Abstract
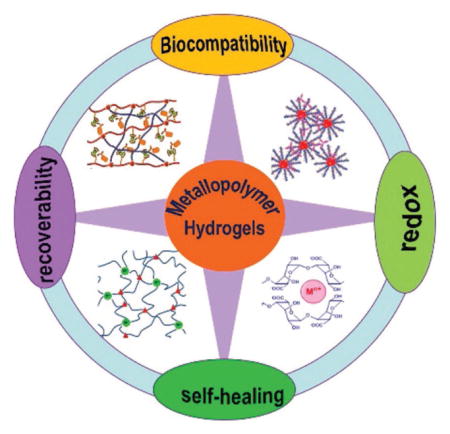
Metal-containing polymer hydrogels have attracted increasing interest in recent years due to their outstanding properties such as biocompatibility, recoverability, self-healing, and/or redox activity. In this short review, methods for the preparation of metal-containing polymer hydrogels are introduced and an overview of these hydrogels with various functionalities is given. It is hoped that this short update can stimulate innovative ideas to promote the research of metal-containing hydrogels in the communities.
Keywords: crosslinked, hydrogels, metal-containing polymers, molecular interactions
Graphical abstract
1. Introduction
Conceptually, a natural or synthetic hydrogel formed by chemical and/or physical crosslinking is a hydrophilic polymeric network with a high ratio (volume or weight) of water to dry gel. Owing to many of outstanding properties such as biocompatibility, tunable mechanical property, and stimuli responsiveness, polymer hydrogels are extensively used in tissue engineering, protein separation, drug delivery, sewage treatment, artificial extracellular materials, etc.[1] Recently, considerable interest has arisen in the preparation and application of metal-containing polymers due to the incorporation of both organic and inorganic components.[2] They combine processing advantages of soft polymers and unique functions provided by hard metal centers. Basically, organic polymers provide flexibility, solubility, and processability, while incorporation of metal elements endows multiple functionalities including redox activity, catalytic, magnetic, responsive, and electronic properties.[3] Therefore, metal-containing polymers with unique and versatile properties should be one of the ideal materials for manufacturing functional hydrogels.
In general, according to the nature of interactions involved in their formation, metal-containing polymer hydrogels can be classified as crosslinked hydrogels via metal coordination,[4] covalently crosslinked hydrogels,[5] and hybrid crosslinked hydrogels.[6] Currently, the most extensively studied metal-containing hydrogels are crosslinked hydrogels via coordination that are held together by metal cations. However, the covalently crosslinked metal-containing hydrogels, especially metallocene-containing polymer hydrogels, are far less studied, partly because of the cytotoxicity of metals and the difficulty in incorporating metal centers into macromolecular networks. Up to now, few review articles have comprehensively summarized metal-containing polymer hydrogels. An earlier review only introduced poly(ferrocenylsilane) (PFS) hydrogels as redox-responsive materials.[5] In this review, we mainly discuss the preparation and application of metal-containing hydrogels developed in recent years. The metal-containing supramolecular hydrogels will not be covered as a stand-alone section here, and readers can find them in relevant reviews.[4,7] Moreover, we will give perspectives on the future development of metal-containing hydrogels.
2. Crosslinked Hydrogels via Metal Coordination
Coordination interactions between metal ions and functional groups in polymer chains are frequently used as crosslinking junctions to fabricate metal-containing hydrogels. Coordination is usually a special chemical bond in which one atom typically supplies electrons, different from a traditional covalent bond in which each atom supplies electrons. Metal coordination resulting from Lewis acid–base interactions is stronger than most noncovalent interactions, but weaker than prototypical covalent bonding interactions (e.g., C–C, C–O, etc.). Thus, hydrogels, like classic Ca-alginate gels,[1f] prepared by metal coordination might possess properties of either physical or chemical gels in different situations. Based on coordination interactions, the formed metal-containing polymer hydrogels exhibited many excellent properties such as biocompatibility, recoverability, self-healing, and redox activity.[3d,8]
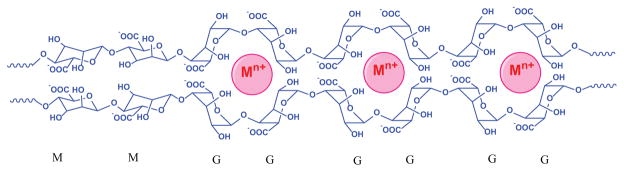
Several metal-coordination hydrogels made from polyvalent metal ions and polysaccharide (alginate) have been reported.[9] Anionic alginate solution can generate hydrogels by using metal cations (Fe3+, Ca2+, Sr2+, Ba2+) as crosslinking agents, which can facilitate coordination with carboxylate groups of alginate. It should be pointed out that the G (guluronic acid) blocks in various alginate chains establish crosslinks through metal coordination, leading to a metal-alginate hydrogel (Figure 1).
Figure 1.
Illustration of cross-linking of alginate by Mn+ metal cations. An alginate chain contains mannuronic acid (M unit) and guluronic acid (G unit). Reproduced with permission.[9e] Copyright 2012, Nature Publishing Group.
Machida-Sano et al. reported a biocompatible metal-coordination hydrogel prepared from ferric ion and alginate, which can be used for cell support.[9a] Ostrowski and co-workers showed that light-responsive hydrogels can be prepared by the coordination of iron(III) and ionic polysaccharides. The physical state of these hydrogels can be changed under light stimuli due to the redox activity of polyvalent metal ions. This kind of light-responsive hydrogels has potential in the area of biomedicine and tissue engineering.[9b,c] Similar iron(III) cross-linked alginate hydrogels were also reported by Melman and co-workers.[9d] Recently, Zhang and co-workers reported metal ion cross-linked alginate hydrogels that can be served as shapeable and versatile templates for insitu metal–organic framework (MOF) growth, and the shapes of hydrogels were easily inherited by their corresponding MOF–alginate composites.[10]
A polymer hydrogel was fabricated by the complexation of ferric ion (Fe3+) and poly(acrylamide-co-acrylic acid) (P(AAm-co-AAc)).[11] Metal coordination between Fe3+ and carboxyl groups of P(AAm-co-AAc) generated physical crosslinks in the gel matrix, and this physical hydrogel possesses high stiffness and toughness, fatigue resistance, and stimuli-triggered healing along with shape memory and processing abilities.
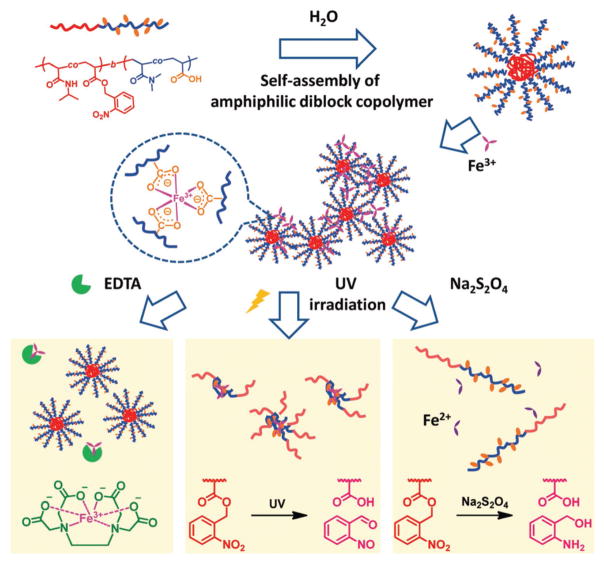
New iron-containing polymer hydrogels were prepared by means of coordination interactions between Fe3+ and carboxylates in an amphiphilic diblock copolymer poly[(N-isopropylacryl-amide-co-2-nitrobenzyl acrylate)-block-(N, N-dimethylacrylamide-co-acrylic acid)] (Figure 2).[12] The obtained hydrogels exhibited sol-gel transition behaviors by UV irradiation, multidentate ligand (EDTA), and redox agent (Na2S2O4).
Figure 2.
Gelation by micelles and complexation of Fe3+ and polymer chains, and sol-gel transition triggered by EDTA, UV irradiation, and Na2S2O4. Reproduced with permission.[12] Copyright 2016, Royal Society of Chemistry.
In addition, coordination involving other metal ions and anionic groups can also be utilized to initiate the gelation process. For instance, Andzelm and co-workers reported that hydrogels can be produced by adding divalent or trivalent cations (Ca2+, Zn2+, Cu2+, Al3+, and Fe3+) into a dispersion of cellulose nanofibrils.[13] They also exploited the ionic interactions of metal ions with carboxylate groups to initiate the gelation process. The hydrogel storage moduli were related to valency of the metal ions, their binding strength with carboxylate groups on the nanofibrils, and the choice of cations.
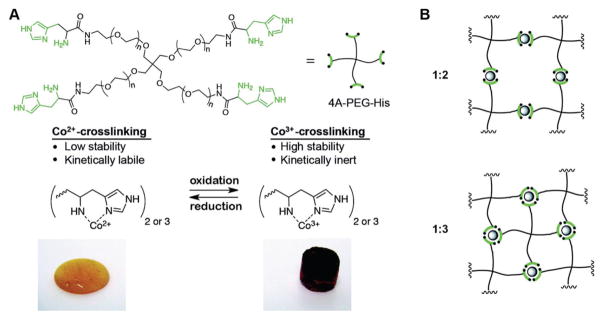
Wegner et al. reported a redox-responsive hydrogel made from 4-arm histidine-modified poly(ethylene glycol) (4A-PEG-His) with cobalt ions as a crosslinker in water (Figure 3).[14] With a simple oxidation step from Co2+ to Co3+, the physical state of the prepared hydrogels can be changed from self-healing viscoelastic liquids to stable elastic solids (Figure 3A). Moreover, different ratios of cobalt ions to histidine end-group (e.g., 1:2 and 1:3) can lead to different connectivity of polymer networks in the cobalt crosslinked hydrogels (Figure 3B). Using the same 4A-PEG-His, Messersmith and co-workers developed novel mussel-inspired metal coordination hydrogels with Zn2+, Cu2+, Co2+, or Ni2+ as a crosslinker.[15]
Figure 3.
A) Cross-linking of the 4A-PEG-His with Co2+ or Co3+ resulted in hydrogels with very different physical properties; B) different ratios of cobalt ions to histidine end-group ratios (1:2 and 1:3) led to different hydrogel connectivity. Reproduced with permission.[14] Copyright 2016, American Chemical Society.
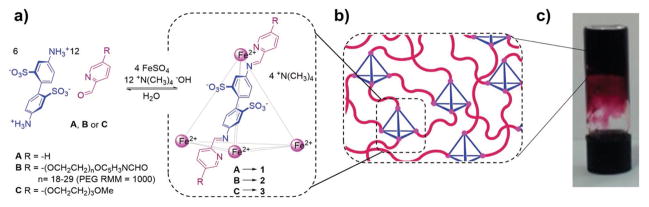
Based on metal coordination, Nitschke and co-workers prepared a new class of metal–organic cage-cross-linked polymeric hydrogels through the self-assembly of formylpyridine functionalized PEG in the presence of Fe2+ (Figure 4).[16] Two distinct internal phases in the hydrogel were formed by selective encapsulation of guest molecules within the cages. Depending on their aptitude for encapsulation within the cages, the two internal phases present in the gels can allow small molecules to be released at different rates and in response to competing guests.
Figure 4.
Metal–organic cage cross-linked hydrogel formation: a) synthesis of cages 1–3 using aldehydes A–C, and b) a schematic view of the structure of cage-cross-linked gel. c) A photograph showing an inverted vial containing a 15 wt % hydrogel formed by cage 2. Reproduced with permission.[16] Copyright 2015, American Chemical Society.
3. Covalently Crosslinked Hydrogels
Over the past six decades, a great number of metal-containing polymers have been synthesized either by integrating metal centers into the main chain or by attaching them as pendant groups at the side chain, and the linkages that couple the metal centers can be strongly covalent bonded or reversibly and dynamically coordinated.[17] These polymers can be directly utilized to prepare metal-containing hydrogels taking advantage of prototypical covalent bond as crosslinking points (metals do not participate the crosslinking).
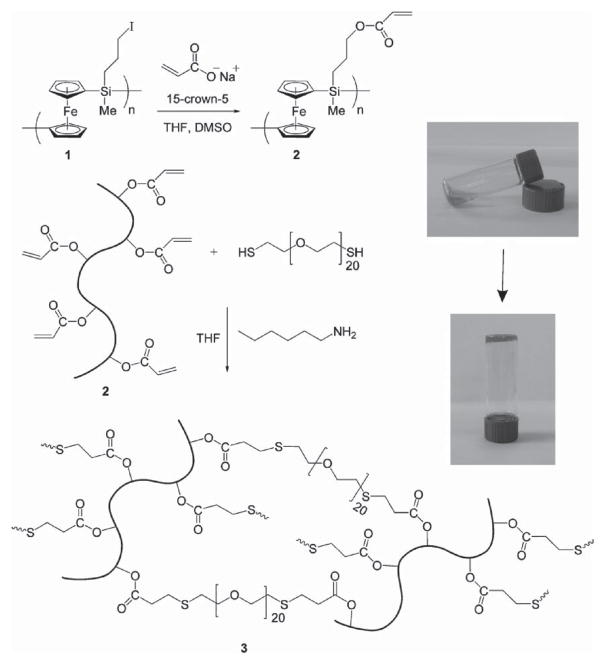
As a star member of the metallocene family, ferrocene can be integrated into the main chain to fabricate metal-containing polymer hydrogels.[5] The first ferrocene-containing polymer hydrogel was reported by Hempenius et al..[18] Two different kinds of poly(ferrocenylsilane) polyion hydrogels, containing positively and negatively charged side groups, respectively, were manufactured by covalent cross-linking methods. The hydrogels owed a high water swelling ratio and displayed reversible redox responsive properties. In 2010, Hempenius and co-workers developed a redox responsive metal-containing hydrogel by a thiol–Michael addition click reaction,[19] using poly(ferrocenylsilane) as a redox-responsive component and poly(ethylene glycol) dithiol as a crosslinker (Figure 5). Recently, they further developed a new class of redox-active hydrogels of anionic poly(ferrocenylsilane) polyelectrolytes by a copper-free azide-alkyne Huisgen cycloaddition reaction. These hydrogels were used for the in situ formation of rather well-defined palladium nanoparticles.[20]
Figure 5.
Synthesis of PFS-PEG-based hydrogel and photographs of the gel formation. Reproduced with permission.[19] Copyright 2010, Wiley-VCH.
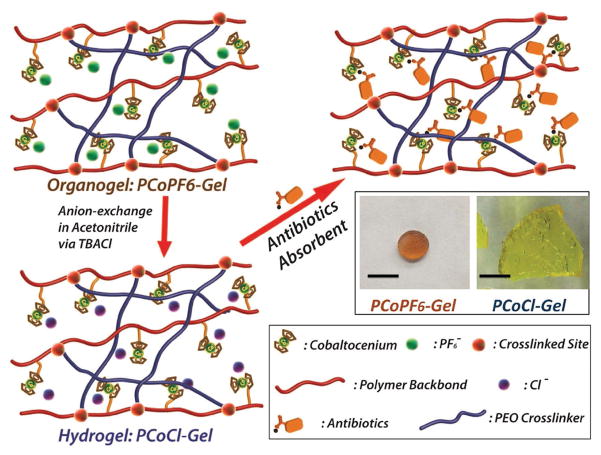
Cationic cobaltocenium-containing polymers have been recently gaining a lot of attention.[21] Taking advantage of antimicrobial activities and hydrophilicity of cationic cobaltocenium,[8b,15,22] Tang and co-workers recently constructed novel cationic cobaltocenium-containing polymer hydrogels (PCoCl-Gel) (Figure 6). They introduced the cationic cobaltocenium moiety into polymeric networks by free radical polymerization of cobaltocenium methacrylate with a PEG functionalized crosslinker.[23] These hydrogels easily absorbed β-lactam antibiotics because of the ability of cobaltocenium to bind anionic antibiotics, which can be applied to treat antibiotic-contaminated water. Moreover, these cationic hydrogels can inhibit the growth of different bacteria, including drug-resistant strains.
Figure 6.
Illustration of anion-paired (Cl−, purple spheres; PF6−, green spheres; and antibiotics) cobaltocenium-containing organogels and hydrogels. Inserted: optical images of two representative gels (Scale Bar: 1 cm). Reproduced with permission.[23] Copyright 2015, Springer Nature.
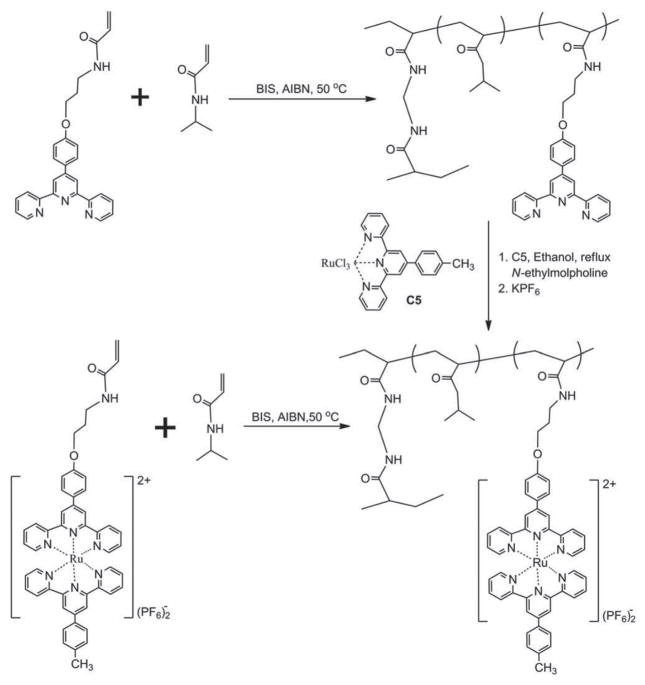
Different from metallocene-containing hydrogels, Zhou et al. reported Ru(II)(Tpy)2-functionalized polymer hydrogels with metal centers linked by coordination interactions.[24] Two synthetic routes were developed to prepare Ru(II)(Tpy)2-functionalized polymer hydrogels. The first one is the direct gelation by copolymerization of acrylamide (as a hydrophilic component) and Ru(II)(Tpy)2 (as the functional group). The second one is that the terpyridine-containing hydrogel was first prepared and then post-functionalized by coordination between Ru(III)(Tpy)Cl3 and terpyridine groups (Figure 7). According to the latter method, Sahiner et al. prepared metal-containing poly(1-vinyl imidazole) hydrogels as a reaction container for catalysis of various organic reactions.[25] Electrostatic interactions between metal complex cations and anionic functional groups of polymer chains can also be used to create metal-containing hydrogels.[26] A typical example is a metal-containing hydrogel prepared by incorporation of positive [Ir(2- phenylpyrine)2 (4,4′-dimethyl-2,2′-bipyridine]Cl into negative poly(2-acrylamido-2-methylpropane sulfonic acid sodium) hydrogel via electrostatic interaction, which exhibited stable phosphorescence (Figure 8).[27]
Figure 7.
A synthetic route for Ru (II) (Tpy)2 -functionalized hydrogels. Reproduced with permission.[24] Copyright 2015, Wiley-VCH.
Figure 8.
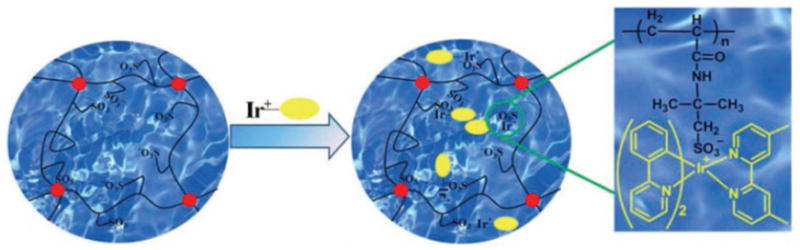
A schematic diagram of electrostatic interaction between the PNaAMPS hydrogel and [Ir(ppy)2(dmbpy)]Cl (red circle: crosslinking point, blank line: polymer network, yellow ellipse: ([Ir(ppy)2(dmbpy)]Cl). Reproduced with permission.[27] Copyright 2012, Wiley-VCH.
4. Hybrid Crosslinked Hydrogels
From a practical point of view in many situations, metal-containing polymer hydrogels should have good mechanical properties. Thus, a combination of ionic interactions and other interactions such as covalent and coordination bonds as crosslinking means is preferred to prepare metal-containing hybrid crosslinked hydrogels with enhanced performance. An example of a metal-containing hydrogel formed by ionic and covalent interactions was given by Xie and co-workers.[28] An iron-containing hydrogel was prepared by one-pot free radical polymerization of acrylic acid (AA) with N, N′-methylenebisacrylamide as the covalent crosslinker and Fe(NO3)3 as the ionic crosslinker. Many reversible crosslinks are formed as a result of coordination interactions between Fe3+ ions and carboxyl groups of PAA within the sparse, covalently crosslinked network (Figure 9a). It is the unique network structure that makes the PAA hydrogel to have high toughness and stretchability (Figure 9b).
Figure 9.

a) Iron-containing PAA hydrogels by electrostatic and covalent interactions; and b) photographs of PAA hydrogels with high stretchability. Reproduced with permission.[28] Copyright 2016, Royal Society of Chemistry.
Meyer and co-workers reported a dually crosslinked iron-containing electroplastic elastomer hydrogels prepared with poly(ethylene glycol) diacrylate as a chemical crosslinker and Fe3+ as a physical crosslinker.[29] These hydrogels can be reversibly switched between hard and soft states while maintaining a 3D shape by sequential switch of oxidative and reductive potentials. Later, the same group reported another dually cross-linked copper-containing electroplastic elastomer hydrogels, which undergo changes in mechanical properties in response to both chemical and electrochemical stimuli.[30]
Recently, Wang and co-workers reported a dual physically crosslinked (DPC) hydrogel by mixing poly(acrylamide-co-acrylic acid), clay nanosheets, and iron (III) chloride hexahydrate.[31] The primary crosslinking junctions were introduced by coordination interactions between Fe3+ and carboxylate groups in P(AM-co-AA) polymer chains. Then, the clay nanosheets were induced to form the secondary crosslinking points through the interaction of hydrogen bonds with P(AM-co-AA) chains. The optimal DPC hydrogels possessed superior mechanical properties such as high tensile strength (≈3.5 MPa), large elongation (≈21 times), remarkable toughness (≈49 MJ m−3), and good self-recoverability.
Gohy and co-workers first synthesized a poly(triethyleneglycol methylether methacrylate)-b-poly-styrene block copolymer (PTEGMA-b-PS) that consisted of a hydrophobic PS block and a soluble PTEGMA block containing terpyridine repeating units. Then, a Ni-containing hybrid crosslinked PTEGMA-b-PS hydrogel was obtained through the hydrophobic interaction of PS blocks and the coordination interactions between the terpyridine groups of PTEGMA and the Ni(II) ions. The moduli of PTEGMA-b-PS hydrogel were much higher than those of PTEGMA homopolymer hydrogel, which has only the coordination interaction.[32]
To further improve the mechanical strength of hydrogels, double network (DN) or interpenetrating network (IPN) polymer structures have also been employed for fabricating metal-containing hydrogels in recent years.[33] Using the DN concept, Suo and coworkers synthesized extremely stretch-able and tough hydrogels by mixing two types of crosslinked polymers:[9e] one polymer network is calcium crosslinked alginate; the other is covalently crosslinked polyacrylamide with N, N-methylenebisacrylamide as the crosslinker. The calcium-containing hydrogels, with a water content of 90%, can be stretched beyond 20 times of their initial length, and have fracture energies of 9000 J m−2.
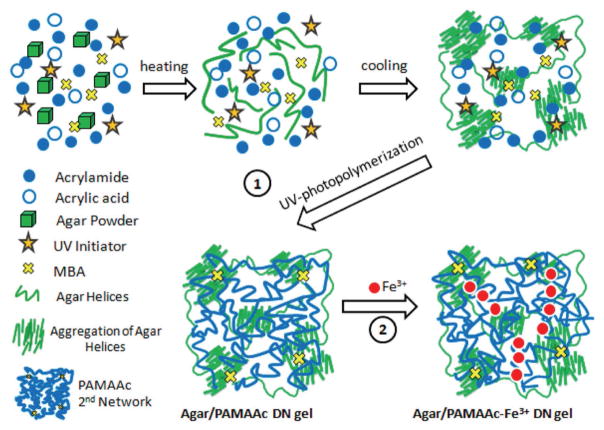
Zheng and co-workers first used an agar gel as the first physical network and a copolymer of acrylamide and acrylic acid (PAMAAc) gel as the second chemical–physical network to form an Agar/PAMAAc DN hydrogel (Figure 10).[34] Then, a new type of Agar/PAMAAc-Fe3+ DN hydrogels was obtained by introducing Fe3+ ions into the second network to form strong coordination interactions. These DN hydrogels possess high mechanical properties, fast self-recovery (50% toughness recovery after 1 min of resting), and good fatigue resistance.
Figure 10.
Synthesis of Agar/PAMAAc-Fe3+ DN gels. Reproduced with permission.[34] Copyright 2016, American Chemical Society.
Waite and co-workers prepared metal-containing IPN hydrogels with rate-dependent stiffness and recovery by incorporating the coordination-based hydrogels into poly(hydroxyethyl acrylamide)-based covalent scaffolds.[35] The coordination-based hydrogels were fabricated by the complexation of trivalent metal cations (In3+, Fe3+, and Al3+) and 4-arm PEG star polymers modified with 3-hydroxy-4-pyridinone.
5. Conclusions and Perspective
In summary, different molecular interactions (metal coordination, prototypical covalent bonding, and a hybrid of both) are used to introduce crosslinking for the formation of metal-containing hydrogels. The presence of metal centers as an integral part of the matrix of a polymer hydrogel can provide a wide range of properties pertained to these soft materials. Many recent examples of preparation and applications of metal-containing polymer hydro-gels may underscore significant advances in the future.
In general, the ionic crosslinks, especially metal coordination, could result in the formation of dynamic and responsive metal-containing polymer hydrogels. It is critical to not only achieve an optimization on the strength of these ionic interactions, but also maintain dynamic and responsive properties. Although the covalent crosslinks can be used to obtain metal-containing polymer hydro-gels, the main challenges are to develop novel monomers containing metal centers that provide more opportunities for fundamental physiochemical properties as well as real applications. Nevertheless, the hybrid crosslinks with coordination and covalent interactions are probably more preferred to fabricate metal-containing hydrogels, such as DN and IPN gels, with high strength and responsive properties. However, shortcomings of these hydrogels lie in their complexity in terms of preparation processes compared to single network metal-containing hydrogels with either a metal-coordination or prototypical covalent crosslink. We hope that more creative ideas will be stimulated to promote research on metal-containing hydrogels with various functionalities.
Acknowledgments
The support from National Institutes of Health (R01AI120987) is acknowledged.
Biographies
 Hui Li is an Associate Professor at Department of Polymer Materials and Engineering at the University of Jinan. He received Ph.D. from Tianjin University under the supervision of Professor Xiaoyan Yuan. He joined Prof. Chuanbing Tang’s group at the University of South Carolina as a research scholar from 2015 to 2016, working on the synthesis of metal-lopolymer hydrogels. His research interests focus on fluorosilicone polymer synthesis, metal-containing polymers, and polymers at interfaces.
Hui Li is an Associate Professor at Department of Polymer Materials and Engineering at the University of Jinan. He received Ph.D. from Tianjin University under the supervision of Professor Xiaoyan Yuan. He joined Prof. Chuanbing Tang’s group at the University of South Carolina as a research scholar from 2015 to 2016, working on the synthesis of metal-lopolymer hydrogels. His research interests focus on fluorosilicone polymer synthesis, metal-containing polymers, and polymers at interfaces.
 Peng Yang received B.S. from Shandong University and Ph.D. from Fudan University with Prof. Changchun Wang. His doctoral research focused on the preparation of multifunctional hollow microspheres and their applications in drug delivery and medical imaging. Since August 2015, he joined Prof. Chuanbing Tang’s group at University of South Carolina as a postdoctoral scholar, working on the synthesis of metallopolymers for antimicrobial application.
Peng Yang received B.S. from Shandong University and Ph.D. from Fudan University with Prof. Changchun Wang. His doctoral research focused on the preparation of multifunctional hollow microspheres and their applications in drug delivery and medical imaging. Since August 2015, he joined Prof. Chuanbing Tang’s group at University of South Carolina as a postdoctoral scholar, working on the synthesis of metallopolymers for antimicrobial application.
 Chuanbing Tang received B.S. from Nanjing University and Ph.D. from Carnegie Mellon University with Prof. Krzyszt of Matyjaszewski and Prof. Tomasz Kowalewski. He was a postdoctoral scholar at the University of California Santa Barbara with Prof. Craig J. Hawker and Prof. Edward J. Kramer. On August 2009, he joined Department of Chemistry and Biochemistry at the University of South Carolina. Currently he is a College of Arts and Sciences Distinguished Professor. His research interests focus on organic polymer synthesis, sustainable polymers from biomass, metal-containing polymers, and polymers for biomedical applications and energy storage.
Chuanbing Tang received B.S. from Nanjing University and Ph.D. from Carnegie Mellon University with Prof. Krzyszt of Matyjaszewski and Prof. Tomasz Kowalewski. He was a postdoctoral scholar at the University of California Santa Barbara with Prof. Craig J. Hawker and Prof. Edward J. Kramer. On August 2009, he joined Department of Chemistry and Biochemistry at the University of South Carolina. Currently he is a College of Arts and Sciences Distinguished Professor. His research interests focus on organic polymer synthesis, sustainable polymers from biomass, metal-containing polymers, and polymers for biomedical applications and energy storage.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Contributor Information
Dr. Hui Li, School of Chemistry and Chemical Engineering and Shandong Key Laboratory of Fluorine Chemistry and Chemical Engineering Materials, University of Jinan, Jinan 250022, China. Department of Chemistry and Biochemistry, University of South Carolina, Columbia, SC 29208, USA
Dr. Peng Yang, Department of Chemistry and Biochemistry, University of South Carolina, Columbia, SC 29208, USA
Parasmani Pageni, Department of Chemistry and Biochemistry, University of South Carolina, Columbia, SC 29208, USA.
Prof. Chuanbing Tang, Department of Chemistry and Biochemistry, University of South Carolina, Columbia, SC 29208, USA
References
- 1.a) Osada Y, Okuzaki H, Hori H. Nature. 1992;355:242. [Google Scholar]; b) Gong JP. Soft Matter. 2010;6:2583. [Google Scholar]; c) Ban K, Park HJ, Kim S, Andukuri A, Cho KW, Hwang JW, Cha HJ, Kim SY, Kim WS, Jun HW. ACS Nano. 2014;8:10815. doi: 10.1021/nn504617g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Banerjee I, Pangule RC, Kane RS. Adv Mater. 2011;23:690. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]; e) Wang XQ, Yang S, Wang CF, Chen L, Chen S. Macromol Rapid Commun. 2016;37:759. doi: 10.1002/marc.201500748. [DOI] [PubMed] [Google Scholar]; f) Yuk H, Zhang T, Lin S, Parada GA, Zhao X. Nat Mater. 2016;15:190. doi: 10.1038/nmat4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Abd-El-Aziz AS. Macromol Rapid Commun. 2002;23:995. [Google Scholar]; b) Astruc D, Ornelas C, Ruiz J. Acc Chem Res. 2008;41:841. doi: 10.1021/ar8000074. [DOI] [PubMed] [Google Scholar]; c) Hadadpour M, Gwyther J, Manners I, Ragogna PJ. Chem Mater. 2015;27:3430. [Google Scholar]; d) Hadadpour M, Liu Y, Chadha P, Ragogna PJ. Macromolecules. 2014;47:6207. [Google Scholar]; e) Novoa S, Paquette JA, Barbon SM, Maar RR, Gilroy JB. J Mater Chem C. 2016;4:3987. [Google Scholar]; f) Wang X, McHale R. Macromol Rapid Commun. 2010;31:331. doi: 10.1002/marc.200900558. [DOI] [PubMed] [Google Scholar]; g) Yang SK, Ambade AV, Weck M. Chem Soc Rev. 2011;40:129. doi: 10.1039/c0cs00073f. [DOI] [PubMed] [Google Scholar]
- 3.a) Bellas V, Rehahn M. Angew Chem, Int Ed. 2007;46:5082. doi: 10.1002/anie.200604420. [DOI] [PubMed] [Google Scholar]; b) Whittell GR, Manners I. Adv Mater. 2007;19:3439. [Google Scholar]; c) Yan Y, Pageni P, Kabir MP, Tang C. Synlett. 2016;27:984. [Google Scholar]; d) Yan Y, Zhang J, Ren L, Tang C. Chem Soc Rev. 2016;45:5232. doi: 10.1039/c6cs00026f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piepenbrock MOM, Lloyd GO, Clarke N, Steed JW. Chem Rev. 2009;110:1960. doi: 10.1021/cr9003067. [DOI] [PubMed] [Google Scholar]
- 5.Hempenius MA, Cirmi C, Savio FL, Song J, Vancso GJ. Macromol Rapid Commun. 2010;31:772. doi: 10.1002/marc.200900908. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Chen H, Zhu L, Zheng J. J Mater Chem B. 2015;3:3654. doi: 10.1039/c5tb00123d. [DOI] [PubMed] [Google Scholar]
- 7.Voorhaar L, Hoogenboom R. Chem Soc Rev. 2016;45:4013. doi: 10.1039/c6cs00130k. [DOI] [PubMed] [Google Scholar]
- 8.a) Yang Y, Ding X, Urban MW. Prog Polym Sci. 2015;49:34. [Google Scholar]; b) Zhang J, Yan Y, Chance MW, Chen J, Hayat J, Ma S, Tang C. Angew Chem, Int Ed. 2013;52:13387. doi: 10.1002/anie.201306432. [DOI] [PubMed] [Google Scholar]
- 9.a) Machida-Sano I, Ogawa S, Ueda H, Kimura Y, Satoh N, Namiki H. Int J Biomater. 2012;2012:820513. doi: 10.1155/2012/820513. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Giammanco GE, Ostrowski AD. Chem Mater. 2015;27:4922. [Google Scholar]; c) Giammanco GE, Sosnofsky CT, Ostrowski AD. ACS Appl Mater Interfaces. 2015;7:3068. doi: 10.1021/am506772x. [DOI] [PubMed] [Google Scholar]; d) Narayanan RP, Melman G, Letourneau NJ, Mendelson NL, Melman A. Biomacromolecules. 2012;13:2465. doi: 10.1021/bm300707a. [DOI] [PubMed] [Google Scholar]; e) Sun JY, Zhao X, Illeperuma WR, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z. Nature. 2012;489:133. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, Zhang Q, Zhu S. ACS Appl Mater Interfaces. 2016;8:17395. doi: 10.1021/acsami.6b04505. [DOI] [PubMed] [Google Scholar]
- 11.Zheng SY, Ding H, Qian J, Yin J, Wu ZL, Song Y, Zheng Q. Macromolecules. 2016;49:9637. [Google Scholar]
- 12.Tao Z, Peng K, Fan Y, Liu Y, Yang H. Polym Chem. 2016;7:1405. [Google Scholar]
- 13.Dong H, Snyder JF, Williams KS, Andzelm JW. Biomacromolecules. 2013;14:3338. doi: 10.1021/bm400993f. [DOI] [PubMed] [Google Scholar]
- 14.Wegner SV, Schenk FC, Witzel S, Bialas F, Spatz JP. Macromolecules. 2016;49:4229. [Google Scholar]
- 15.Fullenkamp DE, He L, Barrett DG, Burghardt WR, Messersmith PB. Macromolecules. 2013;46:1167. doi: 10.1021/ma301791n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster JA, Parker RM, Belenguer AM, Kishi N, Sutton S, Abell C, Nitschke JR. J Am Chem Soc. 2015;137:9722. doi: 10.1021/jacs.5b05507. [DOI] [PubMed] [Google Scholar]
- 17.Whittell GR, Hager MD, Schubert US, Manners I. Nat Mater. 2011;10:176. doi: 10.1038/nmat2966. [DOI] [PubMed] [Google Scholar]
- 18.Hempenius MA, Cirmi C, Song J, Vancso GJ. Macromolecules. 2009;42:2324. [Google Scholar]
- 19.Sui X, van Ingen L, Hempenius MA, Vancso GJ. Macromol Rapid Commun. 2010;31:2059. doi: 10.1002/marc.201000420. [DOI] [PubMed] [Google Scholar]
- 20.Zoetebier B, Hempenius M, Vancso G. Chem Commun. 2015;51:636. doi: 10.1039/c4cc06988a. [DOI] [PubMed] [Google Scholar]
- 21.a) Gu H, Ciganda R, Castel P, Ruiz J, Astruc D. Macromolecules. 2016;49:4763. [Google Scholar]; b) Hardy CG, Ren L, Zhang J, Tang C. Isr J Chem. 2012;52:230. [Google Scholar]; c) Hardy CG, Zhang J, Yan Y, Ren L, Tang C. Prog Polym Sci. 2014;39:1742. [Google Scholar]; d) Mayer UFJ, Gilroy JB, O’Hare D, Manners I. J Am Chem Soc. 2009;131:10382. doi: 10.1021/ja903513e. [DOI] [PubMed] [Google Scholar]; e) Ren L, Hardy CG, Tang C. J Am Chem Soc. 2010;132:8874. doi: 10.1021/ja1037726. [DOI] [PubMed] [Google Scholar]
- 22.a) Zhang J, Chen YP, Miller KP, Ganewatta MS, Bam M, Yan Y, Nagarkatti M, Decho AW, Tang C. J Am Chem Soc. 2014;136:4873. doi: 10.1021/ja5011338. [DOI] [PubMed] [Google Scholar]; b) Zhang J, Pellechia PJ, Hayat J, Hardy CG, Tang C. Macromolecules. 2013;46:1618. [Google Scholar]
- 23.Zhang J, Yan J, Pageni P, Yan Y, Wirth A, Chen YP, Qiao Y, Wang Q, Decho AW, Tang C. Sci Rep. 2015;5 doi: 10.1038/srep11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Yang Y, Xu G, Chen W, Zhang W, Wang Q, Zheng Z, Ding X. J Polym Sci, Part A: Polym Chem. 2015;53:2214. [Google Scholar]
- 25.Sahiner N, Butun S, Ozay O, Dibek B. J Colloid Interface Sci. 2012;373:122. doi: 10.1016/j.jcis.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 26.Sahiner N. Prog Polym Sci. 2013;38:1329. [Google Scholar]
- 27.Li Z, Wei Z, Xu F, Li YH, Lu TJ, Chen YM, Zhou GJ. Macromol Rapid Commun. 2012;33:1191. doi: 10.1002/marc.201200136. [DOI] [PubMed] [Google Scholar]
- 28.Zhong M, Liu YT, Liu XY, Shi FK, Zhang LQ, Zhu MF, Xie XM. Soft Matter. 2016;12:5420. doi: 10.1039/c6sm00242k. [DOI] [PubMed] [Google Scholar]
- 29.Calvo-Marzal P, Delaney MP, Auletta JT, Pan T, Perri NM, Weiland LM, Waldeck DH, Clark WW, Meyer TY. ACS Macro Lett. 2011;1:204. doi: 10.1021/mz2001548. [DOI] [PubMed] [Google Scholar]
- 30.Harris RD, Auletta JT, Motlagh SAM, Lawless MJ, Perri NM, Saxena S, Weiland LM, Waldeck DH, Clark WW, Meyer TY. ACS Macro Lett. 2013;2:1095. doi: 10.1021/mz4004997. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Du Z, Deng X, Wang T, Yang Z, Zhou W, Wang C. Macromolecules. 2016;49:5660. [Google Scholar]
- 32.Jochum FD, Brassinne J, Fustin CA, Gohy JF. Soft Matter. 2013;9:2314. [Google Scholar]
- 33.a) Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Adv Mater. 2003;15:1155. [Google Scholar]; b) Nakajima T, Furukawa H, Tanaka Y, Kurokawa T, Osada Y, Gong JP. Macromolecules. 2009;42:2184. [Google Scholar]; c) Nakajima T, Sato H, Zhao Y, Kawahara S, Kurokawa T, Sugahara K, Gong JP. Adv Funct Mater. 2012;22:4426. [Google Scholar]; d) Suekama TC, Hu J, Kurokawa T, Gong JP, Gehrke SH. ACS Macro Lett. 2013;2:137. doi: 10.1021/mz3006318. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Yan X, Zhu L, Chen H, Jiang B, Wei D, Huang L, Yang J, Liu B, Zheng J. Chem Mater. 2016;28:5710. [Google Scholar]
- 35.Menyo MS, Hawker CJ, Waite JH. ACS Macro Lett. 2015;4:1200. doi: 10.1021/acsmacrolett.5b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]