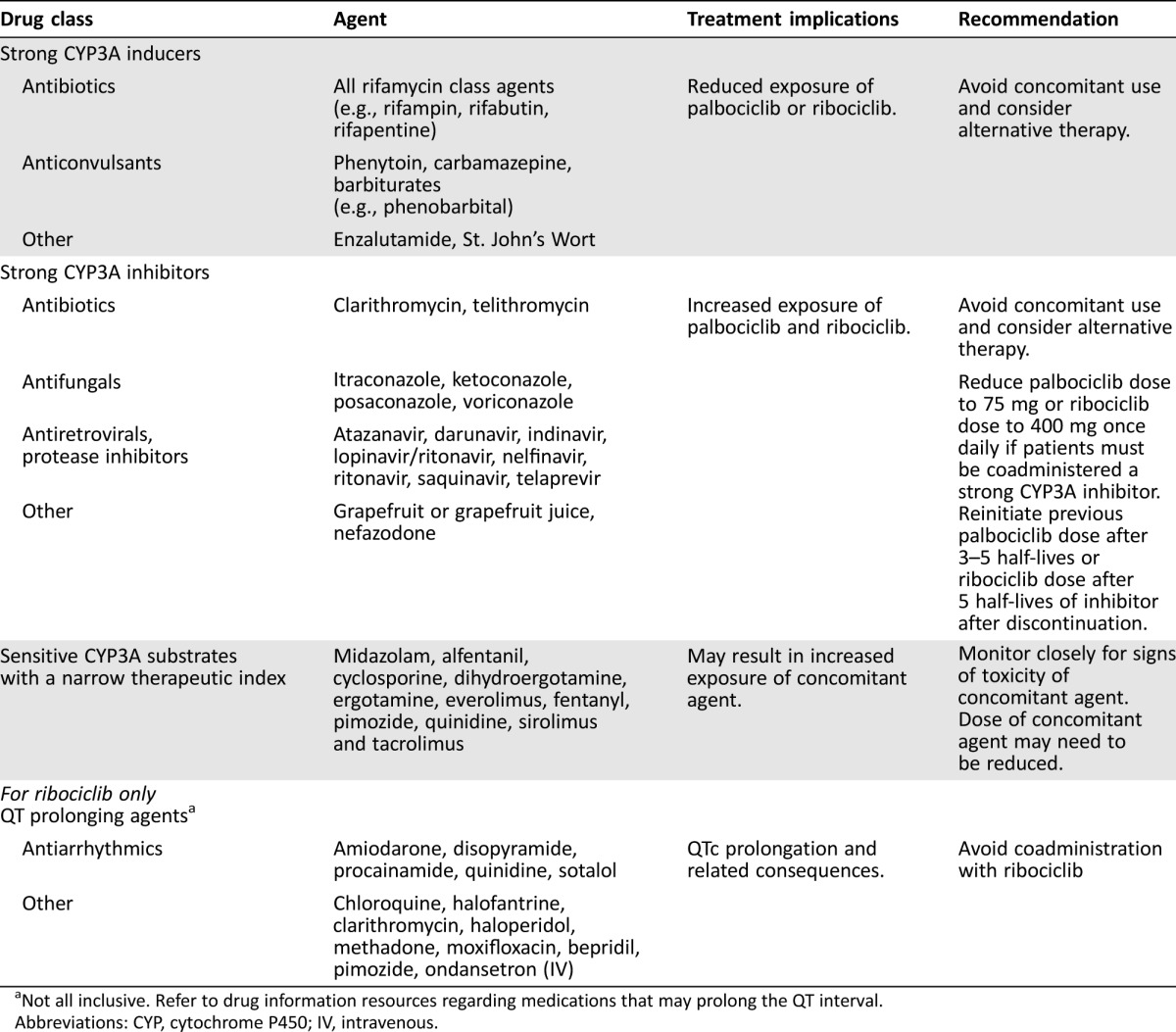
Not all inclusive. Refer to drug information resources regarding medications that may prolong the QT interval.
Abbreviations: CYP, cytochrome P450; IV, intravenous.
Ribociclib.
The recommended initial dose of ribociclib is 600 mg taken orally once daily (preferably in the morning) for 21 consecutive days, followed by 7 days off treatment, constituting a total cycle of 28 days [46]. Absorption is not affected by food; therefore, it may be taken with or without food. The FDA approval for ribociclib for first‐line treatment of metastatic HR‐positive/HER2‐negative breast cancer is in combination with any of the three approved aromatase inhibitors (letrozole, anastrozole, or exemestane), which are given continuously throughout the 28‐day cycle [48].
Ribociclib undergoes extensive hepatic metabolism, the majority of which is mediated by CYP3A4. As such, its elimination may be affected by CYP3A4 inhibitors or inducers [48], [49]. Concomitant use of strong CYP3A inhibitors should be avoided and alternative therapy should be sought. If a strong CYP3A inhibitor must be used concomitantly with ribococlib, the dose of ribociclib should be reduced to 400 mg once daily. If the strong inhibitor is discontinued, the dose of ribociclib should be increased (after 5 half‐lives of the inhibitor) to the dose used prior to the initiation of the strong CYP3A inhibitor [48]. Concomitant use of strong CYP3A inducers should be avoided because plasma exposure of ribociclib was decreased by 89% with coadministration of rifampin (a strong CYP3A4 inducer) in healthy volunteers [48]. Ribociclib also inhibits CYP3A4 in a time‐dependent manner and is a reversible CYP1A2 inhibitor [48]. Concomitant use of CYP3A substrates with a narrow therapeutic index should be done with caution, and may require a dose reduction of the CYP3A substrate, as ribociclib could increase their exposure [48].
Abemaciclib.
Dosing for abemaciclib is 200 mg every 12 hours, given continuously throughout the cycle [45]. Abemaciclib undergoes extensive hepatic metabolism in humans. In vitro and in vivo studies have identified CYP3A as the enzyme responsible for the majority of the CYP‐mediated metabolism of abemaciclib and its metabolites [50]. This suggests that concomitant use of strong CYP3A inducers or inhibitors should be avoided with abemaciclib.
CDK4/6 Inhibitor Toxicity Overview
Palbociclib.
In PALOMA‐1, the most common adverse events reported for the palbociclib plus letrozole group were neutropenia, leukopenia, and fatigue. Overall, 27 (33%) patients in the palbociclib plus letrozole group had dose interruptions because of adverse events, compared with only three (4%) patients in the letrozole group. Adverse events in the PALOMA‐2 trial are outlined in Table 3. In PALOMA‐3, the most common adverse events reported for the palbociclib plus fulvestrant group were neutropenia, leukopenia, fatigue, and nausea [37]. Overall, nine (2.6%) patients in the palbociclib plus fulvestrant group discontinued therapy due to adverse events, compared with three (1.7%) patients in the fulvestrant group.
Table 3. Common adverse events for the palbociclib plus letrozole group in PALOMA‐2 [51] and the ribociclib plus letrozole group in MONALEESA‐2 [44].
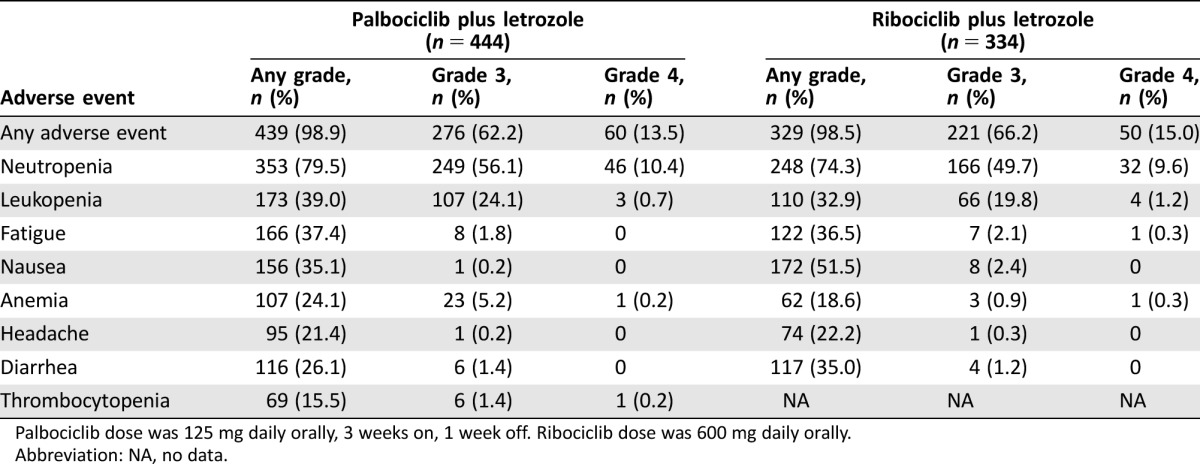
Palbociclib dose was 125 mg daily orally, 3 weeks on, 1 week off. Ribociclib dose was 600 mg daily orally. Abbreviation: NA, no data.
Ribociclib.
As with palbociclib, hematologic adverse events, including neutropenia, are common with ribociclib; therefore, a 1‐week resting period is incorporated into dosing regimens in most trials, although continuous dosing continues to be explored [17], [43]. In MONALEESA‐2, the most common adverse events of any grade were neutropenia, nausea, infections, fatigue, and diarrhea (Table 3) [44]. The majority of the non‐hematologic adverse events were grade 1 or 2 [44]. The most common grade 3 or 4 adverse events were neutropenia, leukopenia, hypertension, increased alanine aminotransferase (ALT) level, lymphopenia, and increased asparatate aminotransferase (AST) level [44]. An increase of more than 60 msec from baseline in the Fridericia's correction formula (QTcF) interval occurred in nine patients (2.7%) in the ribociclib group, and 11 patients (3.3%) had at least one average QTcF interval of more than 480 msec after baseline [44]. No cases of Torsades de Pointes were reported in MONALEESA‐2 [44].
Abemaciclib.
Abemaciclib is structurally distinct from palbociclib and ribociclib, demonstrating greater selectivity for CDK4 compared with CDK6 [29]. Hematologic adverse events, including neutropenia, appear to be less common with abemaciclib, while fatigue and gastrointestinal‐related toxicity are more predominant [17]. The most common treatment‐related adverse events in the MONARCH 1 (NCT02102490) study of abemaciclib monotherapy in women with pretreated metastatic HR‐positive/HER2‐negative breast cancer were creatinine increase (98.5%; grade 3 = 0.8%), diarrhea (90.2%; grade 3 = 19.7%), neutropenia (87.7%; grade 3/4 26.9%), and fatigue (65.2%; grade 3 = 12.9%) [39]. Abemaciclib‐induced diarrhea was manageable with conventional antidiarrheal agents or dose reduction [39]. Diarrhea was experienced early on (median time to onset = 7 days) and generally resolved quickly (median duration of grade 2 = 7.5 days, grade 3 = 4.5 days). Regarding the creatinine increase, abemaciclib is a competitive inhibitor of efflux transporters of creatinine (OCT2, MATE1, and MATE2‐K); therefore, cystatin C calculated glomerular fraction rate was performed to more accurately assess renal function, and this was not raised [39]. In general, dose interruptions and adjustments are typically considered for grade 3 or higher toxicities.
Practical Management Strategies for Palbociclib and Ribociclib Toxicities
Palbociclib and Ribociclib: Hematologic Adverse Events and Management.
Hematologic adverse events are common with CDK4/6 inhibitors. Cyclin‐dependent kinase 6 is particularly important in promoting the proliferation of hematological precursors, and its inhibition is an on‐target effect that results in the cytopenias seen with CDK4/6 inhibition [52], [53]. In general, most hematologic abnormalities seen with CDK4/6 inhibitors can be adequately managed with standard supportive care [40].
A few considerations related to CDK4/6‐induced neutropenia should be noted. First, although neutropenia is a common side effect of cytotoxic agents, the neutropenia associated with CDK4/6 inhibitors is distinct in that it is rapidly reversible, reflecting a cytostatic effect on neutrophil precursors in the bone marrow [17]. Recent work to explore the mechanism of hematologic toxicity induced by palbociclib utilized human bone marrow mononuclear cells to demonstrate that palbociclib‐induced bone marrow suppression occurred through cycle arrest with no apoptosis at clinically relevant concentrations and resumed proliferation following palbociclib withdrawal, thereby demonstrating pharmacologic quiescence [54]. In contrast, exposure of the same cells to chemotherapeutic agents resulted in apoptotic cell death [54]. Accordingly, palbociclib and ribociclib are dosed intermittently to accommodate a break for hematological recovery. Second, while neutropenia is a common adverse event, the incidence of febrile neutropenia is very low (0% in PALOMA‐1, 0.6% in PALOMA‐3, 1.5% in MONALEESA‐2). Third, the median duration of grade ≥3/4 neutropenia is around 7 days, and typically resolves with drug hold [55]. Fourth, neutropenia is proportional to exposure, and often decreases with subsequent cycles, suggesting a lack of cumulative toxicity and effective early dose reductions when indicated.
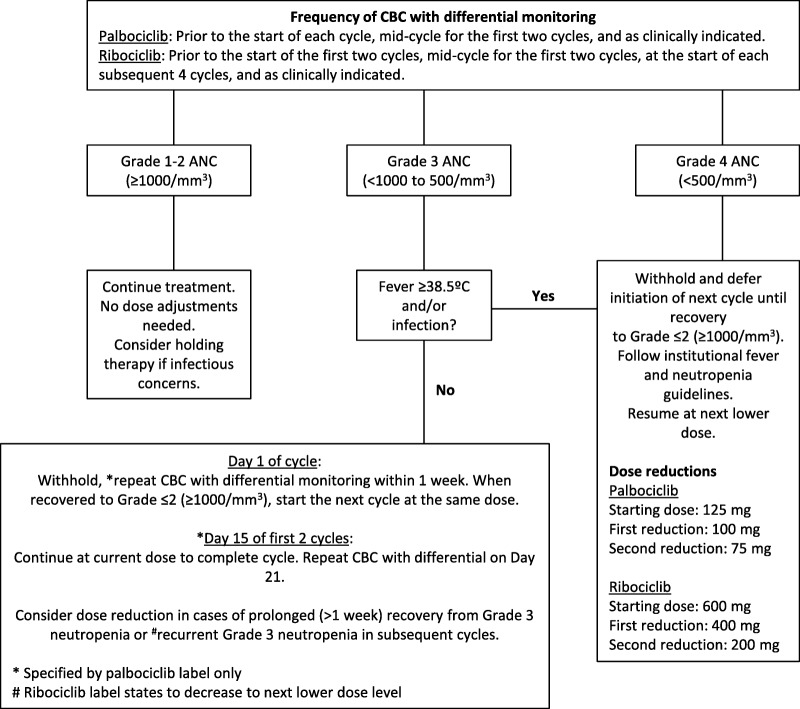
Complete blood count (CBC) with differential needs to be monitored for all patients on CDK4/6 inhibitors. For palbociclib and ribociclib, it is recommended to check a CBC with differential at baseline and every 2 weeks for the first two cycles. For palbociclib, it is recommended to then check prior to each 28‐day cycle and as clinically indicated. For ribociclib, it is recommended to check at the beginning of each subsequent four cycles, and then as clinically indicated. Palbociclib and ribociclib should be held at an absolute neutrophil count (ANC) less than 1,000/mm3 (grade 3) on the first day of each cycle. Further details on managing day 1 and day 15 neutropenia are included in Figure 2. Neutropenia is considered complicated if it is associated with a documented infection or fever (typically greater than or equal to 38.5°C/100.4°F). Criteria for retreatment with palbociclib or ribociclib following treatment interruption for neutropenia has generally been when the ANC is greater than 1,000/mm3 with no fever. Management of palbociclib‐ and ribociclib‐induced neutropenia is outlined in Figure 2. In PALOMA‐3, if a patient was already receiving palbociclib 75 mg per day and required another dose reduction, 75 mg daily on a 2 weeks on, 2 weeks off schedule could be utilized [56]. For grade 3 (<50,000–25,000/mm3) and grade 4 (<25,000 mm3) thrombocytopenia, treatment was held until recovery to 50,000/mm3 and resumed at one dose level lower. If recurrent grade 3 or 4 thrombocytopenia occurred, or if recovery of platelet count to 50,000/mm3 took greater than 2 weeks, two dose reductions were instituted [56]. Similarly, for grade 3 (hemoglobin <8.0 g/dL) and grade 4 (life‐threatening consequences; urgent intervention indicated) anemia, treatment should be held until improvement to grade 2 (hemoglobin <10.0–8.0 g/dL) or better and resumed at one dose level lower. Packed red blood cell transfusions should be per institutional protocol.
Figure 2.
Management of palbociclib and ribociclib‐related neutropenia.
Abbreviations: ANC, absolute neutrophil count; CBC, complete blood count.
Palbociclib and Ribociclib: Gastrointestinal Adverse Events and Management.
Nausea and diarrhea can occur with the use of palbociclib and ribociclib, although rates of grade 3 or 4 gastrointestinal toxicities are low (Table 3). Palbociclib and ribociclib both carry a minimal to low emetic risk, thus prophylactic antiemetics are not routinely indicated [57]. Nausea and vomiting should be treated with routine antiemetics, such as metoclopramide, prochlorperazine, haloperidol, or serotonin 5‐HT3 antagonists on an as‐needed basis [57]. Caution should be taken when coprescribing antiemetics with ribociclib due to the risk of QT prolongation. In the absence of signs of infection, diarrhea can be safely managed with standard non‐pharmacologic (hydration, dietary modification, avoidance of other offending agents) and pharmacologic (anti‐diarrheal agents such as loperamide, deodorized tincture of opium, diphenoxylate/atropine, octreotide) interventions [58]. Prophylactic treatment can be considered for more significant diarrhea.
Nausea and vomiting should be treated with routine antiemetics, such as metoclopramide, prochlorperazine, haloperidol, or serotonin 5‐HT3 antagonists on an as‐needed basis. Caution should be taken when coprescribing antiemetics with ribociclib due to the risk of QT prolongation.
Other Toxicities: General Management.
In general, for non‐hematologic toxicities, no dose hold or dose adjustment are required for grade 1 or 2 toxicities unless they are persistent and bothersome to the patient. For grade 3 or higher toxicities that persist despite medical treatment, palbociclib and ribociclib should be held until symptoms resolve either to grade 1 or less, or to grade 2 if not considered a safety risk for the patient. Palbociclib and ribociclib should then be resumed at the next lower dose.
Palbociclib: Other Toxicities.
A variety of other non‐hematologic and non‐gastrointestinal events have been reported with palbociclib, although the vast majority are grade 1–2 (Table 3). The other most common toxicity seen with palbociclib is fatigue, which is often mild [37]. Thromboembolic events have rarely been reported with palbociclib, although it is notably listed as precaution in the prescribing information. In PALOMA‐1, two patients (0.6%) had a non‐serious event, and four patients (1.2%) had a serious event (three pulmonary emboli and one deep‐vein thrombosis) in the palbociclib plus fulvestrant group, versus none in the fulvestrant alone group [37]. Also, palbociclib may cause embryo‐fetal toxicity and effective contraception is recommended [46]. While typically grade 1, the possibility of alopecia is notable, with grade 1 alopecia seen in 33% of patients in the palbociclib group compared with 16% in the letrozole alone group in PALOMA‐2. As noted above, dose hold and subsequent dose decrease are typically considered for grade 3 or higher toxicities.
Ribociclib: Other Toxicities.
Fatigue, transaminase elevation, and QTc prolongation are other notable toxicities reported with ribociclib [44], [48]. Ribociclib prolongs the QT interval in a concentration‐dependent manner. A baseline electrocardiogram (ECG) should demonstrate a QTcF of less than 450 msec prior to initiating therapy. Patients that have or are at a significant risk of developing QTc prolongation should not receive ribociclib. The QT interval should be assessed via ECG at baseline, approximately day 14, the beginning of cycle two, and then as clinically necessary. Avoidance of QT‐prolonging agents, as well as appropriate supplementation for electrolyte abnormalities, is recommended. Serum electrolytes should be monitored at baseline, prior to the first six cycles, and as clinically indicated.
Ribociclib has been associated with hepatobiliary toxicity, manifesting as transaminase (AST and/or ALT) elevation. Liver function tests (LFTs) should be checked at baseline, every 2 weeks for the first two cycles, prior to each subsequent four cycles, and as clinically indicated thereafter. More frequent monitoring is recommended in the setting of grade ≥2 abnormalities. Treatment modifications for hepatobiliary toxicity are outlined in Table 4. Similar to palbociclib, alopecia is possible with ribociclib and is typically grade 1. Ribociclib may cause embryo‐fetal toxicity and effective contraception is recommended [50].
Table 4. Ribociclib: Dose modification and management for hepatobiliary toxicity [48].
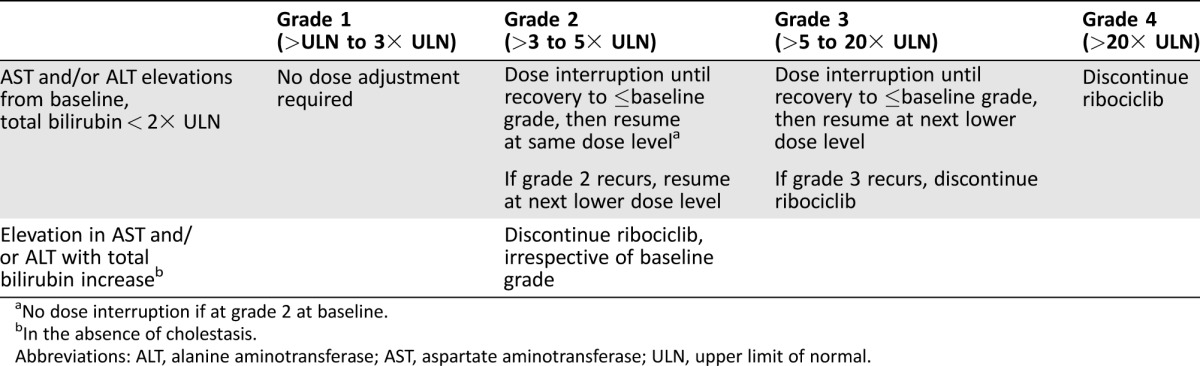
No dose interruption if at grade 2 at baseline.
In the absence of cholestasis.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Palbociclib and Ribociclib: Surgical Procedures.
Count nadir should be avoided at the time of surgery. For palbociclib, it is recommended that patients pursuing surgery hold therapy for 7 days before the surgery and up to 3 weeks following surgery, based on wound healing and recovery. Palbociclib can be resumed once satisfactory wound healing and recovery have occurred. Patients should continue endocrine therapy if palbociclib is held for surgery. There are no specific recommendations for ribociclib surrounding surgery, although we recommend following a similar algorithm to that outlined for palbociclib.
Dose Adjustments for Palbociclib.
Following dose interruption, the palbociclib dose may need to be reduced when treatment is resumed. The first dose reduction is to 100 mg/day and the second and final dose reduction is to 75 mg/day. Once a dose has been reduced for a given patient, dose re‐escalation should typically be avoided. No specific dose adjustments are needed for geriatric use, mild hepatic impairment, or mild to moderate renal impairment. Palbociclib has not been studied in patients with moderate or severe hepatic impairment (total bilirubin >1.5 × ULN and any AST) or in patients with severe renal impairment (CrCl <30 mL/min). Minimal drug is recovered in the urine, suggesting renal impairment would not significantly impact drug levels. A study of palbociclib in renal impairment has been completed, with results pending (NCT02085538).
Dose Adjustments for Ribociclib.
The dose of ribociclib may need to be reduced following dose interruption for toxicity. The first dose reduction is to 400 mg/day and the second dose reduction is to 200 mg/day. Ribociclib should be discontinued if a dose reduction below 200 mg/day is required. Dose re‐escalation is typically not recommended following modification. Ribociclib is commercially available as 200 mg tablets. No specific dose adjustments are needed for geriatric use, mild hepatic impairment, or mild to moderate renal impairment. The recommended starting dose is 400 mg/day for patients with moderate (Child‐Pugh B) or severe (Child‐Pugh C) hepatic impairment. The impact of severe renal impairment (CrCl <30 mL/min) on ribociclib is unknown; however, the renal route contributes to only a small portion of ribociclib elimination. A study of ribociclib in healthy patients with various degrees of renal impairment is currently recruiting participants (NCT02431481).
Conclusion
CDK4/6 inhibitors are generally well‐tolerated oral agents. Palbociclib is currently approved for the first‐line treatment of metastatic HR‐positive breast cancer in combination with letrozole and in the second‐line setting in combination with fulvestrant. Ribociclib is approved for the first‐line treatment of metastatic HR‐positive breast cancer in combination with any aromatase inhibitor. Abemaciclib has been granted Breakthrough Therapy designation by the FDA. The most common adverse event associated with palbociclib and ribociclib is neutropenia; however, febrile neutropenia is rare and the neutropenia is generally easily reversible when the drug is held. Ribociclib is associated with hepatobiliary toxicity and QT prolongation, requiring additional monitoring and dose modification. Gastrointestinal toxicity and fatigue are more prevalent with abemaciclib. Additional research is needed to better understand the toxicity profile and develop management strategies to minimize drug interruptions to optimize the highest possible therapeutic efficacy for patients with breast cancer.
Acknowledgments
Dr. Bardia is supported by National Cancer Institute grant 5K12CA087723‐13. Dr. Spring is supported by National Cancer Institute grant 5T32CA071345‐19.
Contributed equally
Author Contributions
Conception/design: Laura M. Spring, Aditya Bardia
Provision of study material or patients: Laura M. Spring, Mark Zangardi, Aditya Bardia
Collection and/or assembly of data: Laura M. Spring, Mark Zangardi
Data analysis and interpretation: Laura M. Spring, Mark Zangardi, Beverly Moy, Aditya Bardia
Manuscript writing: Laura M. Spring, Mark Zangardi, Aditya Bardia
Final approval of manuscript: Laura M. Spring, Mark Zangardi, Beverly Moy, Aditya Bardia
Disclosures
The authors indicated no financial relationships.
References
- 1. Nurse P, Masui Y, Hartwell L. Understanding the cell cycle. Nat Med 1998;4:1103–1106. [DOI] [PubMed] [Google Scholar]
- 2. Sherr CJ. Cancer cell cycles. Science 1996;274:1672–1677. [DOI] [PubMed] [Google Scholar]
- 3. Malumbres M. Therapeutic opportunities to control tumor cell cycles. Clin Transl Oncol 2006;8:399–408. [DOI] [PubMed] [Google Scholar]
- 4. Hunt T, Nasmyth K, Novák B. The cell cycle. Philos Trans R Soc Lond B Biol Sci 2011;366:3494–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roberts PJ, Bisi JE, Strum JC et al. Multiple roles of cyclin‐dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst 2012;104:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Dev 2013;140:3079–3093. [DOI] [PubMed] [Google Scholar]
- 7. Drapkin R, Le Roy G, Cho H et al. Human cyclin‐dependent kinase‐activating kinase exists in three distinct complexes. Proc Natl Acad Sci U S A 1996;93:6488–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 1995;81:323–330. [DOI] [PubMed] [Google Scholar]
- 9. Wang JY, Knudsen ES, Welch PJ. The retinoblastoma tumor suppressor protein. Adv Cancer Res 1994;64:25–85. [DOI] [PubMed] [Google Scholar]
- 10. Fry DW, Harvey PJ, Keller PR et al. Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427–1438. [PubMed] [Google Scholar]
- 11. Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 2008;8:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosco EE, Knudsen ES. RB in breast cancer: At the crossroads of tumorigenesis and treatment. Cell Cycle 2007;6:667–671. [DOI] [PubMed] [Google Scholar]
- 13. Treré D, Brighenti E, Donati G et al. High prevalence of retinoblastoma protein loss in triple‐negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol 2009;20:1818–1823. [DOI] [PubMed] [Google Scholar]
- 14. Arima Y, Inoue Y, Shibata T et al. Rb depletion results in deregulation of E‐cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial‐to‐mesenchymal transition. Cancer Res 2008;68:5104–5112. [DOI] [PubMed] [Google Scholar]
- 15. Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol 2005;23:4215–4224. [DOI] [PubMed] [Google Scholar]
- 16. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asghar U, Witkiewicz AK, Turner NC et al. The history and future of targeting cyclin‐dependent kinases in cancer therapy. Nat Rev Drug Discov 2015;14:130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butt AJ, McNeil CM, Musgrove EA et al. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: The potential roles of c‐Myc, cyclin D1 and cyclin E. Endocr Relat Cancer 2005;12(suppl 1):S47–S59. [DOI] [PubMed] [Google Scholar]
- 19. Musgrove EA, Lee CS, Buckley MF et al. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci U S A 1994;91:8022–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nair BC, Vadlamudi RK. Regulation of hormonal therapy resistance by cell cycle machinery. Gene Ther Mol Biol: 2008;12:395. [PMC free article] [PubMed] [Google Scholar]
- 21. Altucci L, Addeo R, Cicatiello L et al. Estrogen induces early and timed activation of cyclin‐dependent kinases 4, 5, and 6 and increases cyclin messenger ribonucleic acid expression in rat uterus. Endocrinology 1997;138:978–984. [DOI] [PubMed] [Google Scholar]
- 22. Geum D, Sun W, Paik SK et al. Estrogen‐induced cyclin D1 and D3 gene expressions during mouse uterine cell proliferation in vivo: Differential induction mechanism of cyclin D1 and D3. Mol Reprod Dev 1997;46:450–458. [DOI] [PubMed] [Google Scholar]
- 23. Thangavel C, Dean JL, Ertel A et al. Therapeutically activating RB: Reestablishing cell cycle control in endocrine therapy‐resistant breast cancer. Endocr Relat Cancer 2011;18:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature 2001;411:1017–1021. [DOI] [PubMed] [Google Scholar]
- 25. Toogood PL, Harvey PJ, Repine JT et al. Discovery of a potent and selective inhibitor of cyclin‐dependent kinase 4/6. J Med Chem 2005;48:2388–2406. [DOI] [PubMed] [Google Scholar]
- 26. Rader J, Russell MR, Hart LS et al. Dual CDK4/CDK6 inhibition induces cell‐cycle arrest and senescence in neuroblastoma. Clin Cancer Res 2013;19:6173–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res 2014;20:3379–3383. [DOI] [PubMed] [Google Scholar]
- 28. Zhang YX, Sicinska E, Czaplinski JT et al. Antiproliferative effects of CDK4/6 inhibition in CDK4‐amplified human liposarcoma in vitro and in vivo. Mol Cancer Ther 2014;13:2184–2193. [DOI] [PubMed] [Google Scholar]
- 29. Gelbert LM, Cai S, Lin X et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In‐vivo cell cycle‐dependent/independent anti‐tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tate SC, Cai S, Ajamie RT et al. Semi‐mechanistic pharmacokinetic/pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin‐dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin Cancer Res 2014;20:3763–3774. [DOI] [PubMed] [Google Scholar]
- 31. Kim S, Loo A, Chopra R et al. Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6– Reactivating Rb in cancer. Mol Cancer Ther 2013;12(suppl 11):PR02a. [Google Scholar]
- 32. Finn RS, Dering J, Conklin D et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Witkiewicz AK, Cox D, Knudsen ES. CDK4/6 inhibition provides a potent adjunct to Her2‐targeted therapies in preclinical breast cancer models. Genes Cancer 2014;5:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeMichele A, Sanders Clark A, Heitjan D et al. A phase II trial of an oral CDK 4/6 inhibitor, PD0332991, in advanced breast cancer. J Clin Oncol 2013;31(suppl 15):519–519. [Google Scholar]
- 35. Finn RS, Crown JP, Lang I et al. The cyclin‐dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first‐line treatment of oestrogen receptor‐positive, HER2‐negative, advanced breast cancer (PALOMA‐1/TRIO‐18): A randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 36. Finn RS, Martin M, Rugo HS et al. PALOMA‐2: Primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2– advanced breast cancer (ABC). J Clin Oncol 2016;34(suppl 15)507–507. [Google Scholar]
- 37. Turner NC, Ro J, André F et al. Palbociclib in hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 38. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 39. Dickler MN, Tolaney S, Rugo HS et al. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2‐ breast cancer, after chemotherapy for advanced disease. J Clin Oncol 2016;34(suppl 15):510–510. [Google Scholar]
- 40. Sledge GW, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in patients with HR+/HER2‐ advanced breast cancer who progressed on endocrine therapy. J Clin Oncol 2017;35(suppl 15)1000–1000. [DOI] [PubMed] [Google Scholar]
- 41. Schwartz GK, LoRusso PM, Dickson MA et al. Phase I study of PD 0332991, a cyclin‐dependent kinase inhibitor, administered in 3‐week cycles (Schedule 2/1). Br J Cancer 2011;104:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flaherty KT, Lorusso PM, Demichele A et al. Phase I, dose‐escalation trial of the oral cyclin‐dependent kinase 4/6 inhibitor PD 0332991, administered using a 21‐day schedule in patients with advanced cancer. Clin Cancer Res 2012;18:568–576. [DOI] [PubMed] [Google Scholar]
- 43. Beaver JA, Amiri‐Kordestani L, Charlab R et al. FDA approval: Palbociclib for the treatment of postmenopausal patients with estrogen receptor‐positive, HER2‐negative metastatic breast cancer. Clin Cancer Res 2015;21:4760–4766. [DOI] [PubMed] [Google Scholar]
- 44. Finn R, Crown J, Lang I et al. Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2– advanced breast cancer (PALOMA‐1; TRIO‐18). J Clin Oncol 2017;35:1001a. [Google Scholar]
- 45. Spring L, Bardia A, Modi S. Targeting the cyclin D‐cyclin‐dependent kinase (CDK) 4/6‐retinoblastoma pathway with selective CDK 4/6 inhibitors in hormone receptor‐positive breast cancer: Rationale, current status, and future directions. Discov Med 2016;21:65–74. [PMC free article] [PubMed] [Google Scholar]
- 46. Hortobagyi GN, Stemmer SM, Burris HA et al. Ribociclib as first‐line therapy for HR‐positive, advanced breast cancer. N Engl J Med 2016;375:1738–1748. [DOI] [PubMed] [Google Scholar]
- 47. Patnaik A, Rosen LS, Tolaney SM et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non–small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–753. [DOI] [PubMed] [Google Scholar]
- 48.Palbociclib Prescribing Information . Available at http://labeling.pfizer.com/ShowLabeling.aspx?id= 2191#S14. Accessed May 19, 2017.
- 49.U.S. Food & Drug Administration C for DE and Drug Interactions & Labeling ‐ Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm. Accessed September 25, 2016.
- 50.Kisqali (ribociclib) Prescribing Information. Available at https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. Accessed March 20, 2017.
- 51.U.S. Food & Drug Administration. Oncologic Drugs Advisory Committee > Briefing Information for the November 5, 2013 Meeting of the Pediatric Oncology Subcommittee of the Oncologic Drugs Advisory Committee (PedsODAC). Available at http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/ucm373164.htm. Accessed September 25, 2016.
- 52. Kulanthaivel P, Mahadevan D, Turner PK et al. Abstract CT153: Pharmacokinetic drug interactions between abemaciclib and CYP3A inducers and inhibitors. Cancer Res 2016;76:CT153–CT153. [Google Scholar]
- 53. Finn RS, Martin M, Rugo HS et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 54. Malumbres M, Sotillo R, Santamaría D et al. Mammalian cells cycle without the D‐type cyclin‐dependent kinases Cdk4 and Cdk6. Cell 2004;118:493–504. [DOI] [PubMed] [Google Scholar]
- 55. Hu MG, Deshpande A, Enos M et al. A requirement for cyclin‐dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res 2009;69:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu W, Sung T, Jessen BA et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 2016;22:2000–2008. [DOI] [PubMed] [Google Scholar]
- 57. Finn RS, Crown JP, Ettl J et al. Efficacy and safety of palbociclib in combination with letrozole as first‐line treatment of ER‐positive, HER2‐negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA‐1/TRIO‐18. Breast Cancer Res 2016;18:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Verma S, Bartlett CH, Schnell P et al. Palbociclib in combination with fulvestrant in women with hormone receptor‐positive/HER2‐negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo‐controlled, phase III study (PALOMA‐3). The Oncologist 2016;21:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NCCN Clinical Practice Guidelines in Oncology . Available at https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed September 25, 2016.
- 60. Stein A, Voigt W, Jordan K. Chemotherapy‐induced diarrhea: Pathophysiology, frequency and guideline‐based management. Ther Adv Med Oncol 2010;2:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]