Abstract
Current approaches for topical vaginal administration of nanoparticles result in poor retention and extensive leakage. To overcome these challenges, we developed a nanoparticle-releasing nanofiber delivery platform and evaluated its ability to improve nanoparticle retention in a murine model. We individually tailored two components of this drug delivery system for optimal interaction with mucus, designing (1) mucoadhesive fibers for better retention in the vaginal tract, and (2) PEGylated nanoparticles that diffuse quickly through mucus. We hypothesized that this novel dual-functioning (mucoadhesive/mucus–penetrating) composite material would provide enhanced retention of nanoparticles in the vaginal mucosa. Equivalent doses of fluorescent nanoparticles were vaginally administered to mice in either water (aqueous suspension) or fiber composites, and fluorescent content was quantified in cervicovaginal mucus and vaginal tissue at time points from 24h to 7d. We also fabricated composite fibers containing etravirine-loaded nanoparticles and evaluated the pharmacokinetics over 7d. We found that our composite materials provided approximately 30-fold greater retention of nanoparticles in the reproductive tract at 24h compared to aqueous suspensions. Compared to nanoparticles in aqueous suspension, the nanoparticles in fiber composites exhibited sustained and higher etravirine concentrations after 24h and up to 7d, demonstrating the capabilities of this new delivery platform to sustain nanoparticle release out to 3d and drug retention out to one week after a single administration. This is the first report of nanoparticle-releasing fibers for vaginal drug delivery, as well as the first study of a single delivery system that combines two components uniquely engineered for complementary interactions with mucus.
Keywords: nanoparticles, nanofibers, vaginal drug delivery, microbicides, electrospinning, pharmacokinetics
GRAPHICAL ABSTRACT

INTRODUCTION
Recent progress has been made in engineering nanocarriers to overcome physiological barriers for intravaginal administration. Nanoparticles (NPs) have been designed to rapidly diffuse through the cervicovaginal mucus barrier by PEGylating the nanoparticle surface to impart a net neutral surface charge [1,2]. The variable conditions of the vaginal environment have been employed in the design of pH-responsive Eudragit nanoparticles [3] and hyaluronidase-responsive nanoparticles [4] that can release drug in response to changes in pH or enzyme concentration due to the presence of semen. NPs have also been demonstrated to improve drug delivery to vaginal tissue in vivo compared to free drug delivered as a suspension. For example, a study in mice showed 5-fold higher drug concentration in vaginal tissue when the drug was delivered by nanoparticles compared to free drug in suspension [5]. Greater protection against herpes simplex virus type 2 (HSV-2) has also been documented in vivo for nanoparticles even when administered at a 10-fold lower concentration than free drug [1]. These findings may have application in developing more effective products for the prevention and treatment of STIs, including microbicides for female-initiated HIV prevention.
However, current methods for vaginal mucosal administration of nanoparticles are limited by extensive leakage of the administered dose and poor retention. In vivo studies to date have used aqueous suspensions (water or PBS) to intravaginally administer nanoparticles in mice [1,2,5,6]. Significant leakage has been documented in vivo for aqueous suspensions, with over 50–70% of the administered nanoparticle dose leaking out within 30 minutes, and <1–2% of the total nanoparticle dose retained at 24 hours [5,6]. Such administration methods also often require maintaining mice in an inverted position for 1–10 minutes to reduce leakage [5,6], and practical translation of such methods to clinical use is unlikely. External leakage and messiness from liquid or gel dosage forms have been cited as reasons for poor adherence [7], and gel-based dosage forms used once-daily or pericoitally have been associated with poor adherence in clinical trials [8,9]. To realize the full potential of nanocarrier delivery systems for reproductive health applications, new platforms are needed to increase dose retention of nanoparticles in the reproductive tract and provide a more practical method for their administration.
Electrospun nanofibers have recently been investigated as a platform for vaginal drug delivery [10–18]. Nanofibers (NF) are a solid-state dosage form, which may overcome challenges with user adherence by reducing messiness and leakage that has been associated with liquid or gel-based dosage forms. Proof-of-concept of the application of electrospun fibers to vaginal drug delivery has previously been demonstrated, showing that fibers can deliver active antiretroviral drugs and contraceptive agents with diverse properties [10,11]. Further work has shown that electrospun nanofibers can incorporate a remarkably high drug content up to 60% by mass [14], and that fiber polymer ratios and core-shell structure can be tuned to control drug release kinetics over the time course of days to weeks [15,16,18]. Nanofibers offer many other advantages for vaginal drug delivery, including flexibility in processing parameters (polymer/solvent selection, controllable fiber diameter, thickness), a nearly unlimited material space for electrospinning, the ability to encapsulate diverse agents, and multiple conceptual geometries to achieve practical and user-friendly administration [12,19,20]. Electrospun fibers containing antiretroviral drugs [19,21] or microparticles [22] have also been fabricated using free-surface electrospinning, which is a scalable technique that provides a pathway to clinical translation of fiber-based medical products.
Here, we designed nanoparticle-releasing nanofiber (NP-NF) composites for increased nanoparticle retention in the reproductive tract by individually tailoring each component for optimal interaction with mucus. We hypothesized that because nanofibers are a solid dosage form and offer a high surface area-to-volume ratio for quick dissolution, they would provide enhanced retention and quick release of nanoparticles in the vaginal tract. For the fiber component, we selected two mucoadhesive polymers, poly(vinyl alcohol) (PVA) and poly(vinyl pyrrolidone) (PVP), aimed to increase retention in the vaginal tract through association with the mucosa. Both polymers are hydrophilic and expected to dissolve quickly to release nanoparticles, but PVP is slightly more mucoadhesive than PVA [23–26], providing an interesting comparison to study how mucoadhesivity affects NP delivery. Building on recent work published on mucus-penetrating particles [1], we synthesized PEGylated PLGA nanoparticles designed to rapidly penetrate cervicovaginal mucus and deliver the drug payload to vaginal tissue. Although electrospun fibers containing nanoparticles have been used previously for drug or protein release [27–30], ours is the first study to design and investigate nanofiber composites for optimal delivery and retention of nanoparticles for vaginal applications. In this work, we aim to study the release and biodistribution of nanoparticles from composite fibers after intravaginal administration. To demonstrate the utility of the NP-NF composite platform for application as a topical microbicide for HIV prevention, we also compare the pharmacokinetics of the antiretroviral drug etravirine (ETR) after delivery in nanoparticles from nanofiber composites compared to aqueous suspensions. We show that NP-NF composites dramatically enhance both nanoparticle and drug retention in the reproductive tract relative to aqueous suspensions and sustain nanoparticle release out to three days after a single administration. This composite platform overcomes a major challenge in vaginal drug delivery by significantly prolonging both nanoparticle and drug residence time in the vaginal tract.
MATERIALS AND METHODS
Ethics statement
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Washington (Protocol # 4260-01). All animals were obtained and cared for in accordance with the IACUC guidelines.
Materials
Poly lactic-co-glycolide (PLGA) (50:50 L:G, ester-terminated, inherent viscosity = 0.55–0.75 dL/g in HFIP) was purchased from Lactel Absorbable Polymers. Rhodamine-B, Pluronic® F-127, agar, and poly(vinyl pyrrolidone) (Mw~1.3 MDa) were purchased from Sigma-Aldrich. Poly(vinyl alcohol) (Mw~105 kDa, P1180) was purchased from Spectrum Chemical. Other reagents used include acetone (Fisher), ethanol (Decon Laboratories, Inc.), dimethyl sulfoxide (DMSO) (BDH Solvents), sodium chloride (EMD Millipore), and PBS (1X, Mediattech, Inc.). All solvents used for high performance liquid chromatography (HPLC) were of HPLC grade, including water (Fisher), acetonitrile (ACN) (Fisher), and ammonium acetate (Sigma-Aldrich). Etravirine was purified by and given as a generous gift from I. Suydam at Seattle University (Seattle, WA).
Nanoparticle synthesis
PLGA nanoparticles were synthesized using a nanoprecipitation technique based on modifying previously described methods [31] for passive PEGylation with Pluronic® F-127 [1]. PLGA was dissolved in acetone at 20 mg/mL and added to 0.1% (w/v) Pluronic® F-127 at a 1:11 (v/v) ratio with a syringe pump at a flow rate of 1 mL/min. Acetone was evaporated from the aqueous phase over 4–6 h in a fume hood. Particles were then washed by centrifugation at 10,000g x 20 min and resuspended in water using alternating vortexing and water bath sonication as needed. Aliquots of NP suspension were lyophilized and analyzed for fluorescence or drug content. Remaining suspensions were stored at 4°C until further use.
For fluorescent rhodamine-conjugated PLGA nanoparticles (Rho-NP), we first synthesized a rhodamine-B-PLGA conjugate using methods described by Costantino et al [32]. We determined the conjugation efficiency to be 15.7% (loading of 0.169% (w/w) rhodamine B:PLGA) by dissolving polymer conjugate and measuring fluorescence. Rhodamine-B-PLGA conjugate was dissolved in acetone and NP formed as described above. To synthesize ETR-loaded nanoparticles (ETR-NP), we dissolved ETR at 10% (w/w ETR/PLGA) theoretical loading in the PLGA/acetone solution. ETR was passively loaded in NP during NP synthesis, not directly conjugated to PLGA like rhodamine-B. ETR-NP were synthesized as described above, except that the NP suspension was filtered after evaporation using 2 micron syringe filter to remove any drug precipitate before washing. Characterization of ETR-NP in terms of size, zeta potential, polydispersity index (PDI), drug loading, and encapsulation efficiency is described in the Supporting Information (Supplementary Table S1).
Composite electrospinning
Polymer solutions for electrospinning were prepared by dissolving PVA at 22% (w/v) in water and heating gently for 1–2h, and by dissolving PVP at 24% (w/v) in ethanol (pre-electrospinning polymer concentrations; see schematic in Supplementary Fig. S1). Solutions were placed on a slowly rotating rotisserie apparatus overnight to ensure complete dissolution. Composite NP-NF materials were fabricated by adding equivalent volumes of freshly synthesized NP (resuspended in water at 34.4–37.5 mg/mL) to PVA or PVP polymer solutions. Solutions were slowly rotated for 20–30 minutes prior to electrospinning. The electrospinning set-up consisted of a high voltage power supply (Gamma High Voltage Research) and syringe pump (New Era Pump Systems, Inc.). Polymer solution was loaded into a plastic 6 mL syringe (ThermoScientific) with 18G needle (length = 2 inches) and a rubber stopper (size 10) to focus the electric field. Fibers were collected onto brown waxed paper on a house-made rotating mandrel collector (diameter = 5 inches, speed = 1725 rpm, electrically grounded with a graphite brush) to provide consistent fiber mesh thickness. PVA composite fibers containing PLGA-NP were electrospun at 22.5 kV, 10 cm collector distance, and 10 μL/min flow rate. PVP composite fibers containing PLGA-NP were electrospun at 21 kV, 15 cm collector distance, and 25 μL/min flow rate. All composites were electrospun using a total of ~3.5 mL of polymer solution per mesh. Electrospun composites were stored under vacuum desiccation until further use.
Transmission electron microscopy
Freshly synthesized blank PLGA NP and ETR-PLGA NP were dried on transmission electron microscopy (TEM) sample grids and imaged using TEM with a FEI 200 kV Tecnai G2 TEM at the University of Washington Molecular Analysis Facility.
Scanning electron microscopy
Composite fibers were imaged using scanning electron microscopy (SEM) with a Sirion SEM at the University of Washington Molecular Analysis Facility. Samples were placed on carbon tape and sputtered for 70s with gold/palladium. Images were taken at 5.0 kV, 5 mm working distance, and spot size of 3 at 500x, 5000x, and 20000x magnification from random locations on the sample. Fiber diameter was measured as previously described [11]. Briefly, ImageJ (National Institutes of Health) was used to measure the diameter of at least n=45 fibers per sample.
Confocal microscopy
Fiber samples were collected onto glass slides for 10–15 seconds during composite electrospinning and imaged using a Zeiss 510 META confocal microscope at the Keck Microscopy Facility at the University of Washington. Contrast and gain were adjusted uniformly across an image for optimal imaging of Rho-NP using Fiji/ImageJ [33].
Composite dissolution in vitro
We placed fiber samples on agar hydrogels to visually observe wetting and dissolution time based on previously described methods [13]. Circular punches of PVA or PVP fibers were obtained using a metal die punch to obtain samples with constant area and basis weight (mean of 20 mm diameter, 4.32 mg, 13.8 g/m2 basis weight). Black agar hydrogels were prepared by dissolving agar in water 1.5% (w/v) and adding several drops of India ink to provide sufficient black color contrast to the white fibers. Fiber samples were gently placed on agar hydrogels, and fiber wetting and dissolution was assessed over 2 hours by video recording using an iPhone-5S (Apple).
In vitro nanoparticle release from composites
For in vitro release experiments, 4 mg of PVA or PVP composite fibers containing Rho-NP were placed in 2 mL of deionized water (n=3 per fiber type). At various time points (1.5 min, 10 min, 20 min, 30 min, 1h, 2h, 6h, 24h, and 72h), 100 μL of release media was removed and volume replaced. 500 μL DMSO was added to release samples to dissolve PLGA-NP content, and fluorescence content determined using a TECAN infinite M200PRO fluorescent plate reader (Tecan Austria GmbH) with wavelength set to 570/620 nm Ex/Em.
Nanoparticle integrity post-release
In parallel with in vitro release experiment, dynamic light scattering was used to measure the size and colloidal properties of released nanoparticles from samples taken at 10 – 90 min during the in vitro release study. Samples were diluted in 10 mM NaCl and size, zeta, and PDI were measured using a Zetasizer Nano ZS90 (Malvern Instruments). These methods were also used to measure nanoparticle integrity after release from ETR-NP composites.
Actual loading of Rho-NP composites and ETR-NP composites
To determine the actual loading of nanoparticles within Rho-NP composite fibers, 3 mg fiber samples (n=3) were dissolved in 100 μL H20 for 45 min. 500 μL DMSO was then added, samples were dissolved an additional 1 hour, and fluorescence was measured using a fluorescent plate reader as described above. Fluorescent content in aqueous NP suspensions was measured by adding 75 μL water to 25 μL aqueous suspension of Rho-NP (n=3), and then adding 500 μL DMSO and measuring fluorescence as for the fibers. The concentration of aqueous suspension used for in vivo studies was adjusted to match the fluorescent signal from composite fiber dose to ensure dose equivalency.
Actual drug loading of ETR-NP was measured by dissolving samples (n=3) of lyophilized NP in DMSO and determining drug content using HPLC. Samples of ETR-NP composites were dissolved as described for Rho-NP composites and analyzed using HPLC. PLGA-NP content in drug-loaded composites calculated directly from the actual drug loading of ETR-NP. Equivalent doses of ETR-NP were delivered for fibers and aqueous suspensions for in vivo studies.
Animals
Female C57/Bl6 mice (The Jackson Laboratory, Bar Harbor, ME) 8–9 weeks old were cycled three days prior to intravaginal administration by subcutaneously injecting with 2 mg medroxyprogesertone acetate (Greenstone LLC). Medroxyprogesterone acetate has been shown to induce a diestrus-like state in mice, resulting in a thinned vaginal epithelium layer, thicker mucus barrier, and reduced variability from mouse-to-mouse based on time of cycle [1,5,34].
In vivo methods
Animals were abdominally taped prior to administration to prevent self-grooming. Mice were anaesthetized with isoflurane during abdominal taping and intravaginal administrations. Equivalent doses of NP were administered intravaginally in either aqueous suspensions (25 μL) or 2.8–3.5 mg composite fibers. Composite fibers were cut in the form of circular discs using metal punches (punch size = 16–18 mm). Fibers were then rolled up and folded to fit into modified 10–100 μL positive displacement pipette tips (Rainin), which functioned similarly to tampon applicators for administering fibers in mice. Immediately after intravaginal administration, mice were allowed to recover in the resting prone position. No vaginal pre-treatments, mucus alteration or removal, or inverting mice to prevent leakage were used for these studies. After administration, mice were individually caged to prevent cross-grooming between mice and given ad libitum access to food and water.
Mice were sacrificed at specified time points by cervical dislocation (Rho-NP studies) or cardiac exsanguination under isoflurane overdose for terminal blood collection (ETR-NP studies). Immediately after death, one of two washing methods was used to separate mucus-associated NP and undissolved fibers from vaginal tissue: (1) placing vaginal tissue in washing buffer (5 mL PBS) or (2) cervicovaginal lavage (4 x 50 μL water). Necropsy was performed and specified organs removed, including reproductive tract (cut above cervix to separate uterine horns from vaginal tract), rectum, and liver. Organs were massed and stored at −80°C until processing.
Washing methods for removing mucus-associated NP and undissolved fibers
The most common method for removing vaginal mucus is by cervicovaginal lavage, in which the vagina is washed 2–4 separate times by repeatedly pipetting with 50 μL of water or PBS [1,6,35,36]. We initially tried this method for PVA and PVP fibers, but found that while this method worked for PVA, it did not completely remove undissolved PVP fibers (Supplementary Fig. S2). PVP was so mucoadhesive that it was still visibly stuck to vaginal tissue, even after the lavage. Instead, we developed a more stringent washing method intended to remove any undissolved fibers. After dissection of the reproductive tract, the vaginal tract was separated from the uterine horns and cut open longitudinally up to the cervix. Vaginal tissues were then placed in a wash buffer containing 5 mL PBS and agitated at 37°C on an orbital shaker for 30 min. Wash buffer and vaginal tissue (post-wash) and were analyzed separately for NP content.
Studies were performed to compare the ability of the wash buffer vs. the lavage method to remove undissolved fibers for Rho-NP (PVA and PVP) (Supplementary Fig. S2) and for ETR-NP (PVA only) (Supplementary Fig. S3). For PVA fibers, both the wash buffer and the lavage method worked to remove undissolved fibers, and amounts of NP detected in the wash buffer and lavage were similar. For PVP fibers, neither the wash buffer nor the lavage method worked to fully remove undissolved fibers. We used the wash buffer method for all Rho-NP composite studies, and have discussed limitations resulting from undissolved PVP fibers sticking to tissue. We used the lavage method for ETR-NP composite PK studies, since only PVA fibers were carried forward into PK studies and the lavage method worked to fully remove any undissolved PVA fibers. The lavage method allowed for more direct comparisons of results to be made to previous studies that used the same method [5,6].
Fluorescent nanoparticle in vivo studies
For the 24h Rho-NP study, four groups were compared: untreated/blank fiber control, Rho-NP in aqueous suspension, Rho-NP in PVA composite fibers, and Rho-NP in PVP composite fibers, with n=7 mice per group (n=5 for blank control). Equivalent doses of Rho-NP (~298 μg) were administered intravaginally to mice in either aqueous suspensions (25 μL water) or 2.8 mg composite fibers. Untreated mice received no treatment (abdominal taping only). Blank fibers were PVA composites with a similar loading of blank PLGA NP as Rho-PVA composites. Mice were sacrificed at 24h by cervical dislocation and reproductive tracts excised. Whole reproductive tracts were imaged using Xenogen IVIS® Spectrum imaging system before washing, and then opened vaginal tracts were imaged again after the wash method. Mucus-associated NP and undissolved fibers were removed by cutting open vaginal tract up to the cervix and placing tissue in wash buffer (5 mL PBS) for 30 minutes. Vaginal tracts (post-wash) and uterine horns were weighed and stored at −80°C until analysis. Xenogen images were taken at 570/620 nm ex/em and a 30s exposure time. Images were analyzed using Living Image® 4.0 Software (Caliper Life Sciences, Inc.).
Wash buffer (5 mL PBS) samples were frozen at −80°C and lyophilized for at least 48h. Lyophilized buffer was dissolved in 600 μL 1:5 H2O:DMSO for 1–2h to dissolve NP. Wash buffer samples were then centrifuged at 10,000g for 10 min. The supernatant was analyzed for fluorescent content using a TECAN plate reader at 570/620 nm. Vaginal tracts and uterine horns were thawed and minced with scissors in 500 μL DMSO/organ. Tissues were homogenized using a Precellys 24-Dual bead homogenizer (Bertin Technologies). One mixed zirconium oxide bead kit (Bertin Technologies) was used per tissue, containing ~650 mg 1.4 mm beads and six 2.8 mm beads. Precellys settings were 6,500rpm x 20s x 3 cycles, with a 30s pause in between each cycle. Two homogenization runs completed with these settings to ensure full homogenization, with a 10 minute incubation at 4°C in between runs to allow for cooling. Samples were centrifuged at 5,000 rpm for 15 min. Supernatant was analyzed for fluorescent content in triplicate for each sample using a TECAN plate reader as described above and compared to a standard curve of Rho-NP prepared in blank organ homogenate. Linearity was established from 0.5 μg to 298 μg (0.2–100% total Rho-NP dose).
The same methods were used for the 7d Rho-NP study to evaluate NP retention from PVA composite fibers over a longer time. Two groups were compared at each of four time points (2d, 3d, 5d, and 7d): untreated control (n=1) and Rho-NP in PVA composites (n=3). Equivalent doses of Rho-NP (~368 μg NP in 25 μL water or 3.5 mg composite fibers) were administered for the long-term retention study.
Black paper leakage study
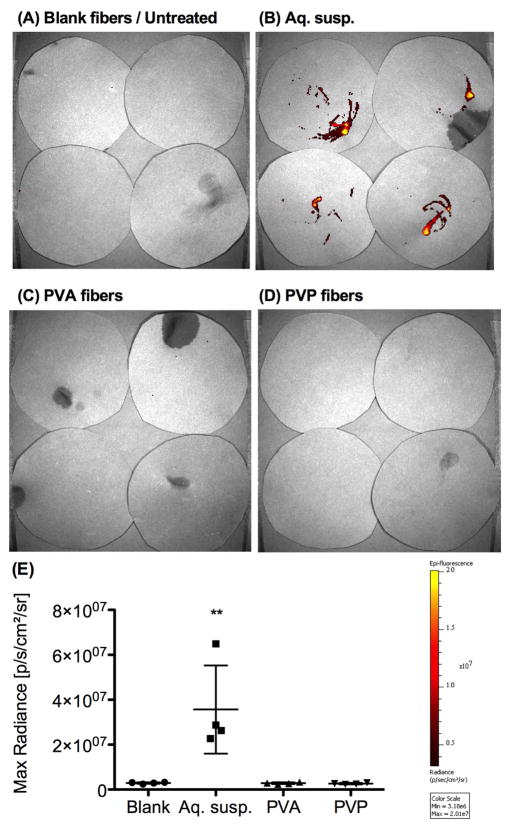
In parallel with the 24h Rho-NP study, we measured external leakage based on methods previously described by Cu et al [6]. Immediately after intravaginal administration of equivalent doses of Rho-NP in either composite fibers or aqueous suspensions, mice (n=4 per group) were placed in plastic beakers lined with circles cut from black paper (Artagain® 400 Series, Strathmore) and allowed to recover normally for 1h. Leakage of Rho-NP on black paper circles was measured using Xenogen imaging to detect fluorescent content. Fluorescent signal was quantified by drawing an ROI defined around each black paper circle and quantifying maximum radiance.
ETR nanoparticle in vivo studies
Two groups were compared for this study: aqueous suspensions versus composite PVA fibers containing equivalent doses of ETR-NP (~446 μg ETR-NP containing 11.42 μg ETR/dose), with n=5 mice per group per time point. Doses were administered intravaginally to mice in either aqueous suspensions (25 μL H20) or in 2.8 mg PVA composite fibers. At various time points (1h, 6h, 12h, 24h, 2d, 3d, 5d-fibers only, 7d-fibers only), mice were sacrificed by cardiac exsanguination under isoflurane overdose. At least 500 μL of blood per mouse was collected in K2EDTA-coated tubes (BD Microtainer®). Blood samples were centrifuged at 1,300g for 10 min and plasma separated. Cervicovaginal lavage was performed immediately after death (4 x 50 μL H20), and organs excised for drug content analysis (vaginal tract, uterine horns, rectum, liver). All samples were massed and kept at −80°C until analysis.
Lavage samples were dissolved in 800 μL DMSO and centrifuged at 13,000g for 10 min. Supernatant was diluted if necessary to be within the linear range for quantification and analyzed for ETR content using HPLC methods described below. Plasma samples were thawed and 25 μL of plasma was added to 50 μL ACN to precipitate protein content, followed by 30 min on a plate shaker. Plasma was centrifuged at 5000g x 10 min, and supernatant analyzed for ETR content using LC-MS/MS methods. Vaginal tissue (post-lavage), uterine horns, and rectum were homogenized in 500 μL ACN/organ using Precellys bead homogenizer as described above. Livers were homogenized in 1 mL ACN/organ using a probe homogenizer (Tissue Tearor, Biospec Products, Inc.) and centrifuged at 4700 rpm for 5 min at 4°C. Supernatant from homogenized organs was filtered through a 0.22 μm syringe filter and analyzed for ETR content using LC-MS/MS methods.
HPLC
HPLC was used to quantify ETR content for drug loading measurements of NP and composites, in vitro release experiments for ETR-NP, and lavage samples from the PK study. A Prominence LC20AD (Shimadzu) HPLC system was used that was fitted with a photodiode array detector and Phenomenex Kinetex C18 column (250x4.6 mm, 5 μm particle size). The mobile phase was 65% ACN:35% ammonium acetate buffer (10 mM) at a flow rate of 1 mL/min under isocratic conditions. The column oven was set to 30°C. ETR was detected at 309 nm at a retention time of 6.3 min. Linearity was established from 0.024 – 100 μg/mL using a 10 μL injection volume (standard curve in DMSO). Blank samples containing homogenized organs from untreated mice were run in parallel with mice from treatment groups to ensure there was no background interference, as well as standards and spiked samples in blank tissue homogenate.
LC-MS/MS
Mass spectrometry methods were used to quantify ETR content in all organs for the PK study except the lavage, including vaginal tissue, uterine horns, rectum, liver, and plasma.
Sample Prep
Etravirine-13C6 was used as an internal standard. All samples were vialed at a volume of 50 μL and spiked with 5 μL of a 10 ng mL−1 solution of the internal standard stock solution (50 pg per sample). A standard curve ranging from 0.01–100 ng mL−1 was prepared in ACN and run before and after unknown sample sets.
Mass Spectrometry
ETR concentration in tissue was analyzed using a LC-MS/MS method on a Waters Xevo TQ-S couple with an I-Class Acquity UPLC (Waters Corporation, Milford, MA, USA). All analytes were acquired in electrospray positive mode and used the following parameters: capillary voltage of 3 kV, source temperature of 150 °C, cone gas flow of 150 L hr−1, desolvation gas flow of 1000 L hr−1, and collision gas flow of 0.15 mL min−1. The spectrometer was analyzing during minutes 2–7 of each run. The drug and internal standard were analyzed at the following m/z transitions: 435.22 to 162.98 (ETR) and 441.20 to 162.98 (ETR-13C6). All species were analyzed as a cone voltage of 50 kV and collision energy of 28 V (full list of parameters can be found at Blakney et al., publication in process).
Liquid Chromatography
A Chromolith Performance RP-18e 100-3mm column was used for analysis, with a gradient method of 10 mM formic acid in H2O (Mobile Phase A) and 10 mM formic acid in 80:20 ACN:MeOH (Mobile Phase B). The gradient consists of 98% A + 2% B for 1 minute, transitioning to 100% B by 6 minutes and holding for an additional 1 minute, and then transitioning back to 98% A + 2% B over 1.5 minutes (see Supplementary Table S2), with a flow rate of 0.5 mL min−1 and an injection volume of 20 μL.
PK parameter analysis
Pharmacokinetic (PK) parameters were calculated for mice receiving aqueous suspensions (sampled from 1h to 72h) and for mice receiving composite fibers (sampled from 1h to 7d), with n=5 mice for each condition tested. Cmax is defined as the maximum observed concentration, Tmax is the time at which Cmax occurred, AUC is the area under the concentration-time curve, and t1/2z is the apparent terminal elimination half-life (estimated from the terminal phase of the log-linear concentration-time curve). Because ETR concentrations were sampled in different animals at each time point (one-point sampling), PK parameters were analyzed using a population approach and noncompartmental analysis by a population-based bootstrap method scripted in the R programming language [37,38]. The apparent terminal elimination rate constant or slope (λz) was estimated from the data by performing a linear regression of concentrations at the last three to five time points (see Supplementary Fig. S4, S5, S6). The apparent terminal elimination half-life (t1/2z) was calculated as 0.693 / λz. AUC0-t was calculated as , and AUC0-∞ was calculated as AUC0-last + (Clast / λz). The ratio of AUC values shown in Table 3 was calculated as AUCfib / AUCsus = AUC0-t, fibers / AUC0-t, suspension. The standard error of the mean (SEM) for PK parameters t1/2z, AUC0-72h, and AUC0-∞ were generated using the bootstrap method with modification [39]. Briefly, using the R program, one of the measured ETR concentrations was randomly imputed at each time point to create 10,000 randomly-generated concentration-time profiles. PK parameters for each of the 10,000 profiles were calculated using the equations above. The 95% confidence intervals (CI) for each parameter were calculated by taking the 2.5 and 97.5% quartiles (i.e., the 251st and 97,501st values of 10,000 bootstrap values); SEMs were calculated using the equation [Estimate ± (1.96)SEM = 95%CI]. Differences in PK parameters were assessed using two-tailed, Student’s t-test, assuming equal variance (GraphPad Version 6.0f).
Table 3.
Effect of nanoparticle dosage platform on pharmacokinetic parameters of ETR using noncompartmental analysis.
| Biological sample | t1/2z (h)a | Tmax (h) | Cmax (μg/g)b | AUC0-72h (hr•μg/g)c | AUCfib/ AUCsus | AUC0-∞ (hr•μg/g)c | AUCfib/AUCsus | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mean (±SEM) | P | Mean (±SEM) | P | Mean (±SEM) | P | Mean (±SEM) | P | ||||
| Plasma | |||||||||||
| Suspension | 8.11 (1.39) | 0.1576 | 1.35 | 0.0038 (0.0006) | 0.0170 | 0.024 (0.003) | 0.0008 | 1.79 | 0.024 (0.003) | 0.0215 | 2.58 |
| Fibers | 55.98 (30.67) | 1.35 | 0.0019 (0.0002) | 0.043 (0.002) | 0.062 (0.013) | ||||||
| Vaginal lavage | |||||||||||
| Suspension | 36.41 (18.77) | 0.6054 | 1.35 | 9.72 (1.96) | <0.0001 | 194.94 (29.54) | <0.0001 | 12.82 | 287.33 (118.81) | 0.0040 | 15.40 |
| Fibers | 54.53 (27.99) | 1.35 | 47.51 (0.94) | 2,498.98 (85.08) | 4,423.60 (1,028.66) | ||||||
| Vagina | |||||||||||
| Suspension | 35.36 (14.92) | 0.9245 | 1.35 | 4.66 (0.35) | 0.0002 | 44.29 (5.69) | 0.1582 | 1.23 | 50.13 (5.96) | 0.0555 | 1.38 |
| Fibers | 33.17 (16.71) | 1.35 | 1.93 (0.24) | 54.26 (2.94) | 69.13 (6.04) | ||||||
| Liver | |||||||||||
| Suspension | 17.17 (4.69) | 0.2592 | 1.35 | 0.028 (0.005) | 0.1247 | 0.266 (0.028) | 0.0007 | 1.81 | 0.278 (0.031) | 0.0020 | 2.59 |
| Fibers | 42.13 (20.01) | 6.47 | 0.018 (0.003) | 0.481 (0.029) | 0.721 (0.093) | ||||||
| Rectum | |||||||||||
| Suspension | 75.41 (68.69) | 0.5190 | 1.35 | 0.049 (0.020) | 0.5213 | 0.63 (0.11) | 0.0253 | 2.27 | 1.32 (0.79) | 0.3774 | 1.61 |
| Fibers | 28.82 (7.26) | 24.38 | 0.033 (0.013) | 1.43 (0.27) | 2.12 (0.33) | ||||||
| Uterine horn | |||||||||||
| Suspension | NA | NA | 1.35 | 0.041 (0.015) | >0.9999 | 0.65 (0.11) | 0.0261 | 2.03 | 1.73 (4.23) | 0.9490 | 1.16 |
| Fibers | 26.83 (9.59) | 12.52 | 0.041 (0.017) | 1.32 (0.22) | 2.01 (0.36) | ||||||
Used data from 0–3 days for suspension, and 0–7 days for fibers.
μg/mL for vaginal lavage and plasma.
hr•μg/mL for vaginal lavage and plasma.
Statistical Methods
Data is presented as mean ± standard deviation unless otherwise indicated, with statistical significance defined as p<0.05. Statistics were calculated using Prism 6.0 (GraphPad Software, Inc.). For Rho-NP studies where there were multiple groups, one-way ANOVA with Bonferroni correction for multiple comparisons was used to compare all groups. For ETR studies, a two-tailed unpaired t-test (equal variance assumed) was used to compare two groups within the same time point. Grubb’s test for determining outliers was used to remove outliers prior to PK analysis (alpha = 0.01).
RESULTS AND DISCUSSION
Fabrication of mucus-penetrating nanoparticle and mucoadhesive nanofiber composites
We optimized NP-NF composite materials for the following design specifications known to promote vaginal drug delivery: (1) nanoparticle diameter of ~200–500 nm, (2) hydrophilic and near neutral nanoparticle surface charge, (3) rapid fiber dissolution (<30 min), (4) fast release of nanoparticles from fiber composites, and (5) retention of original size and surface charge of nanoparticles after release. Nanoparticles with a diameter of 200–500 nm and neutral surface charge have previously been shown to be optimal for mucus-penetration [40]. Since we aimed to deliver intact nanoparticles to tissue as opposed to sustaining drug release from composites, achieving quick fiber dissolution and nanoparticle release was important.
We synthesized both fluorescent Rho-NP and drug-loaded ETR-NP using a nanoprecipitation technique. NP-NF composites were fabricated using the “direct addition” method similar to previously reports [29,41–43]. Suspensions of Rho-NP were directly added to either PVA or PVP polymer solutions and subsequently electrospun to create NP-NF composite materials. Electrospinning parameters were optimized independently for each polymer (PVA or PVP) to result in a stable Taylor cone with minimal polymer dripping. Actual loading of nanoparticles in Rho-NP composites and ETR-NP composites was measured to ensure dose equivalency between composites and aqueous suspensions for in vivo studies. Actual loading of nanoparticles within fibers (wt NP / wt composite fiber) was found to be 10.6% (± 0.1%, n=3) for Rho-NP-PVA composites, 9.7% (± 0.1%, n=3) for Rho-NP-PVP composites, and 15.9% (± 0.4%, n=3) for ETR-NP-PVA composites. NP loading was higher for drug-loaded composites to ensure detectable drug concentrations for PK studies while keeping fiber dose consistent with fluorescent studies. Additional data on characterization of Rho-NP and ETR-NP is found in Supplementary Fig. S7 and Supplementary Table S1.
We visualized the three components of PVA and PVP composite fibers (NP, NF, and NP-NF) using three imaging techniques. TEM micrographs show NP morphology and size, SEM images confirm cylindrical morphology and uniform distribution of fibers, and confocal images confirm incorporation of NPs into fibers visualized as punctate fluorescent deposits (Fig. 1, Supplementary Fig. S8, S9). Uniform morphology is ideal for predictable dissolution and drug release kinetics. Composite fiber diameter and nanoparticle diameter were both around 200–300 nm, with PVA fibers having a mean diameter of 248 ± 88 nm, PVP fibers of 297 ± 125 nm, and PLGA nanoparticles of 172 ± 19 nm (Fig. 1D). Nanoparticle size in the range of 200–500 nm has been found to be optimal for mucus penetration [40], suggesting that our Rho-NP are near the desirable size range that will diffuse quickly through mucus. Minimizing the fiber diameter to match the nanoparticle diameter may facilitate quick dissolution and release of nanoparticles by reducing the thickness of the fiber polymer coating that must dissolve before nanoparticles can be released. These results demonstrate that NP-NF composites were successfully electrospun from both PVA and PVP solutions with similar mean diameter and nanoparticle uniformity, and that this method is suitable for fabricating composite materials.
Figure 1. Fiber composites successfully formed with similar nanoparticle and nanofiber diameter.
(A) TEM micrograph of blank PLGA nanoparticles. (B) SEM micrograph of PVP composite fibers containing Rho-NP. (C) Confocal imaging of Rho-NP electrospun within PVP composite fibers. (D) Diameter (mean ± S.D.) of Rho-NP, Rho-NP-PVP composite fibers, Rho-NP-PVA composite fibers, and ETR-NP-PVA composite fibers. NP diameter was measured using DLS; fiber diameter was measured from SEM images. (E) SEM micrograph of PVA composite fibers containing Rho-NP. (F) Confocal imaging of Rho-NP electrospun within PVA composite fibers.
Fiber composites dissolve quickly in vitro to release mucus-penetrating nanoparticles
A key design constraint for NP-NF composites is that the fibers must wet out rapidly to release nanoparticles, enabling quick NP diffusion to the vaginal tissue target. We observed that both PVA and PVP composite fibers rapidly hydrated within 30s on black agar hydrogels (Fig. 2A). PVP composites wet out immediately upon contact (<1s), whereas PVA composites took slightly longer to wet out (within 30s). Even though the time to fully hydrate is different for PVA and PVP composite fibers, both fibers were observed to dissolve within 30 minutes under in vitro sink conditions in water. We also used a mechanical testing apparatus to quantitatively measure composite disintegration and found consistent results, with a faster mean disintegration time for PVP composites of 0.5s compared to 2.6s for PVA composites (Supplementary Table S3; Supplementary Methods). PVA and PVP composites both resulted in burst release under in vitro sink conditions to give >85% cumulative nanoparticle release in less than 30 min (Fig. 2B). The burst release kinetics of nanoparticles is consistent with our expectation of hydrophilic polymer fibers undergoing rapid dissolution. At early time points (< 30 min), nanoparticle release from PVP fibers was measured to be >100%. We attribute these high values to be from inadvertently including PVP fibers during the sampling procedure at early time points when PVP is not fully dissolved. For PVA fibers, nanoparticles took slightly longer to release out of the hydrated gel/fiber network as it continued to wet and dissolve over 30 minutes.
Figure 2. Fiber composites dissolve quickly in vitro to release nanoparticles.
(A) Composite fiber dissolution on black agar hydrogels used to simulate low fluid volume conditions. White dotted circle represents original area of fiber mesh. Representative images from n=3 replicates per group. (B) Cumulative Rho-NP release from composite fibers under in vitro sink conditions (4 mg composite fibers in 2 mL water). Dotted line at 0.5 h indicates time when both PVA and PVP fibers had fully dissolved. Data plotted as mean ± S.D., with n=3 per group.
Nanoparticles recovered from release studies for PVP composites were found to have similar size and surface charge compared to the original nanoparticles used to make the composites, while nanoparticles released from PVA composites had increased in size by about 125 nm and had a more neutral zeta potential (−0.5 mV as opposed to −5 mV) (Table 1). For PVA composites, these changes may indicate that PVA from fibers is displacing or shielding the Pluronic F127 surface coating on nanoparticles [44]. However, the properties for nanoparticles released from both PVP and PVA composites are still within the range relevant for mucus penetration and within the design targets for this system. Overall, this data is the first to our knowledge that demonstrates that nanoparticles can diffuse out of composite fibers in vitro and retain their physical properties after release.
Table 1.
Physical properties of nanoparticles before and after release from composite fibers.
| Sample | Z-avg size (nm) c | PDI | Zeta potential (mV) |
|---|---|---|---|
| Rho-NP (original) | 171.9 ± 19.1 | 0.089 ± 0.011 | −5.64 ± 0.80 |
| ETR-NP (original) | 175.4 ± 3.4 | 0.056 ± 0.022 | −4.48 ± 0.31 |
| Rho-NP, released from PVP composites | 204.9 ± 39.0 | 0.247 ± 0.060 (***) | −5.56 ± 1.49 |
| Rho-NP, released from PVA composites | 294.9 ± 9.3 (***) | 0.078 ± 0.027 | −0.58 ± 0.06 (***) |
| ETR-NP, released from PVA composites | 302.6 ± 5.4 (***) | 0.177 ± 0.025 (**) | −0.69 ± 0.17 (***) |
Data presented as mean ± S.D. with n ≥ 3 for each sample.
Measured using dynamic light scattering in 10 mM NaCl solution (pH = 5.7).
Significantly different from original NP measurement; ETR- or Rho-NP (released) compared to ETR- or Rho-NP (original)
p<0.01,
p<0.001.
It is important to recognize that in vivo release occurs under much different conditions than the in vitro release study in terms of total fluid volume. In the vaginal lumen, the fibers would dissolve in a low fluid volume comprised of highly viscous mucus and hydrated polymer gel (3 mg fibers/~25–100 μL mucus), instead of in vitro sink conditions of excess water (4 mg fibers/2 mL water). Composite fiber dissolution and nanoparticle release are expected to be much slower in vivo, even for hydrophilic polymers like PVA and PVP. Along with changes in fiber dissolution kinetics, nanoparticle properties may also differ under in vivo conditions in the presence of mucus and hydrated polymer gel. The fiber polymer may interact with the nanoparticle directly to slow or speed its diffusion, as has been previously described for dilute polymer solutions [45], but may also alter the properties of mucus itself. Pretreatment of human cervicovaginal mucus with 1% Pluronic® F-127 has been shown to enhance diffusion of conventional polystyrene nanoparticles, likely due to hydrophobic shielding interactions between Pluronic® F-127 and either the hydrophobic portions of mucus or the nanoparticle surface [46]. Others have shown that the presence of polymer can have dramatic effects on the mucus structure by altering water content, pore size, or packing of mucin fibers [47,48]. The complex interactions between fiber polymer, mucus, and nanoparticles are challenging to recapitulate in vitro and point to the need for in vivo models for evaluation. Improved in vitro-in vivo correlations for nanoparticle transport may be possible by developing ex vivo models that can better mimic the local microenvironment, as has been done in previous studies that have employed multiple particle tracking directly in freshly excised tissues [49,50]. Despite these differences, the in vitro dissolution and release studies confirm that both PVA and PVP composites meet the design constraints of fast fiber dissolution and rapid nanoparticle release.
Composite fibers reduce leakage and promote high retention of NP in vaginal tract
We intravaginally administered equivalent doses of Rho-NP to mice in either aqueous suspensions or composite fibers to study NP leakage and retention. After comparing the amount of external leakage of Rho-NP within one hour of administration, we found that composite fibers resulted in significantly less leakage than aqueous suspensions (Fig. 3). All mice receiving PVA and PVP composite fibers were found to have no detectable leakage above control mice that received blank composite fibers (containing NP with no rhodamine), whereas significant external leakage was visible for all mice that received aqueous suspensions of Rho-NP. To confirm that the reduced leakage provided by the composite fibers resulted in enhanced dose retention, we measured fluorescent content in the reproductive tract at 24h using Xenogen imaging and tissue homogenization techniques. We found that Rho-NP were significantly better retained in the reproductive tract at 24h for both PVA and PVP composite fibers relative to aqueous suspensions (Fig. 4A, B). Xenogen imaging of whole reproductive tracts prior to washing shows greater fluorescence for all mice that received PVA and PVP composite fibers compared to mice that received aqueous suspensions at 24h (Fig. 4A). A mean of only 1.5% of the initially administered Rho-NP dose was recovered for mice receiving aqueous suspensions, while almost half the dose was retained for both PVA fibers (50.3%) and PVP fibers (45.4%) (Fig. 4B; Table 2).
Figure 3. Significantly less external leakage of nanoparticles is observed for PVA and PVP composite fibers compared to aqueous suspensions.
(A–D) Xenogen imaging of black paper circles was used to measured external leakage for 1 h immediately after administration, with n=4 mice per group. In (A), the top two circles represent mice receiving blank PVA composite fibers, and the bottom two circles represent untreated mice. All images were set to the same scale of minimum and maximum radiance. (E) Maximum radiance quantified from Xenogen images in (A-D), mean ± S.D.
Figure 4. Fiber composites result in increased nanoparticle dose retention in the reproductive tract and association with vaginal tissue at 24h.
(A) Whole reproductive tracts imaged after dissection at 24 h, pre-wash. (B) Total dose recovery defined as the sum of dose recovered at 24h in wash buffer, vaginal tissue (post-wash), and uterine horns. (C) Opened vaginal tracts imaged after wash (tissues placed in 5 mL PBS for 30 minutes). (D) Dose recovery of fluorescent nanoparticles at 24h in wash buffer or homogenized vaginal tissue, post-wash. Data plotted as mean ± S.D., with n≥7 per group, n=5 for blank). ***p<0.001 relative to all other groups (one-way ANOVA comparing all four groups within the same tissue type).
Table 2.
Fluorescent nanoparticle recovery in the reproductive tract at 24h.
|
|
||||
|---|---|---|---|---|
| Wash Buffer (% Total Dose NP) | Vaginal Tissue (% Total Dose NP) | Uterine Horns (% Total Dose NP) | Total Dose Recoverya (% Total Dose NP) | |
| Aq. susp. | 1.1 ± 1.1 | 0.3 ± 0.6 | N.D. | 1.5 ± 1.3 |
|
| ||||
| PVA fibers | 43.6 ± 6.6 | 6.8 ± 2.8 | 0.1 ± 0.4 | 50.3 ± 5.5 |
|
| ||||
| PVP fibers | 8.2 ± 15.6 | 36.5 ± 17.2 | N.D. | 45.4 ± 7.0 |
Data shown is mean ± S.D. of n=7 mice per group (n=8 for vaginal sink).
Total dose recovery is the sum of the total dose of nanoparticles administered recovered in the wash buffer, homogenized vaginal tissue (post-wash), and homogenized uterine horns.
N.D. = not detected (signal below blank/untreated control)
This 32- to 35-fold increase in total nanoparticle dose retention at 24h for fibers is very significant in context of previous work on the intravaginal administration of nanoparticles. Cu et al. measured that ~3–13% of total NP dose had leaked externally within 30 min but also report total NP dose recoveries of only 30–49% at 30 minutes, suggesting that an even higher fraction of the dose leaked externally within this time [6]. Das Neves et al. reported that ~70% of the total NP dose was unaccounted for within 30 minutes of administration, which they attributed to external leakage [5]. These studies also report 0.6–2% total NP dose recovery at 24h for suspensions, citing extensive NP external leakage as a major limitation [5,6]. In contrast, our results demonstrate that composite fibers can dramatically reduce dose leakage and increase local retention of NP over a much longer time period than existing methods for nanoparticle delivery, providing a more flexible platform for sustained nanoparticle delivery and greatly enhanced local NP concentration. Additionally, the NP-NF composite dosage form offers a practical solution to cumbersome pre-existing methods for in vivo vaginal dosing that have required maintaining mice in an inverted position to improve vaginal retention and distribution of administered NP. While the final geometry of composite fibers for human application has yet to be determined, we envision that composite fibers could be applied digitally in a form similar to a film or capped tube shape, or using a tampon applicator.
Composite fibers increase nanoparticle association with vaginal tissue at 24h
For many agents of interest that are delivered intravaginally, including antiretroviral (ARV) drugs that act intracellularly like tenofovir and etravirine, the vaginal tissue is the ultimate target for drug action. The ideal dosage form would be able to provide high dose retention in the reproductive tract, and also release nanoparticles that can diffuse through cervicovaginal mucus into the vaginal epithelium. Therefore, we aimed to measure the amount of nanoparticles strictly associated with vaginal tissue by using a wash procedure to remove nanoparticles associated only with the luminal mucus (or still remaining in undissolved fibers). Vaginal tracts were imaged before and after washing in 5 mL PBS for 30 minutes, and the fluorescent NP content in the wash buffer versus associated with homogenized vaginal tissue (post-wash) was measured.
We found that NP association with vaginal tissue was higher for both PVA and PVP composite fibers compared with aqueous suspension (Fig. 4C, D). Xenogen imaging of opened vaginal tracts after the wash procedure showed greater fluorescence dispersed in the vaginal tract for fibers compared to the limited, non-uniform coverage observed for aqueous suspensions (Fig. 4C). After homogenizing vaginal tissue post-wash, a mean of 0.3% of the total dose was recovered for aqueous suspensions, versus 6.8% for PVA fibers and 36.5% for PVP fibers (Table 2). These results show that in addition to enhancing total NP dose retention in the reproductive tract by >30-fold, composite fibers also significantly increase NP delivery to vaginal tissue by 22-fold or higher at 24h.
We tested two fiber materials to study the effect of fiber mucoadhesivity on NP retention and trafficking. We found that at 24h after administration, the less mucoadhesive PVA fibers resulted in greater NP association with the luminal mucus (wash buffer), while the more mucoadhesive PVP fibers resulted in greater NP association with the vaginal tissue (Fig. 4D). Although the wash method efficiently separated undissolved PVA fibers from tissue, which we visualized as large fiber pieces in the buffer, undissolved PVP fibers broke into small fragments and remained highly adherent to the vaginal tissue even after the wash. We speculate that PVP may be sticking to the epithelial cell surface of vaginal tissue. As such, we expect that the 37% NP dose associated with the vaginal tissue for the PVP fiber group also includes NP trapped within highly adherent, undissolved fibers. In contrast, for PVA fibers, we propose that the 7% of total NP dose associated with the vaginal tissue represents only the portion of total NP dose that have exited the fibers and trafficked to vaginal tissue. Continued method development would be needed to separate undissolved PVP fibers from the vaginal tract in order to make any claims concerning in vivo nanoparticle release from PVP fibers (Fig. 4D, PVP only). However, claims about total dose retention in the reproductive tract (Fig. 4B) are still valid for both PVA and PVP fibers. Given this limitation, we chose to move forward with PVA fibers for our NP retention and drug dosing studies since we had greater confidence discerning where nanoparticles are trafficking for PVA than with PVP fibers.
PVA fiber composites sustain nanoparticle retention over three days
Given that nanoparticle retention was >50% of the total dose at 24h for PVA composite fibers, we next evaluated how long nanoparticles could be retained in the vaginal tract after a single administration. We intravaginally administered Rho-NP in composite PVA fibers and measured fluorescent content associated with tissues and fluid as described previously at 2d, 3d, 5d, and 7d after administration. We found by Xenogen fluorescent imaging that nanoparticles were retained in the reproductive tract and associated with vaginal tissue at detectable levels out to 3 days for PVA composite fibers (Fig. 5A, B). At 72h after administration, a mean of 38.5% of the total NP dose was retained in the reproductive tract overall, and 6.0% of the total NP dose was associated with the vaginal tissue (Fig. 5C). The total NP dose recovery decreased slightly from a mean of 47.0% of the total NP dose at 48h to 38.5% at 72h, and then fell below our detection limit (<0.2%) after 5d. These NP release kinetics are consistent with the hypothesis that in low fluid volume conditions in vivo, the fiber polymer wets and dissolves slowly to sustain release of nanoparticles. After release from fibers, some nanoparticles likely diffuse through cervicovaginal mucus to the luminal epithelial surface and/or into vaginal tissue, while some nanoparticles are lost externally due to mucus turnover. This study is significant in that it is the first to our knowledge showing sustained released of nanoparticles for intravaginal delivery out to three days through the use of a fiber dosage form.
Figure 5. Nanoparticles are retained in the reproductive tract out to three days after a single PVA composite fiber administration.
(A) Whole reproductive tracts, pre-wash. (B) Opened vaginal tracts post-wash, cut open to the cervix. Minimum and maximum radiance were set to the same values for all Xenogen images taken in (A) and (B). (C) Dose recovery of fluorescent nanoparticles at 1d, 2d, 3d, 5d, and 7d, measured by tissue homogenization methods, plotted as mean ± S.D. For each time point, n=3 animals received PVA composite fibers and n=1 untreated mouse was used as a control (1d time point from Fig. 4, different study with same methods; n=7 mice per group).
As predicted, we found that both PVA and PVP fibers dissolved more slowly in vivo compared to in vitro fiber dissolution and NP release experiments due to the high fiber dose and low fluid volume. We observed during necropsy that undissolved fibers were still visibly present in the vaginal tract of mice at 24h for both PVA and PVP composites. Even at 72h, we observed that some animals still had a much smaller but visible portion of undissolved PVA fibers inside the vaginal tract. We conducted an initial dosing study in which doses of 1, 3, and 5 mg fibers were administered to mice, and we selected the 3 mg dose to maximize detectability but still allow for fiber dissolution (Supplementary Fig. S10). However, 3 mg is still a large dose for mice relative to what would likely be realistic for human use. Additionally, the low fluid volume conditions and presence of viscous mucus likely slowed the in vivo dissolution of fibers. Even though both PVA and PVP are hydrophilic polymers, their wetting and dissolution requires a sufficient volume of water. The ratio of composite fiber : mucus : vaginal fluid will affect the combined fluid viscosity, and in turn may affect the rate of NP release. The NP release kinetics could potentially be modulated by altering the NP loading in the fibers, changing the total dose of composites administered, or using different polymers and/or excipients for the fiber material.
Cervicovaginal lavage pharmacokinetics
To demonstrate the utility of the composite platform for application as a topical microbicide for HIV prevention, we compared the pharmacokinetics of an 11.42 μg intravaginal dose of the antiretroviral drug ETR over seven days in mice for ETR-NP delivered from either PVA composite fibers or aqueous suspensions. ETR, a non-nucleoside reverse transcriptase inhibitor (NNRTI), was selected for its high potency, excellent resistance profile, and good penetration into the cervicovaginal compartment [51–53]. Here, we aimed to study the drug exposure and the kinetics of that exposure for ETR-NP delivered in either composite fibers or aqueous suspension in the vaginal lavage, vaginal tissue, and selected systemic organs.
Pharmacokinetics in the cervicovaginal lavage were remarkably different for aqueous suspensions compared to composite fibers (Fig. 6A, Tables 3 and 4). For mice that received aqueous suspensions, Cmax occurred at around 1h, with a mean 9.72 μg ETR/mL detected in the lavage (17.0% total ETR dose administered) (Fig. 6A, Table 3). The drug concentration fell rapidly, down to a mean of 0.67 μg ETR /mL (1.2% total dose) by 24h (Fig. 6A). For PVA composite fibers, Cmax also occurred around 1h, with a mean of 47.5 μg ETR/mL detected in the lavage (83.7% total ETR dose) (Fig. 6A, Table 3). However, for fibers, the drug concentration decreased much more slowly in the lavage out through 72h compared to aqueous suspensions (Fig. 6A). At 72h, there was still 33.1 μg ETR/mL (44.0% total dose) detected in the lavage, which is similar to the 32.5% total dose of Rho-NP recovered in the wash buffer at 72h for the PVA composite fibers (Fig. 5C). The ability of composite fibers to significantly enhance the overall drug exposure in the vaginal lumen is reflected by the over 12-fold greater ETR AUC0-72h achieved with fibers compared to aqueous suspension (P < 0.0001) and 15-fold greater ETR AUC0-∞ (P = 0.0040) (Table 3).
Figure 6. PVA composite fibers result enhanced ETR retention over 7 days in the cervicovaginal lavage and vaginal tissue relative to aqueous suspensions.
(A) ETR content in 200 μL cervicovaginal lavage, including any undissolved fibers present. (B) ETR content in homogenized vaginal tissue, post-lavage. Line represents mean of n=5 mice per group at each time point out to 72h, with fiber group only extended to 5d and 7d. Statistical outliers were identified with Grubb’s test (alpha = 0.01) and were not included for PK analysis or displayed in this figure. **p<0.01, ***p<0.001.
At 5d or longer, the drug concentration in the lavage for PVA fibers became more variable, and declined overall to a mean of 8.1 μg ETR/mL (14.3% total dose) at 7d (Fig. 6A). The apparent terminal elimination half-life (t1/2z) values for the two NP dosage platforms show the differences in drug release kinetics, with aqueous suspensions having an t1/2z of 36.4h in the cervicovaginal lavage, versus 54.5h for the PVA fibers (Table 3). The differences in release kinetics in the lavage are likely due to the fast delivery but limited retention of a liquid-based dosage form, compared to the enhanced retention, slow hydration, dissolution, and subsequent release of NP provided by fibers. Because the undissolved fibers and cervicovaginal mucus were both counted as part of the lavage, we did not distinguish between NP/drug within the PVA fibers and NP/drug that had been released into the lumen. However, the data still support our hypothesis that composite fibers did indeed result in much less leakage and greater retention of ETR in the reproductive tract than aqueous suspensions.
Given the extremely high and sustained drug concentrations and exposure (AUC) detected in lavage for the PVA composite fibers (Table 3), the current NP-NF composite platform may be suitable for agents that act as entry inhibitors to HIV-1 like maraviroc, griffithsin, or cyanovirin-N. High concentrations of entry inhibitors are desirable in the vaginal lumen to prevent virus from trafficking further into vaginal tissue and entering cells. Although ETR has not been evaluated in clinical studies as a vaginally applied product, comparisons can be made to dapivirine (DPV). Dapivirine is an antiviral drug with similar physicochemical properties to ETR, and has been more widely studied for vaginal administration. A PK study of women using DPV vaginal rings for 7d reported mean vaginal fluid concentrations of DPV in the range of 0.7–7.1 μg/mL [54]. The DIVA-2 study evaluated ETR concentrations in cervicovaginal fluid (CVF) after oral daily dosing of ETR in HIV-1-infected women, and found good penetration of ETR into the CVF at concentrations of 0.857 μg/mL at 12h after the last dose [52]. In comparison, our PVA fiber composite provided ~10-fold higher ETR concentration in lavage at 7 d after a single dose. Overall, the dramatically enhanced ETR retention and concentration in the vaginal lumen and increased terminal half-life for composite fibers demonstrates the potential of fibers to increase retention and sustain drug release and exposure (AUC) relative to aqueous suspensions. These data suggest that a fiber platform could provide sustained release of both NP and drug in vivo. Since nanofibers are also able to deliver drug in a burst-release manner [13,14], the fiber platform may be able to meet design criteria for new microbicide technologies designed for both “on-demand” or pericoital use and sustained protection [55].
Vaginal tissue pharmacokinetics
We found that PVA composite fibers resulted in slightly increased ETR bioavailability to vaginal tissue over 7 days, with relative bioavailability (AUCfiber/AUCsuspension) of 1.23 for 0–72h and 1.38 for 0-infinity (Table 3). Similar to the cervicovaginal lavage release kinetics, aqueous suspensions resulted in a high Cmax at early times (4.66 μg ETR/g at 1h), but tissue levels decreased rapidly to 0.094 μg ETR/g at 24h (Fig. 6B, Table 3). In contrast, PVA composite fibers provided a lower initial drug concentration but more constant, sustained ETR delivery to vaginal tissue out to 72h. Although not statistically significant, vaginal tissue concentrations were approximately two-fold higher for fibers than suspensions from 24h–72h (Fig. 6B). The prolonged release kinetics is also captured by the increased total drug exposure after a single dose for fibers. The AUC0-∞ was 69.1 hr•μg/g for fibers compared to 50.1 hr•μg/g for suspensions (Table 3) (P = 0.0555). This data is meaningful in that the vaginal tissue is where etravirine would actively inhibit HIV-1 reverse transcriptase.
The two-fold enhancement in ETR content in vaginal tissue provided by the fiber platform from 24–72h is in contrast to the 20-fold enhancement in fluorescent NP at 24h that was observed in our proof-of-concept studies. Importantly, the difference in observations may be attributed to differences in measuring drug content versus NP content. Rapid burst release of ETR from the NP into the lumen and the propensity of ETR to bind proteins in vaginal fluid (as has been seen in plasma [56,57]) may have contributed to the lower drug content observed in vaginal tissues. To evaluate if ETR burst released before reaching vaginal tissue, we measured the in vitro release from ETR-NP under sink conditions and found that >95% of drug had released within 2 h (Supplementary Fig. S11). Though in vitro release does not recapitulate the degree of burst release we would expect to see in the low volume conditions in vivo, these data suggest that ETR is likely surface-associated and may rapidly dissociate from NP surfaces. Burst release of ETR in the lumen would account for the observed high concentrations of drug in the lavage relative to the vaginal tissue. For future studies, this nanoparticle formulation may be further optimized to limit burst release. Testing other ARVs with different physicochemical properties in this system would be useful to understand how drug properties contribute to differences in drug tissue concentrations and elimination kinetics for the composite fiber system. Along with optimizing NP for sustained release, NP should ideally be optimized for trafficking through the vaginal epithelium in addition to mucus. While PEGylated PLGA NP have been shown to diffuse quickly through mucus [1,40], there is limited knowledge on the transport mechanisms of these NP in the vaginal tissue. PEGylation of the nanoparticle surface has been demonstrated to result in decreased cell uptake and reduced efficiency of endosomal escape [58]. Thus, there is need for development of new strategies to create NP that can cross the vaginal epithelial barrier and be taken up intracellularly by target cells for HIV-1. Further studies will investigate how to optimize the composite fiber delivery system for increased diffusion of NP into vaginal tissue, the target for drugs that have intracellular mechanisms of action like ETR or other NNRTIs.
Although we observed low vaginal tissue concentrations of ETR relative to the those in lavage, the concentrations are still relevant in terms of establishing protective drug levels for HIV-1 prevention. The mean vaginal tissue concentrations detected for the PVA fiber group at 72 h was 293 ng/g, nearly 150-fold higher than the in vitro IC50 for ETR of ~2 ng/mL [52]. DPV vaginal ring studies in humans reported vaginal tissue concentrations in the range of 0.3 to 3.5 μg/g [54], similar to the range of ETR detected between 1–72h for the PVA fiber group for our study (0.3–1.9 μg/g). DPV vaginal films have been estimated to provide approximately 152–334 nM DPV (0.05–0.11 μg/mL) to human cervical explant tissues after 6h exposure [59]. NP-NF composites provided concentrations of ETR in vaginal tissue similar to the range reported for vaginal rings, but were also able to sustain exposure out to 1 week, with a mean ETR concentration of 0.06 μg/g at 7d (similar to vaginal film at 6h). NP-NF composites could potentially serve as an alternate dosage platform to vaginal films that could be administered in a similar way, but provide protection for a longer duration. Overall, the PK data for vaginal tissue showed that composite PVA fibers improved ETR delivery to vaginal tissue by about two-fold relative to aqueous suspensions out to three days after a single application and increased tissue exposure over 1 week.
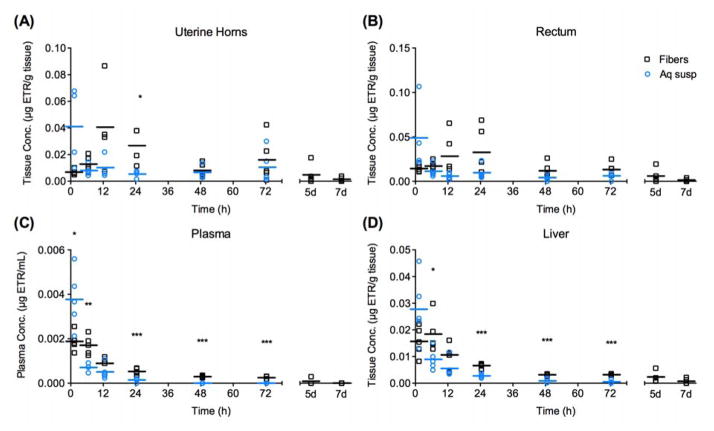
Low systemic drug exposure in secondary organs
We found that systemic exposure to ETR was very low for both delivery platforms (Fig. 7). Maximum ETR concentrations in the uterine horns, rectum, or liver were approximately two orders of magnitude lower (50-fold to 170-fold less) than maximum concentrations in the vaginal tissue (Table 3). Maximum ETR concentrations in the plasma were even lower, about three orders of magnitude (1000-fold to 1200-fold less) than the Cmax in vaginal tissue for both delivery platforms. These data are consistent with previous reports that intravaginal delivery of antiviral drug-loaded NP show low systemic exposure [5], corroborating the rationale for local vaginal drug administration to provide high concentrations of drug in target tissue while limiting systemic exposure and corresponding side effects.
Figure 7. Low systemic drug exposure was observed for both delivery platforms, with more sustained release observed for the PVA composite fibers.
(A)–(D) ETR content detected by LC-MS/MS in homogenized tissues. *p<0.05, **p<0.01, **p<0.001. Line represents mean of n=5 mice per group at each time point out to 72h, with fiber group only extended to 5d and 7d. Statistical outliers were identified with Grubb’s test (alpha = 0.01) and were not included for PK analysis or displayed in this figure.
Even though we observed overall low systemic drug exposure, the pharmacokinetics in the horns, rectum, plasma, and liver for mice that received fibers are consistent with sustained ETR release out to 72 hours. For the secondary organs located anatomically closest to the vaginal tract, the rectum and uterine horns, we observed unique patterns in ETR drug exposure and kinetics for aqueous suspensions compared to composite fibers (Fig. 7A, B). For aqueous suspensions, all secondary organs resulted in Cmax occurring at the earliest time point (1h) and decreasing rapidly to the last time point (72h), consistent with release kinetics observed for lavage and vaginal tissue. For PVA composite fibers, we observed a delay in Tmax, with the uterine horns and rectum reaching Cmax at 12h and 24h, respectively (Table 3). Since Tmax occurs later for fibers in the rectum and uterine horns relative to plasma and liver (Supplementary Fig. S12), this longer apparent distribution phase suggests that drug is not primarily entering these tissues by the blood. Instead, the drug must be distributing to the rectum and uterine horns by a slower means such as drug diffusion through the vaginal tissue. The delay in Cmax for composite fibers is consistent with visual observations of slow fiber dissolution over the time course of days and the hypothesis that fibers would take a longer time to hydrate, dissolve, and release NP. The AUC0-72h was about two-fold higher for fibers than aqueous suspensions for both uterine horns and rectum, showing significantly higher overall drug exposure to tissue for fibers (Table 3). Though low relative to vaginal tissue AUC values, these data also support the claim that the fiber platform is more suitable for sustained release of drug over the course of days, compared to the burst release over minutes or hours seen for the aqueous suspension group.
The drug concentrations measured in liver and plasma follow similar patterns between suspensions and fibers when comparing the total drug exposure and concentration-time profiles, with overall low concentrations detected, but significantly higher drug concentrations for fibers than aqueous suspensions from 24h – 72h (Fig. 7C,D). Total drug exposure (AUC0-∞) in the liver and plasma was significantly higher with fibers versus aqueous suspensions (Table 3) (Liver: P = 0.0020; Plasma: P = 0.0215). Although the difference in values was not statistically significant, t1/2z in liver was longer for the PVA fiber group (42.1h) compared to the suspension (17.2h) (Table 3). Following a similar pattern, the t1/2z in plasma was 56.0h for PVA fibers, compared to 8.1h estimated for the suspensions. This higher ETR exposure and longer t1/2z observed in the liver and plasma for PVA fiber composites parallel the two-fold increase in vaginal tissue ETR concentrations seen for fibers at 24–72h (Fig. 6B). Together, these data support the claim that composite fibers can provide sustained exposure of ETR over the course of three to seven days.
CONCLUSIONS
In this work, we explored electrospun fibers as a novel topical vaginal delivery platform for nanoparticles. We demonstrate the ability of composite fibers to release intact nanoparticles that retained size and surface properties conducive for mucus penetration. Composite fibers were shown to significantly enhance retention of NP and drug in the reproductive tract and sustain release of both NP and drug in vivo out to at least 3 days. This work demonstrates the capability of fibers as a sustained release platform that has potential applications in clinical use as a long-acting microbicide. It is the first report to our knowledge evaluating the in vivo retention of an intravaginal dosage form for NP delivery other than aqueous suspensions. This work is also significant in that two components within a single delivery platform were engineered independently for optimal interactions with cervicovaginal mucus. From the pharmacokinetic study, we observed extremely high drug concentrations retained in the cervicovaginal lavage out to 7 days and a two-fold increase in ETR concentration in vaginal tissue from 24–72h for PVA composite fibers relative to aqueous suspensions.
While there are still many variables left to explore for optimizing drug delivery to vaginal tissue from composite fibers, this work clearly demonstrates that composite fibers substantially improve both nanoparticle and drug retention in the reproductive tract. For this study, we prioritized directly measuring in vivo NP and drug retention in a mouse model where the complex interaction of our composite system with mucus, but also the entire local microenvironment, would collectively measure the impact of our design. Further studies to better delineate the impact of multiple factors on nanoparticle release from composite materials are recommended, perhaps utilizing multiple particle tracking techniques. In particular, further exploration of PVP as a fiber material for vaginal drug delivery is recommended given its high degree of mucoadhesivity. Composite fibers offer a new platform for the intravaginal administration of nanoparticles that is both solid-state and practical to administer. Such a system may also have applications to other target sites for mucosal delivery including pulmonary, nasal, and buccal delivery.
Supplementary Material
Fig. S1—Schematic of NP-NF composite preparation process.
Fig. S2—Lavage method for removing mucus-associated Rho-NP and undissolved PVA and PVP composite fibers.
Fig. S3—Lavage vs. wash buffer method comparison for ETR-NP / PVA composite fibers.
Fig. S4—Terminal elimination half-life estimation by Phoenix WinNonlin for PK parameters for vaginal lavage and vaginal tissue.
Fig. S5—Terminal elimination half-life estimation by Phoenix WinNonlin for PK parameters for plasma and liver.
Fig. S6—Terminal elimination half-life estimation by Phoenix WinNonlin for PK parameters for rectum and uterine horns.
Fig. S7—Release of ETR and rhodamine from PLGA-NP during electrospinning.
Fig. S8—High magnification SEM images of composite fibers.
Fig. S9—Additional confocal fluorescent images of composite fibers.
Fig. S10—Pilot dosing study of 1, 3, 5 mg composite Rho-NP/PVP fibers in mice.
Fig. S11—In vitro release of ETR from PLGA-NP under sink conditions.
Fig. S12—ETR concentration-time profiles following intravaginal administration of ETR-loaded PLGA nanoparticles in aqueous suspension or PVA composite fibers.
Table S1—Physicochemical properties of ETR-PLGA nanoparticles.
Table S2—Gradient method for LC-MS/MS detection of etravirine.
Table S3—Quantitative disintegration time for composite fibers.
Supplementary Methods—Quantitative fiber disintegration
Acknowledgments
We acknowledge J. Park for insightful discussions on in vivo experimental design and L. Chan for discussions on the pharmacokinetic study design. We thank S. Golan-Paz for her assistance with TEM and in vitro ETR-NP release studies, K. Thoreson and L. Habernicht for their assistance with in vivo studies, and S. F. Chou for his assistance with fiber disintegration testing. We thank the I. Suydam Laboratory at Seattle University for the gift of etravirine. We also acknowledge several technology user facilities at the University of Washington: the Molecular Analysis Facility (SEM, TEM), Keck Microscopy Center (confocal microscopy), and Mass Spectroscopy Center (LC-MS/MS). This work was funded by NIH grants AI094412 and AI112002 to KAW. The National Science Foundation provided Graduate Research Fellowships to EK and AB. RR was supported by the University of Washington STD/AIDS Training Fellowship (NIHT32AI07140). The SURP Space Grant Program 6 (ecG1 A68796) provided funding for CN. This work is also supported in part by NIH grants UM1 AI-120176, AI-077390, AI-077390-S1, AI-077390-S2, AI-077390-S3, 1UL1-RR025014, and P510D010425 to RJYH and JCK. In addition, RJYH is supported by the Milo Gibaldi endowment and JCK by the NIH T032 Pharmacological Sciences Training Grant (T32-GM007750).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-Penetrating Nanoparticles for Vaginal Drug Delivery Protect Against Herpes Simplex Virus. Sci Transl Med. 2012;4:138ra79–138ra79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang M, Yu T, Wang YY, Lai SK, Zeng Q, Miao B, Tang BC, Simons BW, Ensign LM, Liu G, Chan KWY, Juang CY, Mert O, Wood J, Fu J, McMahon MT, Wu TC, Hung CF, Hanes J. Vaginal Delivery of Paclitaxel via Nanoparticles with Non-Mucoadhesive Surfaces Suppresses Cervical Tumor Growth. Adv Healthc Mater. 2014;3:1044–1052. doi: 10.1002/adhm.201300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo JW, Giri N, Lee CH. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403:262–267. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Agrahari V, Zhang C, Zhang T, Li W, Gounev TK, Oyler NA, Youan BBC. Hyaluronidase-Sensitive Nanoparticle Templates for Triggered Release of HIV/AIDS Microbicide In Vitro. AAPS J. 2014;16:181–193. doi: 10.1208/s12248-013-9546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.das Neves J, Araújo F, Andrade F, Amiji M, Bahia MF, Sarmento B. Biodistribution and Pharmacokinetics of Dapivirine-Loaded Nanoparticles after Vaginal Delivery in Mice. Pharm Res. 2014;31:1834–1845. doi: 10.1007/s11095-013-1287-x. [DOI] [PubMed] [Google Scholar]
- 6.Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. J Controlled Release. 2011;156:258–264. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, Schwartz K, Laborde N, Soto-Torres L. Women’s Experiences with Oral and Vaginal Pre-Exposure Prophylaxis: The VOICE-C Qualitative Study in Johannesburg, South Africa. PLoS ONE. 2014;9:e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003) 2013 [Google Scholar]
- 9.Rees H. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women. 2015 [Google Scholar]
- 10.Huang C, Soenen SJ, Rejman J, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Stimuli-responsive electrospun fibers and their applications. Chem Soc Rev. 2011;40:2417. doi: 10.1039/c0cs00181c. [DOI] [PubMed] [Google Scholar]
- 11.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-Eluting Fibers for HIV-1 Inhibition and Contraception. PLoS ONE. 2012;7:e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013;100(Supplement):S9–S16. doi: 10.1016/j.antiviral.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Ball C, Woodrow KA. Electrospun Solid Dispersions of Maraviroc for Rapid Intravaginal Preexposure Prophylaxis of HIV. Antimicrob Agents Chemother. 2014;58:4855–4865. doi: 10.1128/AAC.02564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int J Pharm. 2014;475:282–291. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson D, Jiang Y, Woodrow KA. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm Res. 2015 doi: 10.1007/s11095-015-1769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou SF, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Controlled Release. 2015 doi: 10.1016/j.jconrel.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brako F, Raimi-Abraham B, Mahalingam S, Craig DQM, Edirisinghe M. Making nanofibres of mucoadhesive polymer blends for vaginal therapies. Eur Polym J. 2015;70:186–196. doi: 10.1016/j.eurpolymj.2015.07.006. [DOI] [Google Scholar]
- 18.Ball C, Chou SF, Jiang Y, Woodrow KA. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Mater Sci Eng C. 2016;63:117–124. doi: 10.1016/j.msec.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krogstad EA, Rathbone MJ, Woodrow KA. Vaginal Drug Delivery. In: Domb AJ, Khan W, editors. Focal Control Drug Deliv. Springer US; 2014. [accessed March 18, 2014]. pp. 607–651. http://link.springer.com/chapter/10.1007/978-1-4614-9434-8_27. [Google Scholar]
- 20.Ball Cameron, Woodrow Kim A. Electrospun Fibers for Microbicide Drug Delivery. In: das Neves José, Sarmento Bruno., editors. Drug Deliv Dev Anti-HIV Microbicides. Pan Stanford Publishing Pte. Ltd; 2014. pp. 459–508. [Google Scholar]
- 21.Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int J Nanomedicine. 2014;9:2967–2978. doi: 10.2147/IJN.S61664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brettmann BK, Tsang S, Forward KM, Rutledge GC, Myerson AS, Trout BL. Free Surface Electrospinning of Fibers Containing Microparticles. Langmuir. 2012;28:9714–9721. doi: 10.1021/la301422x. [DOI] [PubMed] [Google Scholar]
- 23.Solomonidou D, Cremer K, Krumme M, Kreuter J. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J Biomater Sci Polym Ed. 2001;12:1191–1205. doi: 10.1163/156856201753395743. [DOI] [PubMed] [Google Scholar]
- 24.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 25.de Araújo Pereira RR, Bruschi ML. Vaginal mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 2012;38:643–652. doi: 10.3109/03639045.2011.623355. [DOI] [PubMed] [Google Scholar]
- 26.Asane GS, Nirmal SA, Rasal KB, Naik AA, Mahadik MS, Rao YM. Polymers for Mucoadhesive Drug Delivery System: A Current Status. Drug Dev Ind Pharm. 2008;34:1246–1266. doi: 10.1080/03639040802026012. [DOI] [PubMed] [Google Scholar]
- 27.Qiu K, He C, Feng W, Wang W, Zhou X, Yin Z, Chen L, Wang H, Mo X. Doxorubicin-loaded electrospun poly(L-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J Mater Chem B. 2013 doi: 10.1039/C3TB20636J. [DOI] [PubMed] [Google Scholar]
- 28.Zomer Volpato F, Almodóvar J, Erickson K, Popat KC, Migliaresi C, Kipper MJ. Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 2012;8:1551–1559. doi: 10.1016/j.actbio.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Qiao W, Wang B, Zhang Y, Shao P, Yin T. Electrospun composite nanofibers containing nanoparticles for the programmable release of dual drugs. Polym J. 2011;43:478–483. doi: 10.1038/pj.2011.11. [DOI] [Google Scholar]
- 30.Song B, Wu C, Chang J. Controllable delivery of hydrophilic and hydrophobic drugs from electrospun poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles composite mats. J Biomed Mater Res B Appl Biomater. 2012;100B:2178–2186. doi: 10.1002/jbm.b.32785. [DOI] [PubMed] [Google Scholar]
- 31.Chaowanachan T, Krogstad E, Ball C, Woodrow KA. Drug Synergy of Tenofovir and Nanoparticle-Based Antiretrovirals for HIV Prophylaxis. PLoS ONE. 2013;8:e61416. doi: 10.1371/journal.pone.0061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costantino L, Gandolfi F, Tosi G, Rivasi F, Vandelli MA, Forni F. Peptide-derivatized biodegradable nanoparticles able to cross the blood–brain barrier. J Controlled Release. 2005;108:84–96. doi: 10.1016/j.jconrel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moench TR, Mumper RJ, Hoen TE, Sun M, Cone RA. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471-2334-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.das Neves J, Araújo F, Andrade F, Amiji M, Bahia MF, Sarmento B. Biodistribution and Pharmacokinetics of Dapivirine-Loaded Nanoparticles after Vaginal Delivery in Mice. Pharm Res. 2014;31:1834–1845. doi: 10.1007/s11095-013-1287-x. [DOI] [PubMed] [Google Scholar]
- 36.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mager H, Göller G. Resampling Methods in Sparse Sampling Situations in Preclinical Pharmacokinetic Studies. J Pharm Sci. 1998;87:372–378. doi: 10.1021/js970114h. [DOI] [PubMed] [Google Scholar]
- 38.Efron B, Tibshirani R. An introduction to the bootstrap. Chapman & Hall; New York: 1993. [Google Scholar]
- 39.Lee N, Duan H, Hebert MF, Liang CJ, Rice KM, Wang J. Taste of a Pill: Organic Cation Transporter-3 (OCT3) Mediates Metformin Accumulation and Secretion in Salivary Glands. J Biol Chem. 2014;289:27055–27064. doi: 10.1074/jbc.M114.570564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck-Broichsitter M, Thieme M, Nguyen J, Schmehl T, Gessler T, Seeger W, Agarwal S, Greiner A, Kissel T. Novel “Nano in Nano” Composites for Sustained Drug Delivery: Biodegradable Nanoparticles Encapsulated into Nanofiber Non-Wovens. Macromol Biosci. 2010;10:1527–1535. doi: 10.1002/mabi.201000100. [DOI] [PubMed] [Google Scholar]
- 42.Chen M, Gao S, Dong M, Song J, Yang C, Howard KA, Kjems J, Besenbacher F. Chitosan/siRNA Nanoparticles Encapsulated in PLGA Nanofibers for siRNA Delivery. ACS Nano. 2012;6:4835–4844. doi: 10.1021/nn300106t. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, Tang L, Yang J, Nguyen KT. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013;9:9351–9359. doi: 10.1016/j.actbio.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Lai SK, Yu T, Wang YY, Happe C, Zhong W, Zhang M, Anonuevo A, Fridley C, Hung A, Fu J, Hanes J. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J Controlled Release. 2014;192:202–208. doi: 10.1016/j.jconrel.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mun EA, Hannell C, Rogers SE, Hole P, Williams AC, Khutoryanskiy VV. On the Role of Specific Interactions in the Diffusion of Nanoparticles in Aqueous Polymer Solutions. Langmuir. 2014;30:308–317. doi: 10.1021/la4029035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ensign LM, Lai SK, Wang YY, Yang M, Mert O, Hanes J, Cone R. Pretreatment of Human Cervicovaginal Mucus with Pluronic F127 Enhances Nanoparticle Penetration without Compromising Mucus Barrier Properties to Herpes Simplex Virus. Biomacromolecules. 2014;15:4403–4409. doi: 10.1021/bm501419z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willits RK, Saltzman WM. Synthetic polymers alter the structure of cervical mucus. Biomaterials. 2001;22:445–452. doi: 10.1016/S0142-9612(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 48.Willits RK, Mark Saltzman W. The effect of synthetic polymers on the migration of monocytes through human cervical mucus. Biomaterials. 2004;25:4563–4571. doi: 10.1016/j.biomaterials.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 49.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles Within Brain Tissue. Sci Transl Med. 2012;4:149ra119–149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ensign LM, Henning A, Schneider CS, Maisel K, Wang YY, Porosoff MD, Cone R, Hanes J. Ex Vivo Characterization of Particle Transport in Mucus Secretions Coating Freshly Excised Mucosal Tissues. Mol Pharm. 2013;10:2176–2182. doi: 10.1021/mp400087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schöller-Gyüre DM, Kakuda TN, Raoof A, Smedt GD, Hoetelmans RMW. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine. Clin Pharmacokinet. 2012;48:561–574. doi: 10.2165/10895940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Clavel C, Peytavin G, Tubiana R, Soulié C, Courbon E, Crenn-Hebert C, Ichou H, Blanc C, Schneider L, Katlama C, Marcelin AG, Mandelbrot L. Etravirine Concentrations in the Cervicovaginal Compartment in HIV-1-Infected Women Receiving Etravirine-Containing Antiretroviral Therapy: DIVA 02 Study. Antimicrob Agents Chemother. 2012;56:4018–4020. doi: 10.1128/AAC.06474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson K, Jennings S, Falcon R, Mrus J, Kashuba A. Darunavir, Ritonavir, and Etravirine Pharmacokinetics in the Cervicovaginal Fluid and Blood Plasma of HIV-Infected Women. Antimicrob Agents Chemother. 2011;55:1120–1122. doi: 10.1128/AAC.00889-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, Temmerman M, Verstraelen H, Van Bortel L, Weyers S, Mitchnick M. Safety and Availability of Dapivirine (TMC120) Delivered from an Intravaginal Ring. AIDS Res Hum Retroviruses. 2009;25:483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 55.CAMI Health. MPT Product Prioritization and Gap Analysis: 2014 Summary. Initiative for Multipurpose Prevention Technologies (IMPT); 2014. [accessed November 4, 2015]. http://www.cami-health.org/documents/MPTProductPrioritizationGapAnalysis2014Sum.pdf. [Google Scholar]
- 56.Cottrell ML, Srinivas N, Kashuba AD. Pharmacokinetics of antiretrovirals in mucosal tissue. Expert Opin Drug Metab Toxicol. 2015;11:893–905. doi: 10.1517/17425255.2015.1027682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liedtke M, Rathbun The next generation: etravirine in the treatment of HIV-1 infection in adults refractory to other antiretrovirals. Virus Adapt Treat. 2010:91. doi: 10.2147/VAAT.S6413. [DOI] [Google Scholar]
- 58.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akil A, Devlin B, Cost M, Rohan LC. Increased Dapivirine Tissue Accumulation through Vaginal Film Codelivery of Dapivirine and Tenofovir. Mol Pharm. 2014;11:1533–1541. doi: 10.1021/mp4007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1—Schematic of NP-NF composite preparation process.
Fig. S2—Lavage method for removing mucus-associated Rho-NP and undissolved PVA and PVP composite fibers.
Fig. S3—Lavage vs. wash buffer method comparison for ETR-NP / PVA composite fibers.
Fig. S4—Terminal elimination half-life estimation by Phoenix WinNonlin for PK parameters for vaginal lavage and vaginal tissue.
Fig. S5—Terminal elimination half-life estimation by Phoenix WinNonlin for PK parameters for plasma and liver.
Fig. S6—Terminal elimination half-life estimation by Phoenix WinNonlin for PK parameters for rectum and uterine horns.
Fig. S7—Release of ETR and rhodamine from PLGA-NP during electrospinning.
Fig. S8—High magnification SEM images of composite fibers.
Fig. S9—Additional confocal fluorescent images of composite fibers.
Fig. S10—Pilot dosing study of 1, 3, 5 mg composite Rho-NP/PVP fibers in mice.
Fig. S11—In vitro release of ETR from PLGA-NP under sink conditions.
Fig. S12—ETR concentration-time profiles following intravaginal administration of ETR-loaded PLGA nanoparticles in aqueous suspension or PVA composite fibers.
Table S1—Physicochemical properties of ETR-PLGA nanoparticles.
Table S2—Gradient method for LC-MS/MS detection of etravirine.
Table S3—Quantitative disintegration time for composite fibers.
Supplementary Methods—Quantitative fiber disintegration