Summary
The advent of large-scale in vitro differentiation of human stem cell-derived insulin-producing cells (SCIPC) has brought us closer to treating diabetes using stem cell technology. However, decades of experiences from islet transplantation show that ischemia-induced islet cell death after transplant severely limits the efficacy of the therapy. It is unclear to what extent human SCIPC are susceptible to ischemia. In this study, we show that more than half of SCIPC die shortly after transplantation. Nutrient deprivation and hypoxia acted synergistically to kill SCIPC in vitro. Amino acid supplementation rescued SCIPC from nutrient deprivation, likely by providing cellular energy. Generating SCIPC under physiological oxygen tension of 5% conferred hypoxia resistance without affecting their differentiation or function. A two-pronged strategy of physiological oxygen acclimatization during differentiation and amino acid supplementation during transplantation significantly improved SCIPC survival after transplant.
Keywords: stem cell-derived insulin-producing cells, type 1 diabetes, islet transplant, ischemia, hypoxia, nutrient deprivation, graft survival
Graphical Abstract
Highlights
-
•
Stem cell-derived insulin-producing cells (SCIPC) are susceptible to ischemic injury
-
•
Amino acid supplementation prevents nutrient-deprivation-induced SCIPC death
-
•
Generation of SCIPC at physiological oxygen levels protects them against hypoxia
-
•
Both strategies combined preserve SCIPC graft viability in vivo upon transplant
Cell death after transplant due to ischemic injury is a major obstacle to successful β cell replacement therapy for diabetes. In this issue of Stem Cell Reports, Faleo, Russ et al. analyzed the effects of hypoxia and nutrient deprivation on human stem cell-derived insulin-producing cells and developed effective strategies to promote their survival after transplant.
Introduction
Islet transplantation can be a minimally invasive curative therapy for type 1 diabetes (T1D) (Barton et al., 2012). Although rates of insulin independence after islet transplantation have been steadily improving, wide application of this therapy is limited by the scarcity of eligible deceased donors and the frequent need of more than one donor per recipient (Moassesfar et al., 2016, Wisel et al., 2016). Because islets are detached from the vasculature during isolation, return of blood supply to transplanted islets is delayed relative to whole organ transplants, resulting in prolonged ischemia and reduced graft viability that hinders the efficacy of the therapy.
The high metabolic demand and reliance on aerobic metabolism make β cells highly sensitive to proper oxygenation. Oxygen tension in the native pancreas is estimated to be 5% O2 (Carlsson et al., 1998), but drops below 1% O2 in islet grafts shortly after transplant (Carlsson et al., 2001). Hypoxia induces ER stress (Bensellam et al., 2016) and production of reactive oxygen species in β cells (Clanton, 2007), both of which can lead to β cell death. β cells that survive hypoxia switch from aerobic glucose metabolism to anaerobic glycolysis that impairs β cell function (Cantley et al., 2010, Cantley et al., 2013, Puri et al., 2013). Many strategies are being evaluated to minimize hypoxia-induced β cell death and metabolic reprogramming, including oxygen supplementation, hypoxia preconditioning, promoting neovascularization, and cytoprotection using antioxidants and pro-survival genes (Barkai et al., 2013, Emamaullee et al., 2005a, Emamaullee et al., 2005b, Faleo et al., 2012, Grey et al., 2003, Hogan et al., 2012, Pedraza et al., 2012, Plesner et al., 2005, Yin et al., 2006). However, ischemia also results in nutrient deprivation that can affect cell viability and function. The extent to which nutrient deprivation affects β cell survival, alone or in combination with hypoxia, is unknown.
Recent advances in the generation of functional human stem cell-derived insulin-producing cells (SCIPC) have raised the possibility of providing a renewable source of β cells for transplantation, overcoming the shortage of human donors (Pagliuca et al., 2014, Rezania et al., 2014, Russ et al., 2015, Yoshihara et al., 2016). Currently a clinical trial is ongoing to evaluate the safety and efficacy of human embryonic stem cell-derived pancreatic progenitor cells (NCT02239354). However, how human SCIPC respond to hypoxia and nutrient deprivation has not been investigated. SCIPC are generated in artificial culture media under atmospheric 21% oxygen and are relatively immature when compared with islets isolated from pancreata. Thus, their requirements for nutrients and oxygen may be distinct from those for mature islets. In addition, SCIPC produced using current protocols, while being glucose responsive, are not fully mature β cells, and exposure to ischemia may alter their differentiation state. In this trial, pancreatic progenitor cells are encapsulated to provide an immune-protective barrier and prevent the escape of potentially undifferentiated cells. While encapsulation provides a much-needed safety assurance in early clinical trials using stem cell-derived cellular products, it further exacerbates ischemia and graft failure (Weir, 2013). Thus, there is a pressing need to determine SCIPC response to ischemia to better realize the therapeutic potential of stem cell technology. In this study, we compared the effects of hypoxia and nutrient deprivation on human SCIPC and mature islets. Importantly, we offer strategies to minimize damage by these two insults to optimize engraftment of SCIPC.
Results
Attrition of SCIPC and Mature Islet Grafts after Transplant
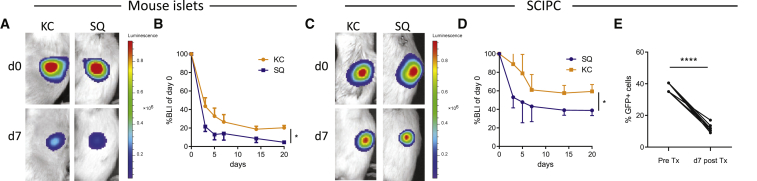
To enable quantitative measurement of SCIPC survival after transplant, we generated a human embryonic stem cell line that expresses a firefly luciferase gene under the control of the constitutive endogenous AAVS1 loci using TALEN technology. For mature islets, we used islets isolated from mice that express a firefly luciferase transgene under the control of the endogenous mouse insulin promoter (B6.MIP-Luc) (Park and Bell, 2009). We transplanted luciferase-expressing SCIPC clusters into NSG mice and B6.MIP-Luc islets into B6 albino mice under the kidney capsules (KC) or in the subcutaneous (SQ) space. We monitored graft survival over time using bioluminescent imaging. Graft mass of both SCIPC and mouse islets dropped sharply within the first 3 days and stabilized by day 7 after transplant (Figure 1). SCIPC grafts survived better than mature islets, and the highly vascularized KC site afforded better graft survival when compared with the SQ site. Nonetheless, approximately 50% of SCIPC graft mass was lost within 1 week after transplant.
Figure 1.
Survival of Mature Mouse Islets and Human SCIPC after Transplant
(A) Representative bioluminescence images of B6.MIP.Luc islets transplanted into non-diabetic B6 albino mice under the kidney capsule (KC, n = 10) or subcutaneously (SQ, n = 3).
(B) Bioluminescent intensity (BLI) of islet grafts over time is shown as percentage of day 0. The data are a compilation of results from three independent experiments.
(C) Representative bioluminescence images of SCIPC.LUC transplanted into non-diabetic NSG mice under KC (n = 8) or SQ (n = 10).
(D) BLI of SCIPC grafts over time are shown as percentage of day 0. The data are a compilation of results from five independent experiments. For (B) and (D), a Wilcoxon signed-rank test is used to determine the statistical significance between each post-transplant data point with a theoretical value of 100%. A t test is used to compare KC and SQ grafts at each time point.
(E) SCIPC grafts were harvested 7 days after transplant, and percentage of GFP+ human cells were quantified using flow cytometry. Data shown are a summary of three independent experiments (n = 10). A paired t test was used to compare the two groups.
For (B), (D), and (E), data are expressed as mean ± SEM. ∗p < 0.05; ∗∗∗∗p < 0.0001.
SCIPC clusters consist of a mix of β cells and pancreatic progenitor cells at different differentiation stages (Russ et al., 2015). To specifically monitor the fate of insulin-producing β cells, we use the Mel1INS-GFP/WT cell line (Micallef et al., 2012) that has a GFP transgene knocked into one allele of the insulin locus, thus specifically marking insulin-producing cells with GFP. We generated SCIPCINS-GFP and determined the percentages of GFP+ β cells before and after transplantation under the KC, a site that showed 60% overall SCIPC survival. The fraction of cells expressing GFP among viable cells significantly decreased by 67% (Figure 1E). Thus, although SCIPC as a whole population are more resistant than mature islets to post-transplant graft loss, the more differentiated insulin-producing cells are selectively lost when compared with pancreatic progenitors within the heterogeneous SCIPC population.
Impact of Nutrient Deprivation on Viability and Function of SCIPC and Islets
Ischemia leads to both hypoxia and nutrient deprivation. It has been well documented that hypoxia is detrimental to mouse (Bensellam et al., 2016) and human islets (Moritz et al., 2002). However, the effect of nutrient deprivation on islets is less clear. To study the impact of nutrient deprivation without the confounding contribution of hypoxia and to provide a system for mechanistic studies and screening for interventions, we developed an in vitro assay of nutrient deprivation for SCIPC and islets. We created an in vitro nutrient-limiting environment by increasing the density of SCIPC or islets cultured in complete medium while keeping oxygen levels constant (Figure S1). We cultured mouse islets expressing GFP under the mouse insulin promoter at various densities and monitored GFP fluorescence over time using time-lapse video (Figures S2A and S2B). The islet-associated GFP signal dropped sharply within 6 hr under high density, particularly in the center of islets (Figures S2A and S2C).
These results are consistent with the interpretation that islets cultured under high density suffer from nutrient deprivation. A hallmark of cellular nutrient deprivation is the activation of autophagy (Fujimoto et al., 2009), which can be detected by the formation of autophagosomes. We thus examined autophagosome formation by using islets from mice expressing an autophagosome reporter transgene, light chain 3-GFP fusion protein (Martino et al., 2012). Microscopic imaging and flow cytometry revealed a significant increase of GFPbright autophagosomes in cells cultured at high density (Figures S3A and S3B), consistent with cells undergoing nutrient deprivation.
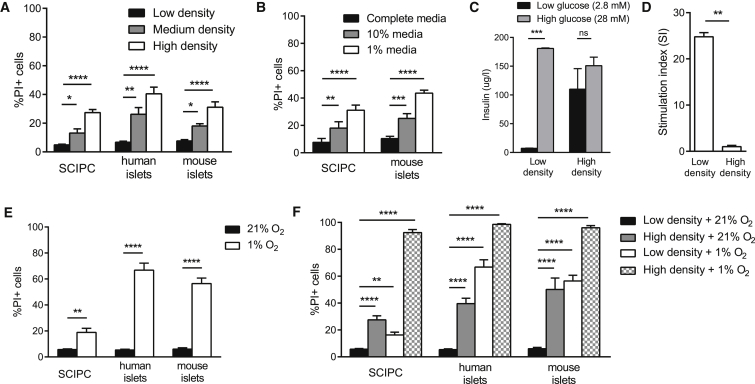
To quantitate cell death and allow analysis of non-transgenic cells, we stained islets and SCIPC clusters with the viability dye propidium iodide (PI) and determined the percentage of PI+ dead cells using flow cytometry. SCIPC, human islets, and mouse islets displayed a density-dependent increase in cell death after 6 hr of incubation (Figure 2A). However, SCIPC were more resilient to increases in cell density, as a 3-fold higher density was necessary to induce similar levels of cell death seen with mature human or mouse islets.
Figure 2.
Impact of Hypoxia and Nutrient Deprivation on Islets and SCIPC Cell Death
(A) SCIPC, human islets, and mouse islets were cultured at different densities for 6 hr as described in Experimental Procedures. Percentages of PI+ dead cells were quantified using flow cytometry. Data are a compilation of results from five independent experiments (SCIPC: n = 12, 10, 6; human islets: n = 6 at each density; mouse islets: n = 9 at each density).
(B) SCIPC and mouse islets were cultured in complete or diluted RPMI medium for 6 hr. Cell viability was quantified as described in (A). Data are a compilation of results from four independent experiments (SCIPC: n = 9 per condition; mouse islets: n = 4 per condition). For (A) and (B), statistical significance of the differences among each cell type at different densities is determined using one-way ANOVA with Holm-Sidak multiple comparisons.
(C) GSIS was measured using mouse islets after 6-hr low-density and high-density cultures. Data are a compilation of results from three independent experiments with paired low- and high-density cultured islets (n = 3 per group). Statistical difference was calculated using two-way ANOVA with Sidak's multiple comparisons test.
(D) Stimulation index of mouse islets shown in (C). A two-tailed paired t test was used to determine statistical significance of the difference (p = 0.0020).
(E) SCIPC, human islets, and mouse islets were cultured for 24 hr in the presence of 21% or 1% oxygen. At the end of the experiment cell viability was measured as described in (A). Data are a compilation of results from three independent experiments (n = 6–7 per condition for each cell type). Statistical significance of the difference between 21% and 1% oxygen for each cell type was calculated using an unpaired t test with Welch's correction.
(F) SCIPC, human islets, and mouse islets cultured for 24 hr at various densities with 1% or 21% oxygen. At the end of the experiment cell viability was measured as described in (A). Data are a compilation of results from three independent experiments (n = 6 per condition for each cell type). Statistical difference was calculated using one-way ANOVA with Holm-Sidak multiple comparisons.
All data are expressed as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
High-density culture may kill cells in ways other than depletion of nutrients, such as localized hypoxia and or accumulation of metabolic waste. Therefore, we simulated nutrient deprivation alternatively by diluting culture media with PBS while keeping the culture density constant. SCIPC and mouse islets showed a significant increase in cell death with media dilution (Figure 2B) comparable with high-density cultures. Lastly, we assessed islet function after exposure to high-density conditions by measuring in vitro glucose-stimulated insulin secretion (GSIS). Mouse islets maintained in low-density conditions had a stimulation index of more than 20, whereas islets cultured at high density showed no increase (Figures 2C and 2D). Together, these results show that short exposure to nutrient deprivation can independently kill mature islets and SCIPC without overt hypoxia.
Impact of Hypoxia on SCIPC and Islet Viability
To assess the effect of hypoxia on cell survival in vitro, we cultured SCIPC, human islets, and mouse islets at 1% oxygen to simulate hypoxic conditions they may encounter post transplant. More than 50% of human and mouse islets died, whereas SCIPC showed less than 20% death (Figure 2E).
We then compared the rate of cell death when SCIPC, human islets, and mouse islets were challenged with hypoxia and nutrient deprivation individually or in combination. While human and mouse islets were vulnerable to either insult alone, and exposure to both resulted in an additive effect resulting in >90% cell death (Figure 2F), SCIPC were more resistant to individual challenges, but the combination of both acted synergistically, killing most SCIPC (Figure 2F). Together, these results suggest that SCIPC are more resilient than mature islets when exposed to individual components of ischemia, but are similarly vulnerable to their combined actions. Thus, optimal therapeutic outcomes using SCIPC will require strategies to mitigate the impacts of nutrient deprivation and hypoxia.
Mitigating Nutrient Deprivation-Induced Cell Death In Vitro
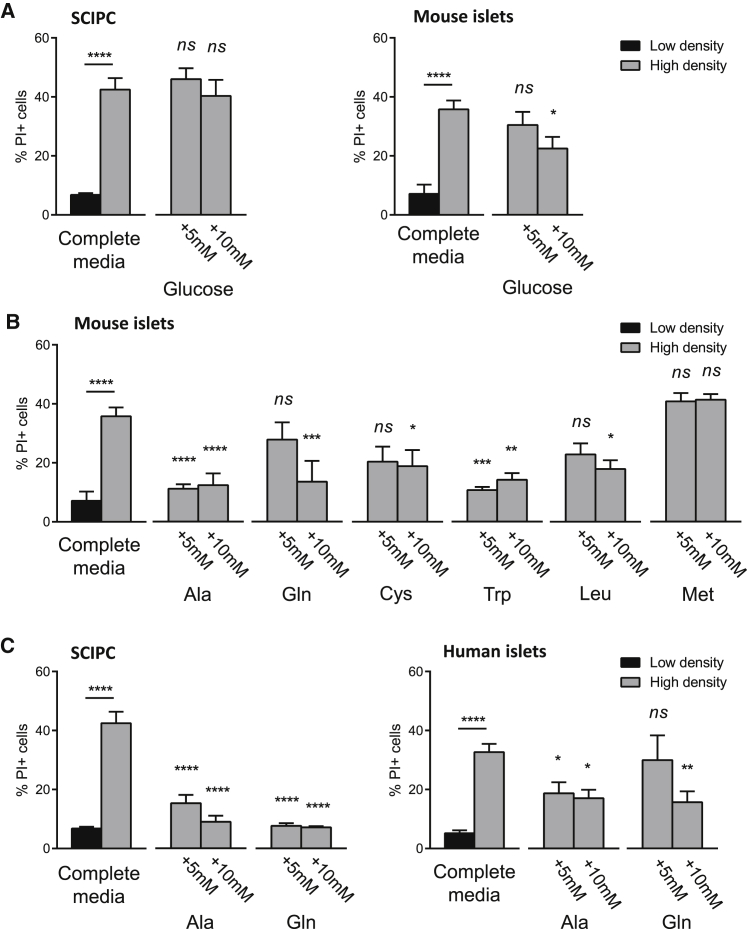
We next set out to identify the limiting nutrients in high-density cultures by adding individual nutrients. Addition of glucose to high-density SCIPC and mouse islet cultures had no effect on cell survival (Figure 3A). Next, we supplemented cultures with amino acids. We selected alanine and glutamine because they are the most abundant amino acids in the interstitial fluid (Curi et al., 2005); cysteine for its cytoprotective effects on islets (Rasilainen et al., 2002), and leucine, tryptophan, and methionine for their ability to inhibit starvation-induced autophagy in hepatocytes (Kanazawa et al., 2004). Supplementation of alanine and tryptophan greatly enhanced mouse islet survival in high-density cultures, glutamine, cysteine, and leucine offered moderate protection, whereas methionine had no effect (Figure 3B). Similarly, alanine and glutamine supplementation preserved SCIPC and human islet survival under high-density conditions (Figure 3C). These results show that addition of single amino acids can efficiently protect islets and SCIPC from nutrient deprivation.
Figure 3.
Effect of Nutrient Supplementation on Islet Cell Death In Vitro
(A) SCIPC and mouse islets were cultured at various densities in complete RPMI with or without addition of glucose. Cell viability was quantified using PI staining via flow cytometry. Data are a compilation of results from three independent experiments (SCIPC: n = 9 per group; mouse: n = 12 per group).
(B) Mouse islet survival in high-density cultures supplemented with selected amino acids. Ala, alanine; Gln, glutamine; Cys, cysteine; Trp, tryptophan; Leu, leucine; Met, methionine. Data are a compilation of results from three independent experiments (n = 9 per condition).
(C) SCIPC and human islet survival in high-density cultures (SCIPC: 3,000 clusters/mL; human islets: 1,000 islets/mL) supplemented with selected amino acids. Cell viability was quantified as described in (A). Data are a compilation of results from three independent experiments (n = 9 per condition).
For all panels, statistical significance of differences from the high-density condition of the same cell type was determined using one-way ANOVA with Holm-Sidak multiple comparisons. All data are expressed as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
Mechanisms of Amino Acid Protection against Nutrient Deprivation
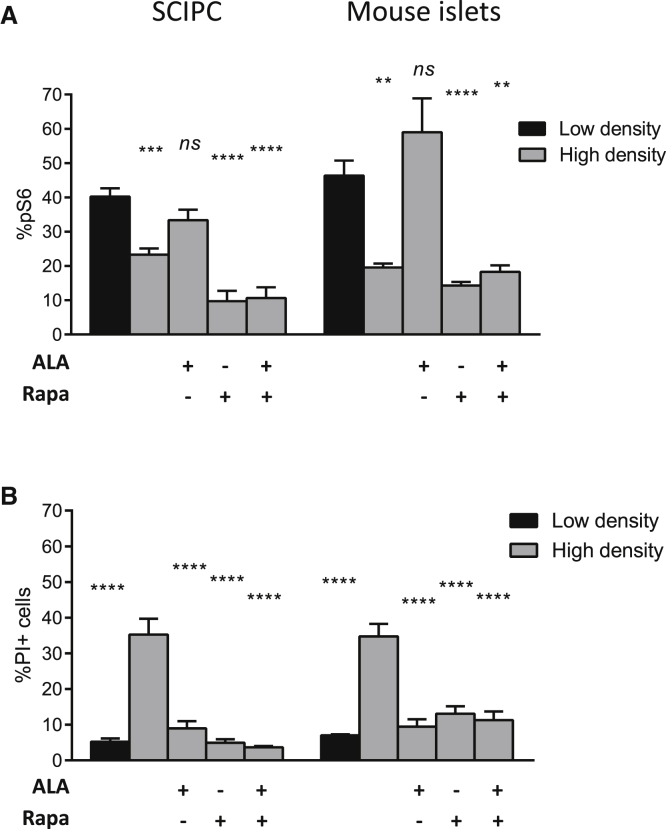
Amino acids have a multitude of cellular effects including serving as precursors of protein synthesis, regulating gene expression, providing energy, and stimulating insulin secretion in β cells (Cunningham et al., 2005, Curi et al., 2005, Dixon et al., 2003, Eto et al., 1999). Amino acids activate protein synthesis via mammalian target of rapamycin (mTOR) (Gulati et al., 2008), which is inhibited under nutrient-limiting conditions (Yu et al., 2010). Thus, we investigated the role of mTOR in amino acid-mediated protection of SCIPC and islets against nutrient deprivation. We found a significantly lower percentage of SCIPC and islet cells with phosphorylated S6 (pS6), a downstream target of mTOR, under nutrient-limiting conditions (Figure 4A), correlating with reduced cell survival (Figure 4B). Alanine supplementation restored pS6 levels in SCIPC and mouse islets cultured at high density and prevented cell death. Addition of rapamycin, an inhibitor of mTOR, reduced pS6 below the levels seen in high-density cultures with or without alanine supplementation, but did not abrogate the protective effect of alanine on SCIPC and islet survival. Moreover, rapamycin alone promoted SCIPC and mouse islet survival in high-density cultures (Figure 4B). These results show that protection by alanine is independent of mTOR- and pS6-regulated cellular processes in SCIPC and mouse islets.
Figure 4.
Effect of mTOR on Amino Acid Rescue of Islet Survival under Nutrient Deprivation
(A) Percentage of cells with phosphorylated S6 (pS6) in SCIPC and mouse islets cultured in various conditions were determined using intracellular staining and flow cytometry (n = 6 per condition for SCIPC; n = 9 per condition for mouse islets).
(B) SCIPC and mouse islet viability in various conditions were measured using PI staining and flow cytometry.
In both panels, data are a compilation of results from three independent experiments (n = 6 per condition for SCIPC; n = 9 per condition for mouse islets). One-way ANOVA with Holm-Sidak multiple comparisons was used to determine statistical significance of differences from the low-density condition (A) or high-density condition (B). All data are expressed as mean ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
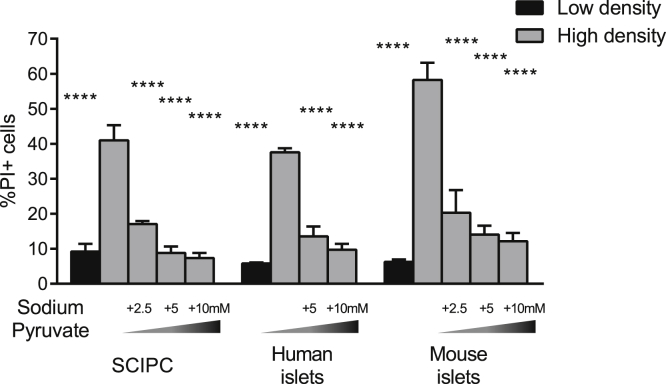
In addition to serving as cellular building blocks, amino acids can help to sustain cellular metabolism via anaplerosis, serving as substrates for replenishing tricarboxylic acid (TCA) cycle intermediates. For example, alanine, tryptophan, and cysteine can be converted into pyruvate and then to the TCA cycle intermediate oxaloacetate. To test whether enhancing anaplerosis can protect islets and SCIPC against nutrient deprivation, we supplemented high-density cultures with pyruvate. We found that pyruvate efficiently rescued SCIPC and mouse islets against cell death, supporting the notion that sustaining the TCA cycle may be essential for β cell survival under nutrient-limiting conditions (Figure 5).
Figure 5.
Effect of Pyruvate on Islet and SCIPC Cell Death during Nutrient Deprivation
Various concentrations of sodium pyruvate were added to high-density SCIPC, human islet, and mouse islet cultures, and viability was measured using PI staining and flow cytometry. Data are expressed as mean ± SEM and are a compilation of results from three independent experiments (SCIPC: n = 9; human islets: n = 9–12; mouse islets: n = 6–9). One-way ANOVA with Holm-Sidak multiple comparisons was used to determine the statistical significance of the differences from the high-density condition. ∗∗∗∗p < 0.0001.
Mitigating Hypoxia-Induced Cell Death In Vitro
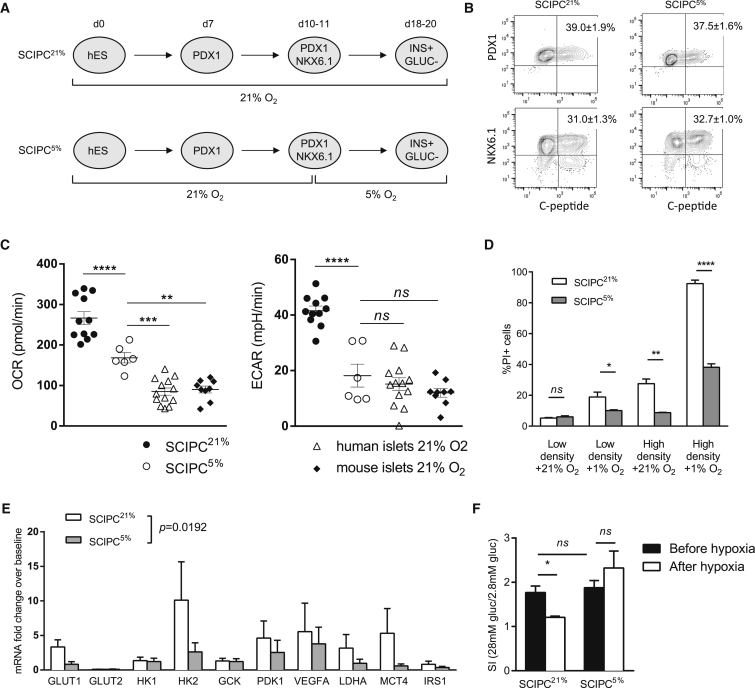
Upon transplantation, grafted cells experience a sudden decrease in oxygen availability, from atmospheric oxygen concentration of 21% to less than 1% in the grafts (Carlsson et al., 2001). Oxygen levels in the native pancreas are estimated to be 5% (Carlsson et al., 1998). We hypothesize that adjusting to physiological oxygen levels of 5% during SCIPC differentiation may reduce the impact of hypoxia after transplantation. We therefore transitioned differentiating cultures from 21% to 5% oxygen on day 10, before the induction of endocrine differentiation (Figure 6A) (Russ et al., 2015). Percentages of pancreatic and duodenal homeobox 1 (PDX1)+ C-peptide+ and Homeobox protein 6.1 (NKX6.1)+ C-peptide+ cells were comparable between SCIPC differentiated at 21% oxygen throughout (SCIPC21%), and SCIPC differentiated with 5% oxygen from day 10 onward (SCIPC5%) (Figure 6B), indicating that transition to 5% oxygen at the progenitor stage of direct differentiation did not affect the differentiation efficiency of SCIPC.
Figure 6.
Generation of SCIPC at 5% Oxygen
(A) Schematic for the differentiation of SCIPC with 21% or 5% oxygen (referred to as SCIPC21% and SCIPC5%, respectively).
(B) Representative flow plots of SCIPC21% and SCIPC5% from three independent experiments are shown, and mean ± SEM of percentages of cells are listed in the upper right quadrants (n = 6 per condition).
(C) OCR and ECAR of SCIPC21% (n = 11), SCIPC5% (n = 6), human islet (n = 13), and mouse islet (n = 9) cultures were measured at 21% oxygen level. Data are a summary of three independent experiments with individual data points, mean, and SEM shown. Statistical significance of the differences of the means was determined using one-way ANOVA Holm-Sidak multiple comparisons test.
(D) SCIPC21% and SCIPC5% were cultured in high- or low-density (20 and 3,000 clusters/mL) with 21% or 1% oxygen for 24 hr. Cell viability was measured using PI staining and flow cytometry. Data are expressed as mean ± SEM and are a compilation of results from three independent experiments (SCIPC21%: n = 6–10; SCIPC5%: n = 10–16). An unpaired t test with Welch's correction was used to compare the two groups.
(E) qPCR analysis of expression of hypoxia-induced gene transcripts in SCIPC21% or SCIPC5% after 18-hr exposure to 1% oxygen. Results are expressed as fold change over pre-exposure baseline after normalization to the housekeeping gene GAPDH. Values are mean ± SEM with n = 3 for each cell type. Statistical significance of the difference between the two cell types across all 10 genes was determined using a paired t test (p = 0.0192).
(F) GSIS of SCIPC21% and SCIPC5% before and after 18-hr exposure to 1% oxygen. Stimulation indexes are shown (n = 3 per group). Statistical significance of the differences among all conditions was determined using one-way ANOVA with Holm-Sidak multiple comparisons test.
All data are expressed as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
We next compared the metabolic profiles SCIPC5%, SCIPC21%, human islets, and mouse islets. We quantified oxygen consumption rate (OCR), a measurement of mitochondrial respiration, and extracellular acidification rate (ECAR), a measurement of glycolysis. SCIPC21% exhibited significantly elevated basal OCR and ECAR when compared with mouse and human islets. SCIPC5% showed reduced OCR and ECAR when compared with SCIPC21% (Figure 6C). These results show that the metabolic state of SCIPC5% more closely resembles that of mature islets when compared with SCIPC21%. Moreover, SCIPC5% were significantly more resistant to challenges with 1% oxygen, high-density cultures, and the combination of the two (Figure 6D). These results suggest that SCIPC5% may survive better in vivo after transplantation.
Extreme hypoxia induces permanent changes in glucose sensing of β cells by altering expression of genes involved in glucose metabolism, leading to decoupling of glucose exposure and insulin secretion (Cantley et al., 2013). Thus, we examined the expression of hypoxia-induced genes in SCIPC5% and SCIPC21% after exposure to 1% oxygen for 18 hr. Consistent with previous reports, SCIPC21% exposed to 1% oxygen showed dramatic increase of hypoxia-induced gene transcripts GLUT1, HK2, PDK1, VEGF-A, LDHA, and MCT4 (Figure 6C). In comparison, SCIPC5% exhibited a significantly blunted response to 1% oxygen challenge, indicating an increased resistance to hypoxic reprograming. In vitro functional assessments showed similar GSIS by SCIPC21% and SCIPC5% before hypoxic exposure (Figure 6E). However, after 18 hr of hypoxia, SCIPC21% failed to respond to glucose stimulation, whereas functionality of SCIPC5% was preserved (Figure 6E). Taken together, these results indicate that differentiation of SCIPC at a physiological oxygen concentration of 5% results in a protective adaptation without impairing differentiation or function.
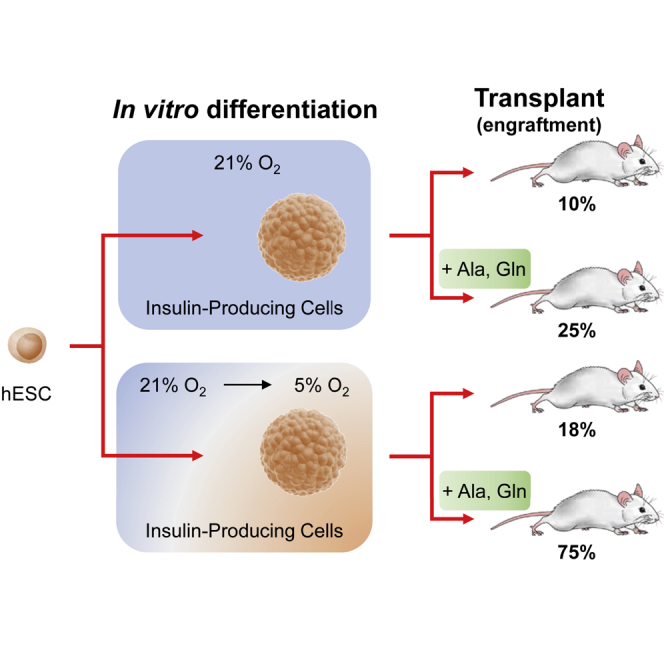
Promoting SCIPC Survival after Transplant
Our in vitro results thus far suggest that generation of SCIPC at 5% oxygen alone or in combination with amino acid supplementation may protect against ischemia after transplantation. Hence, we transplanted SCIPC5% or SCIPC21% with or without amino acid supplementation in SQ space and monitored graft survival using bioluminescent imaging (Figure 7A). In these experiments, we used a stringent criterion of maintenance of more than 80% of the initial cell mass to define graft survival. Most of the control SCIPC21% without amino acid supplementation rapidly dropped below this threshold. SCIPC5% without amino acid supplementation showed improved survival early after transplant, but the protective effect faded by day 10, resulting in overall survival similar to that of the SCIPC21% control (Figure 7B). Glutamine and alanine supplementation to SCIPC21% also showed improvement early after transplant, but this protective effect did not persist. Combining SCIPC5% with amino acid supplementation significantly improved graft survival, with 75% of grafts surviving to day 20 (Figure 7B). The SCIPC5% + amino acid group not only have better graft mass but also better preservation of insulin-producing cells when compared with the other groups (Figure 7C). Taken together, these results suggest that hypoxia and nutrient deprivation are two major contributors of SCIPC graft loss after transplantation and mitigating both factors is needed to preserve SCIPC graft mass after transplant.
Figure 7.
Effect of Amino Acid Supplementation and Oxygen Acclimatization on SCIPC Survival after Transplantation
(A) Representative bioluminescence images of SCIPC transplanted subcutaneously into non-diabetic NSG mice at days 0 and 20.
(B) Graft survival over time after transplantation of SCIPC21% (n = 10), SCIPC5% (n = 11), SCIPC21% + amino acid (aa) (n = 8), and SCIPC5% + aa (n = 8). Grafts were considered “failed” when the BLI dropped below 80% of day 0. Significance of differences in survival from SCIPC21% without supplement was determined for each experimental condition using a log-rank (Mantel-Cox) test.
(C) Top: representative images of insulin staining of SCIPC grafts in (B) collected on day 20. Scale bars, 200 μm. Bottom: magnified views of the insets in the larger images. Scale bars, 50 μm. DAPI (blue), insulin (red).
Discussion
Graft survival is a major barrier to islet transplantation, and its impact on stem cell-derived β cell replacement therapy is unclear. In this study, we demonstrate a significant decline of SCIPC graft mass shortly after transplant with a preferential loss of more mature insulin-producing cells. Although SCIPC are more resistant to hypoxia and nutrient deprivation individually when compared with mature islets, they are vulnerable to their combined action. We have developed strategies for mitigating the impact of ischemia to achieve greater retention of SCIPC graft mass after transplant. Our results suggest that effective therapeutic applications of SCIPC should incorporate strategies to mitigate the impact of hypoxia and nutrient deprivation for promoting graft survival after transplant. Importantly, the concepts and the approaches presented in this study may be applicable to other stem cell-based therapies.
It is well known that β cells in mature islets are sensitive to ischemia and that the majority of them die shortly after transplantation (Emamaullee and Shapiro, 2007, Potter et al., 2014, Rickels et al., 2005). Previous studies comparing adult islets and fetal islet-like cells have shown better survival of fetal cells due to their ability to withstand ischemia and to replenish graft mass by proliferation (Beattie et al., 1997, Hayek and Beattie, 1997). These findings have kindled the hope that SCIPC would be similarly resistant to ischemia, thus providing a solution to the islet graft survival problem. Indeed, survival and continued differentiation of pancreatic progenitor cells after transplantation has been demonstrated (Bruin et al., 2013, Kroon et al., 2008, Vegas et al., 2016). However, some experimental evidence suggests that consistent engraftment of pancreatic progenitor cells is challenging to achieve (Courtney et al., 2010, Matveyenko et al., 2010). Our side-by-side quantitative comparisons showed that SCIPC indeed survived better than mature islets after transplant, although substantial graft mass was still lost. Moreover, the more mature insulin-producing cells among SCIPC preparations were preferentially lost after transplant. The increased vulnerability of more mature insulin-producing cells may be due to preferential death or de-differentiation of more mature cells under ischemia. Previous studies have shown that hypoxia can induce de-differentiation (Puri et al., 2015). The combined effect of cell death and de-differentiation may explain the inconsistent results with SCIPC grafts and the long duration needed to achieve graft function reported previously (Kroon et al., 2008, Matveyenko et al., 2010).
There are currently two general experimental approaches to the control of hypoxia-induced death of mature β cells from islets: preconditioning before transplant or provision of oxygen after transplant. Preconditioning is based on the concept that cells can adapt to survive under low oxygen tensions if the change is introduced gradually (Abaci et al., 2010, Hals et al., 2015). Moreover, transient hypoxia induces hypoxia-induced factor (HIF) that promotes angiogenesis (Pugh and Ratcliffe, 2003), which benefits long-term graft function. Artificial induction of HIF-1α improves transplant outcomes (Faleo et al., 2008, Stokes et al., 2013). The current process of islet transplantation extracts islets from native pancreas with 5% O2 and rests them at atmospheric 21% O2 for a few days before infusing them into the portal vein with 1–2% O2. The abruptness of going from supraphysiological oxygen tension to hypoxia deprives the cells time to adapt. Preconditioning the islets using pulsatile cycles of 5–21% oxygen levels for 1 hr helps to maintain islet function during later exposure to hypoxia in vitro (Lo et al., 2012). These results argue that exposure to hypoxia can be beneficial for long-term survival and function of islet and SCIPC grafts. However, dropping oxygen tension too low for too long may kill islets and change the metabolic program of surviving cells, leading to long-term dysfunction (Cantley et al., 2013, Smith et al., 2017). Thus, the challenge is to define the proper conditions under which to prepare the cells to withstand hypoxia without metabolic reprogramming.
Current stem cell differentiation protocols are carried out at supraphysiological oxygen levels of 21% instead of the physiological oxygen tension in the naive pancreas of 5%. We reasoned that acclimatizing SCIPC to physiological 5% oxygen tension will provide protection from hypoxia-induced cell death upon transplantation. Encouragingly, transitioning cultures to 5% oxygen after 10 days of differentiation did not negatively affect SCIPC generation, and adjusted their metabolic profile to those observed in mature islets. Lower metabolic demand of SCIPC5% could be responsible for their higher resilience under conditions of hypoxia and nutrient deprivation. Importantly, SCIPC5% showed much-attenuated response to later encounter of extreme hypoxia of 1% oxygen and, most importantly, SCIPC5% remain functional after hypoxic exposure while SCIPC21% do not. Thus, generating SCIPC at physiological oxygen tension appears to achieve a balance of inducing protection against hypoxia without impairing the SCIPC developmentally or functionally.
One other major conclusion from this study is that nutrient deprivation, another consequence of ischemia, can also kill islets and SCIPC and contribute to poor outcomes after transplant. In searching for a strategy to mitigate the impact of nutrient deprivation on islet and SCIPC survival, we found some amino acids to be effective. It is important to note that this newly identified role of amino acids in sustaining β cell viability under nutrient deprivation is distinct from the previously reported effect of amino acids in potentiating β cell insulin secretion upon glucose stimulation (Newsholme et al., 2007, Prentki et al., 2013). Two features of amino acid-mediated rescue are important: single amino acid supplementation can be sufficient and not all amino acids work. Furthermore, our study suggests that amino acids likely protect β cells by sustaining mitochondria oxidation via anaplerosis instead of mTOR-dependent anabolism. However, most amino acids can enter the TCA cycle, but not all equally protect islets and SCIPC against nutrient deprivation. In addition, glucose also has anaplerotic effects, but did not confer any protection at a wide range of concentrations tested. Entrance to cells and mitochondria and activities of specific anaplerotic enzymes may impose limitations on some nutrients to become TCA intermediates. Definitive demonstration of anaplerosis and/or amino acid oxidation in islets and SCIPC will require comprehensive metabolic profiling that is currently ongoing in our laboratories. These efforts may shed light on the mechanisms of amino acid rescue of islets and SCIPC under nutrient deprivation and help to guide optimized strategy to support islet and SCIPC survival after transplant.
The surprising finding that rapamycin alone sustained SCIPC and islet survival under nutrient deprivation suggests that limiting protein synthesis under nutrient deprivation may be protective by reserving the cellular pool of amino acids. In this regard, short 2-hr rapamycin exposure has been shown to improve cell viability of human liver carcinoma HepG2 cells and human embryonic kidney HEK293 cells via induction of autophagy (Kapuy et al., 2014). Autophagy is a cell-intrinsic adaptation strategy to starvation by breaking down proteins to fuel basal survival needs (Mizushima and Komatsu, 2011). However, longer 24- to 48-hr treatment of rapamycin impairs islet survival despite induction of autophagy (Tanemura et al., 2012). Thus, although islets have the ability to adapt to nutrient deprivation by autophagy, this catabolic survival mechanism is likely unsustainable. Supplementation of critical nutrients will likely be a more effective approach to sustain islets and SCIPC survival after transplant until revascularization.
Glutamine and alanine are the two most abundant amino acids in plasma (Curi et al., 2005). The highest levels of alanine have been found in the endocrine system, including islets (Ota et al., 2014). Islets normally have a high demand for glutamine and alanine (Dixon et al., 2003). During whole-body starvation, muscle breakdown provides a source of glutamine and alanine to support cellular functions (Chang and Goldberg, 1978). Therefore, supplementation of glutamine and alanine to transplanted islets and SCIPC may mimic the physiological response to endure nutrient deprivation.
Our in vivo results of greatly improved SCIPC survival by combining physiological oxygen acclimatization and amino acid supplementation suggest that hypoxia and nutrient deprivation are likely the two most important contributors to SCIPC death after transplant. These experiments mainly focused on cell viability, because cell survival is a prerequisite to function and needs to be addressed first. It is encouraging that this approach not only sustained cell viability but also insulin expression, suggesting functional preservation. Future studies are needed to evaluate this strategy in a diabetes model. Overall, our study demonstrates that by addressing individual contributors of ischemia-induced SCIPC death, dramatic improvement of SCIPC engraftment can be achieved. The general strategy of physiological oxygen acclimatization and nutrient supplementation may be applicable to other stem cell-derived grafts to improve their survival and therapeutic efficacy.
Experimental Procedures
All animal procedures were performed under approved protocols and in accordance with ethical guidelines by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Francisco.
In Vitro Ischemia Simulations
SCIPC, human islets, and mouse islets were cultured at increasing densities in RPMI media with 5% fetal calf serum (R5) for 6–24 hr to simulate decreasing nutrients. SCIPC were cultured in concentrations of 200 clusters/mL for low density (LD), 1,500 clusters/mL for medium density (MD), and 3,000 clusters/mL for high density (HD). Mouse and human islets were cultured at concentration of 200 islets/mL for LD, 500 islets/mL for MD, and 1,000 islets/mL for HD. Alternatively, SCIPC, human islets, and mouse islets were cultured with 1% and 10% R5 diluted in PBS for 6–24 hr to simulate nutrient deprivation. For the hypoxia challenges, SCIPC, human islets, and mouse islets were cultured in R5 with 1% oxygen in incubators with adjustable oxygen levels (Heracell 240, Thermo Fisher Scientific, Waltham, MA).
Islet and SCIPC Transplantation
Transplantation of mouse islets and SCIPC into renal capsules or the subcutaneous space was performed as reported previously (Halberstadt et al., 2005, Szot et al., 2007). For amino acid supplementation, additional 10 mM alanine and 10 mM glutamine were supplemented to SCIPC culture medium 2 hr before the cells were collected for transplant. SCIPC were transplanted subcutaneously together with 50 μL of the alanine- and glutamine-supplemented media.
Other experimental procedures are described in Supplemental Experimental Procedures.
Author Contributions
Conceptualization, Q.T.; Methodology, G.F., H.A.R., and Q.T.; Writing – Original Draft, G.F. and Q.T.; Investigation, G.F., H.A.R., V.N., S.W., A.V.P., G.G.N., J.E.F., K.E.V., and G.L.S.; Writing – Review & Editing, G.F., H.A.R., M.H., and Q.T.; Funding Acquisition and Supervision, M.H. and Q.T.
Acknowledgments
We thank Dr. J. Greenland for his advice on statistical analyses, A. Albright and J. Maynard for their help as summer interns, and M. Pauli, V. Dang, N. Lescano, J. Wang, and R. Guerrero-Moreno for mouse colony management. The project is supported by the Foundation for Diabetes Research and the American Diabetes Association (grant no. 1-15-BS-179). G.F. was supported by the Larry Hillblom Foundation Fellowship and the UCSF Program for Breakthrough Biomedical Research. H.A.R. was supported by a postdoctoral fellowship from the JDRF (3-2012-266). S.W. was supported by fellowships from Eli and Edy the Broad Regeneration Medicine and Stem Cell Fellowship and a T32 grant (T32AI125222). Work in the M.H. laboratory was supported by NIH grants (DK105831, DK108666). M.H. is a consultant for Semma Therapeutics. The work benefited from NIH/NIDDK Diabetes and Endocrinology Core Research grant (P30DK063720). LC3 mice were a gift from Noboru Mizushima. PURO-CAS9-Donor, AAVS1-TALEN-L, and AAVS1-TALEN-R plasmids were gifts from Danwei Huangfu (Addgene plasmids 58409, 59025, and 59026).
Published: August 10, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.07.012.
Supplemental Information
References
- Abaci H.E., Truitt R., Luong E., Drazer G., Gerecht S. Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 2010;298:C1527–C1537. doi: 10.1152/ajpcell.00484.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkai U., Weir G.C., Colton C.K., Ludwig B., Bornstein S.R., Brendel M.D., Neufeld T., Bremer C., Leon A., Evron Y. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22:1463–1476. doi: 10.3727/096368912X657341. [DOI] [PubMed] [Google Scholar]
- Barton F.B., Rickels M.R., Alejandro R., Hering B.J., Wease S., Naziruddin B., Oberholzer J., Odorico J.S., Garfinkel M.R., Levy M. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie G.M., Otonkoski T., Lopez A.D., Hayek A. Functional beta-cell mass after transplantation of human fetal pancreatic cells: differentiation or proliferation? Diabetes. 1997;46:244–248. doi: 10.2337/diab.46.2.244. [DOI] [PubMed] [Google Scholar]
- Bensellam M., Maxwell E.L., Chan J.Y., Luzuriaga J., West P.K., Jonas J.C., Gunton J.E., Laybutt D.R. Hypoxia reduces ER-to-Golgi protein trafficking and increases cell death by inhibiting the adaptive unfolded protein response in mouse beta cells. Diabetologia. 2016;59:1492–1502. doi: 10.1007/s00125-016-3947-y. [DOI] [PubMed] [Google Scholar]
- Bruin J.E., Rezania A., Xu J., Narayan K., Fox J.K., O'Neil J.J., Kieffer T.J. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56:1987–1998. doi: 10.1007/s00125-013-2955-4. [DOI] [PubMed] [Google Scholar]
- Cantley J., Grey S.T., Maxwell P.H., Withers D.J. The hypoxia response pathway and beta-cell function. Diabetes Obes. Metab. 2010;12(Suppl 2):159–167. doi: 10.1111/j.1463-1326.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- Cantley J., Walters S.N., Jung M.H., Weinberg A., Cowley M.J., Whitworth T.P., Kaplan W., Hawthorne W.J., O'Connell P.J., Weir G. A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant. 2013;22:2147–2159. doi: 10.3727/096368912X658728. [DOI] [PubMed] [Google Scholar]
- Carlsson P.O., Liss P., Andersson A., Jansson L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes. 1998;47:1027–1032. doi: 10.2337/diabetes.47.7.1027. [DOI] [PubMed] [Google Scholar]
- Carlsson P.O., Palm F., Andersson A., Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- Chang T.W., Goldberg A.L. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J. Biol. Chem. 1978;253:3685–3693. [PubMed] [Google Scholar]
- Clanton T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. (1985) 2007;102:2379–2388. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- Courtney M.L., Jones P.M., Burns C.J. Importance of quantitative analysis in the generation of insulin-expressing cells from human embryonic stem cells. Pancreas. 2010;39:105–107. doi: 10.1097/MPA.0b013e3181b79d3c. [DOI] [PubMed] [Google Scholar]
- Cunningham G.A., McClenaghan N.H., Flatt P.R., Newsholme P. L-Alanine induces changes in metabolic and signal transduction gene expression in a clonal rat pancreatic beta-cell line and protects from pro-inflammatory cytokine-induced apoptosis. Clin. Sci. (Lond) 2005;109:447–455. doi: 10.1042/CS20050149. [DOI] [PubMed] [Google Scholar]
- Curi R., Lagranha C.J., Doi S.Q., Sellitti D.F., Procopio J., Pithon-Curi T.C., Corless M., Newsholme P. Molecular mechanisms of glutamine action. J. Cell Physiol. 2005;204:392–401. doi: 10.1002/jcp.20339. [DOI] [PubMed] [Google Scholar]
- Dixon G., Nolan J., McClenaghan N., Flatt P.R., Newsholme P. A comparative study of amino acid consumption by rat islet cells and the clonal beta-cell line BRIN-BD11-the functional significance of L-alanine. J. Endocrinol. 2003;179:447–454. doi: 10.1677/joe.0.1790447. [DOI] [PubMed] [Google Scholar]
- Emamaullee J., Liston P., Korneluk R.G., Shapiro A.M., Elliott J.F. XIAP overexpression in islet beta-cells enhances engraftment and minimizes hypoxia-reperfusion injury. Am. J. Transplant. 2005;5:1297–1305. doi: 10.1111/j.1600-6143.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- Emamaullee J.A., Rajotte R.V., Liston P., Korneluk R.G., Lakey J.R., Shapiro A.M., Elliott J.F. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54:2541–2548. doi: 10.2337/diabetes.54.9.2541. [DOI] [PubMed] [Google Scholar]
- Emamaullee J.A., Shapiro A.M. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16:1–8. [PubMed] [Google Scholar]
- Eto K., Tsubamoto Y., Terauchi Y., Sugiyama T., Kishimoto T., Takahashi N., Yamauchi N., Kubota N., Murayama S., Aizawa T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- Faleo G., Neto J.S., Kohmoto J., Tomiyama K., Shimizu H., Takahashi T., Wang Y., Sugimoto R., Choi A.M., Stolz D.B. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- Faleo G., Fotino C., Bocca N., Molano R.D., Zahr-Akrawi E., Molina J., Villate S., Umland O., Skyler J.S., Bayer A.L. Prevention of autoimmune diabetes and induction of beta-cell proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes. 2012;61:1769–1778. doi: 10.2337/db11-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K., Hanson P.T., Tran H., Ford E.L., Han Z., Johnson J.D., Schmidt R.E., Green K.G., Wice B.M., Polonsky K.S. Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. J. Biol. Chem. 2009;284:27664–27673. doi: 10.1074/jbc.M109.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey S.T., Longo C., Shukri T., Patel V.I., Csizmadia E., Daniel S., Arvelo M.B., Tchipashvili V., Ferran C. Genetic engineering of a suboptimal islet graft with A20 preserves beta cell mass and function. J. Immunol. 2003;170:6250–6256. doi: 10.4049/jimmunol.170.12.6250. [DOI] [PubMed] [Google Scholar]
- Gulati P., Gaspers L.D., Dann S.G., Joaquin M., Nobukuni T., Natt F., Kozma S.C., Thomas A.P., Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt C.R., Williams D., Emerich D., Goddard M., Vasconcellos A.V., Curry W., Bhatia A., Gores P.F. Subcutaneous transplantation of islets into streptozocin-induced diabetic rats. Cell Transplant. 2005;14:595–605. doi: 10.3727/000000005783982792. [DOI] [PubMed] [Google Scholar]
- Hals I.K., Bruerberg S.G., Ma Z., Scholz H., Bjorklund A., Grill V. Mitochondrial respiration in insulin-producing beta-cells: general characteristics and adaptive effects of hypoxia. PLoS One. 2015;10:e0138558. doi: 10.1371/journal.pone.0138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek A., Beattie G.M. Experimental transplantation of human fetal and adult pancreatic islets. J. Clin. Endocrinol. Metab. 1997;82:2471–2475. doi: 10.1210/jcem.82.8.4151. [DOI] [PubMed] [Google Scholar]
- Hogan A.R., Doni M., Molano R.D., Ribeiro M.M., Szeto A., Cobianchi L., Zahr-Akrawi E., Molina J., Fornoni A., Mendez A.J. Beneficial effects of ischemic preconditioning on pancreas cold preservation. Cell Transplant. 2012;21:1349–1360. doi: 10.3727/096368911X623853. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Taneike I., Akaishi R., Yoshizawa F., Furuya N., Fujimura S., Kadowaki M. Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J. Biol. Chem. 2004;279:8452–8459. doi: 10.1074/jbc.M306337200. [DOI] [PubMed] [Google Scholar]
- Kapuy O., Vinod P.K., Banhegyi G. mTOR inhibition increases cell viability via autophagy induction during endoplasmic reticulum stress - an experimental and modeling study. FEBS Open Bio. 2014;4:704–713. doi: 10.1016/j.fob.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Lo J.F., Wang Y., Blake A., Yu G., Harvat T.A., Jeon H., Oberholzer J., Eddington D.T. Islet preconditioning via multimodal microfluidic modulation of intermittent hypoxia. Anal. Chem. 2012;84:1987–1993. doi: 10.1021/ac2030909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino L., Masini M., Novelli M., Beffy P., Bugliani M., Marselli L., Masiello P., Marchetti P., De Tata V. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS One. 2012;7:e36188. doi: 10.1371/journal.pone.0036188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveyenko A.V., Georgia S., Bhushan A., Butler P.C. Inconsistent formation and nonfunction of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude rats. Am. J. Physiol. Endocrinol. Metab. 2010;299:E713–E720. doi: 10.1152/ajpendo.00279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef S.J., Li X., Schiesser J.V., Hirst C.E., Yu Q.C., Lim S.M., Nostro M.C., Elliott D.A., Sarangi F., Harrison L.C. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia. 2012;55:694–706. doi: 10.1007/s00125-011-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Moassesfar S., Masharani U., Frassetto L.A., Szot G.L., Tavakol M., Stock P.G., Posselt A.M. A comparative analysis of the safety, efficacy, and cost of islet versus pancreas transplantation in nonuremic patients with type 1 diabetes. Am. J. Transplant. 2016;16:518–526. doi: 10.1111/ajt.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz W., Meier F., Stroka D.M., Giuliani M., Kugelmeier P., Nett P.C., Lehmann R., Candinas D., Gassmann M., Weber M. Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB J. 2002;16:745–747. doi: 10.1096/fj.01-0403fje. [DOI] [PubMed] [Google Scholar]
- Newsholme P., Bender K., Kiely A., Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007;35:1180–1186. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- Ota N., Rubakhin S.S., Sweedler J.V. D-Alanine in the islets of Langerhans of rat pancreas. Biochem. Biophys. Res. Commun. 2014;447:328–333. doi: 10.1016/j.bbrc.2014.03.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Bell G.I. Noninvasive monitoring of changes in pancreatic beta-cell mass by bioluminescent imaging in MIP-luc transgenic mice. Horm. Metab. Res. 2009;41:1–4. doi: 10.1055/s-0028-1087209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza E., Coronel M.M., Fraker C.A., Ricordi C., Stabler C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesner A., Liston P., Tan R., Korneluk R.G., Verchere C.B. The X-linked inhibitor of apoptosis protein enhances survival of murine islet allografts. Diabetes. 2005;54:2533–2540. doi: 10.2337/diabetes.54.9.2533. [DOI] [PubMed] [Google Scholar]
- Potter K.J., Westwell-Roper C.Y., Klimek-Abercrombie A.M., Warnock G.L., Verchere C.B. Death and dysfunction of transplanted beta-cells: lessons learned from type 2 diabetes? Diabetes. 2014;63:12–19. doi: 10.2337/db12-0364. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F.M., Madiraju S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Puri S., Akiyama H., Hebrok M. VHL-mediated disruption of Sox9 activity compromises beta-cell identity and results in diabetes mellitus. Genes Dev. 2013;27:2563–2575. doi: 10.1101/gad.227785.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S., Folias A.E., Hebrok M. Plasticity and dedifferentiation within the pancreas: development, homeostasis, and disease. Cell Stem Cell. 2015;16:18–31. doi: 10.1016/j.stem.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasilainen S., Nieminen J.M., Levonen A.L., Otonkoski T., Lapatto R. Dose-dependent cysteine-mediated protection of insulin-producing cells from damage by hydrogen peroxide. Biochem. Pharmacol. 2002;63:1297–1304. doi: 10.1016/s0006-2952(02)00864-x. [DOI] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Rickels M.R., Schutta M.H., Markmann J.F., Barker C.F., Naji A., Teff K.L. {beta}-Cell function following human islet transplantation for type 1 diabetes. Diabetes. 2005;54:100–106. doi: 10.2337/diabetes.54.1.100. [DOI] [PubMed] [Google Scholar]
- Russ H.A., Parent A.V., Ringler J.J., Hennings T.G., Nair G.G., Shveygert M., Guo T., Puri S., Haataja L., Cirulli V. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.E., Kelly A.C., Min C.G., Weber C.S., McCarthy F.M., Steyn L.V., Badarinarayana V., Stanton J.B., Kitzmann J.P., Strop P. Acute ischemia induced by high density culture increases cytokine expression and diminishes the function and viability of highly purified human islets of Langerhans. Transplantation. 2017 doi: 10.1097/TP.0000000000001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes R.A., Cheng K., Deters N., Lau S.M., Hawthorne W.J., O'Connell P.J., Stolp J., Grey S., Loudovaris T., Kay T.W. Hypoxia-inducible factor-1alpha (HIF-1alpha) potentiates beta-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 2013;22:253–266. doi: 10.3727/096368912X647180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot G.L., Koudria P., Bluestone J.A. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J. Vis. Exp. 2007;404:e404. doi: 10.3791/404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemura M., Ohmura Y., Deguchi T., Machida T., Tsukamoto R., Wada H., Kobayashi S., Marubashi S., Eguchi H., Ito T. Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am. J. Transplant. 2012;12:102–114. doi: 10.1111/j.1600-6143.2011.03771.x. [DOI] [PubMed] [Google Scholar]
- Vegas A.J., Veiseh O., Gurtler M., Millman J.R., Pagliuca F.W., Bader A.R., Doloff J.C., Li J., Chen M., Olejnik K. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G.C. Islet encapsulation: advances and obstacles. Diabetologia. 2013;56:1458–1461. doi: 10.1007/s00125-013-2921-1. [DOI] [PubMed] [Google Scholar]
- Wisel S.A., Braun H.J., Stock P.G. Current outcomes in islet versus solid organ pancreas transplant for beta-cell replacement in type 1 diabetes. Curr. Opin. Organ Transplant. 2016;21:399–404. doi: 10.1097/MOT.0000000000000332. [DOI] [PubMed] [Google Scholar]
- Yin D., Ding J.W., Shen J., Ma L., Hara M., Chong A.S. Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning. Am. J. Transplant. 2006;6:60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- Yoshihara E., Wei Z., Lin C.S., Fang S., Ahmadian M., Kida Y., Tseng T., Dai Y., Yu R.T., Liddle C. ERRgamma is required for the metabolic maturation of therapeutically functional glucose-responsive beta cells. Cell Metab. 2016;23:622–634. doi: 10.1016/j.cmet.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., McPhee C.K., Zheng L., Mardones G.A., Rong Y., Peng J., Mi N., Zhao Y., Liu Z., Wan F. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.