Abstract
Background
Antibiotic prophylaxis (AP) administered prior to invasive procedures in patients at risk of developing infective endocarditis (IE) has historically been the focus of IE prevention. Recent changes in AP guidelines in the US and Europe have substantially reduced the numbers for whom AP is recommended. In the UK, the National Institute for Health and Care Excellence (NICE) guidelines recommended complete cessation of AP in March 2008. We report the impact of these guidelines on AP prescribing; in addition, IE incidence was examined following the introduction of the guidelines.
Methods
We analyzed English AP prescribing data from January 2004 to March 2013 and hospital discharge episode statistics for patients with a primary diagnosis of IE from January 2000 to March 2013.
Findings
AP prescribing rates fell dramatically after introduction of the NICE guidance (10,935 prescriptions/month vs. 2,236 prescriptions/month, p<0·0001). Commencing in March 2008, there was also a significant increase in the number of IE cases/month (0·11 cases/10million/month, CI 0·05–0·16, p<0·0001) above the projected historical trend. By March 2013, there were an additional 35 cases/month than would have been expected if the previous trend had continued. This increase in IE incidence was significant for both ‘high-risk’ and ‘lower-risk’ individuals.
Interpretation
Although our data do not establish a causal relationship, there has been a substantial reduction in AP prescribing and a significant increase in IE incidence in England since introduction of the NICE guidelines in 2008.
Funding
Different aspects of this study were supported by Heart Research UK and Simplyhealth [Grant Ref: RG2632/13/14] and NIDCR R03 grant [Ref: 1R03DE023092-01] from the National Institutes for Health.
Introduction
Infective endocarditis (IE) is uncommon, but has high morbidity and mortality.1 Oral viridans group streptococci (VGS) are implicated as causal organisms in 35–45% of cases.2–5 Antibiotic prophylaxis (AP) prior to invasive dental procedures has been the focus for preventing IE for over 50 years and remains the standard of care for ‘high-risk’ patients in most parts of the world.6,7 The aim of AP is to reduce or eliminate bacteremia8–11 that can cause IE in susceptible individuals. There has never been a randomized clinical trial of AP12 and there is little evidence to support its effectiveness.2,4,9
Until recently, it was the standard of care in most parts of the world to provide AP to patients at ‘high-risk’ (previous IE, prosthetic heart valves or valves repaired with prosthetic material, unrepaired cyanotic congenital heart disease, or certain repaired congenital heart defects) and ‘moderate-risk’ (previous rheumatic fever, heart murmur, or evidence of native valve disease) of IE. In March 2008, the UK National Institute for Health and Care Excellence (NICE) produced new guidance recommending complete cessation of AP.13–15 In contrast, the American Heart Association (AHA)7 and European Society of Cardiology (ESC)6 produced new guidelines in 2007 and 2009, respectively, recommending cessation of AP for ‘moderate-risk’ patients only.
The NICE guidance provided an opportunity for a retrospective secular trend study, analyzed as an interrupted time series, of the effect of AP versus no prophylaxis on the incidence of IE in the entire population of England (population 53·7 million). In a preliminary study, just two years after the introduction of the NICE guidelines, no significant increase in IE incidence was seen despite a 78% reduction in AP prescribing.16 However, concerns were expressed that two years was too soon to detect a clinically significant change.17 Moreover, 2,500 AP prescriptions/month were still being issued at this point, with evidence of targeting of ‘high-risk’ individuals.18 Therefore, the aim of the present study was to examine the effect of the NICE guidelines on AP prescribing and IE incidence over a longer time frame.
Methods
Prior to introduction of the NICE guidelines, a single 3g dose of oral amoxicillin (or a 600mg dose of oral clindamycin in penicillin allergic individuals) was prescribed before invasive dental procedures as AP to those at ‘moderate-risk’ or ‘high-risk’ of developing IE. This dose, timing and mode of administration of amoxicillin and clindamycin are almost uniquely associated with AP prescribing to cover invasive dental procedures in the UK.16 Data on AP prescribing from January 2004 to March 2013 were obtained from the National Health Service Business Services Authority (www.ppa.org.uk/ppa/ppa_main.htm).
IE incidence data and associated in-hospital mortality from January 2000 until March 2013 were obtained using national hospital episode statistics (HES) of inpatient hospital activity, as previously described.16 All patients admitted to United Kingdom hospitals have standard data recorded, including their primary discharge diagnosis (and up to 12 secondary diagnoses) using the ICD-10 coding system (http://apps.who.int/classifications/apps/icd/icd10online). These anonymized data are reported to the warehouse of the Secondary Uses Service (www.connectingforhealth.nhs.uk/systemsandservices/sus).
All patients (including those who died in hospital) with a primary diagnosis of “acute or subacute infectious endocarditis” in any episode (ICD-10 code I33.0) were identified. Patients admitted to one hospital and transferred to another as part of their management (so called “superspells”) were identified using standardized methodology19 and counted only once. Hospital admissions are recorded as emergency or elective. Elective admissions are subdivided into booked (patient admitted having been given a date at the time the decision to admit was made, determined mainly on the grounds of resource availability), waiting list (patient admitted electively from a waiting list having been given no date of admission at a time a decision was made to admit) and planned (patient admitted, having been given a date or approximate date at the time that the decision to admit was made). We included only emergency, booked and planned admissions.
Incidence of IE was corrected for changes in the size of the English population and compared before and after introduction of the NICE guideline using segmented regression analysis of the interrupted time series20 using R.21 Examining the partial autocorrelation function for the dataset confirmed that no adjustment for seasonality was required. To allow for autocorrelation in the data, the segmented regression described20 was fitted using R’s gls function from the nlme package.22 This package allows for the regression model to be estimated under the condition of autocorrelation. The order of autocorrelation was obtained by examining both the autocorrelation and partial autocorrelation functions. To confirm robustness of the segmented regression, change-point-analysis was used to calculate the optimal positioning and number of data change-points using the R change-point package that implements the Hinkley algorithm.23
Analysis of secondary codes and correlation with preceding hospital admissions at the individual patient level were used to identify cases that had been at ‘high-risk’ of developing IE, as defined by AHA7 and ESC6 guidelines. All other patients were regarded as having been at ‘lower-risk’ (i.e. ‘moderate’ or ‘low-risk’). Additional details are provided in the Appendix. Secondary and supplemental codes were also used to try and identify the causal organisms for each case of IE (see Appendix for methodology)
HES data were used to identify and quantify other variables that might influence the incidence of IE over time. Thus, annual data were collected regarding (i) the number of individuals undergoing valve replacement, valve repair, or percutaneous valve implantation, (ii) new in-patient diagnoses of cyanotic congenital heart disease, (iii) surgical or percutaneous procedures in congenital heart disease patients, and (iv) the number of cardiovascular implantable electronic devices (CIEDs).24 Finally, data were obtained from the NHS Business Authority to demonstrate the number of individuals accessing primary care dental services between March 2006 and December 2013 (expressed as a percentage of the adult and child [under 18] population of England).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. MT and MD had access to the prescribing data and SJ and MD had access to the hospital episode statistics data. All the authors had final responsibility for the decision to submit for publication.
Results
Change in antibiotic prophylaxis prescribing
Before 2008, the rate of AP prescribing had remained relatively constant for many years. However, following the introduction of NICE guidelines concerning cessation of AP, there was a highly significant reduction in the mean number of AP prescriptions/month (pre-NICE: 10,900, post-NICE: 2,236; p<0·0001). In the last six months studied, this fell further to an average of 1,307 prescriptions/month (Figure 1). The vast majority of prescriptions were for amoxicillin (Figure 1a) with approximately 90% issued by dentists (Figure 1b).
Figure 1.
The total number of prescriptions for antibiotic prophylaxis (AP) dispensed each month: (a) Division by prescription (single 3g oral amoxicillin, BLUE; single 600mg oral clindamycin, PURPLE) (b) Division by prescriber (dentists, MAGENTA; general medical practitioners, BLUE; nurse practitioners, RED; hospitals, BLACK). The grey bar indicates March 2008, the month in which cessation of AP for infective endocarditis (IE) was recommended by NICE.
Change in the incidence of infective endocarditis
19,804 patients with a primary diagnosis of IE were identified between January 2000 and March 2013. 17,031 (86%) were emergency admission and 2,773 (14%) were booked or planned admissions (usually because of lack of beds, because the patient needed to make arrangements before admission or because the general practitioner had discussed the patient directly with a hospital specialist and the patient had been booked for admission without the patient passing through the emergency department).
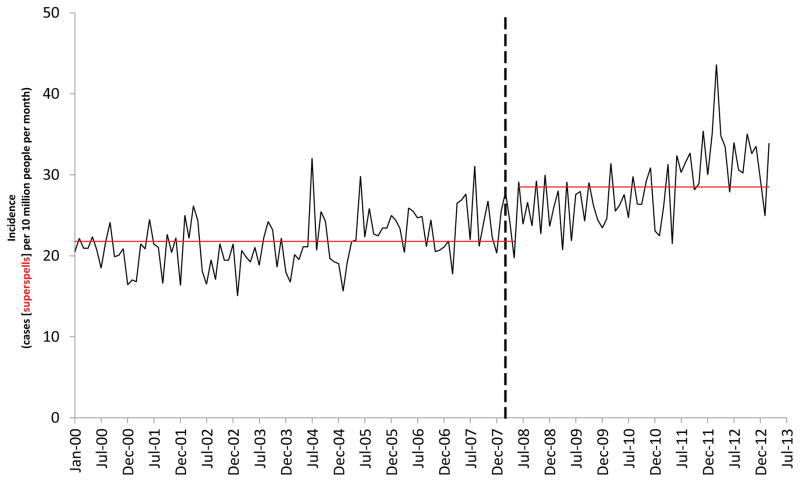
Before March 2008, there was a consistent upward trend in the population-corrected IE incidence in England (Figure 2). However, soon after implementation of the NICE guidelines there was a significant increase in the slope of this trend line (0·11 cases/10million/month, CI 0·05–0·16, p<0·0001) that was also seen in the uncorrected incidence data (Appendix Figure S1). By March 2013, we estimate that there were 34.9 (95% CI, 7·9 – 61·9) more IE cases/month than would have been expected if the previous trend had continued. Because AP prescribing had fallen from a pre-NICE mean of 10,900 to 1,235 by March 2008, a fall of 9,665 (89%), we can approximate that 276.8 (95% CI, 156·1 – 1217·3) AP prescriptions would be required to prevent one case of IE. Even with the March 2012 outlier value removed, the upward change in the trend line slope remained significant for the population-corrected (Figure 2) and uncorrected data (Appendix Figure S1). Both ‘high-risk’ and ‘lower-risk’ individuals were affected by this increase (Figure 3) with a statistically significant increase in both trend lines (p=0·025, p=0·0002, respectively). A significant change was also seen in the uncorrected ‘high-risk’ and ‘lower-risk’ data (Appendix Figure S3). A break down of IE incidence in different ‘high-risk’ categories is provided in Figure 4 (and Appendix Table S2).
Figure 2.
The number of infective endocarditis (IE) cases (superspells) recorded each month (solid BLACK line) and associated in-patient mortality (solid RED line). The data are corrected for change in the size of the English population. The vertical dashed black line indicates March 2008, the month in which cessation of antibiotic prophylaxis (AP) for IE was recommended by NICE. The trend lines for IE incidence (dashed BLACK lines) and associated in-patient mortality (dashed RED lines) before and after introduction of the NICE guidelines are also shown. NOTE: with the IE cases March 2012 outlier value removed the change in the IE incidence trend line remains statistically significant (change in level = −0·28, CI −2·27–1·7, p=0·78; change in slope = 0·09, CI 0·04–0·14, p<0·001)
Figure 3.
The number of infective endocarditis (IE) cases recorded each month affecting ‘lower-risk’ (solid BLACK line) and ‘high-risk’ (solid RED line) individuals. The data are corrected for change in the size of the total English population (not for change in the size of population at ‘high-risk’ or ‘lower-risk’ of IE). The vertical dashed black line indicates March 2008, the month in which cessation of antibiotic prophylaxis (AP) for IE was recommended by NICE. The trend lines for ‘lower-risk’ (dashed BLACK lines) and ‘high-risk’ (dashed RED lines) cases before and after introduction of the NICE guidelines are also shown.
Figure 4.
The number of IE cases recorded each month in individuals at ‘high-risk’ of developing IE, according to category of ‘high-risk’ status (previous IE, previous prosthetic replacement of heart valve, previous repair of heart valve, pre-existing congenital heart disease repaired with prosthetic material [only within the previous 6 months], pre-existing congenital heart disease with surgical shunt or conduit, pre-existing unrepaired congenital cyanotic heart condition, pre-existing artificial heart or ventricular assist device). The vertical dashed black line indicates March 2008, the month in which cessation of antibiotic prophylaxis for IE was recommended by NICE.
At the same time, there was a non-significant increase in the slope of population-corrected IE-associated mortality (0·01 cases/10 million/month, CI −0·01–0·02, p=0·394; Figure 2) that was replicated in the uncorrected data (Appendix Figure S1).
‘Change point analysis’ of the population-corrected (Figure 5) and uncorrected (Appendix Figure S2) IE incidence data shows that the change in IE incidence occurred in June 2008, three months after the change in AP guidelines. This three months lag is plausible since the incubation period of IE is usually less than six weeks and HES data capture the discharge diagnosis – in 2008 the median duration of hospital stay for patients in this study was 25 days.
Figure 5.
The number of population-corrected IE cases per 10 million population per month (solid BLACK line). The vertical dashed black line indicates March 2008, the month in which cessation of antibiotic prophylaxis for IE was recommended by NICE. The red lines show the result of change-point analysis, indicating that the change occurred in June 2008.
Comparing patients diagnosed with IE before and after March 2008 however, there was no significant change in the sex distribution (before - male 10,606 (69%), female 4,823 (31%); after - male 2963 (68%), female 1411 (32%), (p=0·394)), age (mean±SD, 59·0 years ±20·3 before; 59·3 years ±20·8 after (p=0·139)) or median length of stay in hospital (24 days before; 25 days after (p=0·224)).
Pathogen-specific secondary or supplementary causal organism coding of IE cases was unevenly distributed and increased from 30% to 49% over the study period. The rate of increase was also uneven and diminished over the last 3 years of the study. Furthermore, there were no specific codes that could be used to identify oral viridans group streptococci (OVGS). As a consequence it was impossible to draw any meaningful information from this data with regard to the effect of the change in AP prescribing on the nature of the organisms responsible for cases of IE.
Other factors that could have influenced the incidence of infective endocarditis
HES data were used to quantify several other variables that might influence the incidence of IE over time (further details provided in the Appendix), including; (i) the number of individuals undergoing valve replacement, valve repair, or percutaneous valve implantation (Appendix Figure S4a), (ii) new in-patient diagnoses of cyanotic congenital heart disease (Appendix Figure S4b), (iii) surgical or percutaneous procedures in congenital heart disease patients (Appendix Figure S4b), (iv) the number of cardiovascular implantable electronic devices (CIEDs) (Appendix Figure S4c) and (v) the number of individuals accessing primary care dental services (Appendix Figure S4d).
Discussion
Summary of the findings
Since introduction of the NICE guidelines in March 2008, which recommended cessation of AP to prevent IE, there has been both a highly significant fall in AP prescribing and a significant increase in the incidence of IE in England. This rise has affected both ‘high-risk’ and ‘lower-risk’ individuals. Although there was an increase in IE-associated in-hospital mortality, this did not reach statistical significance, possibly because of the lower mortality associated with IE due to oral streptococci, the general fall in IE mortality and the lack of statistical power resulting from the smaller number of mortalities.
Possible implications
Of paramount importance is whether the fall in AP prescribing was responsible for the increase in IE incidence. Although there was a temporal association, we were not able to prove a causal relationship.
We previously analyzed these data two years after the introduction of the NICE guidelines. At that time, a significant increase in IE incidence was not demonstrable despite a highly significant 78.6% reduction (p<0·0001) in AP prescribing.16
The impact of the 2007 AHA guidelines was examined in four investigations and no increase in IE incidence was observed following their implementation.25–28 However, all of these US studies involved either a smaller population size and/or a shorter period of follow up than the present assessment. One study was performed only nine months after the change in guidelines and included only 396 cases.27 Another, performed three years after the introduction of the guidelines, was restricted to children and included 1157 cases of IE.26 The third used Medicare records to identify the incidence of IE in approximately 75% of older adult Medicare beneficiaries for around 2·5 years after introduction of the AHA guidelines.28 The fourth25 contained two different cohorts. First, an in depth study on the incidence of IE in Olmsted County (adult population <150,000) over three years following introduction of the guidelines. Second, a much larger study using ICD-9 coding data from the Nationwide Inpatient Sample (NIS) database, that contains a ~20% stratified sample of US community hospitals.25 This study more closely matched our own, but only examined IE incidence for two years following the AHA guidelines update. Although our initial assessment, two years after the introduction of the NICE guidelines, demonstrated no change in IE incidence,16 the present more sophisticated re-analysis after five years has detected a significant change.
Similarly, Duval et al reported a follow up study in three French regions (population ~11 million adults)29 where a guideline change in 2002 restricted AP to patients at ‘high-risk’ (approximately 10% of the total). They found no significant increase in the incidence of oral streptococcal IE in 2008 compared with their findings in 1991 and 1999. Although these data were collected six years after the guideline change, the methodology was different and the population size studied smaller than the current study. Moreover, AP remained the standard of care for ‘high-risk’ patients in both the US and French studies.
Our estimate of the number of AP prescription needed to prevent one case of IE is considerably lower than other estimates.30,31 Our data is based on prescribing data and IE incidence data obtained from a large population. Nonetheless, it is associated with large CI and assumes a link between the prescribing and incidence data that may not be true. Other estimates also make assumptions and are generally derived from complex calculations using estimated figures derived from relatively small sample size populations. Such calculations based on multiple estimates tend to multiply the uncertainty but are nonetheless valid.
On the positive side, dental management of patients at risk of IE has been simplified by the NICE guidelines and the fall in AP prescribing will have reduced AP prescribing costs and the number of AP related adverse drug reactions.
Limitations
There are several limitations to this study. The data rely on UK hospital coding and may not be generalizable to other populations. In the UK, data are collected on every patient admitted to hospital by trained and accredited coders. Although these data are subject to error, they have been shown, for example, to provide more reliable and complete data capture for vascular surgery than a UK national research database specifically designed for that purpose.32 Furthermore, as the coding was undertaken independently of our study, it was not subject to bias or influenced in any other way by introduction of the NICE guidelines. Moreover, the size and scale of the data set and consistency of the underlying coding process are likely to negate the impact of any systematic error. Although IE may present to different hospital specialties and cause difficulties in initial diagnosis, HES data record the final diagnosis for each episode, and should reflect as accurately as possible the number of IE cases treated. Nonetheless, the diagnosis of IE is sometimes uncertain and will not always have been based on the Duke criteria.33 Furthermore, because of the high mortality and morbidity associated with IE, clinicians may treat some cases as IE even when the diagnosis is uncertain. Undoubtedly, therefore, some cases will have been miscoded.
ICD-10 and OPCS-4 codes were used to identify episodes of IE occurring in individuals at ‘high-risk’ of IE. This required us to look backwards in time from the index case of IE to identify previous episodes of IE, pre-existent cyanotic congenital heart disease and previous operative procedures (such as valve surgery) that would have defined the individual as ‘high-risk’. However, since some of these searches were limited and reliant on accurate recording of risk factors, it is likely we underestimated the number of ‘high-risk’ individuals. We also assumed that IE cases that did not arise in a ‘high-risk’ individual must have occurred in individuals who were at ‘moderate-risk’ or ‘low-risk’. Since we could not distinguish these groups using HES data, we clustered them together as ‘lower-risk’ cases. It is possible that a small number of ‘high-risk’ individuals were erroneously included resulting in overestimation of the size of the lower-risk group.
The pathogen-specific causal organism data had major limitations: (i) secondary/supplementary coding was unreliable, (ii) relevant codes were recorded in only 30–49% of cases and we cannot be certain that these represented a random subset of the entire IE population, (iii) the rate of improvement in secondary/supplementary coding was uneven (iv) there are no pathogen-specific ICD-10 codes that identify OVGS, (v) we could not be certain that the organism coded was the pathogen responsible for IE and not some other intercurrent infection, (vi) because of the small amount of data for each type of organism it was underpowered to detect a significant change.
Given these limitations, it was impossible to draw any conclusions from the organism-specific data with regard to the change in AP prescribing.
Although we have demonstrated a rise in the number of cases of IE, there are many factors other than the change in the AP guidelines in March 2008 that could be responsible. For example, there may have been a sudden large increase in the number of individuals at risk of IE. However, for many of those factors that put an individual at ‘high-risk’ from IE we have demonstrated that this is unlikely to be the case (see Appendix for details). Using HES data, an overall annual increase in the number of prosthetic heart valve and valve repair procedures was demonstrated over the study period, but no sudden change in procedural volume that could account for the increase in IE incidence. Similarly, the number of surgical procedures for congenital heart disease was almost constant over the period, and while there was a dramatic increase in the number of percutaneous procedures performed for congenital heart disease in 2005–6, this fell subsequently from 2009/2010 onwards. There was no sudden change in the annual rates of pacemaker or cardioverter-defibrillator insertion. However, we were unable to obtain data for other groups of individuals potentially at risk of developing IE such as diabetics, the elderly or those living in residential care. Nonetheless, publically-available data on the prevalence of diabetes in England shows a steady rise in the number of individuals with diabetes from 2,088,335 in 2007/8 to 2,455,937 in 2010/11 and this appears to be part of a long term trend.34 Similarly, the number of individuals age 65 or over living in residential care in England has remained static between 2001 (290,000) and 2011 (291,000) but fallen as a proportion of the total population over 65 from 3·5% in 2001 to 3·2% in 2011.35
Alternatively, IE incidence could have increased due to susceptible individuals being exposed to more risk prone procedures and bacteremias. Although we identified no significant change in the proportion of the English population receiving dental treatment, we were unable to study more subtle changes in the pattern of dental care, standards of oral hygiene, or patterns of oral disease that might influence the size and frequency of VGS related bacteremia. Nonetheless, dental statistics for England show dental extractions, at ~2·2 million per year, have remained fairly constant for many years while there has been a slow increase in the 12–12·8 million scale and polish courses of treatment per year.36 We cannot, however, know if this pattern of care is as applicable to those at risk of IE as it is to the general population. We also don’t know if the interest caused by the NICE guidelines, or growing knowledge about bacteremia resulting from daily habits such as tooth brushing, will have changed the behavior of patients at risk of IE in favor of seeking/avoiding dental care or improving/neglecting oral hygiene. A change in the frequency of other potentially risk prone procedures such as colonoscopy, renal dialysis, intravenous drug therapy and wound management, could also have affected IE incidence. Data is not available on all of these but HES data for England37 show no sudden increase in colonoscopies and data from the UK Renal Registry show only a gradual increase in hemodyalisis between 2007–2009 and then a fall. This data also shows a fall in MRSA bacteremia rates for all dialysis patients from 2007–2012.38 Nonetheless, we cannot exclude the possibility that a combination of factors affecting risk prone individuals and the number of episodes of bacteremia to which they are exposed could have caused the increase the IE incidence had they occurred at the right time.
Although we have corrected for changes in the size of the English population, changes in the age and sex distribution and more subtle population changes e.g. immigration from parts of the world with high rates of rheumatic heart disease or poor oral hygiene, might explain the change in incidence of IE if large in size and coincident with the change in AP guidelines. Furthermore, other changes in health policy could be possible confounders e.g. new or amended policies that altered the rate of transient bacteremia resulting from procedures such as colonoscopy, intravenous line placement or others, could have brought about a systematic change in IE incidence. In addition, use of more sophisticated diagnostic tools, improved diagnostic tool performance or changes in diagnostic strategy could have increased the number of IE diagnoses. If such changes occurred in the same time frame as the cessation of AP, it would be difficult if not impossible to distinguish these effects.
Conclusions
We have demonstrated both a significant reduction in AP prescribing and a significant rise in the rate of new IE cases in England since introduction of the NICE guidelines recommending cessation of AP in 2008. While demonstrating a temporal relationship between the two our data do not establish a causal link.
Supplementary Material
Research in Context.
Updated guidelines concerning the role of antibiotic prophylaxis to prevent infective endocarditis vary in different nations but all share a common theme – the number of patients in whom antibiotic prophylaxis is recommended has been markedly reduced since there are no robust data to support its efficacy and some concerns regarding safety. Ongoing surveillance has been recommended to ensure that reduced use of antibiotic prophylaxis does not result in an increase in the incidence of infective endocarditis. Population-based surveys are required and the data outlined in the present study represent an extremely large database upon which to base conclusions. Although we are unable to prove a causal link between the cessation of antibiotic prophylaxis and the increase in incidence of infective endocarditis, further investigation is now warranted to define an explanation and determine whether other populations demonstrate similar trends.
Acknowledgments
Different aspects of this study were supported by Heart Research UK and Simplyhealth [Grant Ref: RG2632/13/14] and NIDCR R03 grant [Ref: 1R03DE023092-01] from the National Institutes for Health. Dr Prendergast’s work is also supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centre’s funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health or any other funder.
Footnotes
Contributions:
All the authors contributed to the study design, writing of the paper and decision to publish. Prescribing data were gathered, analyzed and vouched for by MT. Incidence data were gathered, analyzed and vouched for by SJ. MD contributed to both. Statistical analyses were performed by SJ. MD and MT created the figures.
Declaration of interests:
LB and PL are members of the American Heart Association’s Committee on Rheumatic Fever, Endocarditis, Kawasaki Disease and were involved in producing the 2007 American Heart Association guideline on Prevention of Infective Endocarditis. BP was a member of the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC) that produced the 2009 ESC guidelines on the prevention, diagnosis and treatment of infective endocarditis. BP also acted as a consultant to the committee that produced the NICE clinical guideline 64 on Prophylaxis Against Infective Endocarditis. We declare no other competing interests.
Bibliography
- 1.Prendergast BD. The changing face of infective endocarditis. Heart. 2006;92(7):879–85. doi: 10.1136/hrt.2005.067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacassin F, Hoen B, Leport C, et al. Procedures associated with infective endocarditis in adults. A case control study. Eur Heart J. 1995;16(12):1968–74. doi: 10.1093/oxfordjournals.eurheartj.a060855. [DOI] [PubMed] [Google Scholar]
- 3.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345(18):1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 4.Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med. 1998;129(10):761–9. doi: 10.7326/0003-4819-129-10-199811150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. Jama. 2005;293(24):3022–8. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 6.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30(19):2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 7.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 8.Lockhart PB, Brennan MT, Kent ML, Norton HJ, Weinrib DA. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation. 2004;109(23):2878–84. doi: 10.1161/01.CIR.0000129303.90488.29. [DOI] [PubMed] [Google Scholar]
- 9.Lockhart PB, Loven B, Brennan MT, Fox PC. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J Am Dent Assoc. 2007;138(4):458–74. doi: 10.14219/jada.archive.2007.0198. quiz 534–5, 437. [DOI] [PubMed] [Google Scholar]
- 10.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118–25. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140(10):1238–44. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durack DT. Prevention of infective endocarditis. N Engl J Med. 1995;332(1):38–44. doi: 10.1056/NEJM199501053320107. [DOI] [PubMed] [Google Scholar]
- 13.Connaughton M. Commentary: Controversies in NICE guidance on infective endocarditis. BMJ. 2008;336(7647):771. doi: 10.1136/bmj.39512.666412.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Clinical Excellence. [accessed March 2008];Prophylaxis against infective endocarditis. 2008 http://www.nice.org.uk/CG064. [PubMed]
- 15.Richey R, Wray D, Stokes T. Prophylaxis against infective endocarditis: summary of NICE guidance. BMJ. 2008;336(7647):770–1. doi: 10.1136/bmj.39510.423148.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. Bmj. 2011;342:d2392. doi: 10.1136/bmj.d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers JB, Shanson D, Venn G, Pepper J. NICE v world on endocarditis prophylaxis. BMJ. 2011;342:d3531. doi: 10.1136/bmj.d3531. [DOI] [PubMed] [Google Scholar]
- 18.Dayer MJ, Chambers JB, Prendergast B, Sandoe JA, Thornhill MH. NICE guidance on antibiotic prophylaxis to prevent infective endocarditis: a survey of clinicians’ attitudes. Qjm. 2013 doi: 10.1093/qjmed/hcs235. [DOI] [PubMed] [Google Scholar]
- 19.Health and Social Care Information Centre. [accessed 09/06/2014];Methodology to create provider and CIP spells from HES APC data. 2014 http://www.hscic.gov.uk/SHMI.
- 20.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of clinical pharmacy and therapeutics. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 21.R Core Development Team. R: A language and environment for statistical computing. Vienne, Austria: R Foundation for statistical computing; 2008. [Google Scholar]
- 22.Fox J. Time-series regression and generalized least squares. [accessed 09/06/2014];Appendix to: An R and S-PLUS Companion to Applied Regression. 2002 http://cran.r-project.org/doc/contrib/Fox-Companion/appendix-timeseries-regression.pdf.
- 23.Hinkley DV. Inference about the change-point in a sequence of random variables. Biometrika. 1970;57:1–17. [Google Scholar]
- 24.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–77. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 25.Desimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association’s endocarditis prevention guidelines. Circulation. 2012;126(1):60–4. doi: 10.1161/CIRCULATIONAHA.112.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasquali SK, He X, Mohamad Z, et al. Trends in endocarditis hospitalizations at US children’s hospitals: impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J. 2012;163(5):894–9. doi: 10.1016/j.ahj.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers AM, Schiller NB. Impact of the first nine months of revised infective endocarditis prophylaxis guidelines at a university hospital: so far so good. J Am Soc Echocardiogr. 2008;21(6):775. doi: 10.1016/j.echo.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Bikdeli B, Wang Y, Kim N, Desai MM, Quagliarello V, Krumholz HM. Trends in hospitalization rates and outcomes of endocarditis among Medicare beneficiaries. J Am Coll Cardiol. 2013;62(23):2217–26. doi: 10.1016/j.jacc.2013.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol. 2012;59(22):1968–76. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;42(12):e102–7. doi: 10.1086/504385. [DOI] [PubMed] [Google Scholar]
- 31.Pallasch TJ. Antibiotic prophylaxis: problems in paradise. Dental clinics of North America. 2003;47(4):665–79. doi: 10.1016/s0011-8532(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 32.Aylin P, Lees T, Baker S, Prytherch D, Ashley S. Descriptive study comparing routine hospital administrative data with the Vascular Society of Great Britain and Ireland’s National Vascular Database. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2007;33(4):461–5. doi: 10.1016/j.ejvs.2006.10.033. discussion 6. [DOI] [PubMed] [Google Scholar]
- 33.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;30(4):633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 34.The Information Center for Health and Social Care. Diabetes prevalence in England. Association of Public Health Observatories (APHO); http://data.gov.uk/dataset/diabetes_prevalence. [Google Scholar]
- 35.Office of National Statistics. Changes in the older resident care home population between 2001 and 2011. United Kingdom: Office of National Statistics; 2014. http://www.ons.gov.uk/ons/dcp171776_373040.pdf. [Google Scholar]
- 36.Health and Social Care Information Center. NHS Dental Statistics for England. United Kingdom: Health and Social Care Information Center; http://data.gov.uk/dataset/nhs_dental_statistics_for_england. [Google Scholar]
- 37.Health and Social Care Information Centre. Hospital Episode Statistics. United Kingdom: Health and Social Care Information Centre; http://www.hscic.gov.uk/hes. [Google Scholar]
- 38.UK Renal Registry. UK Renal Registry 16th Annual Report. 2013 https://http://www.renalreg.org/reports/2013-the-sixteenth-annual-report/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.