Increasing concern over multidrug-resistant organisms (MDROs), especially vancomycin-resistant enterococci (VRE), Clostridium difficile, and multidrug-resistant gram-negative bacteria (MDR-GNB), has led to increasing attention being paid to the role of high touch environmental surfaces in transmission. Our current understanding of the roles of environmental surfaces in MDRO transmission include the following: 1) a primary role with transmission from source patient to environmental surface to subsequent patient and, 2) a secondary role from source patient to environmental surface to hands of healthcare personnel to subsequent patient. Either a prior room occupant or a contemporaneous patient sharing reusable medical equipment is the source patient in most primary transmission events.
Standard environmental cleaning and disinfection entails manual cleaning and application of a disinfectant, often utilizing a detergent disinfectant. In addition to new disinfectants with greater potency and shorter contact times, new technological advances include ‘non-touch disinfection’ (NTD) methods, the most developed of which are hydrogen peroxide vapor (HPV), and automated germicidal ultraviolet irradiation. Both methods appear highly efficacious in inactivating the microbial bioburden present on surfaces and both remove much of the variance inherent in human cleaning activity via a high degree of automation and feedback loops for verification that contact or irradiation times are adequate.1–3
Despite these advances, demonstrating the clinical impact of both old and new environmental cleaning and disinfection technologies remains challenging. We propose an evidentiary hierarchy for assessing any environmental disinfection strategy (Figure) beginning with a foundation (i.e. level I) of laboratory efficacy studies similar to those required for registration by the Environmental Protection Agency.4 There are numerous patient and practice factors that confound the relationship between environmental bioburden reductions and MDRO transmission interruption, spanning from the number of patients on antibiotics with wounds, devices, and diarrhea (rendering them either more contagious or susceptible to colonization), to rates of compliance with hand hygiene and isolation, to interventions aimed at source control such as chlorhexidine bathing. Because only a small proportion of all MDRO acquisitions lead to eventual infection, linking infection reductions to environmental bioburden reductions (i.e. level V of Figure) is even more challenging. However, because infections correlate more closely than colonization with mortality, excess length of stay, and cost, such linkage will eventually become necessary to calculate the cost effectiveness of new technologies.
Figure 1.
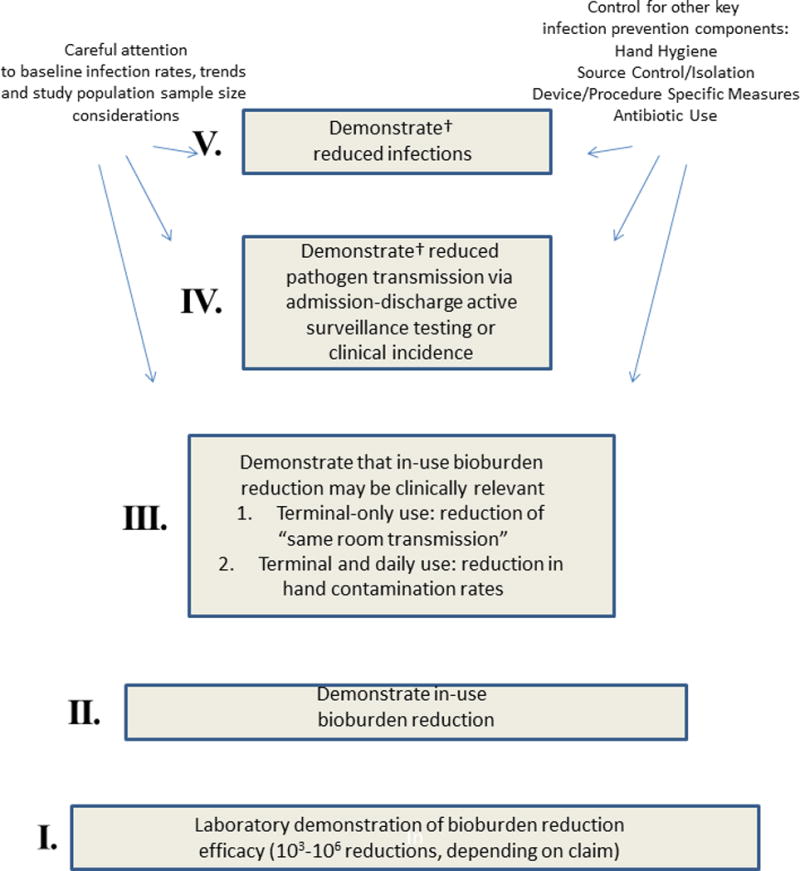
Evidence hierarchy for increasing patient safety through healthcare environmental surface cleaning and disinfection.
†Prioritize cluster randomization over interrupted time series design
Such a hierarchy can assist the development of a new disinfection technology, guiding industry in demonstrating achievement at a lower level in the hierarchy before investment is made at a higher level. It also highlights the need for tools to link achievements at lower levels (e.g. achievable log10 reductions in the laboratory or as part of an in-use study) to the likelihood of success at a higher level. Standardized methods for environmental and hand sampling, microbiologic cultures, and assessment of adherence to standard environmental cleaning, hand hygiene, and isolation precautions will all be important to make the climbing of this hierarchy more efficient.
The report by Passaretti et al. in this issue of Clinical Infectious Diseases, in which investigators found that HPV-decontamination of MDRO patient rooms was associated with a 45% reduction in environmental contamination and 80% reduction in acquisition of VRE among patients with a prior MDRO-colonized room occupant, fits in the middle of this proposed hierarchy (i.e. level III of Figure).5 The focus on possible transmission from a prior room occupant follows from HPV-decontamination being practical only for terminal and not daily room cleaning and disinfection. In another recent study using performance-improved standard cleaning and disinfection methods, MRSA acquisition was reduced by 62% and VRE by 22% in patients with a prior room occupant colonized by the respective MDRO.6
In previous studies 19% of all MRSA, 18–38% of VRE, and 11% of C. difficile acquisitions or infections occurred in patients where the prior room occupants were known to be colonized or infected by the respective MDRO.7–9 By extrapolating the unadjusted data in table 2 of the report by Passaretti et al. it appears that, had HPV-decontamination not been used, approximately 25% of all VRE, 23% of MRSA, 29% of all MDR-GNB, and 28% C. difficile acquisitions or infections would have occurred in patients with a prior room occupant colonized or infected with one or more, but not necessarily respective, MDROs.5 However, only a fraction of these MDRO acquisitions are the result of primary environmental transmission. Huang et al. estimated that the excess risk for acquisition from a colonized or infected prior room occupant represented only 5.1% of the overall risk for MRSA acquisition and 6.8% of the risk for VRE.7 Other data show that, despite being highly efficacious in reducing bioburden, recontamination occurs quickly following HPV room decontamination.1 If NTD or other new technologies feasible only for terminal decontamination are going to climb to higher evidentiary levels and demonstrate impact on overall MDRO transmission (i.e. level IV of Figure) they will probably need to be coupled with more reliable methods of daily cleaning and disinfection.
Intervention and control wards in the study by Pasaretti et al. were located all in a single hospital and it is unclear whether the assignment of the intervention to certain units was random.5 Moreover, there was mixing across time periods, with transmission opportunities on all the wards during the pre-intervention phase, along with the opportunities on the control wards during the intervention phase, serving as collective controls to the opportunities on the HPV wards during the intervention phase. While this design was adopted to increase the size of the study, secular trends in rates across all units could result in a significant association with the intervention introduced late in the overall study period. However, the modeling performed by these investigators controlled for rates and time and still found a significant association with HPV-decontamination.
Although there was a mortality risk index included in their model, there was no direct measure of factors such as invasive devices, antibiotic exposures, presence of wounds, or diarrhea. In addition, there was no reported measure of institutional factors such as compliance with hand hygiene or isolation precautions. It is possible, though not probable, that because HPV decontamination involves use of sophisticated equipment and processes, there was greater awareness by healthcare personnel of the importance of infection control, leading to higher levels of compliance with hand hygiene and isolation precautions.
Finally, there was no report on the adequacy of standard cleaning and disinfection and the method used to assess adequacy, direct observation by study personnel, is severely limited by the Hawthorne effect. This is probably one of the greatest limitations of this study; while it demonstrates superiority of HPV-decontamination in preventing a minor subset of transmission events, the reader is left with the question “compared to what?” Future studies should include measures of the adequacy of cleaning and disinfection in a control based on more standardized, reliable methods.10
Other data helpful in understanding these findings would have been full characterization (i.e. strain type) of both patient and environmental MDRO isolates including those from prior and subsequent room occupants when transmission was assumed to have occurred. Importantly, discordant MDRO transmission events (i.e. prior occupant with one MDRO, subsequent occupant found with another MDRO species) were included in this study to assess clinical effectiveness of HPV-room decontamination. The frequent finding of MDRO environmental contaminants that differed from the recent room occupant would appear to support this inclusion.
Surprisingly 13.9% of rooms were still contaminated after HPV decontamination (i.e. Table 5 in report by Passaretti et al.), despite the remarkable efficacy of HPV-decontamination.1, 2 Although the culture methods used may have been overly sensitive (i.e. broth amplifying as little as 1 colony forming unit), this may have been offset by a relatively small, and therefore relatively insensitive, surface area sampled (25cm2). Because environmental contamination has a probabilistic relationship to transmission, the sampling of larger surface areas using quantitative culture methods will allow better correlation of in-practice bioburden reductions to the interruption of transmission.11
Despite these limitations, this is an important study that further elucidates the role of environmental surfaces in transmission. Not only is this the first controlled study showing the potential advantage of a NTD intervention, its focus on ‘prior-to-subsequent room occupant’ transmission was well planned and implemented to achieve sufficient power. Though limited, the environmental culture results show directionality in support of transmission reductions. The investigators are to be commended for their seminal work that will serve as an important guide for future studies.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Hardy KJ, Gossain S, Henderson N, et al. Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour. J Hosp Infect. 2007 Aug;66(4):360–368. doi: 10.1016/j.jhin.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Boyce JM, Havill NL, Otter JA, et al. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol. 2008 Aug;29(8):723–729. doi: 10.1086/589906. [DOI] [PubMed] [Google Scholar]
- 3.Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis. 2010;10:197. doi: 10.1186/1471-2334-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EPA. Antimicrobial Science Policies. 2012 May 9; http://www.epa.gov/oppad001/sciencepolicy.htm. Accessed September 9, 2012.
- 5.Passaretti CL, Otter JA, Reich NG, Myers J, Shepard J, Ross T, Carroll KC, Lipsett P, Perl TM. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug resistant organisms. Clin Infect Dis. 2012;N(N):N. doi: 10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- 6.Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med. 2011 Mar 28;171(6):491–494. doi: 10.1001/archinternmed.2011.64. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006 Oct 9;166(18):1945–1951. doi: 10.1001/archinte.166.18.1945. [DOI] [PubMed] [Google Scholar]
- 8.Martinez JA, Ruthazer R, Hansjosten K, Barefoot L, Snydman DR. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch Intern Med. 2003 Sep 8;163(16):1905–1912. doi: 10.1001/archinte.163.16.1905. [DOI] [PubMed] [Google Scholar]
- 9.Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011 Mar;32(3):201–206. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- 10.Guh A, Carling P. Options for Evaluating Environmental Cleaning. 2010 Dec 6; http://www.cdc.gov/HAI/toolkits/Evaluating-Environmental-Cleaning.html. Accessed September 5, 2012.
- 11.Carlsen TM, MacQueen DH, Krauter PW. Sampling Requirements for Chemical and Biological Agent Decontamination Efficacy Verification. U.S. Department of Energy Lawrence Livermore National Laboratory; 2001. [Google Scholar]


